A Review on the Catalytic Hydrogenation of Bromate in Water Phase
Abstract
:1. Introduction
2. Catalysts for the Bromate Hydrogenation
2.1. Influence of the Metallic Active Species
2.2. Influence of the Support
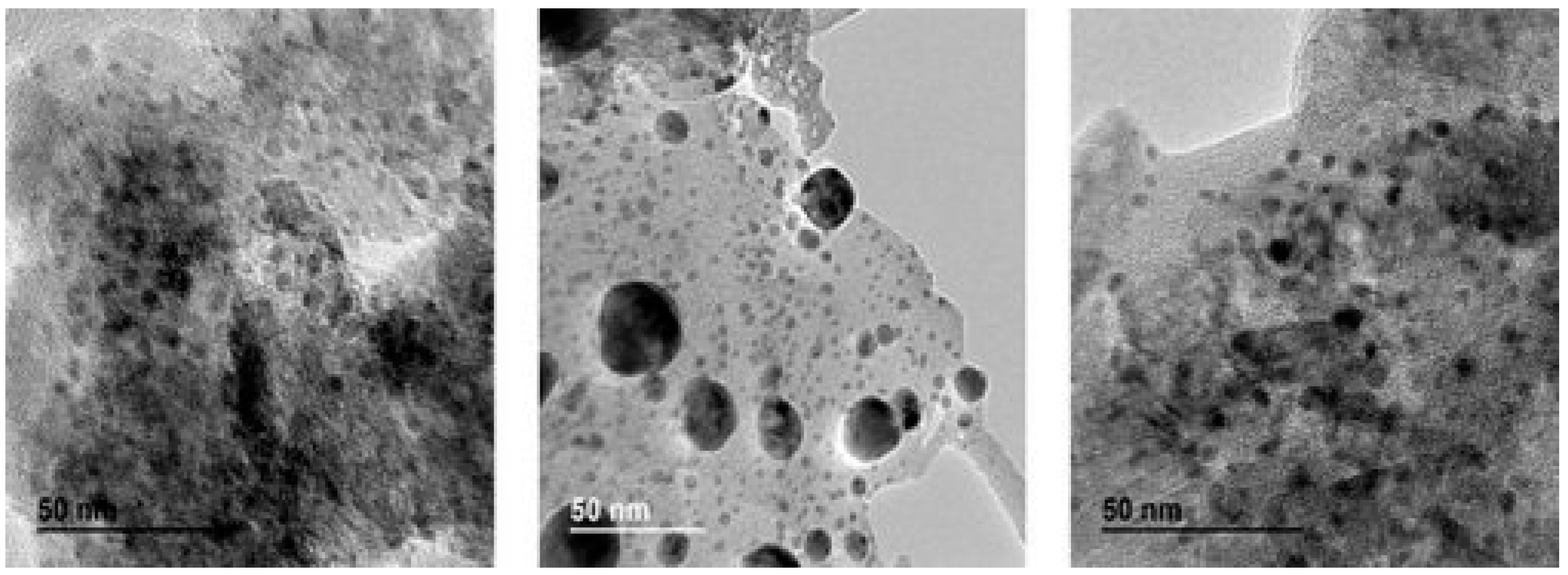
3. Catalyst Characterization and Active Sites
4. Reaction Mechanism
5. Influence of the Water Composition and Stability Tests
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Armor, J.N. Environmental catalysis. Appl. Catal. B Environ. 1992, 1, 221–256. [Google Scholar] [CrossRef]
- Armor, J. Catalytic solutions to reduce pollutants. Catal. Today 1997, 38, 163–167. [Google Scholar] [CrossRef]
- Moreno-González, M.; Blasco, T.; Góra-Marek, K.; Palomares, A.; Corma, A. Study of propane oxidation on Cu-zeolite catalysts by in-situ EPR and IR spectroscopies. Catal. Today 2014, 227, 123–129. [Google Scholar] [CrossRef]
- Cucciniello, R.; Intiso, A.; Siciliano, T.; Palomares, A.E.; Martínez-Triguero, J.; Cerrillo, J.L.; Proto, A.; Rossi, F. Oxidative Degradation of Trichloroethylene over Fe2O3–doped Mayenite: Chlorine Poisoning Mitigation and Improved Catalytic Performance. Catalysts 2019, 9, 747. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Labaki, M.; Giraudon, J.-M.; Lamonier, J.-F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef]
- Zhang, N.; Ye, C.; Yan, H.; Li, L.; He, H.; Wang, D.; Li, Y. Single-atom site catalysts for environmental catalysis. Nano Res. 2020, 13, 1–18. [Google Scholar] [CrossRef]
- Jablonska, M.; Palomares Gimeno, A.E.; Wegrzyn, A.; Chmielarz, L. A short review about NOx storage/reduction catalysts based on metal oxides and hydrotalcite-type anionic clays. Acta Geodyn. Geomater. 2014, 11, 175–186. [Google Scholar] [CrossRef]
- Blanch-Raga, N.; Palomares, A.E.; Martínez-Triguero, J.; Puche, M.; Fetter, G.; Bosch, P. The oxidation of trichloroethylene over different mixed oxides derived from hydrotalcites. Appl. Catal. B Environ. 2014, 160, 129–134. [Google Scholar] [CrossRef]
- Chaplin, B.P.; Reinhard, M.; Schneider, W.F.; Schuth, C.; Shapley, J.R.; Strathmann, T.J.; Werth, C.J. Critical Review of Pd-Based Catalytic Treatment of Priority Contaminants in Water. Environ. Sci. Technol. 2012, 46, 3655–3670. [Google Scholar] [CrossRef]
- Hu, M.; Liu, Y.; Yao, Z.; Ma, L.; Wang, X. Catalytic reduction for water treatment. Front. Environ. Sci. Eng. 2018, 12, 3. [Google Scholar] [CrossRef]
