Thermochemical Energy Storage Performance Analysis of (Fe,Co,Mn)Ox Mixed Metal Oxides
Abstract
:1. Introduction
2. Result and Discussion
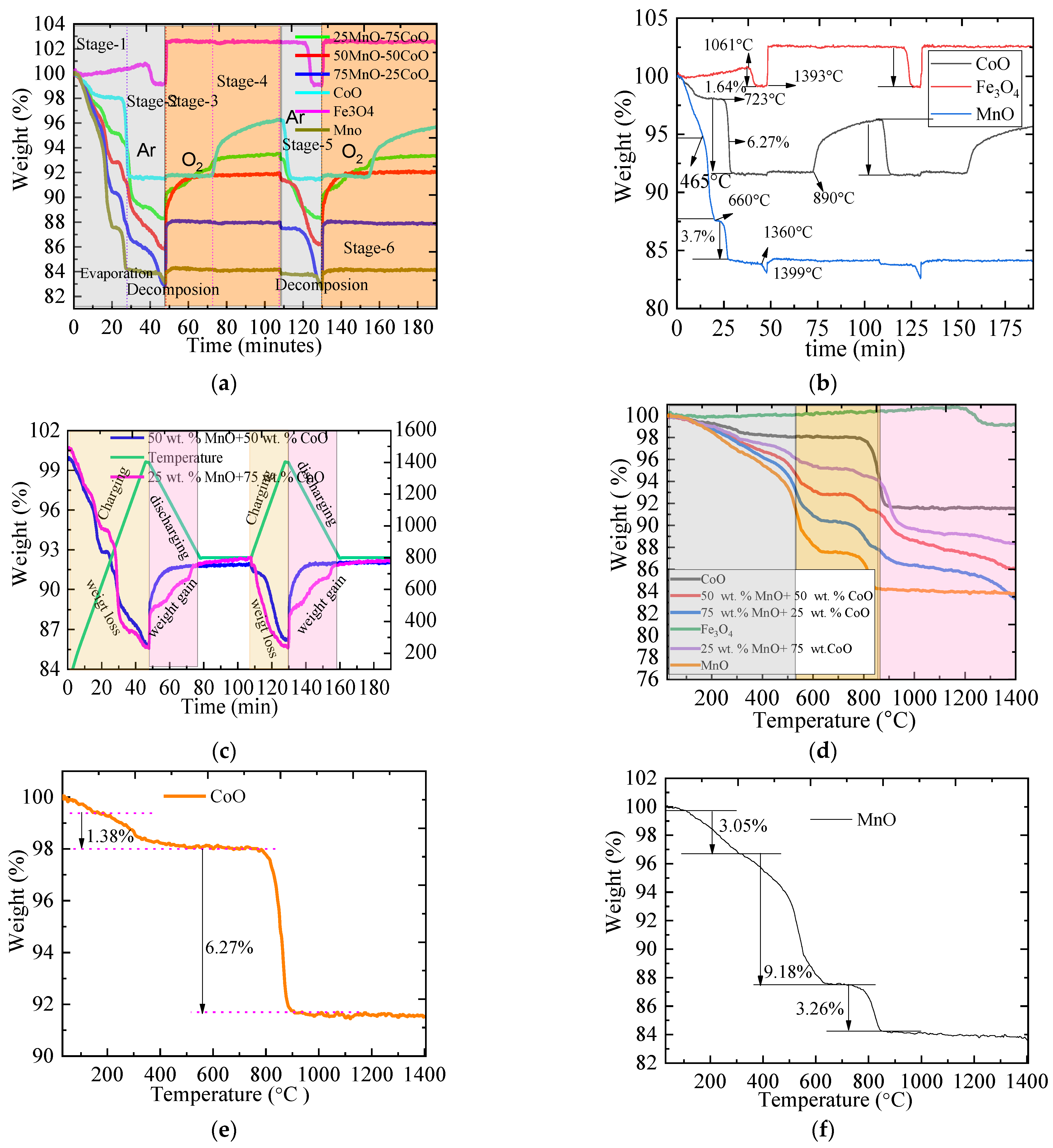
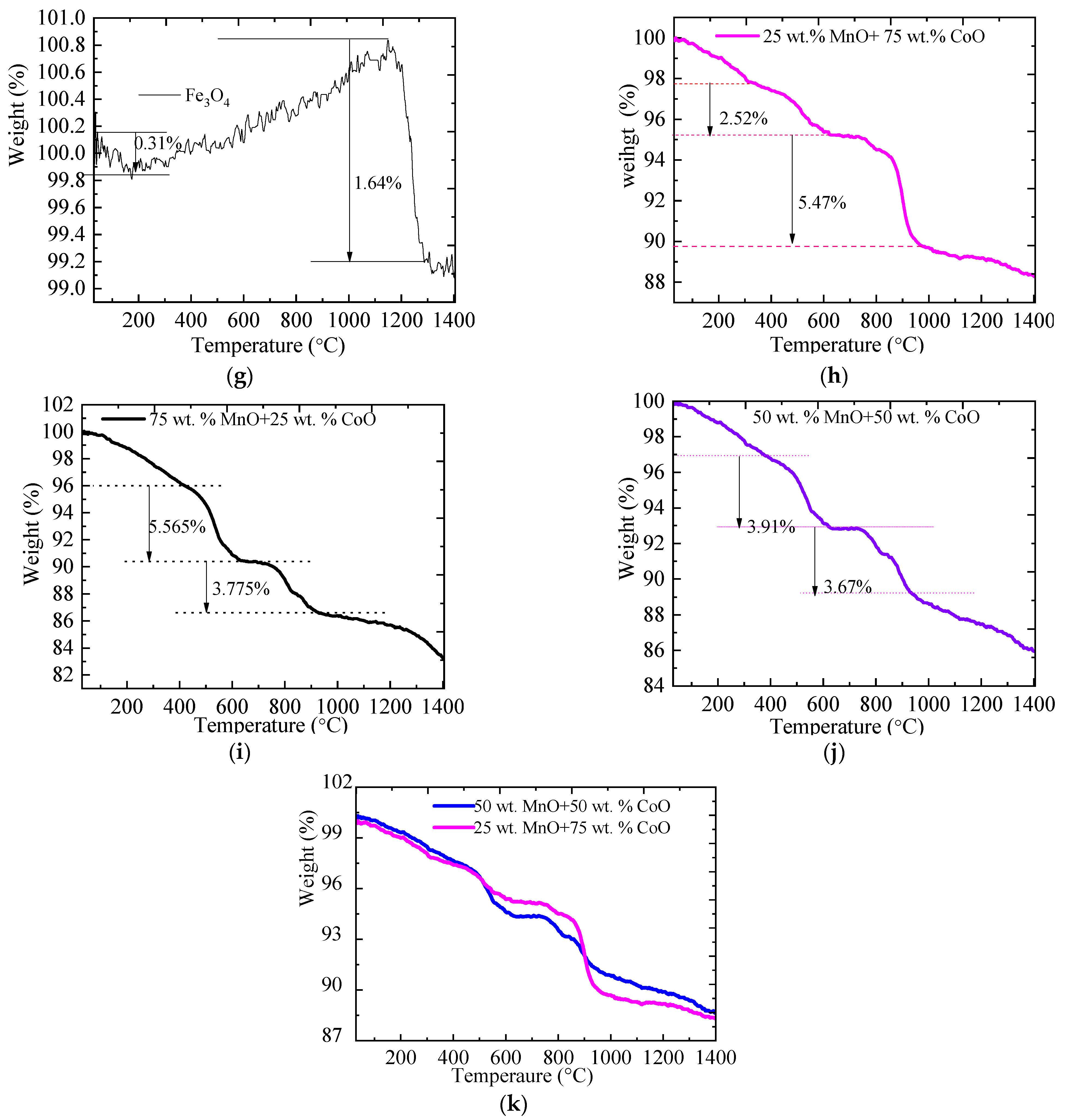
2.1. The TGA of Metallic Oxides during Redox Reactions
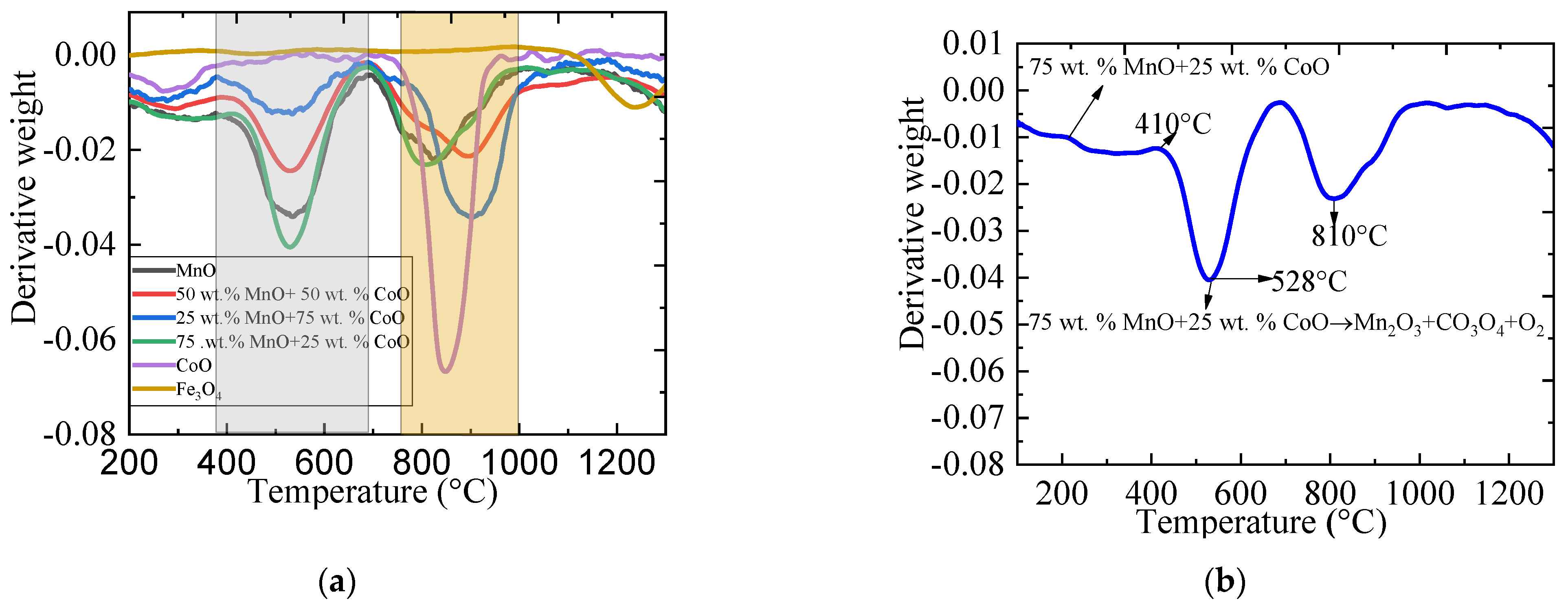
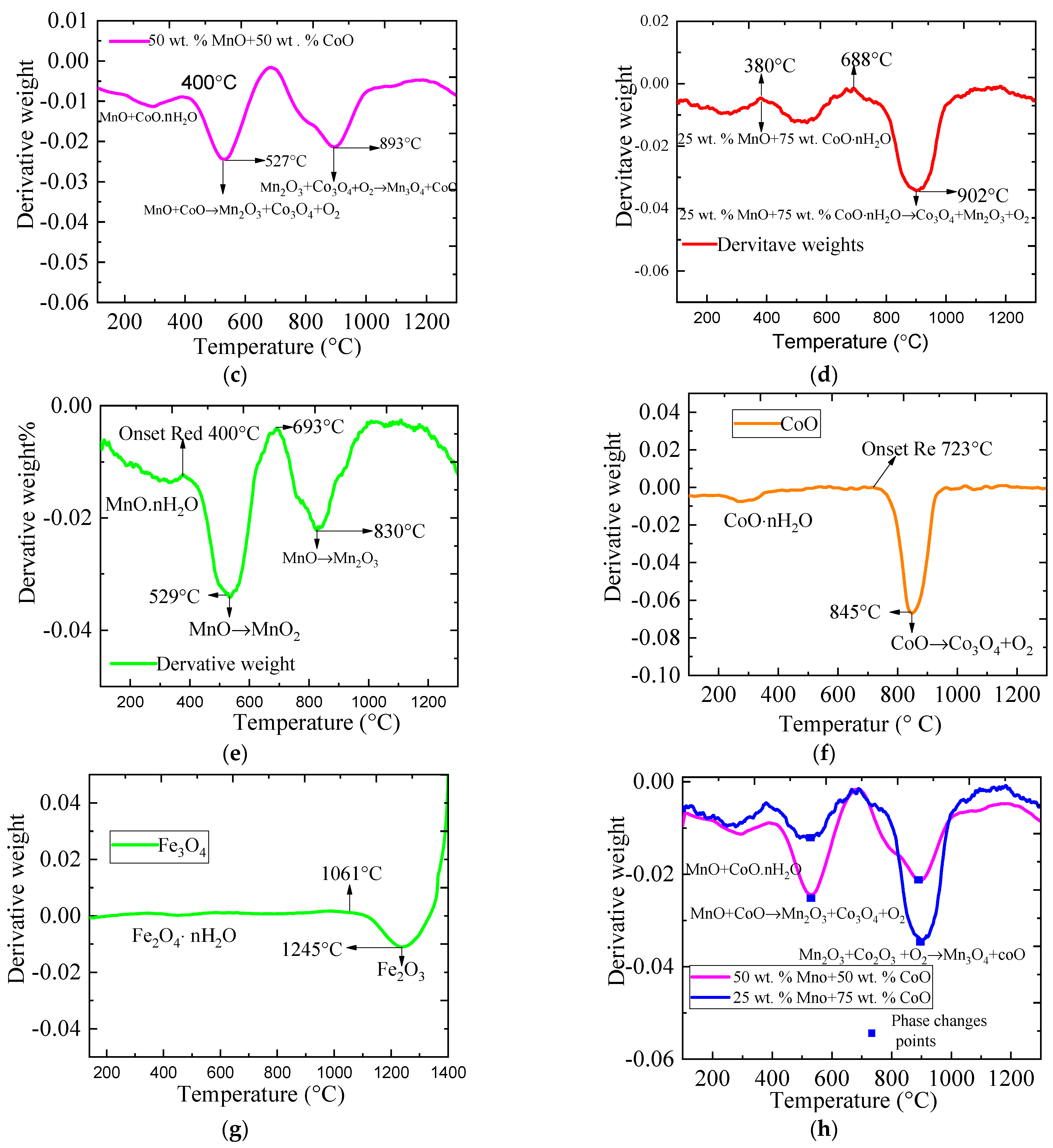
2.2. The Phase Change Conditions Using Weight Derivative Graphs (WDG) on Metallic Oxides during the Redox Reaction
2.3. The Thermal Performance and the Oxygen Uptake of Metallic Oxides
2.3.1. The Thermal Performance of Metallic Oxides
2.3.2. The Oxygen Storage Capacity of Metallic Oxides
2.4. The Impact of Gas Flow Rate on the Redox Reaction
2.5. The Effect of Heating Rate on Redox Reaction
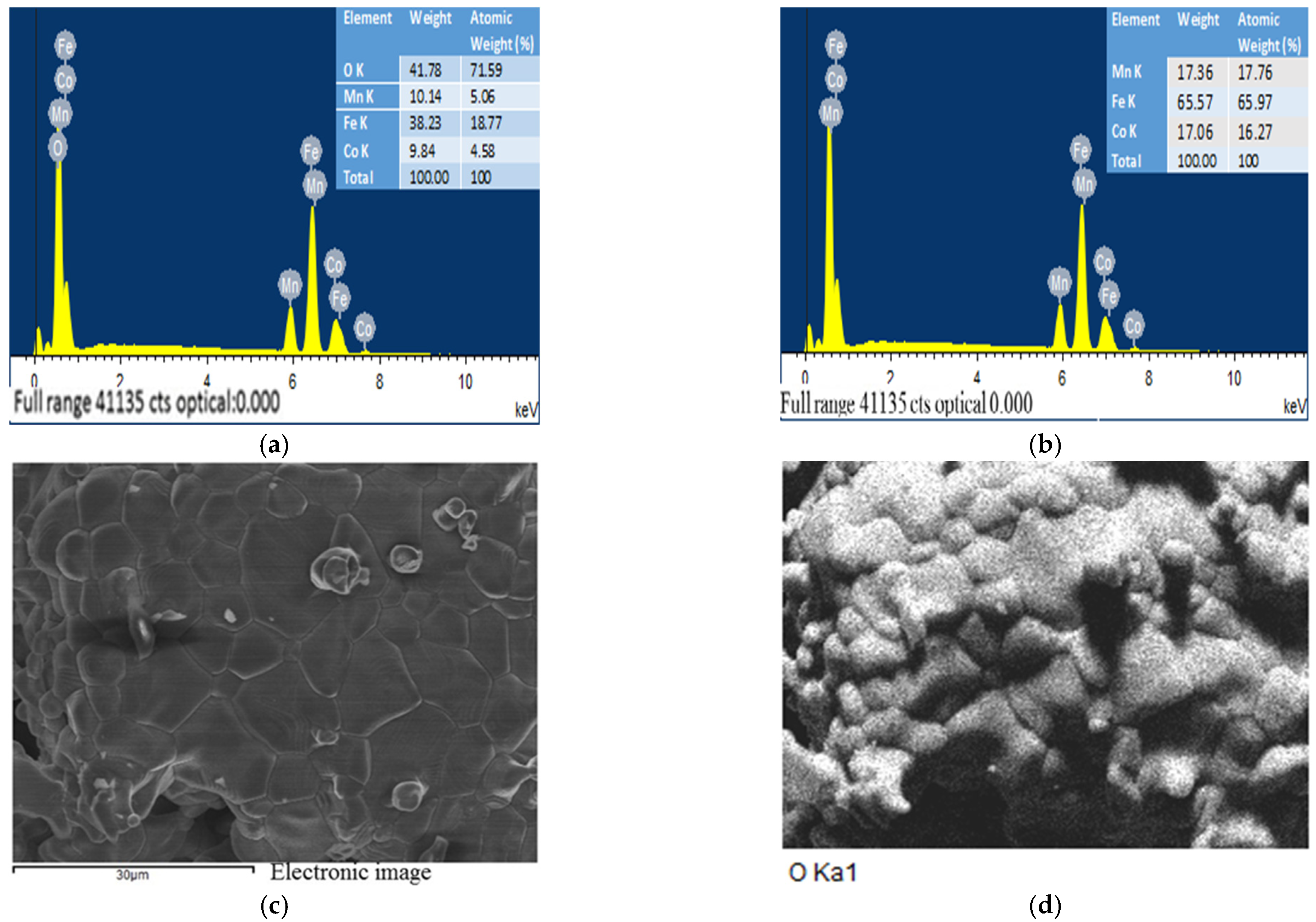
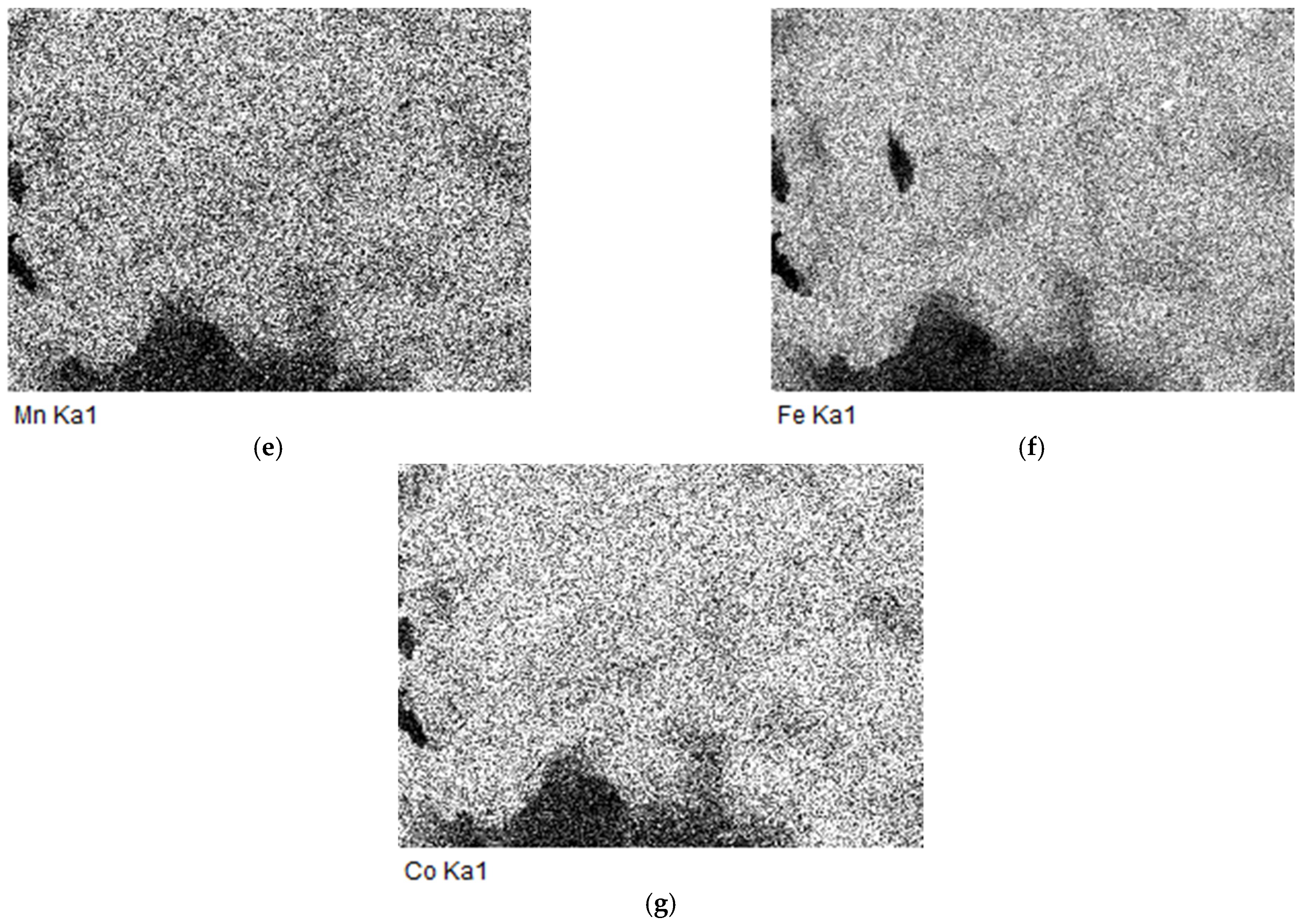
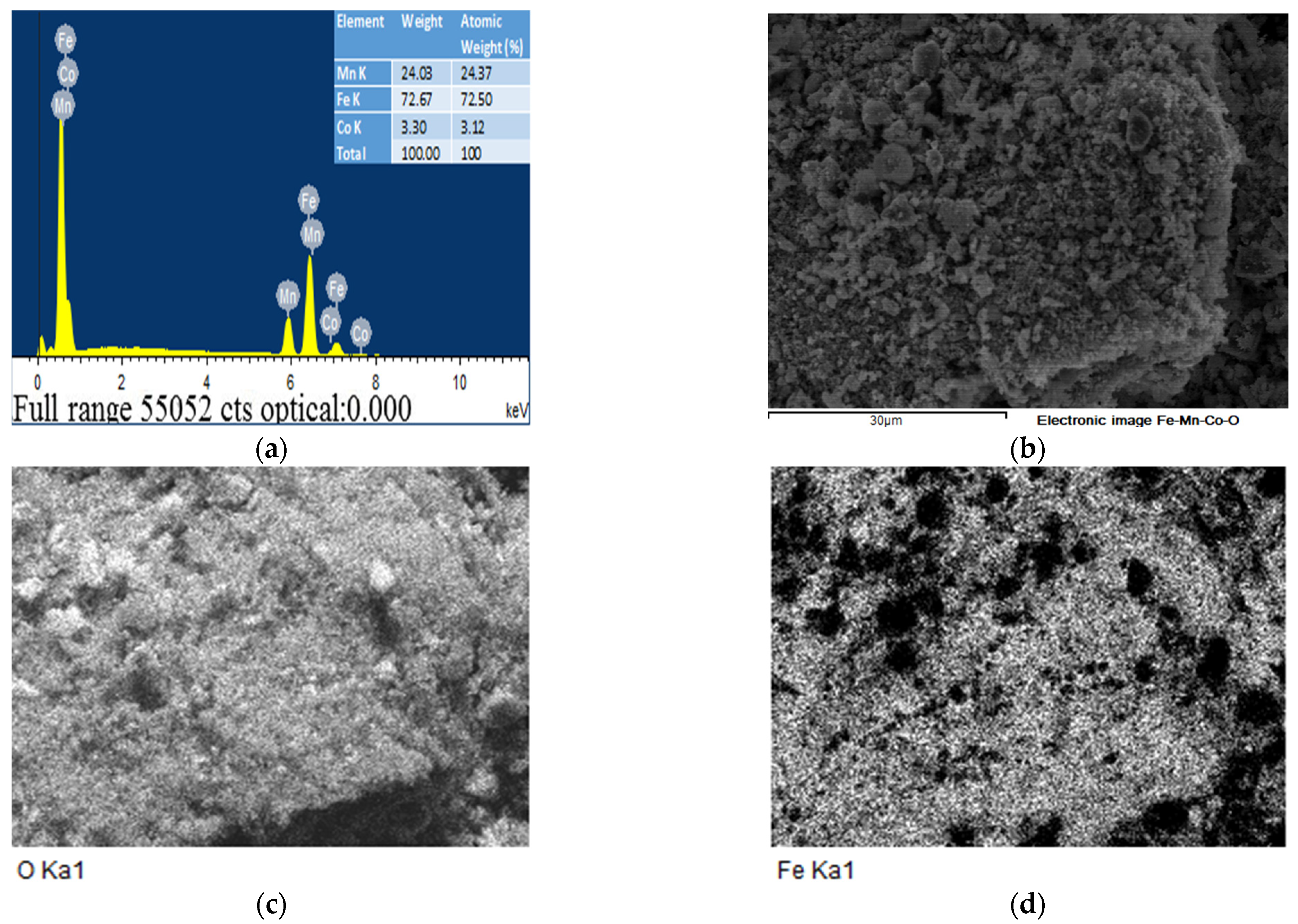
2.6. The EDS and SEM analysis of the (Fe,Co,Mn)Ox Material
EDS of the (Fe,Co,Mn)Ox Material
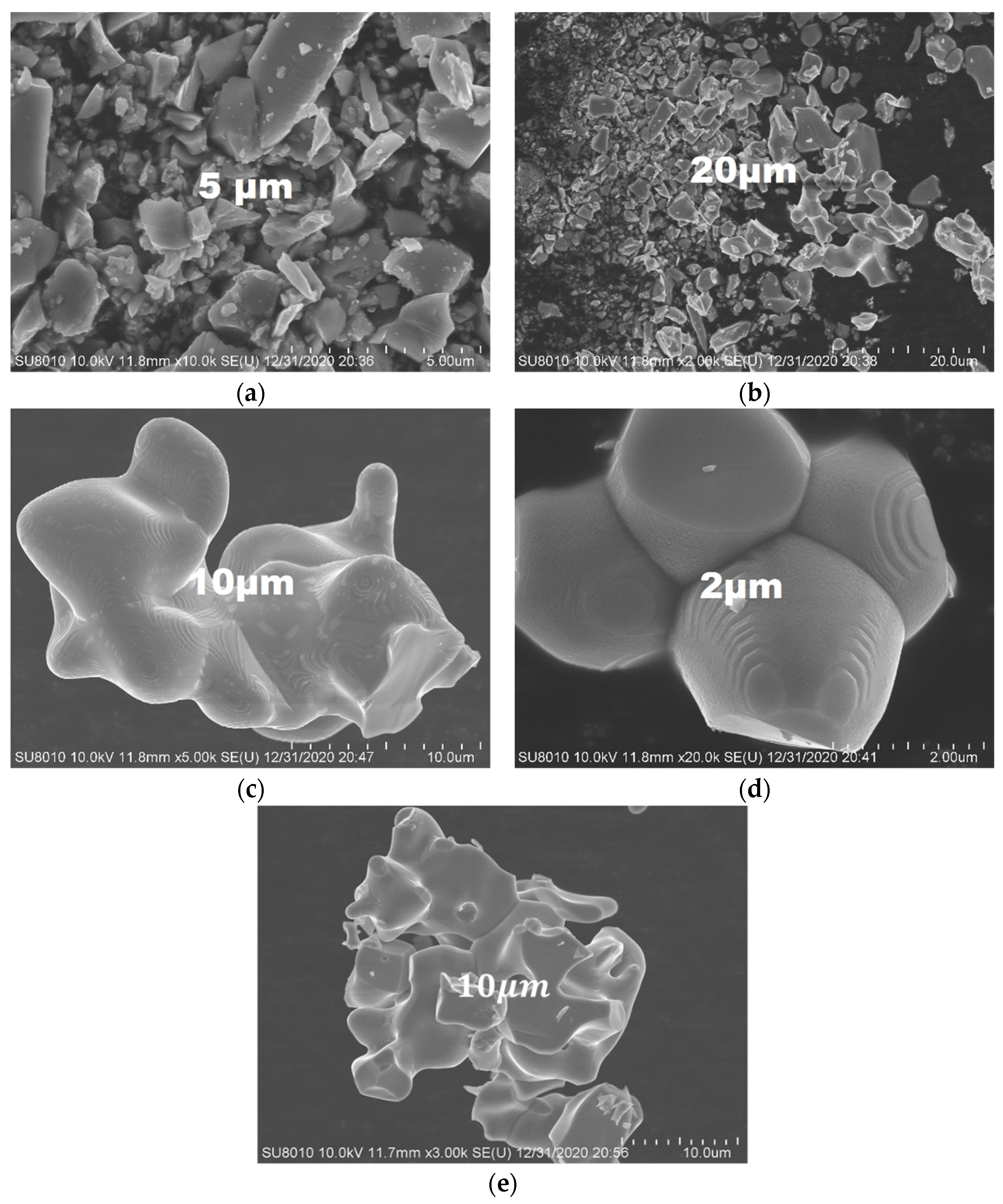
2.7. The SEM Analysis
3. Material and Methods
3.1. Material Synthesis and Characterization
3.2. Experimental Procedure for Thermochemical Redox Cycling
3.3. Thermodynamic Calculations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Medrano, M.; Gil, A.; Martorell, I.; Potau, X.; Cabeza, L.F. State of the art on high-temperature thermal energy storage for power generation. Part 2-Case studies. Renew. Sustain. Energy Rev. 2010, 14, 56–72. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A review of promising candidate reactions for chemical heat storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Farulla, G.A.; Cellura, M.; Guarino, F.; Ferraro, M. A review of the Thermochemical Emergy Storage Systems for Power Grid Support. Appl. Sci. Rev. 2020, 10, 3142. [Google Scholar] [CrossRef]
- Pelay, U.; Luo, L.; Fan, Y.; Stitou, D.; Rood, M. Thermal energy storage systems for concentrated solar power plants. Renew. Sustain. Energy Rev. 2017, 79, 82–100. [Google Scholar] [CrossRef]
