Direct Self-Assembly of Hierarchically Grown Rhodium Thin Films for Electrocatalytic Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Deposition Process
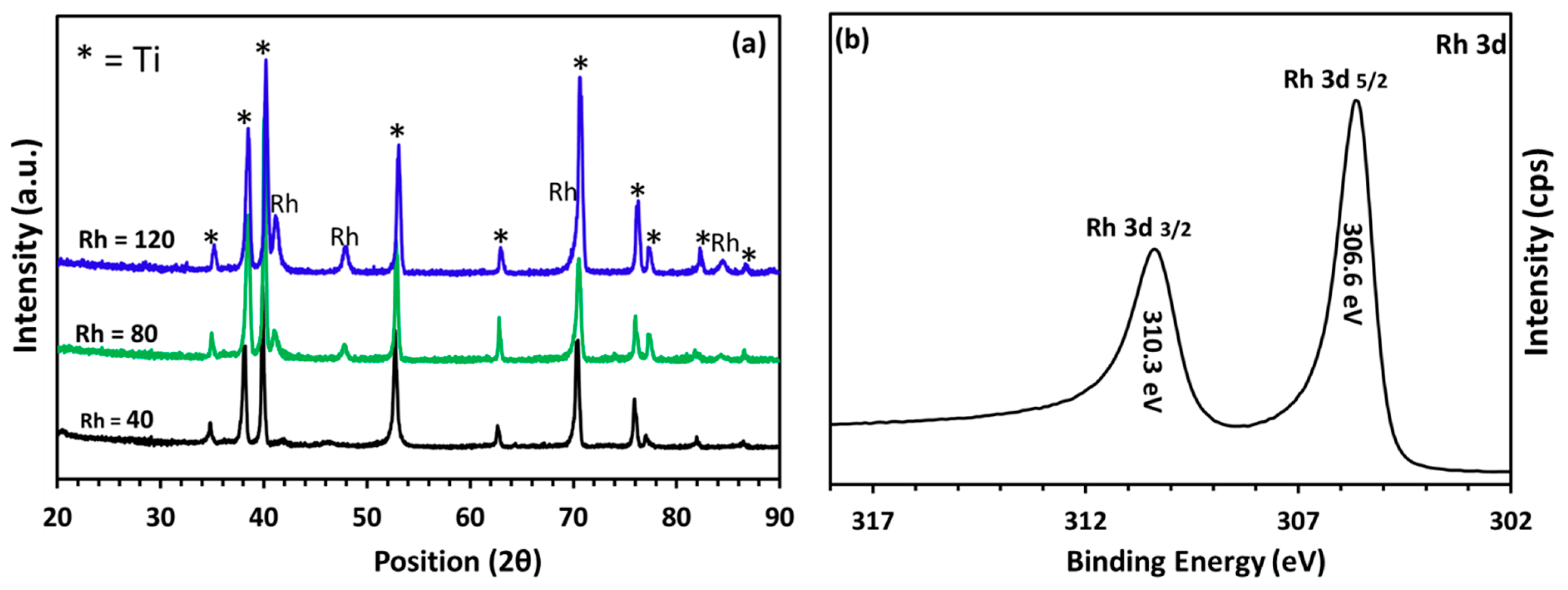
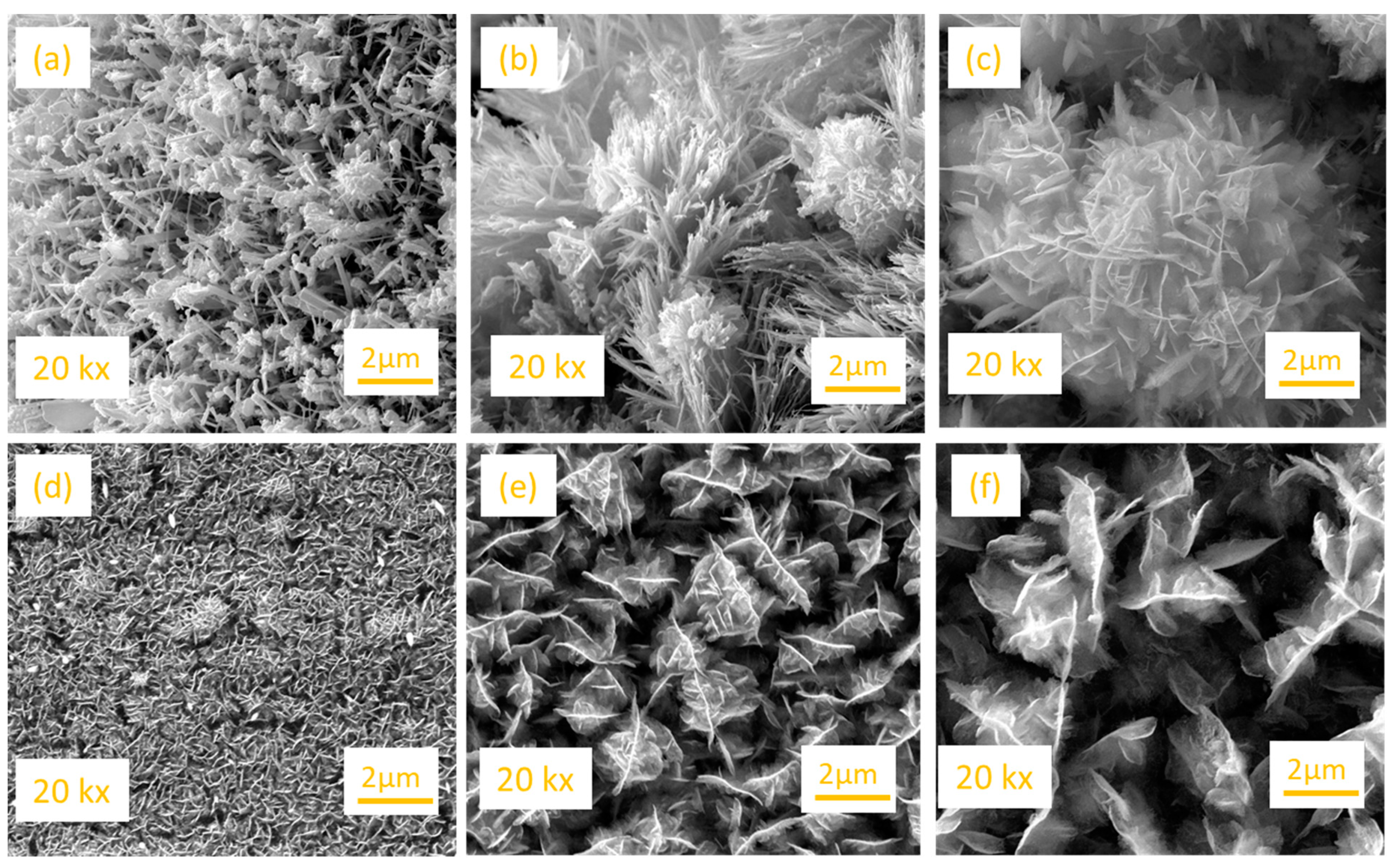
2.2. Structural Characterization
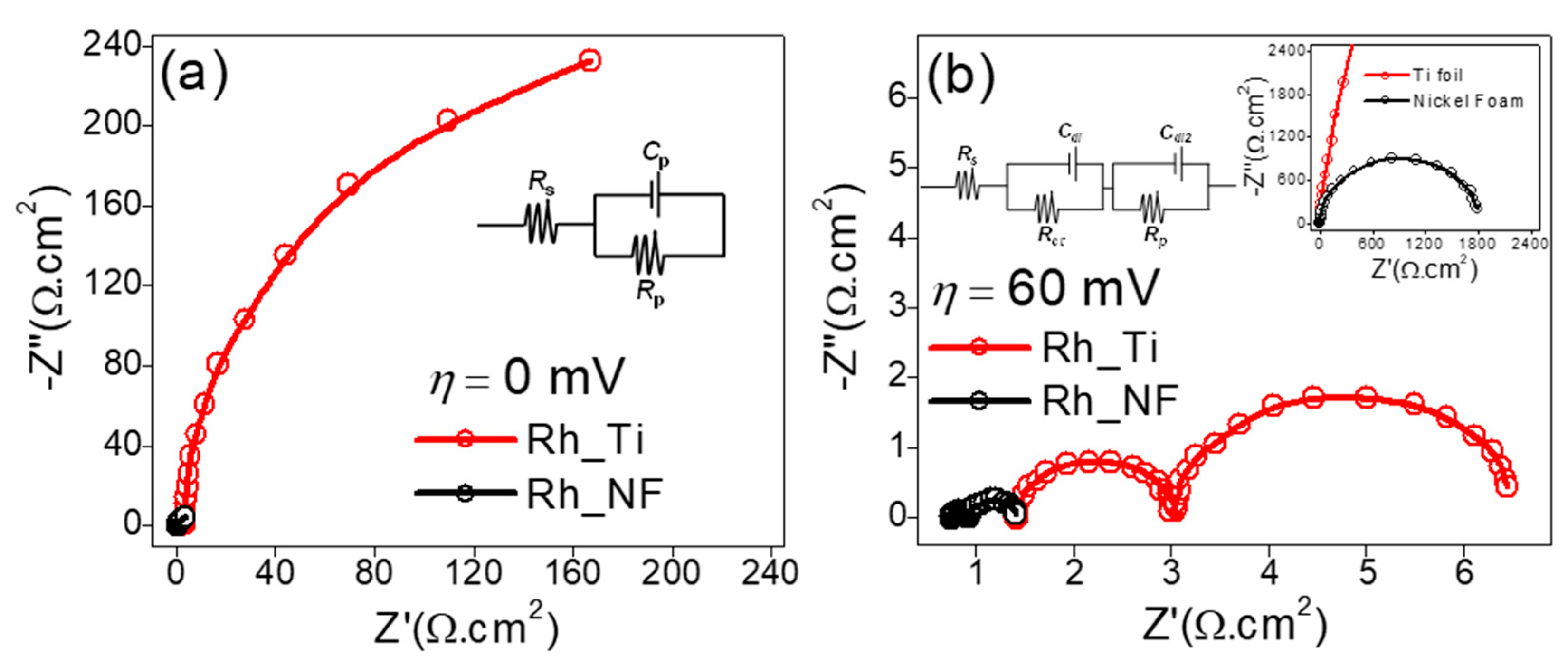
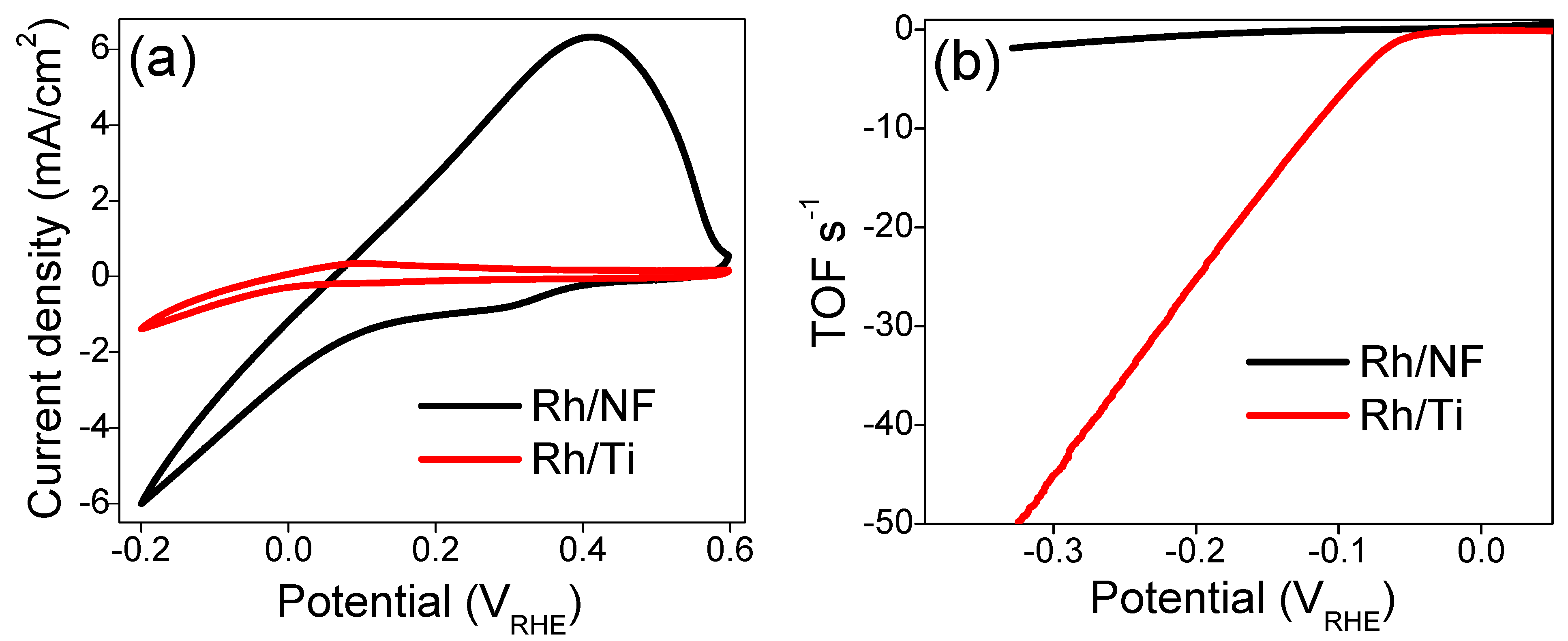
2.3. Evaluation of the Hydrogen Evolution Reaction (HER)
3. Experimental
3.1. Chemicals for Precursor Synthesis
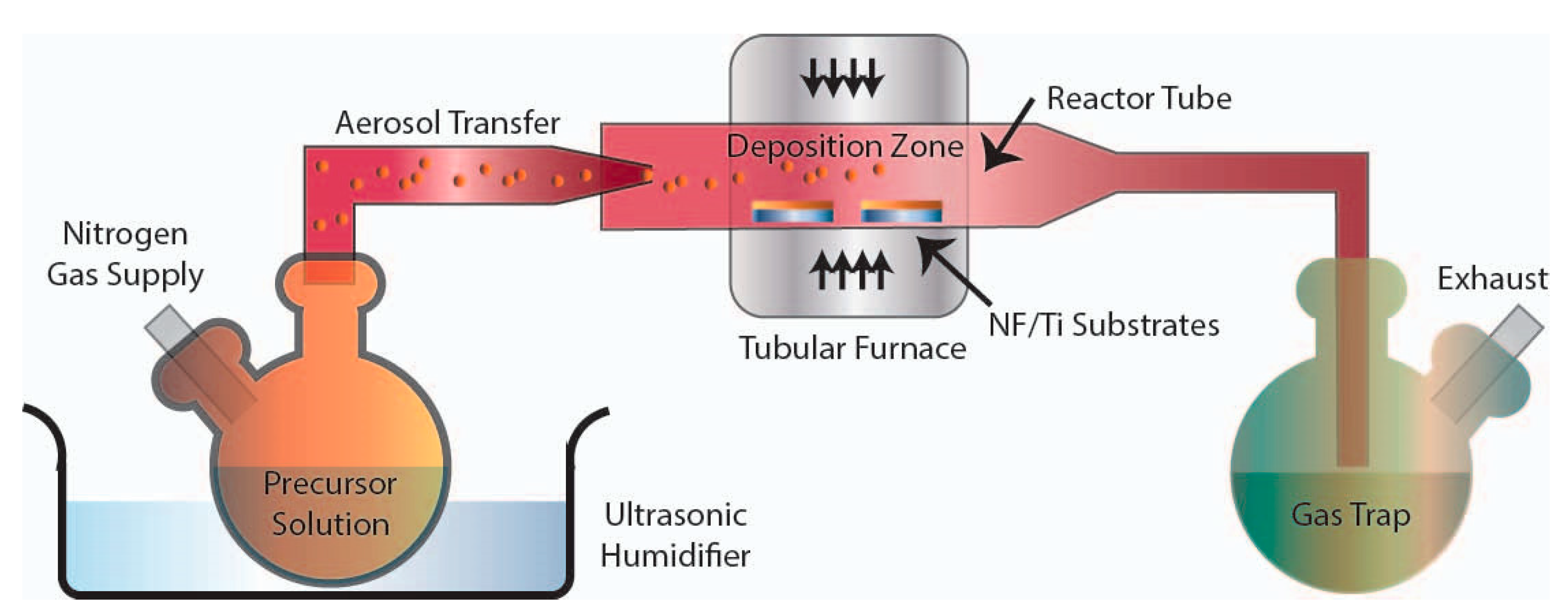
3.2. Aerosol-Assisted Chemical Vapor Deposition (AACVD) Experiments
3.3. Instrumentation
3.4. Electrocatalytic HER Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2019, 120, 851–918. [Google Scholar] [CrossRef]
- Dubouis, N.; Grimaud, A. The hydrogen evolution reaction: From material to interfacial descriptors. Chem. Sci. 2019, 10, 9165–9181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suliman, M.H.; Adam, A.; Li, L.; Tian, Z.; Siddiqui, M.N.; Yamani, Z.H.; Qamar, M. FeP/MoS2 Enriched with Dense Catalytic Sites and High Electrical Conductivity for the Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 17671–17681. [Google Scholar] [CrossRef]
- Suliman, M.H.; Adam, A.; Siddiqui, M.N.; Yamani, Z.H.; Qamar, M. Facile synthesis of ultrathin interconnected carbon nanosheets as a robust support for small and uniformly-dispersed iron phosphide for the hydrogen evolution reaction. Carbon 2019, 144, 764–771. [Google Scholar] [CrossRef]
- Khoobi, A.; Ghoreishi, S.M.; Behpour, M.; Shaterian, M.; Salavati-Niasari, M. Design and evaluation of a highly sensitive nanostructure-based surface modification of glassy carbon electrode for electrochemical studies of hydroxychloroquine in the presence of acetaminophen. Colloids Surf. B Biointerfaces 2014, 123, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Nazir, R.; Fageria, P.; Basu, M.; Pande, S. Decoration of carbon nitride surface with bimetallic nanoparticles (Ag/Pt, Ag/Pd, and Ag/Au) via galvanic exchange for hydrogen evolution reaction. J. Phys. Chem. C. 2017, 121, 19548–19558. [Google Scholar] [CrossRef]
