Photocatalytic Reduction of CO2 to Methanol Using a Copper-Zirconia Imidazolate Framework
Abstract
:1. Introduction
2. Results
2.1. Phase Analysis of Catalyst Components
2.2. Photocatalytic Activity
2.3. Optimization Study
2.4. Effect of Process Parameters on Methanol Yield
2.5. Optimum Reaction Conditions
3. Discussion
4. Materials and Methods
4.1. Synthesis of CuZrIm Catalyst
4.2. Photocatalytic Study
4.3. Optimization Study
4.4. Characterization of CuZrIm Catalyst
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Nadeem, S.; Mumtaz, A.; Mumtaz, M.; Mutalib, M.A.; Shaharun, M.S.; Abdullah, B. Visible light driven CO2 reduction to methanol by Cu-porphyrin impregnated mesoporous Ti-MCM-48. J. Mol. Liq. 2018, 272, 656–667. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Yang, D.-A.; Ahn, W.-S. Carbon dioxide adsorption over zeolite-like metal organic frameworks (ZMOFs) having a sod topology: Structure and ion-exchange effect. Chem. Eng. J. 2011, 168, 1134–1139. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-like metal–organic frameworks (ZMOFs): Design, synthesis, and properties. Chem. Soc. Rev. 2015, 44, 228–249. [Google Scholar] [CrossRef] [Green Version]
- McGuire, C.V.; Forgan, R.S. The surface chemistry of metal–organic frameworks. Chem. Commun. 2015, 51, 5199–5217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-C.; Li, J.-R.; Lv, X.-L.; Zhang, Y.-Q.; Guo, G. Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Meyer, K.; Ranocchiari, M.; van Bokhoven, J.A. Metal organic frameworks for photo-catalytic water splitting. Energy Environ. Sci. 2015, 8, 1923–1937. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Multifunctional metal–organic frameworks for photocatalysis. Small 2015, 11, 3097–3112. [Google Scholar] [CrossRef]
- Gao, S.T.; Liu, W.H.; Shang, N.Z.; Feng, C.; Wu, Q.H.; Wang, Z.; Wang, C. Integration of a plasmonic semiconductor with a metal–organic framework: A case of Ag/AgCl@ZIF-8 with enhanced visible light photocatalytic activity. Rsc Adv. 2014, 4, 61736–61742. [Google Scholar] [CrossRef]
- Liu, Q.; Low, Z.-X.; Li, L.; Razmjou, A.; Wang, K.; Yao, J.; Wang, H. ZIF-8/Zn2GeO 4 nanorods with an enhanced CO 2 adsorption property in an aqueous medium for photocatalytic synthesis of liquid fuel. J. Mater. Chem. A 2013, 1, 11563–11569. [Google Scholar] [CrossRef]