- Ströhle, S.; Haselbacher, A.; Jovanovic, Z.R.; Steinfeld, A. The effect of the gas-solid contacting pattern in a high-temperature thermochemical energy storage on the performance of a concentrated solar power plant. Energy Environ. Sci. 2016, 9, 1375–1389. [Google Scholar] [CrossRef]
- Bayon, A.; Bader, R.; Jafarian, M.; Fedunik-Hofman, L.; Sun, Y.; Hinkley, J.; Miller, S.; Lipiński, W. Techno-economic assessment of solid-gas thermochemical energy storage systems for solar thermal power applications. Energy 2018, 149, 473–484. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Fujii, I.; Tsuchiya, K.; Higano, M.; Yamada, J. Studies of an energy storage system by use of the reversible chemical reaction: CaO + H2OCa(OH)2. Sol. Energy 1985, 34, 367–377. [Google Scholar] [CrossRef]
- Pardo, P.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cognet, P.; Cabassud, M. Ca(OH)2/CaO reversible reaction in a fluidized bed reactor for thermochemical heat storage. Sol. Energy 2014, 107, 605–616. [Google Scholar] [CrossRef] [Green Version]
- Linder, M.; Roßkopf, C.; Schmidt, M.; Wörner, A. Thermochemical energy storage in kW-scale based on CaO/Ca(OH)2. Energy Procedia 2013, 49, 888–897. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Long, X.F.; Lou, B.; Wu, J. Thermal cycling stability of thermochemical energy storage system Ca(OH)2/CaO. Appl. Therm. Eng. 2018, 133, 261–268. [Google Scholar] [CrossRef]
- Criado, Y.A.; Huille, A.; Rougé, S.; Abanades, J.C. Experimental investigation and model validation of a CaO/Ca(OH)2 fluidized bed reactor for thermochemical energy storage applications. Chem. Eng. J. 2017, 313, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Zhao, C.Y. First-principle study of CaO/Ca(OH)2 thermochemical energy storage system by Li or Mg cation doping. Chem. Eng. Sci. 2014, 117, 293–300. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Enhancement of a CaO/Ca(OH)2 based material for thermochemical energy storage. Sol. Energy 2016, 135, 800–809. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, C.; Romano, M.C.; Valverde, J.M.; Binotti, M.; Chacartegui, R. Process integration of Calcium-Looping thermochemical energy storage system in concentrating solar power plants. Energy 2018, 155, 535–551. [Google Scholar] [CrossRef]
