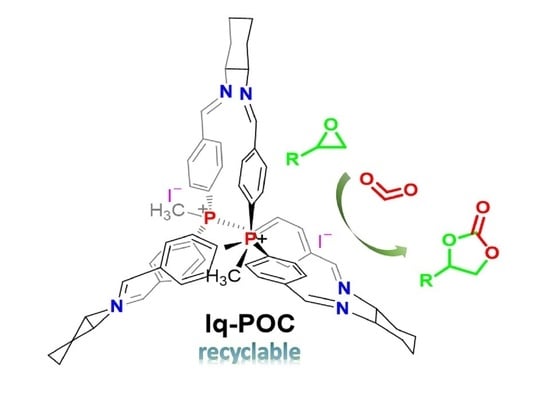
The Ionic Organic Cage: An Effective and Recyclable Testbed for Catalytic CO2 Transformation
Abstract
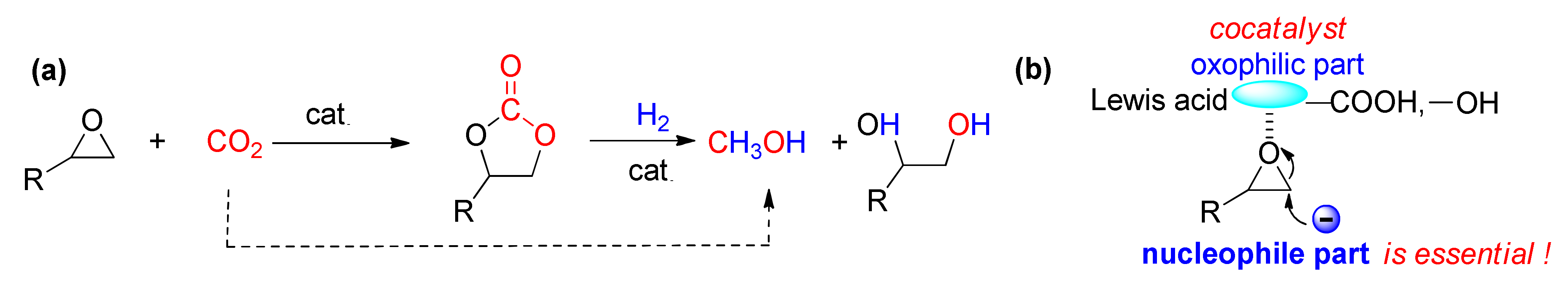
1. Introduction
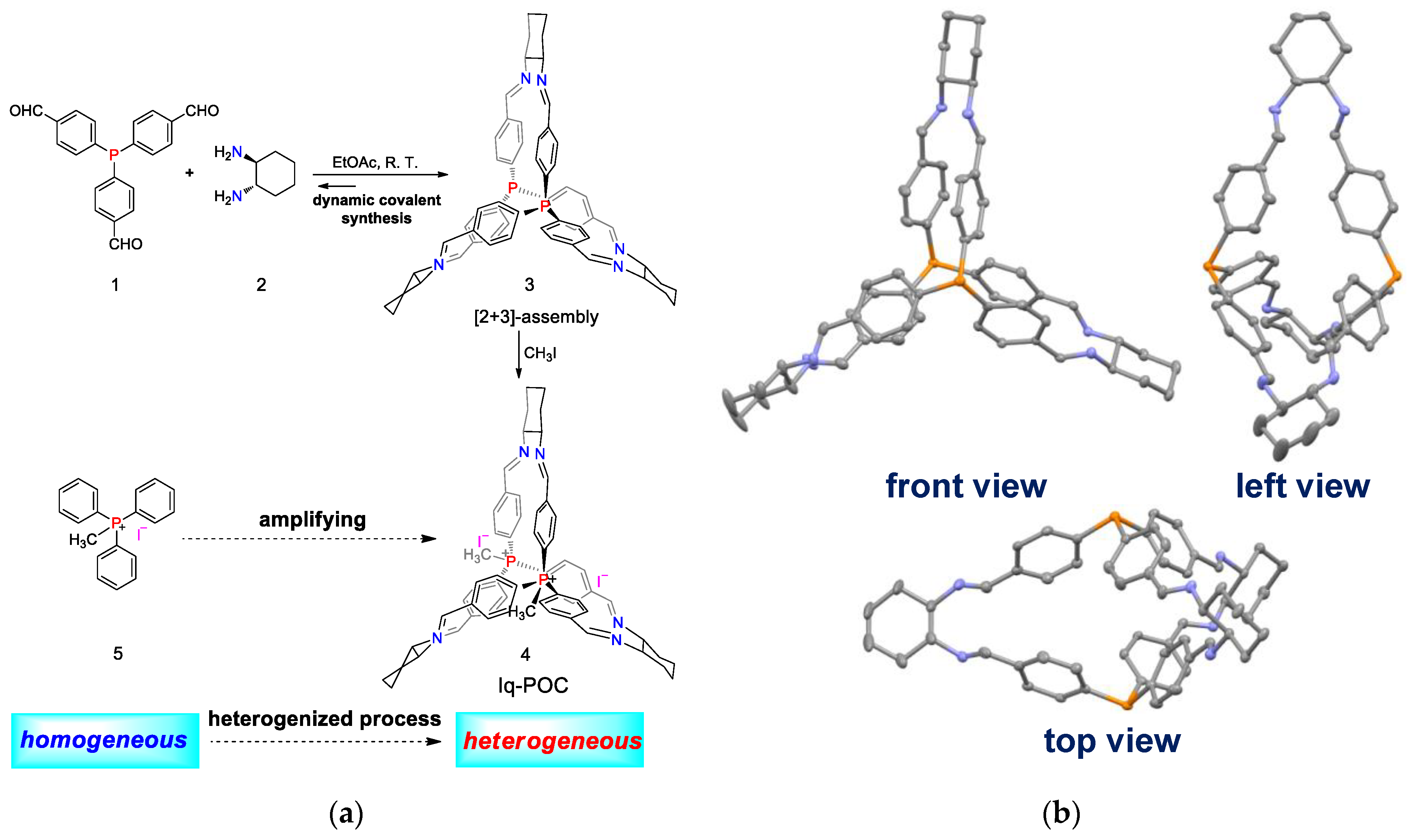
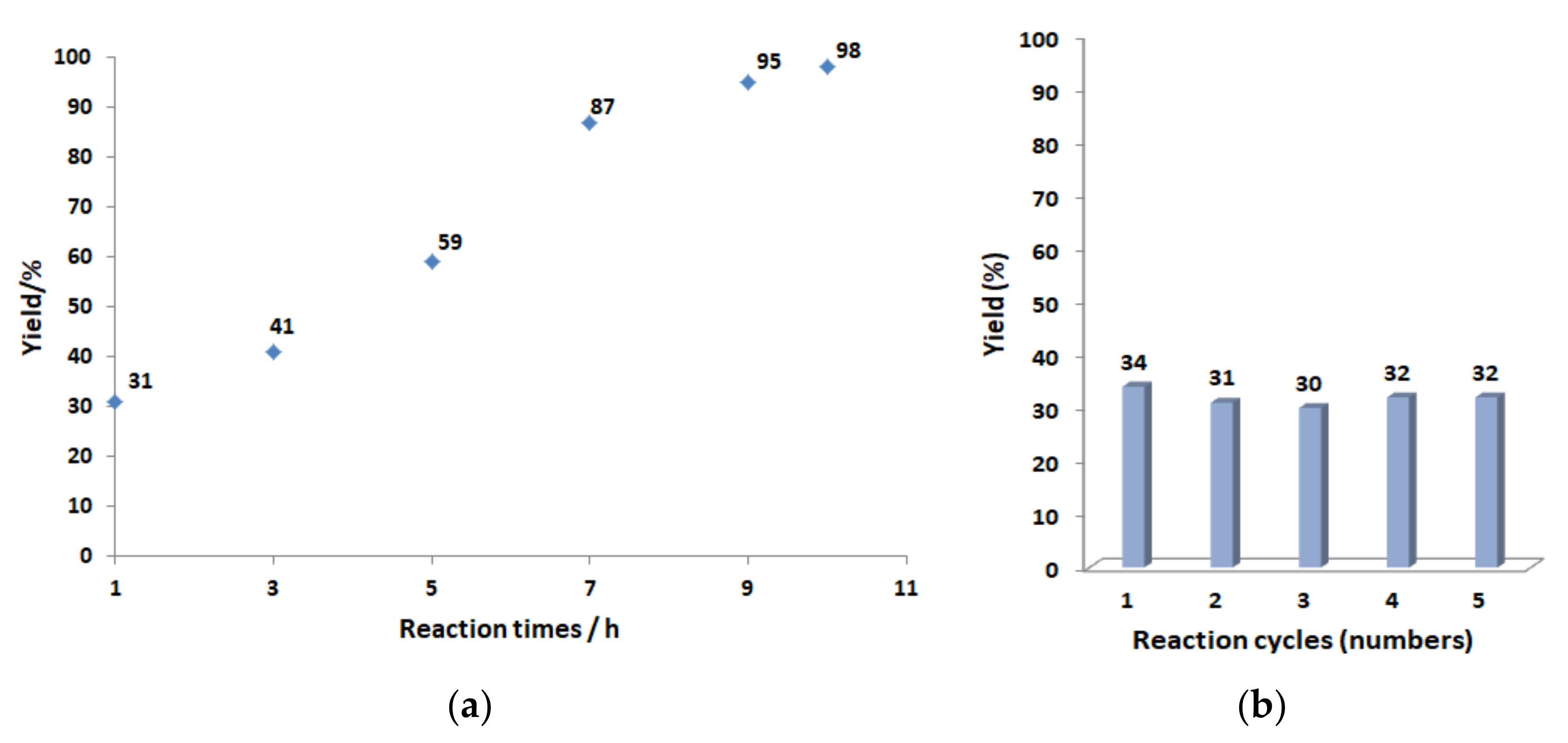
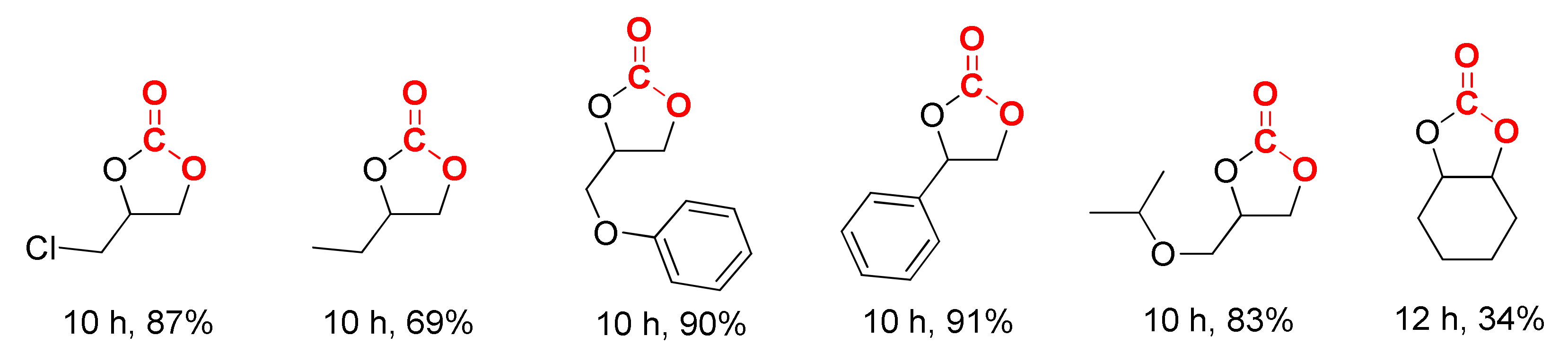
2. Results and Discussion
3. Materials and Experiments
3.1. General Remarks
3.2. Synthesis of the Cage 3
3.3. Synthesis of Iq-POC
3.4. General Procedure of Catalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tozawa, T.; Jones, J.T.A.; Swamy, S.I.; Jiang, S.; Adams, D.J.; Shakespeare, S.; Clowes, R.; Bradshaw, D.; Hasell, T.; Chong, S.Y.; et al. Porous organic cages. Nat. Mater. 2009, 8, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Little, M.A.; Cooper, A.I. The chemistry of porous organic molecular materials. Adv. Funct. Mater. 2020, 30, 1909842. [Google Scholar] [CrossRef]
- Mastalerz, M. Porous shape-persistent organic cage compounds of different size, geometry, and function. Acc. Chem. Res. 2018, 51, 2411–2422. [Google Scholar] [CrossRef]
- Cooper, A.I. Porous molecular solids and liquids. ACS Cent. Sci. 2017, 3, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.E.; Cooper, A.I. A perspective on the synthesis, purification, and characterization of porous organic cages. Chem. Mater. 2017, 29, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hasell, T.; Cooper, A.I. Porous organic cages: Soluble, modular and molecular pores. Nat. Rev. Mater. 2016, 1, 16053. [Google Scholar] [CrossRef]
- Yu, N.; Ding, H.; Wang, C. Synthesis and application of organic molecular cages. Prog. Chem. 2016, 28, 1721–1731. [Google Scholar]
- Slater, A.G.; Cooper, A.I. Function-led design of new porous materials. Science 2015, 348, aaa8075. [Google Scholar] [CrossRef]
- Evans, J.D.; Sumby, C.J.; Doonan, C.J. Synthesis and applications of porous organic cages. Chem. Lett. 2015, 44, 582–588. [Google Scholar] [CrossRef]
- Zhang, G.; Mastalerz, M. Organic cage compounds – from shape-persistency to function. Chem. Soc. Rev. 2014, 43, 1934–1947. [Google Scholar] [CrossRef]
- Mastalerz, M. Shape-persistent organic cage compounds by dynamic covalent bond formation. Angew. Chem. Int. Ed. 2010, 49, 5042–5053. [Google Scholar] [CrossRef]