- Yuan, M.; Xu, H.; Wang, Y.; Jin, L.; Wang, C.; Chen, C.; Wang, Y.; Shang, H.; Du, Y. Three-dimensional PdCuRu Alloy Porous Nanosheets as Efficient Electrocatalysts for Hydrogen Evolution Reaction in Varied Electrolytes. ChemElectroChem 2020, 7, 3135–3139. [Google Scholar] [CrossRef]
- Paul, R.; Zhu, L.; Chen, H.; Qu, J.; Dai, L. Recent Advances in Carbon-Based Metal-Free Electrocatalysts. Adv. Mater. 2019, 31, 1806403. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.-B. Recent advances in noble metal (Pt, Ru, and Ir)-based electrocatalysts for efficient hydrogen evolution reaction. ACS Omega 2019, 5, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ming, M.; Niu, S.; Zhang, Y.; Fan, G.; Hu, J.S. Scalable Solid-State Synthesis of Highly Dispersed Uncapped Metal (Rh, Ru, Ir) Nanoparticles for Efficient Hydrogen Evolution. Adv. Energy Mater. 2018, 8, 1801698. [Google Scholar] [CrossRef]
- Yin, H.-J.; Zhou, J.-H.; Zhang, Y.-W. Shaping well-defined noble-metal-based nanostructures for fabricating high-performance electrocatalysts: Advances and perspectives. Inorg. Chem. Front. 2019, 6, 2582–2618. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, B.; Zhang, N.; Sun, M.; Shao, Q.; Huang, X. Channel-Rich RuCu Nanosheets for pH-Universal Overall Water Splitting Electrocatalysis. Angew. Chem. Int. Ed. 2019, 58, 13983–13988. [Google Scholar] [CrossRef]
- Shao, F.-Q.; Feng, J.-J.; Yang, Z.-Z.; Chen, S.-S.; Yuan, J.; Wang, A.-J. Cytosine assisted aqueous synthesis of AgPt hollow alloyed nanostructures as highly active electrocatalyst for ethylene glycol oxidation and hydrogen evolution. Int. J. Hydrog. Energy 2017, 42, 24767–24775. [Google Scholar] [CrossRef]