- Chen, X.; Jin, X.; Liu, Z.; Ling, X.; Wang, Y. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping. Energy 2018, 155, 128–138. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Multicycle activity of natural CaCO3 minerals for thermochemical energy storage in Concentrated Solar Power plants. Sol. Energy 2017, 153, 188–199. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Evaluation and performances comparison of calcium, strontium and barium carbonates during calcination/carbonation reactions for solar thermochemical energy storage. J. Energy Storage 2017, 13, 193–205. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Nellore, R.; Moghtaderi, B. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle. Energy Convers. Manag. 2018, 168, 421–453. [Google Scholar] [CrossRef]
- Bulfin, B.; Vieten, J.; Agrafiotis, C.; Roeb, M.; Sattler, C. Applications and limitations of two step metal oxide thermochemical redox cycles: A review. J. Mater. Chem. A 2017, 5, 18951–18966. [Google Scholar] [CrossRef]
- Alonso, E.; Pérez-Rábago, C.; Licurgo, J.; Fuentealba, E.; Estrada, C.A. First experimental studies of solar redox reactions of copper oxides for thermochemical energy storage. Sol. Energy 2015, 115, 297–305. [Google Scholar] [CrossRef]
- Haseli, P.; Jafarian, M.; Nathan, G.J. High temperature solar thermochemical process for production of stored energy and oxygen based on CuO/Cu2O redox reactions. Sol. Energy 2017, 153, 1–10. [Google Scholar] [CrossRef]
- Hänchen, M.; Stiel, A.; Jovanovic, Z.R.; Steinfeld, A. Thermally driven copper oxide redox cycle for the separation of oxygen from gases. Ind. Eng. Chem. Res. 2012, 51, 7013–7021. [Google Scholar] [CrossRef]
- Clayton, C.K.; Whitty, K.J. Measurement and modeling of decomposition kinetics for copper oxide-based chemical looping with oxygen uncoupling. Appl. Energy 2014, 116, 416–423. [Google Scholar] [CrossRef]