- Acharyya, K.; Mukherjee, P.S. Organic imine cages: Molecular marriage and applications. Angew. Chem. Int. Ed. 2019, 58, 8640–8653. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Wu, G.; Zhang, Y.; Shen, L.; Lei, Y.; Wang, G.-Y.; Fahrenbach, A.C.; Li, H. Self-assembly in water with N-substituted imines. Angew. Chem. Int. Ed. 2020, 59, 18350–18367. [Google Scholar] [CrossRef] [PubMed]
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Z.-T. A dynamic route for structure and function: Recent advances in imine-based organic nanostructured materials. Aust. J. Chem. 2013, 66, 9–22. [Google Scholar] [CrossRef]
- Schneider, M.W.; Oppel, I.M.; Ott, H.; Lechner, L.G.; Hauswald, H.-J.S.; Stoll, R.; Mastalerz, M. Periphery-substituted [4+6] salicylbisimine cage compounds with exceptionally high surface areas: Influence of the molecular structure on nitrogen sorption properties. Chem. Eur. J. 2012, 18, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wang, X.; Clowes, R.; Cui, P.; Chen, L.; Little, M.A.; Cooper, A.I. 3D Cage COFs: A dynamic three-dimensional covalent organic framework with high-connectivity organic cage nodes. J. Am. Chem. Soc. 2020, 142, 16842–16848. [Google Scholar] [CrossRef]
- Hasell, T.; Wu, X.; Jones, J.T.A.; Bacsa, J.; Steiner, A.; Mitra, T.; Trewin, A.; Adams, D.J.; Cooper, A.I. Triply interlocked covalent organic cages. Nat. Chem. 2010, 2, 750–755. [Google Scholar] [CrossRef]
- Zhang, G.; Presly, O.; White, F.; Oppel, I.M.; Mastalerz, M. A permanent mesoporous organic cage with an exceptionally high surface area. Angew. Chem. Int. Ed. 2014, 53, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Elbert, S.M.; Regenauer, N.I.; Schindler, D.; Zhang, W.-S.; Rominger, F.; Schröder, R.R.; Mastalerz, M. Shape-persistent tetrahedral [4+6] boronic ester cages with different degrees of fluoride substitution. Chem. Eur. J. 2018, 24, 11438–11443. [Google Scholar] [CrossRef]
- Brutschy, M.; Schneider, M.W.; Mastalerz, M.; Waldvogel, S.R. Porous organic cage compounds as highly potent affinity materials for sensing by quartz crystal microbalances. Adv. Mater. 2012, 24, 6049–6052. [Google Scholar] [CrossRef]
- Brutschy, M.; Schneider, M.W.; Mastalerz, M.; Waldvogel, S.R. Direct gravimetric sensing of GBL by molecular recognition process in organic cage compounds. Chem. Commun. 2013, 49, 8398–8400. [Google Scholar] [CrossRef]
- Hähsler, M.; Mastalerz, M. A giant [8+12] boronic ester cage with 48 terminal alkene units in the periphery for postsynthetic alkene metathesis. Chem. Eur. J. 2021, 27, 233–237. [Google Scholar] [CrossRef]