- Yin, Y.B.; Guo, S.; Heck, K.N.; Clark, C.A.; Coonrod, C.L.; Wong, M.S. Treating water by degrading oxyanions using metallic nanostructures. Acs Sustain. Chem. Eng. 2018, 6, 11160–11175. [Google Scholar] [CrossRef] [Green Version]
- Frierdich, A.J.; Shapley, J.R.; Strathmann, T.J. Rapid reduction of N-nitrosamine disinfection byproducts in water with hydrogen and porous nickel catalysts. Environ. Sci. Technol. 2008, 42, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, A.S.; Zaki, A.; Troncea, S.; Casale, S.; Vinod, C.; Dacquin, J.; Granger, P. Enhanced selectivity of 3-D ordered macroporous Pt/Al2O3 catalysts in nitrites removal from water. Appl. Catal. A Gen. 2018, 564, 26–32. [Google Scholar] [CrossRef]
- Palomares, A.; Franch, C.; Corma, A. A study of different supports for the catalytic reduction of nitrates from natural water with a continuous reactor. Catal. Today 2011, 172, 90–94. [Google Scholar] [CrossRef]
- Yaseneva, P.; Marti, C.F.; Palomares, E.; Fan, X.; Morgan, T.; Perez, P.S.; Ronning, M.; Huang, F.; Yuranova, T.; Kiwi-Minsker, L. Efficient reduction of bromates using carbon nanofibre supported catalysts: Experimental and a comparative life cycle assessment study. Chem. Eng. J. 2014, 248, 230–241. [Google Scholar] [CrossRef]
- Butler, R.; Godley, A.; Lytton, L.; Cartmell, E. Bromate environmental contamination: Review of impact and possible treatment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 193–217. [Google Scholar] [CrossRef]
- IARC. Potassium Bromate (Summary of Data Reported and Evaluation); International Agency for Research on Cancer: Lyon, France, 1999. [Google Scholar]
- McCann, B. By-products blues. Water 1999, 21, 15–18. [Google Scholar]
- von Gunten, U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- von Gunten, U. The basics of oxidants in water treatment. Part B: Ozone reactions. Water Sci. Technol. 2007, 55, 25–29. [Google Scholar] [CrossRef]
- Buffle, M.O.; Galli, S.; von Gunten, U. Enhanced Bromate Control during Ozonation: The Chlorine-Ammonia Process. Environ. Sci. Technol. 2004, 38, 5187–5195. [Google Scholar] [CrossRef]
- Pinkernell, U.; von Gunten, U. Bromate Minimization during Ozonation: Mechanistic Considerations. Environ. Sci. Technol. 2001, 35, 2525–2531. [Google Scholar] [CrossRef]
- Song, R.; Minear, R.; Westerhoff, P.; Amy, G. Bromate formation and control during water ozonation. Environ. Technol. 1996, 17, 861–868. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Amy, G.L. Factors affecting DBP Formation during ozone-bromine reactions. J. Am. Water Work. Assoc. 1993, 85, 63–72. [Google Scholar] [CrossRef]