- Alonso, E.; Pérez-Rábago, C.; Licurgo, J.; Gallo, A.; Fuentealba, E.; Estrada, C.A. Experimental aspects of CuO reduction in solar-driven reactors: Comparative performance of a rotary kiln and a packed bed. Renew. Energy 2017, 105, 665–673. [Google Scholar] [CrossRef]
- Schrader, A.J.; Muroyama, A.P.; Loutzenhiser, P.G. Solar electricity via an Air Brayton cycle with an integrated two-step thermochemical cycle for heat storage based on Co3O4/CoO redox reactions: Thermodynamic analysis. Sol. Energy 2015, 118, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Tescari, S.; Lantin, G.; Agrafiotis, C.; Roeb, M.; Sattler, C. Solar thermochemical heat storage via the Co3O4/CoO looping cycle: Storage reactor modelling and experimental validation. Sol. Energy 2017, 144, 453–465. [Google Scholar] [CrossRef]
- Abedin, A.H.; Rosen, M.A. A critical review of thermochemical energy storage systems. Open Renew. Energy 2011, 4, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Ruan, Z.-H.; Huang, X.; Jiang, Y.-Q.; Tan, H.-P. Energy-absorption-based explanation of the TiO2/C photocatalytic activity enhancement mechanism. J. Catal. 2017, 348, 246–255. [Google Scholar] [CrossRef]
- Fuqiang, W.; Ziming, C.; Jianyu, T.; Yuan, Y.; Yong, S.; Linhua, L. Progress in concentrated solar power technology with parabolic trough collector system: A comprehensive review. Renew. Sustain. Energy. Rev. 2017, 79, 1314–1328. [Google Scholar] [CrossRef]
- Block, T.; Knoblauch, N.; Shmücker, M. The cobalt-oxide/iron-oxide binary system for use as high temperature thermochemical energy storage material. Thermochim. Acta 2014, 577, 25–32. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Schücker, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solar heat. Part 2: Redox oxide-coated porous ceramic structures as integrated thermochemical reactors/heat exchangers. Sol. Energy 2015, 114, 440–458. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Tescari, S.; Roeb, M.; Schücker, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solarheat.Part 3: Cobaltoxide monolithic porous structures as integrated thermochemical reactors/heat exchanger. Sol. Energy 2015, 114, 459–475. [Google Scholar] [CrossRef]