- Levy, O.; Chepulskii, R.V.; Hart, G.L.; Curtarolo, S. The new face of rhodium alloys: Revealing ordered structures from first principles. J. Am. Chem. Soc. 2010, 132, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Teeriniemi, J.; Huisman, J.; Taskinen, P.; Laasonen, K. First-principles modelling of solid Ni–Rh (nickel–rhodium) alloys. J. Alloys Compd. 2015, 652, 371–378. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Hsu, C.-Y.; Chen, S.S.; Ahamad, T.; Alshehri, S.M.; Tsang, D.C.; Wu, K.C.-W. Selective hydrogenation of furfural to tetrahydrofurfuryl alcohol over a Rh-loaded carbon catalyst in aqueous solution under mild conditions. Sustain. Energ. Fuels 2020, 4, 293–301. [Google Scholar] [CrossRef]
- Bhuvanendran, N.; Balaji, S.; Zhang, W.; Xu, Q.; Pasupathi, S.; Su, H. Improved activity and stability of Ag–Rh network-like nanoalloy as support-free electrocatalyst for oxygen reduction in alkaline fuel cells. J. Renew. Sustain. Energy 2020, 12, 044301. [Google Scholar] [CrossRef]
- Arandiyan, H.; Wang, Y.; Scott, J.; Mesgari, S.; Dai, H.; Amal, R. In situ exsolution of bimetallic Rh–Ni nanoalloys: A highly efficient catalyst for CO2 methanation. ACS Appl. Mater. Interfaces 2018, 10, 16352–16357. [Google Scholar] [CrossRef]
- Grass, M.E.; Zhang, Y.; Butcher, D.R.; Park, J.Y.; Li, Y.; Bluhm, H.; Bratlie, K.M.; Zhang, T.; Somorjai, G.A. A reactive oxide overlayer on rhodium nanoparticles during CO oxidation and its size dependence studied by in situ ambient-pressure X-ray photoelectron spectroscopy. Angew. Chem. Int. Ed. 2008, 47, 8893–8896. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-J.; Chen, L.-X.; Ma, X.; Yuan, J.; Chen, J.-R.; Wang, A.-J.; Xu, Q.-Q. Bimetallic AuPt alloy nanodendrites/reduced graphene oxide: One-pot ionic liquid-assisted synthesis and excellent electrocatalysis towards hydrogen evolution and methanol oxidation reactions. Int. J. Hydrog. Energy 2017, 42, 1120–1129. [Google Scholar] [CrossRef]
- Yin, X.P.; Wang, H.J.; Tang, S.F.; Lu, X.L.; Shu, M.; Si, R.; Lu, T.B. Engineering the coordination environment of single-atom platinum anchored on graphdiyne for optimizing electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2018, 57, 9382–9386. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [PubMed]