- Prados-Ramirez, M.; Ciba, N.; Bourbigot, M.M. Available techniques for reducing bromate in drinking water. Water Supply 1995, 13, 61–70. [Google Scholar]
- Zeino, A.; Abulkibash, A.; Khaled, M.; Atieh, M. Bromate removal from water using doped iron nanoparticles on multiwalled carbon nanotubes (CNTS). J. Nanomater. 2014. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, A.; Choi, Y.-H.; Yoon, Y.-J.; Shin, Y.; Jeon, B.-H.; Kang, J.-W. Bromate removal from water by granular ferric hydroxide (GFH). J. Hazard. Mater. 2009, 170, 134–140. [Google Scholar] [CrossRef]
- Liu, G.; You, S.; Zhang, Y.; Huang, H.; Spanjers, H. Conjugated donor-acceptor (DA) supramolecule catalyst for visible-light-driven photocatalytic removal of bromate in water. J. Colloid Interface Sci. 2019, 553, 666–673. [Google Scholar] [CrossRef]
- van Ginkel, C.G.; van Haperen, A.M.; van der Togt, B. Reduction of bromate to bromide coupled to acetate oxidation by anaerobic mixed microbial cultures. Water Res. 2005, 39, 59–64. [Google Scholar] [CrossRef]
- Lin, D.; Liang, H.; Li, G. Factors affecting the removal of bromate and bromide in water by nanofiltration. Environ. Sci. Pollut. Res. 2020, 27, 24639–24649. [Google Scholar] [CrossRef]
- Han, P.; Xia, Y. Thiol-functionalized metal-organic framework for highly efficient removal of bromate from water. J. Environ. Chem. Eng. 2018, 6, 3384–3391. [Google Scholar] [CrossRef]
- Siddiqui, M.; Amy, G.; Ozekin, K.; Zhai, W.; Westerhoff, P. Alternative strategies for removing bromate. J. Am. Water Work. Assoc. 1994, 86, 81–96. [Google Scholar] [CrossRef]
- Gordon, G.; Gauw, R.D.; Emmert, G.L.; Walters, B.D.; Bubnis, B. Treatment technologies: Chemical reduction methods for bromate ion removal. J. Am. Water Work. Assoc. 2002, 94, 91–98. [Google Scholar] [CrossRef]
- Xie, L.; Shang, C. Effects of copper and palladium on the reduction of bromate by Fe(0). Chemosphere 2006, 64, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, R.; Wang, H.; Kong, Z.; Dai, D.; Jing, Z.; Jiang, W.; Hou, Y. Reduction of bromate from water by zero-valent iron immobilized on functional polypropylene fiber. Chem. Eng. J. 2016, 292, 190–198. [Google Scholar] [CrossRef]
- Chiu, Y.-T.; Lee, P.-Y.; Wi-Afedzi, T.; Lee, J.; Lin, K.-Y.A. Elimination of bromate from water using aluminum beverage cans via catalytic reduction and adsorption. J. Colloid Interface Sci. 2018, 532, 416–425. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Lin, C.-H.; Yang, H. Enhanced bromate reduction using zero-valent aluminum mediated by oxalic acid. J. Environ. Chem. Eng. 2017, 5, 5085–5090. [Google Scholar] [CrossRef]