- Kumar, R.R.R.S.; Kumar, S.S.; Kulandainathan, M.A. Highly selective electrochemical reduction of carbon dioxide using Cu based metal organic framework as an electrocatalyst. Electrochem. Commun. 2012, 25, 70–73. [Google Scholar] [CrossRef]
- Lee, D.Y.; Shin, C.Y.; Yoon, S.J.; Lee, H.Y.; Lee, W.; Shrestha, N.K.; Lee, J.K.; Han, S.-H. Enhanced photovoltaic performance of Cu-based metal-organic frameworks sensitized solar cell by addition of carbon nanotubes. Sci. Rep. 2014, 4, 3930. [Google Scholar] [CrossRef]
- Li, J.; Luo, D.; Yang, C.; He, S.; Chen, S.; Lin, J.; Zhu, L.; Li, X. Copper (II) imidazolate frameworks as highly efficient photocatalysts for reduction of CO2 into methanol under visible light irradiation. J. Solid State Chem. 2013, 203, 154–159. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Sun, D.; Fu, Y.; Liu, W.; Ye, L.; Wang, D.; Yang, L.; Fu, X.; Li, Z. Studies on photocatalytic CO2 reduction over NH2-Uio-66 (Zr) and its derivatives: Towards a better understanding of photocatalysis on metal–organic frameworks. Chem. A Eur. J. 2013, 19, 14279–14285. [Google Scholar] [CrossRef] [PubMed]
- Laurier, K.G.; Vermoortele, F.; Ameloot, R.; de Vos, D.E.; Hofkens, J.; Roeffaers, M.B. Iron (III)-based metal–organic frameworks as visible light photocatalysts. J. Am. Chem. Soc. 2013, 135, 14488–14491. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An amine-functionalized titanium metal–organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. 2012, 124, 3420–3423. [Google Scholar] [CrossRef]
- Wang, S.; Yao, W.; Lin, J.; Ding, Z.; Wang, X. Cobalt imidazolate metal–organic frameworks photosplit CO2 under mild reaction conditions. Angew. Chem. 2014, 126, 1052–1056. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Ma, W.X.; Wang, M.Y.; Yang, X.J.; Xu, X.Y. Structure, photoluminescent properties and photocatalytic activities of a new Cd (II) metal–organic framework. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 657–662. [Google Scholar] [CrossRef]
- Muüller, M.; Hermes, S.; Kaähler, K.; van den Berg, M.W.; Muhler, M.; Fischer, R.A. Loading of MOF-5 with Cu and ZnO nanoparticles by gas-phase infiltration with organometallic precursors: Properties of Cu/ZnO@ MOF-5 as catalyst for methanol synthesis. Chem. Mater. 2008, 20, 4576–4587. [Google Scholar]