- Sait, E.; Beelders, W.; Bradley, S.J.; Kroon, R.; Laufersky, G.; Andersson, M.; Nann, T. Platinum Terpyridine Metallopolymer Electrode as Cost-Effective Replacement for Bulk Platinum Catalysts in Oxygen Reduction Reaction and Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2017, 5, 10206–10214. [Google Scholar]
- Ji, J.; Zhang, Y.; Tang, L.; Liu, C.; Gao, X.; Sun, M.; Zheng, J.; Ling, M.; Liang, C.; Lin, Z. Platinum single-atom and cluster anchored on functionalized MWCNTs with ultrahigh mass efficiency for electrocatalytic hydrogen evolution. Nano Energy 2019, 63, 103849. [Google Scholar] [CrossRef]
- Han, X.; Wu, X.; Deng, Y.; Liu, J.; Lu, J.; Zhong, C.; Hu, W. Ultrafine Pt nanoparticle-decorated pyrite-type CoS2 nanosheet arrays coated on carbon cloth as a bifunctional electrode for overall water splitting. Adv. Energy Mater. 2018, 8, 1800935. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Suliman, M.H.; Rehman, A.; Hakeem, A.S.; Al Ghanim, A.; Qamar, M. Fabrication of platinum thin films for ultra-high electrocatalytic hydrogen evolution reaction. Int. J. Hydrog. Energy 2020, 45, 15076–15085. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Suliman, M.H.; Rehman, A.; Hakeem, A.S.; Yamani, Z.H.; Qamar, M. Direct deposition of a nanoporous palladium electrocatalyst for efficient hydrogen evolution reaction. New J. Chem. 2020, 44, 7795–7801. [Google Scholar] [CrossRef]
- Muench, F.; Neetzel, C.; Kaserer, S.; Brötz, J.; Jaud, J.-C.; Zhao-Karger, Z.; Lauterbach, S.; Kleebe, H.-J.; Roth, C.; Ensinger, W. Fabrication of porous rhodium nanotube catalysts by electroless plating. J. Mater. Chem. 2012, 22, 12784–12791. [Google Scholar] [CrossRef]
- Falini, G.; Gualandi, A.; Savoia, D. Rhodium/graphite-catalyzed hydrogenation of carbocyclic and heterocyclic aromatic compounds. Synthesis 2009, 14, 2440–2446. [Google Scholar]
- Maegawa, T.; Akashi, A.; Sajiki, H. A mild and facile method for complete hydrogenation of aromatic nuclei in water. Synlett 2006, 9, 1440–1442. [Google Scholar] [CrossRef]