- Neises, M.; Tescari, S.; DeOliveira, L.; Roeb, M.; Sattler, C.; Wong, B. Solar-heated rotary kilnfor thermochemical energy storage. Sol. Energy 2012, 86, 3040–3048. [Google Scholar] [CrossRef]
- Tescari, S.; Agrafiotis, C.; Breuer, S.; DeOliveira, L.; Nieses-vonPuttkamer, M.; Roeb, M.; Sattler, C. Thermochemical solar energy storage varied oxides: Materials and reactor/heat exchanger concepts. Energy Procedia 2014, 49, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Karagiannakis, G.; Pagkoura, C.; Zygogianni, A.; Lorentzou, S.; Konstandopoulos, A.G. Monolithic ceramic redox materials for thermochemical heat storage applications in CSP plants. Energy Procedia 2014, 49, 820–829. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Improving the thermo- chemical energy storage performance of the Mn2O3/Mn3O4 redox couple by the incorporation of iron. ChemSusChem 2015, 8, 1947–1954. [Google Scholar] [CrossRef]
- Pagkoura, C.; Karagiannakis, G.; Zygogianni, A.; Lorentzou, S.; Kostoglou, M.; Konstandopoulos, A.G.; Rattenburry, M.; Woodhead, W.J. Cobalt oxide based structured bodies as redox thermochemical heat storage medium for future CSP plants. Sol. Energy 2014, 108, 146–163. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical heat storage based on the Mn2O3/Mn3O4 redox couple: Influence of the initial particle on the morphological evolution and cyclability. J. Mater. Chem. A 2014, 2, 19435–19443. [Google Scholar] [CrossRef]
- Jung, I.H.; Decterov, S.A.; Pelton, A.D.; Kim, H.M.; Kang, Y.B. Thermodynamic evaluation and modeling of the Fe-Co-O system. Acta Mater. 2004, 52, 507–519. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Chen, M. Thermodynamic modeling of the Co–Fe–O system. Calphad 2013, 41, 76–88. [Google Scholar] [CrossRef]
- Kang, Y.B.; Jung, I.H. Thermodynamic modeling of oxide phases in the Fe-Mn-O system. J. Phys. Chem. Solids 2016, 98, 237–246. [Google Scholar] [CrossRef]
- Kjellqvist, L.; Selleby, M. Thermodynamic assessment of the Fe-Mn-O system. J. Phase Equilibria Diffus. 2010, 31, 113–134. [Google Scholar] [CrossRef]