- Hamid, S.; Abudanash, D.; Han, S.; Kim, J.R.; Lee, W. Strategies to enhance the stability of nanoscale zero-valent iron (NZVI) in continuous BrO3- reduction. J. Environ. Manag. 2019, 231, 714–725. [Google Scholar] [CrossRef]
- Westerhoff, P. Reduction of Nitrate, Bromate, and Chlorate by Zero Valent Iron (Feo). J. Environ. Eng. 2003, 129, 10–16. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Liu, J.; He, H.; Yang, M.; Yu, J.; Ma, Z.; Jiang, F. Removal of bromate ion using powdered activated carbon. J. Environ. Sci. 2010, 22, 1846–1853. [Google Scholar] [CrossRef]
- Mustapha, C.; Benamar, D. Comparison of the bromate ions removal by nanofiltration membranes made from different polymers at different conditions. Chem. Rev. Lett. 2019, 2, 118–122. [Google Scholar]
- Gyparakis, S.; Diamadopoulos, E. Formation and reverse osmosis removal of bromate ions during ozonation of groundwater in coastal areas. Sep. Sci. Technol. 2007, 42, 1465–1476. [Google Scholar] [CrossRef]
- Wisniewski, J.A.; Kabsch-Korbutowicz, M.; Lakomska, S. Removal of bromate ions from water in the processes with ion-exchange membranes. Sep. Purif. Technol. 2015, 145, 75–82. [Google Scholar] [CrossRef]
- Matos, C.T.; Velizarov, S.; Reis, M.A.M.; Crespo, J.G. Removal of Bromate from Drinking Water Using the Ion Exchange Membrane Bioreactor Concept. Environ. Sci. Technol. 2008, 42, 7702–7708. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Amy, G.L.; Cooper, W.J.; Kurucz, C.N.; Waite, T.D.; Nickelsen, M.G. Bromate ion removal by HEEB irradiation. J. Am. Water Work. Assoc. 1996, 88, 90–101. [Google Scholar] [CrossRef]
- Siddiqui, M.; Amy, G.; Zhai, W.; McCollum, L. Removal of bromate after ozonation during drinking water treatment. In Disinfection By-Products in Water Treatment. The Chemistry of Their Formation and Control; Minear, A., Amy, G., Eds.; CRC Press: Boca Raton, FL, USA, 1995; p. 520. [Google Scholar]
- Noguchi, H.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Removal of bromate ion from water using TiO2 and alumina-loaded TiO2 photocatalysts. Water Sci. Technol. 2002, 46, 27–31. [Google Scholar] [CrossRef]
- Noguchi, H.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Design of a photocatalyst for bromate decomposition: Surface modification of TiO2 by pseudo-boehmite. Environ. Sci. Technol. 2003, 37, 153–157. [Google Scholar] [CrossRef]