- Vermisoglou, E.C.; Romanos, G.E.; Karanikolos, G.N.; Kanellopoulos, N.K. Catalytic NOx removal by single-wall carbon nanotube-supported Rh nanoparticles. J. Hazard. Mater. 2011, 194, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Beyer, H.; Köhler, K. NOx removal by rhodium catalysts supported on carbon nanotubes: Evidence for the stoichiometric reduction of NO2 and NO by the carbon support. Appl. Catal. B 2010, 96, 110–116. [Google Scholar] [CrossRef]
- Kanuru, V.K.; Humphrey, S.M.; Kyffin, J.M.; Jefferson, D.A.; Burton, J.W.; Armbrüster, M.; Lambert, R.M. Evidence for heterogeneous Sonogashira coupling of phenylacetylene and iodobenzene catalyzed by well defined rhodium nanoparticles. Dalton Trans. 2009, 7602–7605. [Google Scholar] [CrossRef] [PubMed]
- Sathe, B.R. Methanol electro-oxidation on nanostructured rhodium network. Energy Environ. 2015, 4, 196–200. [Google Scholar] [CrossRef]
- Sathe, B.R.; Balan, B.K.; Pillai, V.K. Enhanced electrocatalytic performance of interconnected Rh nano-chains towards formic acid oxidation. Energy Environ. Sci. 2011, 4, 1029–1036. [Google Scholar] [CrossRef]
- Biacchi, A.J.; Schaak, R.E. The solvent matters: Kinetic versus thermodynamic shape control in the polyol synthesis of rhodium nanoparticles. ACS Nano 2011, 5, 8089–8099. [Google Scholar] [CrossRef]
- Mizuno, T.; Matsumura, Y.; Nakajima, T.; Mishima, S. Effect of support on catalytic properties of Rh catalysts for steam reforming of 2-propanol. Int. J. Hydrog. Energy 2003, 28, 1393–1399. [Google Scholar] [CrossRef]
- Han, Y.-F.; Kahlich, M.; Kinne, M.; Behm, R. CO removal from realistic methanol reformate via preferential oxidation—performance of a Rh/MgO catalyst and comparison to Ru/γ-Al2O3, and Pt/γ-Al2O3. Appl. Catal. B 2004, 50, 209–218. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Hickey, N. Automotive catalytic converters: Current status and some perspectives. Catal. Today 2003, 77, 419–449. [Google Scholar] [CrossRef]