- Takata, H.; Ono, K.; Iwasawa, N. Controlled release of the guest molecule via borate formation of fluorinated boronic ester cage. Chem. Commun. 2020, 56, 5613–5616. [Google Scholar] [CrossRef]
- Li, H.-G.; Li, L.; Xu, H.; Wang, G.-W. Mechanochemical synthesis and properties of boronic ester cage compounds. Curr. Org. Chem. 2018, 22, 923–929. [Google Scholar] [CrossRef]
- Takahagi, H.; Fujibe, S.; Iwasawa, N. Guest-induced dynamic self-assembly of two diastereomeric cage-like boronic esters. Chem. Eur. J. 2009, 15, 13327–13330. [Google Scholar] [CrossRef]
- Tan, C.; Jiao, J.; Li, Z.; Liu, Y.; Han, X.; Cui, Y. Design and assembly of a chiral metallosalen-based octahedral coordination cage for supramolecular asymmetric catalysis. Angew. Chem. Int. Ed. 2018, 57, 2085–2090. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Little, M.A.; Kapil, V.; Ceriotte, M.; Yang, S.; Ding, L.; Holden, D.L.; Balderas-Xicohténcatl, R.; He, D.; et al. Barely porous organic cages for hydrogen isotope separation. Science 2019, 366, 613–620. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Chen, S.; Duan, J.; Jin, W. Mixed-matrix membranes with soluble porous organic molecular cage for highly efficient C3H6/C3H8 separation. J. Memb. Sci. 2020, 611, 118288. [Google Scholar] [CrossRef]
- Lucero, J.M.; Carreon, M.A. Separation of light gases from xenon over porous organic cage membranes. ACS Appl. Mater. Interfaces 2020, 12, 32182–32188. [Google Scholar] [CrossRef]
- Kunde, T.; Nieland, E.; Schroder, H.V.; Schalley, C.A.; Schmidt, B.M. A porous fluorinated organic [4+4] imine cage showing CO2 and H2 adsporption. Chem. Commun. 2020, 56, 4761–4764. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.D.; Bloch, E.D. High-pressure methane storage and selective gas adsorption in a cyclohexane-functionalised porous organic cage. Supramol. Chem. 2019, 31, 508–513. [Google Scholar] [CrossRef]
- Jin, Y.; Voss, B.A.; Jin, A.; Long, H.; Noble, R.D.; Zhang, W. Highly CO2-selective organic molecular cages: What determines the CO2 selectivity. J. Am. Chem. Soc. 2011, 133, 6650–6658. [Google Scholar] [CrossRef]
- Wang, Z.; Sikdar, N.; Wang, S.-Q.; Li, X.; Yu, M.; Bu, X.-H.; Chang, Z.; Zou, X.; Chen, Y.; Cheng, P.; et al. Soft porous crystal based upon organic cages that exhibit guest-induced breathing selective gas separation. J. Am. Chem. Soc. 2019, 141, 9408–9414. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Holden, D.; Wang, R.; Cheng, Y.; Well, M.; Cooper, A.I.; Ding, L. Understanding the effect of host flexibility on the adsorption of CH4, CO2 and SF6 in porous organic cages. Kristallogr. Cryst. Mater. 2019, 234, 547–555. [Google Scholar] [CrossRef]