- Cunha, G.S.; Santos, S.G.; Souza-Chaves, B.M.; Silva, T.F.; Bassin, J.P.; Dezotti, M.W.; Boaventura, R.A.; Dias, M.M.; Lopes, J.C.B.; Vilar, V.J. Removal of bromate from drinking water using a heterogeneous photocatalytic mili-reactor: Impact of the reactor material and water matrix. Environ. Sci. Pollut. Res. 2019, 26, 33281–33293. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Liu, H.; Lu, T. Graphene oxide and F co-doped TiO2 with (0 0 1) facets for the photocatalytic reduction of bromate: Synthesis, characterization and reactivity. Chem. Eng. J. 2017, 307, 860–867. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, G.; Zhang, Z. TiO2-based catalysts for photocatalytic reduction of aqueous oxyanions: State-of-the-art and future prospects. Environ. Int. 2020, 136, 105453. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Voogt, R.; Veenendaal, H.R.; van der Jagt, H.; van der Kooij, D. Bromate reduction by denitrifying bacteria. Appl. Environ. Microbiol. 1995, 61, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Duonghong, D.; Erbs, W.; Shuben, L.; Grätzel, M. Efficient redox catalysis by RuO2 in the generation of oxygen and bromine from aqueous bromate solutions. Chem. Phys. Lett. 1983, 95, 266–268. [Google Scholar] [CrossRef]
- Dong, Z.; Dong, W.; Sun, F.; Zhu, R.; Ouyang, F. Effects of preparation conditions on catalytic activity of Ru/AC catalyst to reduce bromate ion in water. React. Kinet. Mech. Catal. 2012, 107, 231–244. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, F.; Dong, W.; Jiang, C. Catalytic bromate removal from water by using activated carbon supported with ruthenium (AC/Ru) catalyst. Environ. Eng. Sci. 2018, 35, 176–184. [Google Scholar] [CrossRef]
- Pintar, A.; Batista, J.; Levec, J. Catalytic denitrification: Direct and indirect removal of nitrates from potable water. Catal. Today 2001, 66, 503–510. [Google Scholar] [CrossRef]
- Yuranova, T.; Franch, C.; Palomares, A.; Garcia-Bordejé, E.; Kiwi-Minsker, L. Structured fibrous carbon-based catalysts for continuous nitrate removal from natural water. Appl. Catal. B Environ. 2012, 123, 221–228. [Google Scholar] [CrossRef]
- Thakur, D.B.; Tiggelaar, R.M.; Weber, Y.; Gardeniers, J.G.E.; Lefferts, L.; Seshan, K. Ruthenium catalyst on carbon nanofiber support layers for use in silicon-based structured microreactors. Part II: Catalytic reduction of bromate contaminants in aqueous phase. Appl. Catal. B 2011, 102, 243–250. [Google Scholar] [CrossRef]