- Guene Lougou, B.; Shuai, Y.; Guohua, Z.; Chaffa, G.; Ahouannou, C.; Tan, H. Analysis of H2 and CO production via solar thermochemical reacting system of NiFe2O4 redox cycles combined with CH4 partial oxidation. Int. J. Hydrog. Energy 2018, 43, 5996–6010. [Google Scholar] [CrossRef]
- Lougou, B.G.; Shuai, Y.; Chaffa, G.; Ahouannou, C.; Pan, R.; Zhang, H.; Tan, H. Analysis of two-step solar thermochemical looping reforming of Fe3O4 redox cycles for synthesis gas production. Energy Technol. 2018, 7, 1800588. [Google Scholar] [CrossRef]
- Lougou, B.G.; Shuai, Y.; Pan, R.; Chaffa, G.; Tan, H. Heat transfer and fluid flow analysis of porous medium solar thermochemical reactor with quartz glass cover. Int. J. Heat. Mass. Transf. 2018, 127, 61–74. [Google Scholar] [CrossRef]
- Lougou, B.G.; Shuai, Y.; Pan, R.; Chaffa, G.; Ahouannou, C.; Zhang, H.; Tan, H. Radiative heat transfer and thermal characteristics of Fe-based oxides coated SiC and Alumina RPC structures as integrated solar thermochemical reactor. Sci. China Technol. Sci. 2018, 61, 1788–1801. [Google Scholar] [CrossRef]
- Guene Lougou, B.; Han, D.; Zhang, H.; Jiang, B.; Anees, J.; Ahouannou, C.; Zhao, J.; Shuai, Y. Numerical and experimental analysis of reactor optimum design and solar thermal-chemical energy conversion for multidisciplinary applications. Energy Convers. Manag. 2020, 213, 112870. [Google Scholar] [CrossRef]
- Shuai, Y.; Zhang, H.; Guene Lougou, B.; Jiang, B.; Mustafa, A.; Wang, C.-H.; Wang, F.; Zhao, J. Solar-driven thermochemical redox cycles of ZrO2 supported NiFe2O4 for CO2 reduction into chemical energy. Energy 2021, 223, 120073. [Google Scholar] [CrossRef]
- Shuai, Y.; Guene Lougou, B.; Zhang, H.; Zhao, J.; Ahouannou, C.; Tan, H. Heat transfer analysis of solar-driven high-temperature thermochemical reactor using NiFe-Aluminate RPCs. Int. J. Hydrog. Energy 2021, 46, 10104–10118. [Google Scholar] [CrossRef]
- Getahun Dessie, Y.; Guene Lougou, B.; Qi, H.; Tan, H. Reactor Design and Thermal Performance Analysis for Solar Thermal Energy Storage Application. Energies 2020, 13, 3186. [Google Scholar] [CrossRef]