- Little, M.A.; Chong, S.Y.; Schmidtmann, M.; Hasell, T.; Copper, A. Guest control of structure in porous organic cages. Chem. Commun. 2014, 50, 9465–9468. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Verma, A.; Tomar, K.; Bharadwaj, P.K.; Sadhu, K.K. Regioisomeric cryptand stabilized gold supraspheres and elongated dodecahedron supraparticles for reversible host-guest chemistry. Chem. Commun. 2018, 54, 12836–12839. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, X.; Zhong, Y.; Hu, Y.; Li, G.; Zhang, R. Carbon dot-decorated porous organic cage as fluorescent sensor for rapid discrimination of nitrophenol isomers and chiral alcohols. Anal. Chim. Acta 2019, 1050, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-J.; Duan, A.-H.; Zhang, J.-H.; Xie, S.-M.; Cao, Q.-E.; Yuan, L.-M. An Enantioselective Potentiometric Sensor for 2-Amino-1-Butanol Based on Chiral Porous Organic Cage CC3-R. Molecules 2019, 24, 420. [Google Scholar] [CrossRef] [PubMed]
- Duan, A.-H.; Wang, B.-J.; Xie, S.-M.; Zhang, J.-H.; Yuan, L.-M. A chiral, porous, organic cage-based, enantioselective potentiometric sensor for 2-aminobutanol. Chirality 2017, 29, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Xie, T.-P.; Yan, K.-Q.; Xie, S.-M.; Wang, B.-J.; Zhang, J.-H.; Yuan, L.-M. A hydroxyl-functionalized homochiral porous organic cage for gas chromatography. Microchim. Acta 2020, 187, 269. [Google Scholar] [CrossRef]
- Li, H.-X.; Xie, T.-P.; Xie, S.-M.; Wang, B.-J.; Zhang, J.-H.; Yuan, L.-M. Enantiomeric separation on a homochiral porous organic cage-based chiral stationary phase by gas chromatography. Chromatographia 2020, 83, 703–713. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Xie, S.-M.; Wang, B.-J.; He, P.-G.; Yuan, L.-M. A homochiral porous organic cage with large cavity and pore windows for the efficient gas chromatography separation of enantiomers and positional isomers. J. Sep. Sci. 2018, 41, 1385–1394. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Zhu, P.-J.; Xie, S.-M.; Zi, M.; Yuan, L.-M. Homochiral porous organic cage used as stationary phase for open tubular capillary electrochromatography. Anal. Chim. Acta 2018, 999, 169–175. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, R.; Long, H.; Jin, Y.; Sanders, A.; Park, W.; Zhang, W. Template synthesis of gold nanoparticles with an organic molecular cage. J. Am. Chem. Soc. 2014, 136, 1782–1785. [Google Scholar] [CrossRef] [PubMed]