- Sathe, B.R. Rhodium nanoparticle–carbon nanosphere hybrid material as an electrochemical hydrogen sensor. RSC Adv. 2013, 3, 5361–5365. [Google Scholar] [CrossRef]
- N’Diaye, J.; Poorahong, S.; Hmam, O.; Izquierdo, R.; Siaj, M. Facile synthesis rhodium nanoparticles decorated single layer graphene as an enhancement hydrogen peroxide sensor. J. Electroanal. Chem. 2017, 789, 85–91. [Google Scholar] [CrossRef]
- Aaltonen, T.; Ritala, M.; Leskelä, M. ALD of rhodium thin films from Rh (acac) 3 and oxygen. Electrochem. Solid-State Lett. 2005, 8, C99. [Google Scholar] [CrossRef]
- Lu, J.-P.; Chu, P.W.; Raj, R.; Gysling, H. Ultrahigh vacuum chemical vapor deposition of rhodium thin films on clean and TiO2-covered Si (111). Thin Solid Films 1992, 208, 172–176. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Hakeem, A.S.; Sharif, M.; Rehman, A. Direct Deposition of Amorphous Cobalt–Vanadium Mixed Oxide Films for Electrocatalytic Water Oxidation. ACS Omega 2019, 4, 12671–12679. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, M.A.; Hasan, M.M.; Islam, T.; Hossain, M.D.; Aziz, M.A.; Ahammad, A.S. Fabrication of nanostructured Pd thin films using aerosol-assisted chemical vapor deposition for the nonenzymatic electrochemical detection of H2O2. ACS Appl. Electron. Mater. 2019, 1, 417–429. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Sohail, M.; Jamil, R.; Hakeem, A.S. Single-Step Fabrication of Nanostructured Palladium Thin Films via Aerosol-Assisted Chemical Vapor Deposition (AACVD) for the Electrochemical Detection of Hydrazine. Electrocatalysis 2019, 10, 214–221. [Google Scholar] [CrossRef]
- Joya, K.S.; Ehsan, M.A.; Sohail, M.; Yamani, Z.H. Nanoscale palladium as a new benchmark electrocatalyst for water oxidation at low overpotential. J. Mater. Chem. A 2019, 7, 9137–9144. [Google Scholar] [CrossRef]
- Marsh, E.P.; Uhlenbrock, S. Solvated Ruthenium Precursors for Direct Liquid Injection of Ruthenium and Ruthenium Oxide and Method of Using Same. Google Patents 2003. [Google Scholar]