- Liu, Y.; Yang, Y.; Sun, Q.; Wang, Z.; Huang, B.; Dai, Y.; Qin, X.; Zhang, X. Chemical adsorption enhanced CO2 capture and photoreduction over a copper porphyrin based metal organic framework. Acs Appl. Mater. Interfaces 2013, 5, 7654–7658. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Bal, M.; Behera, S.K.; Sen, T.K.; Meikap, B.C. Process optimization study of Zn2+ adsorption on biochar-alginate composite adsorbent by response surface methodology (RSM). Water 2019, 11, 325. [Google Scholar] [CrossRef] [Green Version]
- Espinós, J.; Morales, J.; Barranco, A.; Caballero, A.; Holgado, J.; González-Elipe, A. Interface effects for Cu, CuO, and Cu2O deposited on SiO2 and ZrO2. XPS determination of the valence state of copper in Cu/SiO2 and Cu/ZrO2 catalysts. J. Phys. Chem. B 2002, 106, 6921–6929. [Google Scholar] [CrossRef]
- Durán-Martín, D.; Ojeda, M.; Granados, M.L.; Fierro, J.; Mariscal, R. Stability and regeneration of Cu–ZrO2 catalysts used in glycerol hydrogenolysis to 1, 2-propanediol. Catal. Today 2013, 210, 98–105. [Google Scholar] [CrossRef]
- Liu, Z.; Amiridis, M.D.; Chen, Y. Characterization of CuO supported on tetragonal ZrO2 catalysts for N2O decomposition to N2. J. Phys. Chem. B 2005, 109, 1251–1255. [Google Scholar] [CrossRef]
- Battaglin, G.; Bertoncello, R.; Boscolo-Boscoletto, A.; Caccavale, F.; Mazzoldi, P.; Polato, P. Heavy metal fluoride glasses: Modifications induced by ion implantation. J. Non-Cryst. Solids 1989, 111, 36–42. [Google Scholar] [CrossRef]
- Bosman, H.; Pijpers, A.; Jaspers, A. An X-Ray Photoelectron Spectroscopy Study of the Acidity of SiO2–ZrO2 Mixed Oxides. J. Catal. 1996, 161, 551–559. [Google Scholar] [CrossRef]
- Damyanova, S.; Grange, P.; Delmon, B. Surface characterization of zirconia-coated alumina and silica carriers. J. Catal. 1997, 168, 421–430. [Google Scholar] [CrossRef]
- Damyanova, S.; Petrov, L.; Grange, P. XPS characterization of zirconium-promoted CoMo hydrodesulfurization catalysts. Appl. Catal. A Gen. 2003, 239, 241–252. [Google Scholar] [CrossRef]