- Nihei, M.; Ida, H.; Nibe, T.; Moeljadi, A.M.P.; Trinh, Q.T.; Hirao, H.; Ishizaki, M.; Kurihara, M.; Shiga, T.; Oshio, H. Ferrihydrite particle encapsulated within a molecular organic cage. J. Am. Chem. Soc. 2018, 140, 17753–17759. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.-K.; Kitta, M.; Pang, H.; Xu, Q. Encapsulating highly catalytically active metal nanoclusters inside porous organic cages. Nat. Catal. 2018, 1, 214–220. [Google Scholar] [CrossRef]
- Mondal, B.; Mukherjee, P.S. Cage Encapsulated Gold Nanoparticles as heterogeneous photocatalyst for facile and selective reduction of nitroarenes to azo compounds. J. Am. Chem. Soc. 2018, 140, 12592–12601. [Google Scholar] [CrossRef]
- Mondal, B.; Acharyya, K.; Howlader, P.; Mukherjee, P.S. Molecular cage impregnated palladium nanoparticles: Efficient, additive-free heterogeneous catalysts for cyanation of aryl halides. J. Am. Chem. Soc. 2016, 138, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; McCaffrey, R.; Jin, Y.; Gong, Y.; Hu, Y.; Sun, H.; Park, W.; Zhang, W. Cage-templated synthesis of highly stable palladium nanoparticles and their catalytic activities in Suzuki-Miyaura coupling. Chem. Sci. 2018, 9, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; De, D.; Saha, R.; Chattaraj, P.K.; Bharadwaj, P.K. Flexibility induced encapsulation of ultrafine palladium nanoparticles into organic cages for Tsuji-Trost allylation. ACS Appl. Mater. Interfaces 2020, 12, 8539–8546. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Tomar, K.; Bharadwaj, P.K. Nanosized bispyrazole-based cryptand-stabilized palladium (0) nanoparticles: A resuable heterogeneous catalyst for the Suzuki-Miyaura coupling reaction in water. Inorg. Chem. 2019, 58, 1003–1006. [Google Scholar] [CrossRef]
- Sharma, V.; Bharadwaj, P.K. Organic cage supported metal nanoparticles for applications. Dalton Trans. 2020, 49, 15574–15586. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-K.; Zhan, W.-W.; Akita, T.; Xu, Q. Toward homogenization of heterogeneous metal nanoparticle catalysts with enhanced catalytic performance: Soluble porous organic cage as a stabilizer and homogenizer. J. Am. Chem. Soc. 2015, 137, 7063–7066. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Ge, J.; Lin, R.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Porous organic cage stabilised palladium nanoparticles: Efficient heterogeneous catalysts for carbonylation reaction of aryl halides. Chem. Commun. 2018, 54, 2796–2799. [Google Scholar] [CrossRef]