- Swanson, H.E.; Fuyat, R.K.; Ugrinic, G.M. International Centre for Diffraction Data, Powder Diffraction File, Entry. US Natl. Bur. Stand. Circ. 1996, 5, 685. [Google Scholar]
- Abe, Y.; Kato, K.; Kawamura, M.; Sasaki, K. Rhodium and rhodium oxide thin films characterized by XPS. Surf. Sci. Spectra 2001, 8, 117–125. [Google Scholar] [CrossRef]
- Adam, A.; Suliman, M.H.; Dafalla, H.; Al-Arfaj, A.R.; Siddiqui, M.N.; Qamar, M. Rationally dispersed molybdenum phosphide on carbon nanotubes for the hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2018, 6, 11414–11423. [Google Scholar] [CrossRef]
- Adam, A.; Suliman, M.H.; Siddiqui, M.N.; Yamani, Z.H.; Merzougui, B.; Qamar, M. Interconnected hollow cobalt phosphide grown on carbon nanotubes for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2018, 10, 29407–29416. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Ahn, S.H. Electrodeposited rhodium phosphide with high activity for hydrogen evolution reaction in acidic medium. ACS Sustain. Chem. Eng. 2019, 7, 14041–14050. [Google Scholar] [CrossRef]
- Smiljanic, M.; Rakocevic, Z.; Maksic, A.; Strbac, S. Hydrogen evolution reaction on platinum catalyzed by palladium and rhodium nanoislands. Electrochim. Acta 2014, 117, 336–343. [Google Scholar] [CrossRef]
- Shen, W.; Ge, L.; Sun, Y.; Liao, F.; Xu, L.; Dang, Q.; Kang, Z.; Shao, M. Rhodium nanoparticles/F-doped graphene composites as multifunctional electrocatalyst superior to Pt/C for hydrogen evolution and formic acid oxidation reaction. ACS Appl. Mater. Interfaces 2018, 10, 33153–33161. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, M.A.; Adam, A.; Rehman, A.; Hakeem, A.S.; Isab, A.A.; Qamar, M. Morphologically controlled rapid fabrication of rhodium sulfide (Rh2S3) thin films for superior and robust hydrogen evolution reaction. Sustain. Energy Fuels 2021, 5, 459–468. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, Y.; Du, Y.; Chen, Y.; Cheng, G.; Chen, S.; Luo, W. A Monodisperse Rh2P-Based Electrocatalyst for Highly Efficient and pH-Universal Hydrogen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1703489. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, H.; Li, Y.; Liao, F.; Lifshitz, Y.; Sheng, M.; Lee, S.-T.; Shao, M. A rhodium/silicon co-electrocatalyst design concept to surpass platinum hydrogen evolution activity at high overpotentials. Nat. Commun. 2016, 7, 12272. [Google Scholar] [CrossRef] [Green Version]


| Catalyst | Synthesis Approach | Electrode | Overpotential η50 (mV) @50 mA cm−2 | Tafel Slope (mV dec−1) | Reference |
|---|---|---|---|---|---|
| Nanoporous Pd | AACVD | Nickel Foam | ~65 | ~29 | [28] |
| Pt thin films | AACVD | Nickel foam | ~100 | ~31 | [27] |
| Rh2S3 | AACVD | Nickel foam | ~72 | 36 | [57] |
| Rh nanoparticles | Solvent reduction method | Glassy carbon | ˂250 | 120 | [58] |
| Rh nanoparticles | Etching | Glassy carbon | ˂250 | 40 | [59] |
| Rh thin films | AACVD | Ti-foil | 67 | 42 | This work |
| Rh thin films | AACVD | Nickel foam | 127 | 52 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehsan, M.A.; Adam, A.; Rehman, A.; Qamar, M. Direct Self-Assembly of Hierarchically Grown Rhodium Thin Films for Electrocatalytic Hydrogen Evolution Reaction. Catalysts 2021, 11, 338. https://doi.org/10.3390/catal11030338
Ehsan MA, Adam A, Rehman A, Qamar M. Direct Self-Assembly of Hierarchically Grown Rhodium Thin Films for Electrocatalytic Hydrogen Evolution Reaction. Catalysts. 2021; 11(3):338. https://doi.org/10.3390/catal11030338
Chicago/Turabian StyleEhsan, Muhammad Ali, Alaaldin Adam, Abdul Rehman, and Mohammad Qamar. 2021. "Direct Self-Assembly of Hierarchically Grown Rhodium Thin Films for Electrocatalytic Hydrogen Evolution Reaction" Catalysts 11, no. 3: 338. https://doi.org/10.3390/catal11030338
APA StyleEhsan, M. A., Adam, A., Rehman, A., & Qamar, M. (2021). Direct Self-Assembly of Hierarchically Grown Rhodium Thin Films for Electrocatalytic Hydrogen Evolution Reaction. Catalysts, 11(3), 338. https://doi.org/10.3390/catal11030338