- Liu, Z.; Jing, X.; Wang, L. Luminescence of native defects in Zn2GeO4. J. Electrochem. Soc. 2007, 154, H500–H506. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, K.; Shi, R.; Tang, A. Sol–gel synthesis and photocatalytic activity of CeO2/TiO2 nanocomposites. J. Am. Ceram. Soc. 2007, 90, 1370–1374. [Google Scholar] [CrossRef]
- George, E.P.B. Statistics for Experiments: An Introduction to Design, Data Analysis, and Model Building; Wiley: New York, NY, USA, 1978; Volume 18, p. 653. [Google Scholar]
- Hasan, S.H.; Srivastava, P. Biosorptive abatement of Cd2+ by water using immobilized biomass of arthrobacter sp.: Response surface methodological approach. Ind. Eng. Chem. Res. 2010, 50, 247–258. [Google Scholar] [CrossRef]
- Hu, C.-C.; Bai, A. Optimization of hydrogen evolving activity on nickel–phosphorus deposits using experimental strategies. J. Appl. Electrochem. 2001, 31, 565–572. [Google Scholar] [CrossRef]
- Francis, F.; Sabu, A.; Nampoothiri, K.M.; Ramachandran, S.; Ghosh, S.; Szakacs, G.; Pandey, A. Use of response surface methodology for optimizing process parameters for the production of α-amylase by Aspergillus oryzae. Biochem. Eng. J. 2003, 15, 107–115. [Google Scholar] [CrossRef]
- Chen, D.; Li, F.; Ray, A.K. Effect of mass transfer and catalyst layer thickness on photocatalytic reaction. Aiche J. 2000, 46, 1034–1045. [Google Scholar] [CrossRef]
- Aurian-Blajeni, B.; Halmann, M.; Manassen, J. Photoreduction of carbon dioxide and water into formaldehyde and methanol on semiconductor materials. Sol. Energy 1980, 25, 165–170. [Google Scholar] [CrossRef]
- Hussain, M.; Akhter, P.; Saracco, G.; Russo, N. Nanostructured TiO2/KIT-6 catalysts for improved photocatalytic reduction of CO2 to tunable energy products. Appl. Catal. B Environ. 2015, 170, 53–65. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Z.; Wang, Z. Photocatalytic reduction of carbon dioxide using sol–gel derived titania-supported CoPc catalysts. Photochem. Photobiol. Sci. 2007, 6, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Ruthiya, K.C. Mass transfer and hydrodynamics in catalytic slurry reactors. Chem. React. Eng. 2005, 34, 229. [Google Scholar]
- Tseng, I.-H.; Chang, W.-C.; Wu, J.C. Photoreduction of CO2 using sol–gel derived titania and titania-supported copper catalysts. Appl. Catal. B Environ. 2002, 37, 37–48. [Google Scholar] [CrossRef]