- Song, Q.; Wang, W.D.; Hu, X.; Dong, Z. Ru nanoclusters confined in porous organic cages for catalytic hydrolysis of ammonia borane and tandem hydrogenation reaction. Nanoscale 2019, 11, 21513–21521. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cox, H.J.; Papaioannou, E.I.; Tang, C.; Liu, H.; Murdoch, B.J.; Gibson, E.K.; Metcalfe, I.S.; Evan, J.S.O.; Beaumont, S.K. Shape-persistent porous organic cage supported palladium nanoparticles as heterogeneous catalytic materials. Nanoscale 2019, 11, 14929–14936. [Google Scholar] [CrossRef]
- Chen, G.-J.; Xin, W.-L.; Wang, J.-S.; Cheng, J.-Y.; Dong, Y.-B. Visible-light triggered selective reduction of nitroarenes to azo compounds catalysed by Ag@organic molecular cages. Chem. Commun. 2019, 55, 3586–3589. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, C.; Wang, H.; Yang, L.; Jin, P.; Zhang, W.; Jiang, J. Multifunctional tubular organic cage-supported ultrafine palladium nanoparticles for sequential catalysis. Angew. Chem. Int. Ed. 2019, 58, 18011–18016. [Google Scholar] [CrossRef]
- Smith, P.T.; Benke, B.P.; Cao, Z.; Kim, Y.; Nichols, E.V.; Kim, K.; Chang, C.J. Iron porphyrin embedded into a supramolecular porous organic cage for electrochemical CO2 reduction in water. Angew. Chem. Int. Ed. 2018, 57, 9684–9688. [Google Scholar] [CrossRef]
- Liu, C.; Liu, K.; Wang, C.; Liu, H.; Wang, H.; Su, H.; Li, X.; Chen, B.; Jiang, J. Elucidating heterogeneous photocatalytic superiority of microporous porphyrin organic cage. Nat. Commun. 2020, 11, 1047. [Google Scholar] [CrossRef]
- Koo, J.; Kim, I.; Kim, Y.; Cho, D.; Hwang, I.-C.; Mukhopadhyay, R.D.; Song, H.; Ko, Y.H.; Dhamija, A.; Lee, H.; et al. Gigantic porphyrinic cages. Chem 2020, 6, 3374–3384. [Google Scholar] [CrossRef]
- Hussain, M.D.W.; Giri, A.; Patra, A. Organic nanocages: A promising testbed for catalytic CO2 conversion. Sustain. Energy Fuels 2019, 3, 2567–2571. [Google Scholar] [CrossRef]
- Shih, C.F.; Zhang, T.; Li, J.; Bai, C. Powering the future with liquid sunshine. Joule 2018, 2, 1–25. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Artz, J.; Mueller, T.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable conversion of carbon dioxide: An integrated review of catalysis and life cycle assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef] [PubMed]