- Andrew Lin, K.-Y.; Chen, S.-Y. Bromate reduction in water by catalytic hydrogenation using metal-organic frameworks and sodium borohydride. RSC Adv. 2015, 5, 43885–43896. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, S.-Y. Catalytic Reduction of Bromate Using ZIF-Derived Nanoscale Cobalt/Carbon Cages in the Presence of Sodium Borohydride. ACS Sustain. Chem. Eng. 2015, 3, 3096–3103. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Wan, H.; Zheng, J.; Yin, D.; Zheng, S. Aqueous bromate reduction by catalytic hydrogenation over Pd/Al2O3 catalysts. Appl. Catal. B 2010, 96, 307–313. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, Y.; Guo, Y.; Shi, W.; Wang, W.; Zhang, B.; Zhang, R.; Bao, X.; Wu, S.; Cui, F. Pd and Pt nanoparticles supported on the mesoporous silica molecular sieve SBA-15 with enhanced activity and stability in catalytic bromate reduction. Chem. Eng. J. 2018, 344, 114–123. [Google Scholar] [CrossRef]
- Chen, X.; Huo, X.; Liu, J.; Wang, Y.; Werth, C.J.; Strathmann, T.J. Exploring beyond palladium: Catalytic reduction of aqueous oxyanion pollutants with alternative platinum group metals and new mechanistic implications. Chem. Eng. J. 2017, 313, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Restivo, J.; Soares, O.S.G.P.; Orfao, J.J.M.; Pereira, M.F.R. Metal assessment for the catalytic reduction of bromate in water under hydrogen. Chem. Eng. J. 2015, 263, 119–126. [Google Scholar] [CrossRef]
- Franch, C.; Rodríguez-Castellón, E.; Reyes-Carmona, Á.; Palomares, A.E. Characterization of (Sn and Cu)/Pd catalysts for the nitrate reduction in natural water. Appl. Catal. A Gen. 2012, 425, 145–152. [Google Scholar] [CrossRef]
- Barrabés, N.; Just, J.; Dafinov, A.; Medina, F.; Fierro, J.; Sueiras, J.; Salagre, P.; Cesteros, Y. Catalytic reduction of nitrate on Pt-Cu and Pd-Cu on active carbon using continuous reactor: The effect of copper nanoparticles. Appl. Catal. B Environ. 2006, 62, 77–85. [Google Scholar] [CrossRef]
- Restivo, J.; Soares, O.S.G.P.; Orfao, J.J.M.; Pereira, M.F.R. Bimetallic activated carbon supported catalysts for the hydrogen reduction of bromate in water. Catal. Today 2015, 249, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Soares, O.S.G.P.; Freitas, C.M.A.S.; Fonseca, A.M.; Orfao, J.J.M.; Pereira, M.F.R.; Neves, I.C. Bromate reduction in water promoted by metal catalysts prepared over faujasite zeolite. Chem. Eng. J. 2016, 291, 199–205. [Google Scholar] [CrossRef]