- Getahun Dessie, Y.; Guene Lougou, B.; Qi, H.; Tan, H.; Zhang, J.; Gao, B.; Islam, M.A. Thermal Performance Analysis of a Solar Reactor Designed for Syngas Production. Energies 2020, 13, 3405. [Google Scholar] [CrossRef]
- Guene Lougou, B.; Shuai, Y.; Zhang, H. Thermochemical CO2 reduction over NiFe2O4@alumina filled reactor heated by high-flux solar simulator. Energy 2020, 197, 117267. [Google Scholar] [CrossRef]
- Serra, R.; Nomen, R.; Sempere, J. The non-parametric kinetics a new method for the kinetic study of thermoanalytical data. J. Thermal. Anal. Calorim. 1998, 52, 933–943. [Google Scholar] [CrossRef]
- Serra, R.; Sempere, J.; Nomen, R. A new method for the kinetic study of thermoanalytical data: The nonparametric kinetics method. Thermochim. Acta 1998, 316, 37–45. [Google Scholar] [CrossRef]
- Sempere, J.; Nomen, R.; Serra, R.; Soravilla, J. The NPK method—An innovative approach for kinetic analysis of data from thermal analysis and calorimetry. Thermochim. Acta 2002, 388, 407–414. [Google Scholar] [CrossRef]
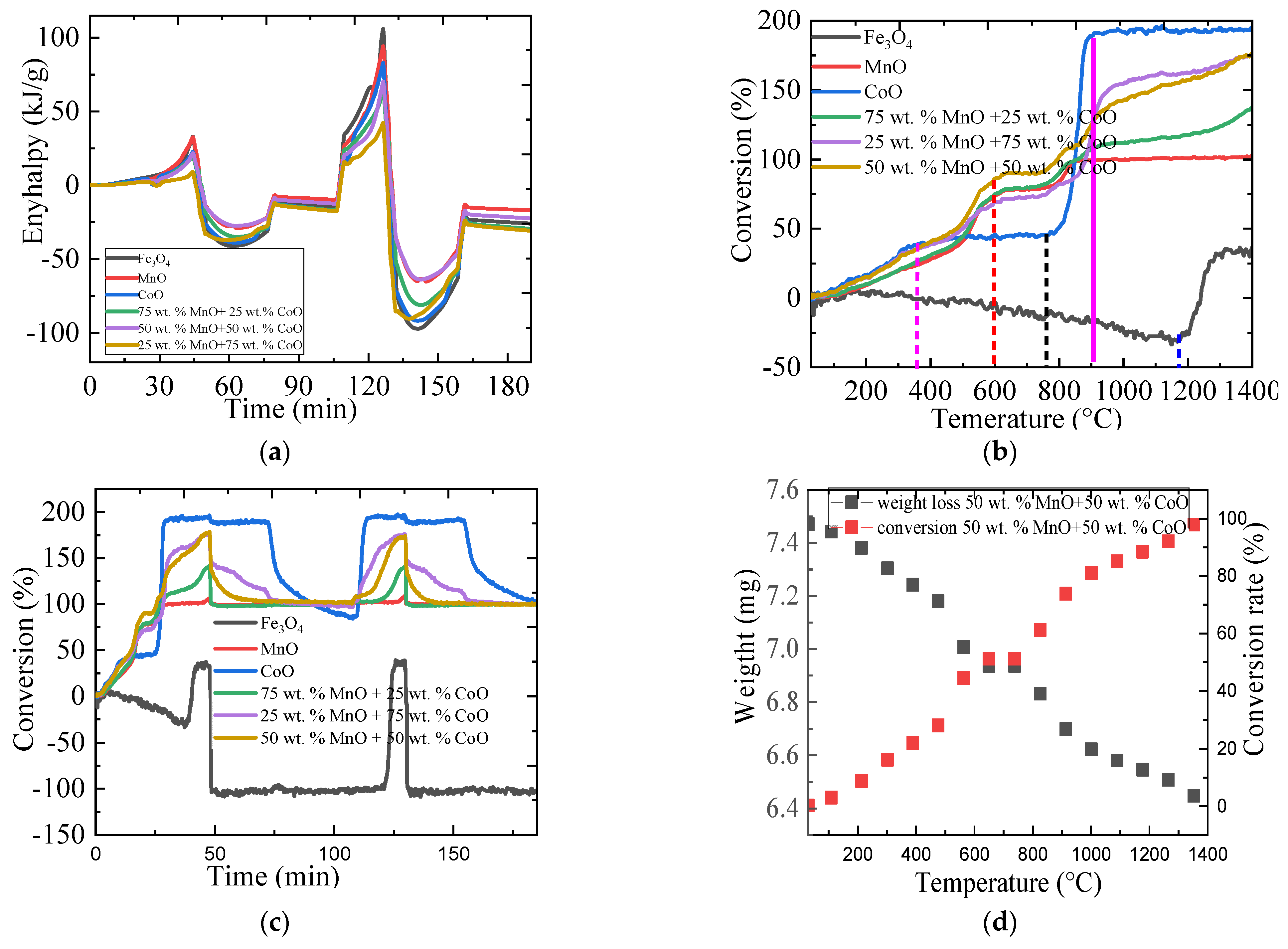

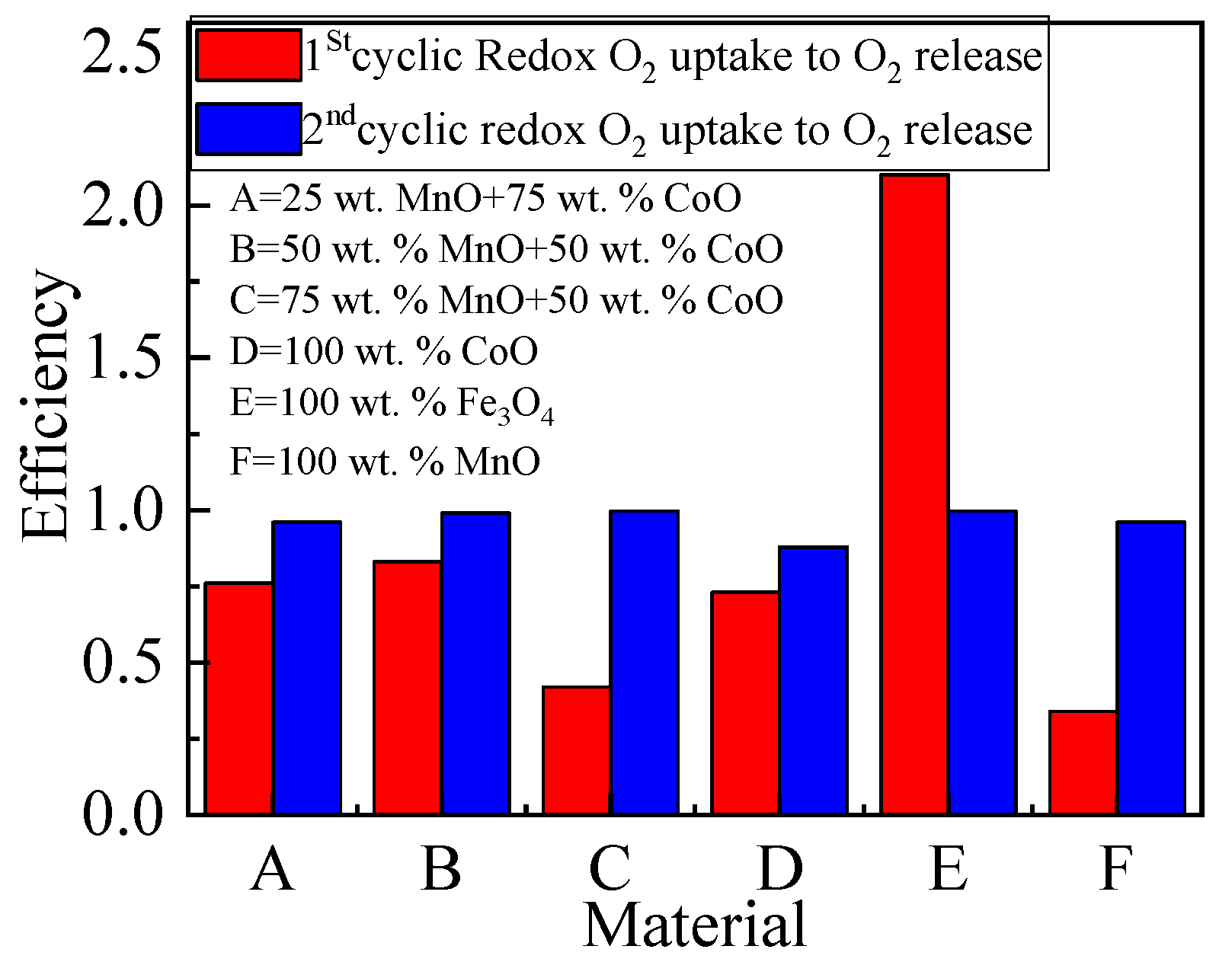
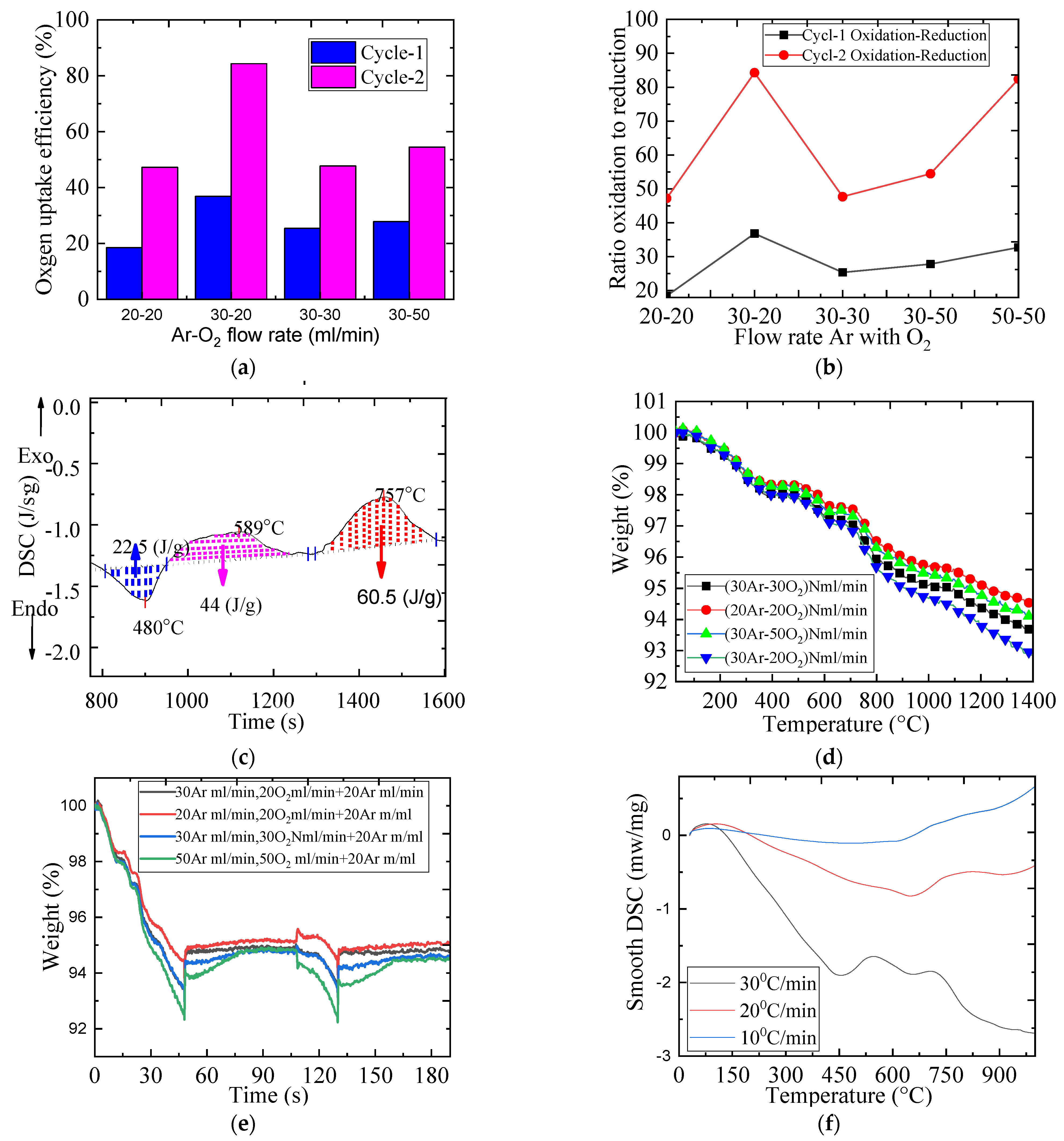
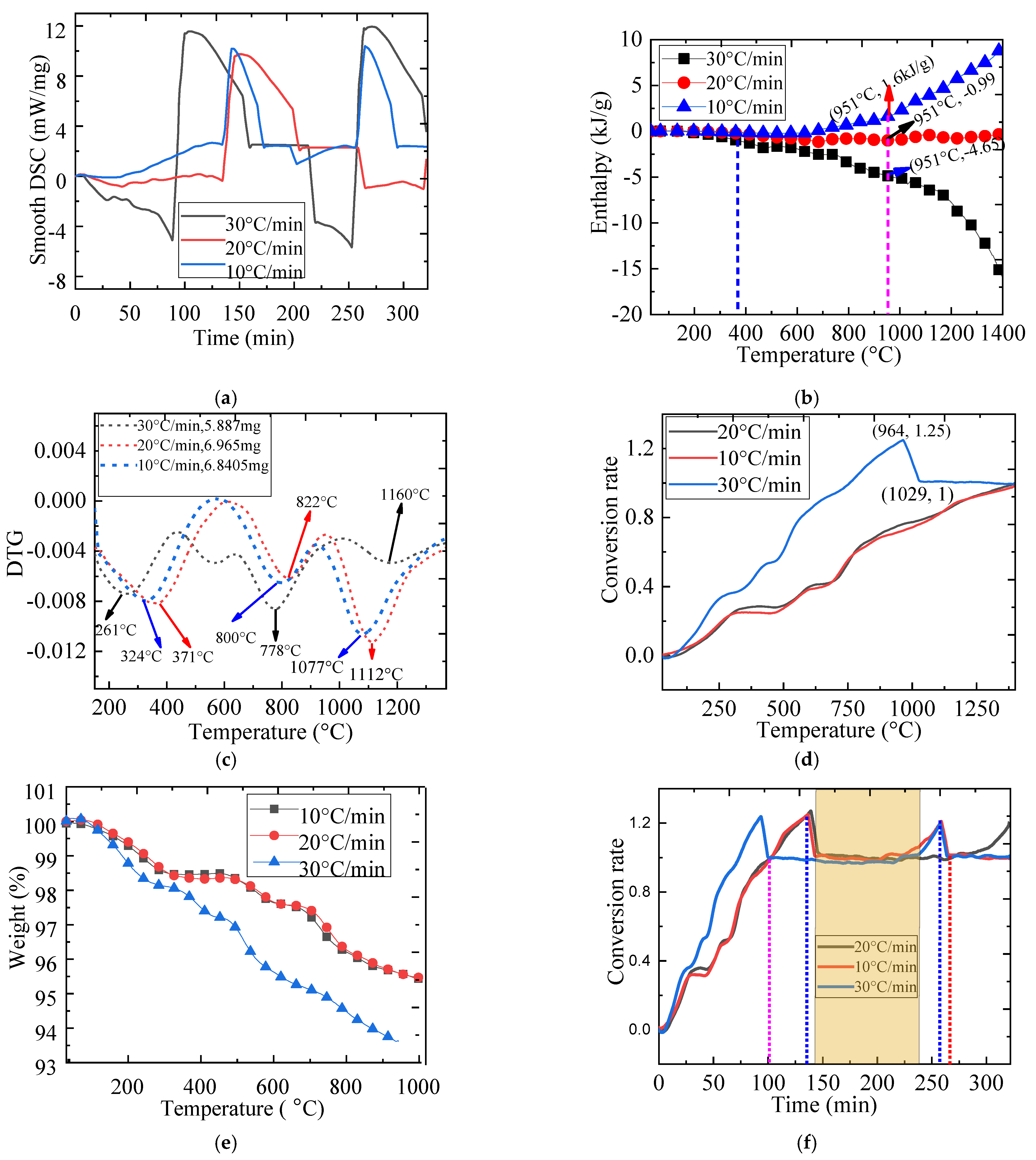


| Material | Onset Temperature Reduction (°C) | Conversion | Enthalpy (J/g) | Phase Changing Temperature (°C) | |
|---|---|---|---|---|---|
| Crystallization | Melting | ||||
| 50 wt.% MnO + 50 wt.% CoO | 406 | 92.340 | 28 | 30 | 530–895 |
| 25 wt.% MnO + 75 wt.% CoO | 395 | 87.500 | No observation | 403 | 518–905 |
| 75 wt.% MnO + 25 wt.% CoO | 425 | 87.600 | 13 | 8 | 529–808 |
| CoO | 720 | 86.00 | No observation | 284.70 | 850 |
| MnO | 400 | 83.800 | No observation | 33 | 525–834 |
| Fe3O4 | 1063 | 90.400 | No observation | 28 | 1245 |
| Material | Energy Content in kJ/g at 952 °C | O2/O2 Ratio of Energy Uptake to Energy Release at 952 °C in % | ||||
|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | |||||
| Reduction | Oxidation | Reduction | Oxidation | Cycle 1 | Cycle 2 | |
| 50 wt.% MnO + 50 wt.% CoO | 4.4 | 26.300 | 23.800 | 57.300 | 16.700 | 41.500 |
| 25 wt.% MnO + 75 wt.% CoO | 2.2 | 33 | 16.400 | 71 | 6.700 | 23.100 |