- Nam, S.; Tratnyek, P.G. Reduction of azo dyes with zero-valent iron. Water Res. 2000, 34, 1837–1845. [Google Scholar] [CrossRef]
- Goyal, S.; Shaharun, M.; Kait, C.; Abdullah, B.; Ameen, M. Photoreduction of carbon dioxide to methanol over copper based zeolitic imidazolate framework-8: A new generation photocatalyst. Catalysts 2018, 8, 581. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, K.; Shen, Y.; Wang, X.; Guo, Y.; Zhou, X.; Bai, R. Enhanced photocatalytic degradation for organic pollutants by a novel m-Bi2O4/Bi2O2CO3 photocatalyst under visible light. Res. Chem. Intermed. 2018, 44, 3061–3079. [Google Scholar] [CrossRef]


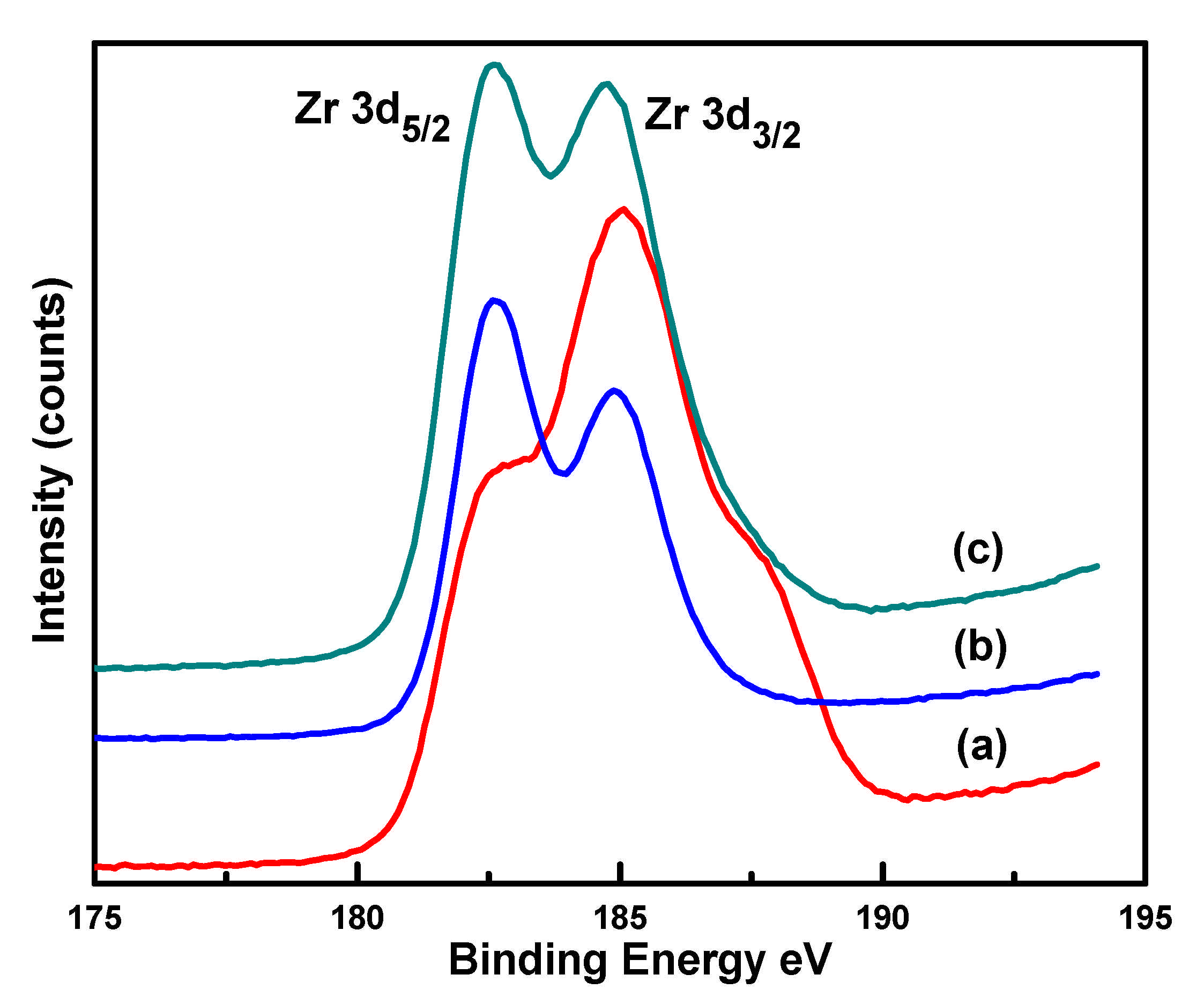
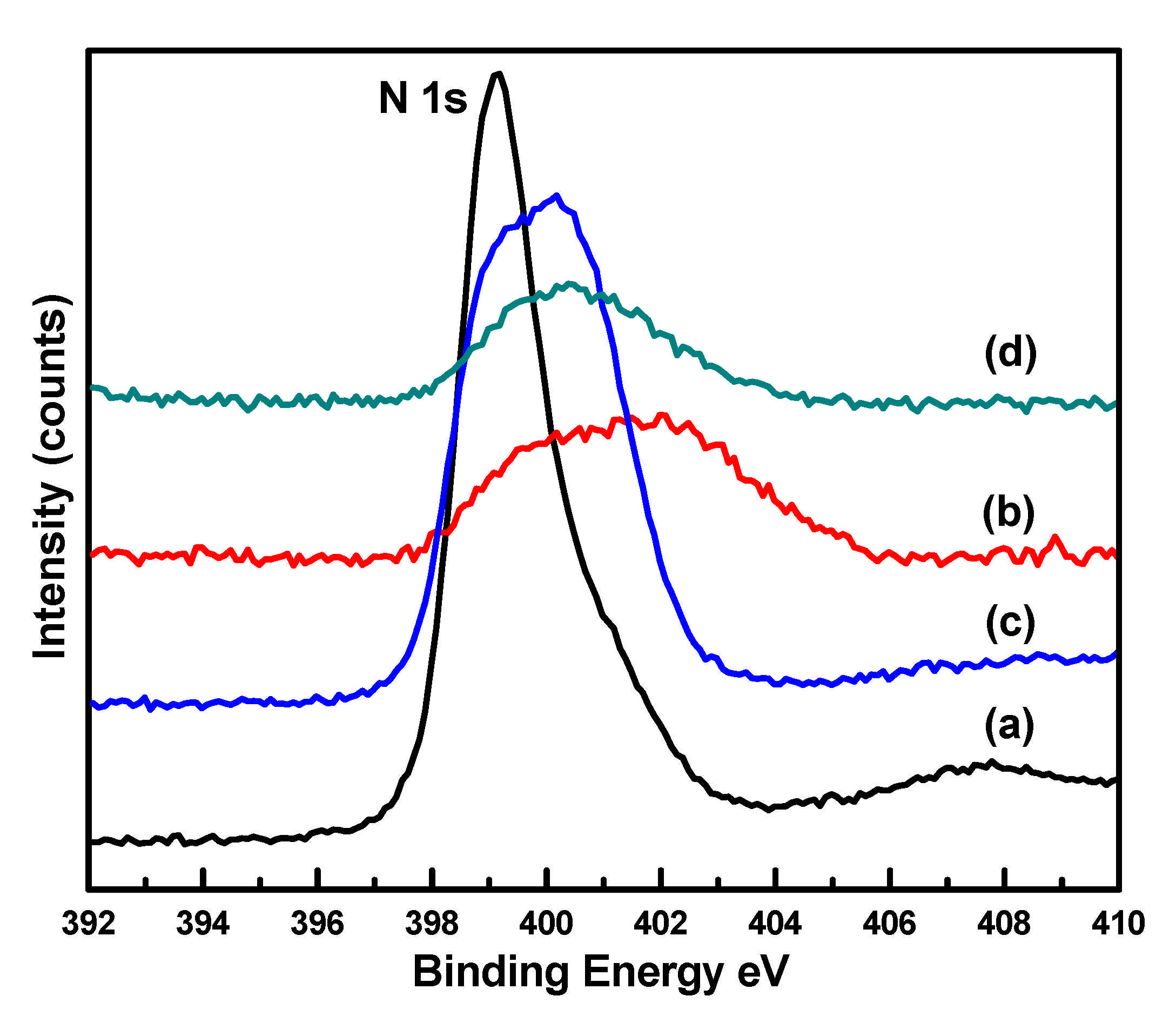












| Catalyst | Binding Energies (eV) | FWHM (eV) | Binding Energies (eV) N 1s | ||
|---|---|---|---|---|---|
| Cu 2p3/2 | Zr 3d5/2 | Cu 2p3/2 | Zr 3d5/2 | ||
| CuIm | 933.2 | - | 4.22 | - | 399.3 |
| CuZrIm0.5 | 936.4 | 185 | 4.39 | 5.51 | 401.5 |
| CuZrIm1 | 934.3 | 182.6 | 4.17 | 4.36 | 400.2 |
| CuZrIm1.5 | 934.6 | 182.7 | 4.28 | 4.40 | 400.5 |
| Variable | Symbol | Unit | Ranges and Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Intensity of light | A | W/m2 | 200 | 270 | 340 |
| Stirring rate | B | rpm | 400 | 550 | 700 |
| Catalyst loading | C | g/L | 1 | 1.5 | 2 |
| Runs | Intensity of Light (W/m2) | Stirring Rate (rpm) | Catalyst Loading (g/L) | Methanol Activity (µmol/L.g) |
|---|---|---|---|---|
| 1. | 270 | 550 | 1.5 | 1053.04 |
| 2. | 270 | 550 | 1.5 | 1033.95 |
| 3. | 270 | 550 | 1.5 | 1034 |
| 4. | 270 | 700 | 2 | 885.14 |
| 5. | 200 | 550 | 1 | 854.13 |
| 6. | 340 | 550 | 1 | 845.65 |
| 7. | 200 | 700 | 1.5 | 648.35 |
| 8. | 200 | 550 | 2 | 679.47 |
| 9. | 200 | 400 | 1.5 | 772.84 |
| 10. | 340 | 550 | 2 | 898.32 |
| 11. | 340 | 400 | 1.5 | 771.28 |
| 12. | 340 | 700 | 1.5 | 818.15 |
| 13. | 270 | 550 | 1.5 | 1054.1 |
| 14. | 270 | 400 | 2 | 825.59 |
| 15. | 270 | 550 | 1.5 | 1054 |
| 16. | 270 | 700 | 1 | 902.43 |
| 17. | 270 | 400 | 1 | 948.28 |
| Source | Sequential p-Value | Lack of Fit p-Value | Adjusted R-Squared | Predicted R-Squared |
|---|---|---|---|---|
| Linear | 0.6987 | <0.0001 | −0.1070 | −0.4279 |
| 2FI | 0.7890 | <0.0001 | −0.3017 | −1.2893 |