- Freitas, C.M.A.S.; Soares, O.S.G.P.; Orfao, J.J.M.; Fonseca, A.M.; Pereira, M.F.R.; Neves, I.C. Highly efficient reduction of bromate to bromide over mono and bimetallic ZSM5 catalysts. Green Chem. 2015, 17, 4247–4254. [Google Scholar] [CrossRef]
- Yuranova, T.; Kiwi-Minsker, L.; Franch, C.; Palomares, A.E.; Armenise, S.; Garcia-Bordeje, E. Nanostructured Catalysts for the Continuous Reduction of Nitrates and Bromates in Water. Ind. Eng. Chem. Res. 2013, 52, 13930–13937. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Gao, S.; Gao, Y.; Sun, C.; Li, Q. Catalytic reduction of aqueous bromate by a non-noble metal catalyst of CoS2 hollow spheres in drinking water at room temperature. Sep. Purif. Technol. 2020, 251, 117353. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, W.; Yang, W.; Li, Q. Creation of Pd/Al2O3 Catalyst by a Spray Process for Fixed Bed Reactors and Its Effective Removal of Aqueous Bromate. Sci. Rep. Nat. 2017, 7, 41797. [Google Scholar] [CrossRef] [Green Version]
- Cerrillo, J.L.; Lopes, C.W.; Rey, F.; Agostini, G.; Kiwi-Minsker, L.; Palomares, A.E. Nature and evolution of Pd catalysts supported on activated carbon fibers during the catalytic reduction of bromate in water. Catal. Sci. Technol. 2020, 10, 3646–3653. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Fu, H.; Wan, H.; Wan, Y.; Qu, X.; Xu, Z.; Yin, D.; Zheng, S. Enhanced catalytic hydrogenation reduction of bromate on Pd catalyst supported on CeO2 modified SBA-15 prepared by strong electrostatic adsorption. Appl. Catal. B 2018, 229, 32–40. [Google Scholar] [CrossRef]
- Sun, W.; Li, Q.; Gao, S.; Shang, J.K. Highly efficient catalytic reduction of bromate in water over a quasi-monodisperse, superparamagnetic Pd/Fe3O4 catalyst. J. Mater. Chem. A 2013, 1, 9215–9224. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, P.; Tan, W.; Jiang, F.; Tang, R. Palladium supported on amino functionalized magnetic MCM-41 for catalytic hydrogenation of aqueous bromate. RSC Adv. 2014, 4, 38743–38749. [Google Scholar] [CrossRef]
- Cerrillo, J.L.; Lopes, C.W.; Rey, F.; Palomares, A.E. The Influence of the Support Nature and the Metal Precursor in the Activity of Pd-based Catalysts for the Bromate Reduction Reaction. ChemCatChem 2021, 13, 1230–1238. [Google Scholar] [CrossRef]
- Perez-Coronado, A.M.; Soares, O.S.G.P.; Calvo, L.; Rodriguez, J.J.; Gilarranz, M.A.; Pereira, M.F.R. Catalytic reduction of bromate over catalysts based on Pd nanoparticles synthesized via water-in-oil microemulsion. Appl. Catal. B 2018, 237, 206–213. [Google Scholar] [CrossRef]