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- Martin, C.; Fiorani, G.; Kleij, A.W. Recent advances in the catalytic preparation of cyclic organic carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic strategies for the cycloaddition of pure, diluted, and waste CO2 to epoxides under ambient conditions. ACS Catal. 2018, 8, 419–450. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, J.-Y.; Liu, F.; Dai, S. Synthesis of porous polymeric catalysts for the conversion of carbon dioxide. ACS Catal. 2018, 8, 9079–9102. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Han, Z.; Ding, K.; Liu, H.; Xia, C.; Chen, J. Efficient production of methanol and diols via the hydrogenation of cyclic carbonates using copper-silica nanocomposite catalysts. Green Chem. 2015, 17, 4281–4290. [Google Scholar] [CrossRef]
- Han, Z.; Rong, L.; Wu, J.; Zhang, L.; Wang, Z.; Ding, K. Catalytic hydrogenation of cyclic carbonates: A practical approach from CO2 and epoxides to methanol and diols. Angew. Chem. Int. Ed. 2012, 51, 13041–13045. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Yan, L.; Wang, Y.; Jiang, M.; Ding, Y. Ionic liquid/Zn-PPh3 integrated porous organic polymers featuring multifunctional sites: Highly active heterogeneous catalyst for cooperative conversion of CO2 to cyclic carbonates. ACS Catal. 2016, 6, 6091–6100. [Google Scholar] [CrossRef]
- Li, F.; Xiao, L.; Xia, C.; Hu, B. Chemical fixation of CO2 with highly efficient ZnCl2/[BMIm]Br catalyst system. Tetrahedron Lett. 2004, 45, 8307–8310. [Google Scholar] [CrossRef]
- Xu, B.-H.; Wang, J.-Q.; Sun, J.; Huang, Y.; Zhang, J.-P.; Zhang, X.-P.; Zhang, S.-J. Fixation of CO2 into cyclic carbonates catalyzed by ionic liquids: A multi-scale approach. Green Chem. 2015, 17, 108–122. [Google Scholar] [CrossRef]
- Cheng, W.; Su, Q.; Wang, J.; Sun, J.; Ng, F.T.T. Ionic liquids: The synergistic catalytic effect in the synthesis of cyclic carbonates. Catalysts 2013, 3, 878–901. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Fanizzi, A. Cyclic carbonate formation from carbon dioxide and oxiranes in tetrabutylammonium halides as solvents and catalysts. Org. Lett. 2002, 4, 2561–2563. [Google Scholar] [CrossRef] [PubMed]