| Quadratic | <0.0001 | 0.1186 | 0.9849 | 0.9195 |
| Cubic | 0.1186 | 0.9930 |
| Factor | F-Value | p-Value |
|---|---|---|
| Model | 116.99 | <0.0001 |
| A-Intensity of light | 70.75 | <0.0001 |
| B-Stirring rate | 2.02 | 0.1986 |
| C-Catalyst loading | 33.87 | 0.0007 |
| AB | 28.98 | 0.0010 |
| AC | 51.01 | 0.0002 |
| BC | 10.97 | 0.0129 |
| A2 | 551.05 | <0.0001 |
| B2 | 205.19 | <0.0001 |
| C2 | 32.71 | 0.0007 |
| Parameters | Intensity of Light (W/m2) | Stirring Rate (rpm) | Catalyst Loading (g/L) |
|---|---|---|---|
| Optimum conditions | 275 | 540 | 1.3 |
| Sr. No. | Catalyst | Reactant | UV Cut-off Filter Wavelength (nm) | Xenon Lamp | Methanol (µmol/L.g) | Year |
|---|---|---|---|---|---|---|
| 1. | Cu porphyrin-based MOF | CO2 and a solution of the triethylamine | λ ≥ 420 | 300 W | 5.97 | 2013 |
| 2. | ZIF-8/Zn2GeO4 | CO2 and a solution of sodium sulfite | No filter used | 500 W | 2.44 | 2013 |
| 3. | CuIm | CO2 and a solution of sodium hydroxide and sodium sulfite | λ > 400 | 500 W | 1712.7 | 2013 |
| 4. | Ti-MCM-48(25)CuTPP | CO2 and a solution of sodium hydroxide and sodium sulfite | No filter used | 500 W | 297.06 | 2018 |
| 5. | CuZrIm1 | CO2 and a solution of sodium hydroxide and sodium sulfite | No filter used | 500 W | 1054.0 | Present study |
| Process | Variables |
|---|---|
| Intensity of light (W/m2) | 200, 270, 340 |
| Stirring rate (rpm) | 400, 550, 700 |
| Catalyst loading (g/L) | 1, 1.5, 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, S.; Shaharun, M.S.; Jayabal, G.S.; Kait, C.F.; Abdullah, B.; Wei, L.J. Photocatalytic Reduction of CO2 to Methanol Using a Copper-Zirconia Imidazolate Framework. Catalysts 2021, 11, 346. https://doi.org/10.3390/catal11030346
Goyal S, Shaharun MS, Jayabal GS, Kait CF, Abdullah B, Wei LJ. Photocatalytic Reduction of CO2 to Methanol Using a Copper-Zirconia Imidazolate Framework. Catalysts. 2021; 11(3):346. https://doi.org/10.3390/catal11030346
Chicago/Turabian StyleGoyal, Sonam, Maizatul Shima Shaharun, Ganaga Suriya Jayabal, Chong Fai Kait, Bawadi Abdullah, and Lim Jun Wei. 2021. "Photocatalytic Reduction of CO2 to Methanol Using a Copper-Zirconia Imidazolate Framework" Catalysts 11, no. 3: 346. https://doi.org/10.3390/catal11030346