| 75 wt.% MnO + 25 wt.% CoO | 4.2 | 32.400 | 25.500 | 73 | 12.960 | 34.900 |
| CoO | 6.4 | 36.700 | 23.900 | 80.90 | 17.400 | 29.500 |
| MnO | 6 | 27.300 | 32 | 58 | 24.200 | 55.200 |
| Fe3O4 | 6.4 | 40 | 39.500 | 82 | 16 | 48.200 |
| Materials | O2 Released (μmol/g) | O2 Uptake (μmol/g) | O2 Uptake/O2 Released Ratio | |||
|---|---|---|---|---|---|---|
| 1st Cycle | 2nd Cycle | 1st Cycle | 2nd Cycle | 1st Cycle | 2nd Cycle | |
| 25 wt.% Mno + 75 wt.% CoO | 2125 | 1625 | 1625 | 1563 | 0.760 | 0.960 |
| 50 wt.% Mno + 50 wt.% CoO | 2250 | 1843 | 1875 | 1819 | 0.830 | 0.990 |
| 75 wt.% Mno + 50 wt.% CoO | 3972 | 1569 | 1650 | 1561 | 0.420 | 0.996 |
| 100 wt.% CoO | 2032 | 1413 | 1475 | 1238 | 0.730 | 0.880 |
| 100 wt.% Fe3O4 | 516 | 1066 | 1097 | 1063 | 2.100 | 0.997 |
| 100 wt.% Mno | 1372 | 391 | 469 | 375 | 0.340 | 0.960 |
| Materials | O2 RELEASED (μmol/g) | O2 Uptake (μmol/g) | O2 Uptake/O2 Released Ratio | |||
|---|---|---|---|---|---|---|
| Argon and O2 Flow | 1st Cycle | 2nd Cycle | 1st Cycle | 2nd Cycle | 1st Cycle | 2nd Cycle |
| 20–20 | 1017 | 338 | 188 | 159 | 18.440 | 47.200 |
| 30–20 | 1097 | 388 | 404 | 327 | 36.810 | 84.270 |
| 30–30 | 1159 | 393 | 293 | 187 | 25.350 | 47.690 |
| 30–50 | 1129 | 463 | 314 | 243 | 27.810 | 54.465 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dessie, Y.G.; Hong, Q.; Lougou, B.G.; Zhang, J.; Jiang, B.; Anees, J.; Tegegne, E.B. Thermochemical Energy Storage Performance Analysis of (Fe,Co,Mn)Ox Mixed Metal Oxides. Catalysts 2021, 11, 362. https://doi.org/10.3390/catal11030362
Dessie YG, Hong Q, Lougou BG, Zhang J, Jiang B, Anees J, Tegegne EB. Thermochemical Energy Storage Performance Analysis of (Fe,Co,Mn)Ox Mixed Metal Oxides. Catalysts. 2021; 11(3):362. https://doi.org/10.3390/catal11030362
Chicago/Turabian StyleDessie, Yabibal Getahun, Qi Hong, Bachirou Guene Lougou, Juqi Zhang, Boshu Jiang, Junaid Anees, and Eyale Bayable Tegegne. 2021. "Thermochemical Energy Storage Performance Analysis of (Fe,Co,Mn)Ox Mixed Metal Oxides" Catalysts 11, no. 3: 362. https://doi.org/10.3390/catal11030362