- Tiffner, M.; Gonglach, S.; Haas, M.; Schofberger, W.; Waser, M. CO2 Fixation with epoxides under mild conditions with a cooperative metal corrole/quaternary ammonium salt catalyst system. Chem. Asian J. 2017, 12, 1048–1051. [Google Scholar] [CrossRef]
- Zhao, L.-Y.; Chen, J.-Y.; Li, W.-C.; Lu, A.-H. B2O3: A heterogeneous metal-free Lewis acid catalyst for carbon dioxide fixation into cyclic carbonates. J. CO2 Util. 2019, 29, 172–178. [Google Scholar] [CrossRef]
- Sun, J.; Ren, J.; Zhang, S.; Cheng, W. Water as an efficient medium for the synthesis of cyclic carbonate. Tetrahedron Lett. 2009, 450, 423–426. [Google Scholar] [CrossRef]
- Wang, W.; Cui, L.; Sun, P.; Shi, L.; Yue, C.; Li, F. Reusable N-heterocyclic carbene complex catalysts and beyond: A perspective on recycling strategies. Chem. Rev. 2018, 118, 9843–9929. [Google Scholar] [CrossRef] [PubMed]
- Chalier, F.; Berchadsky, Y.; Finet, J.-P.; Gronchi, G.; Marque, S.; Tordo, P. Synthesis, X-ray geometry, and anodic behavior of tris[2 -(hydroxymethyl)phenyl]phosphane. J. Phys. Chem. 1996, 100, 4323–4330. [Google Scholar] [CrossRef]
- Bartlett, P.A.; Bauer, B.; Singer, S.J. Synthesis of water-soluble undecagold cluster compounds of potential importance in electron microscopic and other studies of biological systems. J. Am. Chem. Soc. 1978, 100, 5085–5089. [Google Scholar] [CrossRef]


| Entry | Catalyst | Gas Chromatography (GC) Yield (%) | TOF (h−1) |
|---|---|---|---|
| 1 | Iq-POC/ZnBr2 | 31 | 1550 |
| 2 | Iq-POC/ZnCl2 | 21 | 1050 |
| 3 | Iq-POC/ZnI2 | 28 | 1400 |
| 4 | ZnBr2 | trace | — |
| 5 | Iq-POC | 2 | 100 |
| 6 | CH3P+Ph3I−/ZnBr2 | 38 | 1900 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Mao, Y.; Jin, J.; Huo, Y.; Cui, L. The Ionic Organic Cage: An Effective and Recyclable Testbed for Catalytic CO2 Transformation. Catalysts 2021, 11, 358. https://doi.org/10.3390/catal11030358
Wang W, Mao Y, Jin J, Huo Y, Cui L. The Ionic Organic Cage: An Effective and Recyclable Testbed for Catalytic CO2 Transformation. Catalysts. 2021; 11(3):358. https://doi.org/10.3390/catal11030358
Chicago/Turabian StyleWang, Wenlong, Yuanyou Mao, Jutao Jin, Yanping Huo, and Lifeng Cui. 2021. "The Ionic Organic Cage: An Effective and Recyclable Testbed for Catalytic CO2 Transformation" Catalysts 11, no. 3: 358. https://doi.org/10.3390/catal11030358
APA StyleWang, W., Mao, Y., Jin, J., Huo, Y., & Cui, L. (2021). The Ionic Organic Cage: An Effective and Recyclable Testbed for Catalytic CO2 Transformation. Catalysts, 11(3), 358. https://doi.org/10.3390/catal11030358