- Restivo, J.; Soares, O.S.G.P.; Orfao, J.M.J.; Pereira, M.F.R. Catalytic reduction of bromate over monometallic catalysts on different powder and structured supports. Chem. Eng. J. 2017, 309, 197–205. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, F.; Chen, H. Enhanced catalytic hydrogenation of aqueous bromate over Pd/mesoporous carbon nitride. Chem. Eng. J. 2013, 234, 195–202. [Google Scholar] [CrossRef]
- Palomares, A.E.; Franch, C.; Yuranova, T.; Kiwi-Minsker, L.; Garcia-Bordeje, E.; Derrouiche, S. The use of Pd catalysts on carbon-based structured materials for the catalytic hydrogenation of bromates in different types of water. Appl. Catal. B 2014, 146, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, J.; Wang, P.; Werth, C.J.; Strathmann, T.J. Palladium Nanoparticles Encapsulated in Core-Shell Silica: A Structured Hydrogenation Catalyst with Enhanced Activity for Reduction of Oxyanion Water Pollutants. Acs Catal. 2014, 4, 3551–3559. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.; Sun, J.; Fu, H.; Qu, X.; Xu, Z.; Zheng, S. Highly effective bromate reduction by liquid phase catalytic hydrogenation over Pd catalysts supported on core-shell structured magnetites: Impact of shell properties. Sci. Total Environ. 2019, 663, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, J.; Luo, Y.; Shi, W.; Wang, W.; Zhang, B.; Zhang, R.; Bao, X.; Guo, Y.; Cui, F. Pt nanoparticles supported on amino-functionalized SBA-15 for enhanced aqueous bromate catalytic reduction. Catal. Commun. 2018, 105, 11–15. [Google Scholar] [CrossRef]
- Marco, Y.; Garcia-Bordeje, E.; Franch, C.; Palomares, A.E.; Yuranova, T.; Kiwi-Minsker, L. Bromate catalytic reduction in continuous mode using metal catalysts supported on monoliths coated with carbon nanofibers. Chem. Eng. J. 2013, 230, 605–611. [Google Scholar] [CrossRef]
- Amali, A.J.; Rana, R.K. Stabilisation of Pd (0) on surface functionalised Fe3O4 nanoparticles: Magnetically recoverable and stable recyclable catalyst for hydrogenation and Suzuki–Miyaura reactions. Green Chem. 2009, 11, 1781–1786. [Google Scholar] [CrossRef]
- Sandoval, V.H.; Gigola, C.E. Characterization of Pd and Pd-Pb/α-Al2O3 catalysts. A TPR-TPD study. Appl. Catal. A Gen. 1996, 148, 81–96. [Google Scholar] [CrossRef]
- Lopes, C.W.; Cerrillo, J.L.; Palomares, A.E.; Rey, F.; Agostini, G. An in situ XAS study of the activation of precursor-dependent Pd nanoparticles. Phys. Chem. Chem. Phys. 2018, 20, 12700–12709. [Google Scholar] [CrossRef] [PubMed]
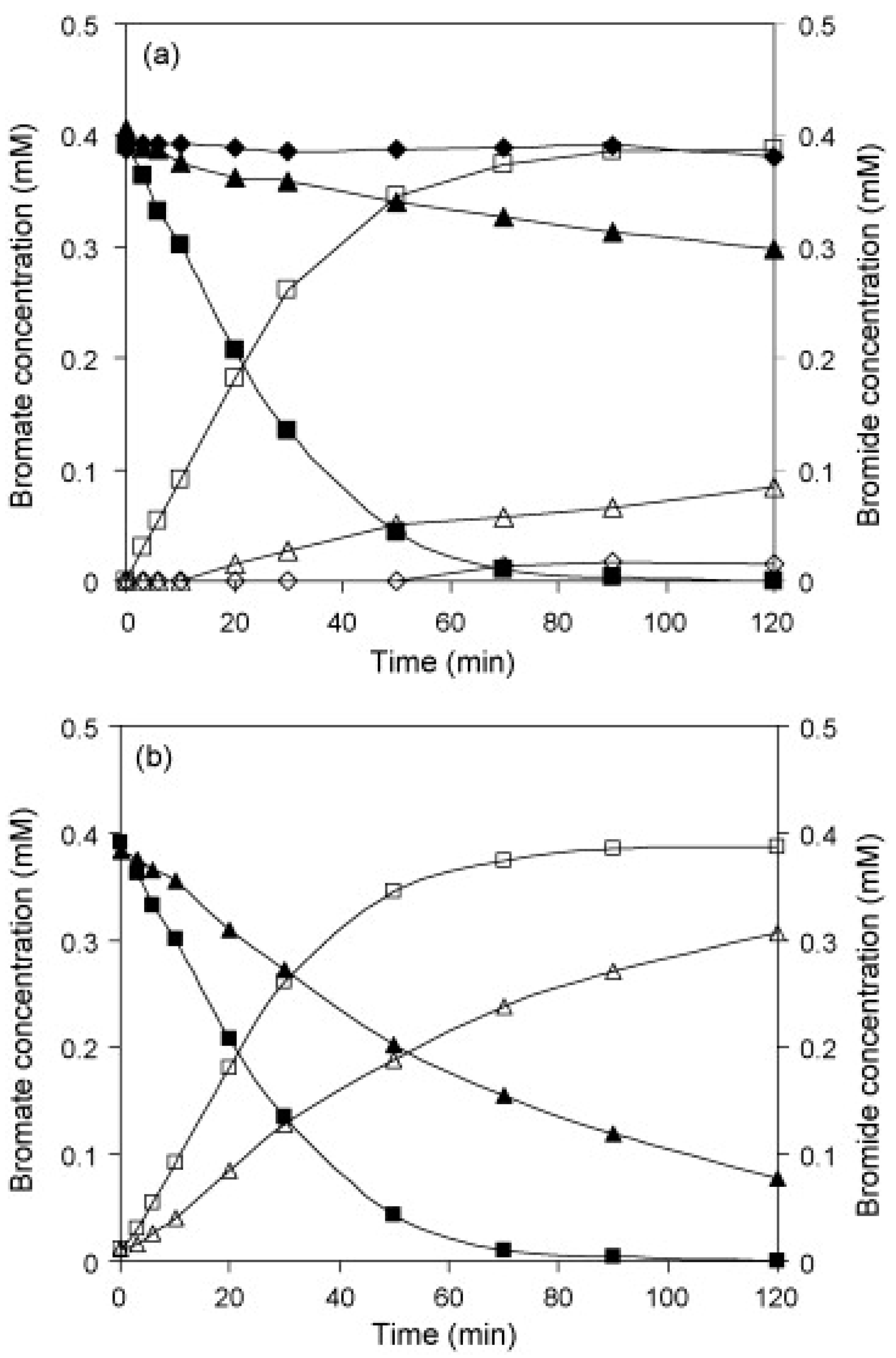

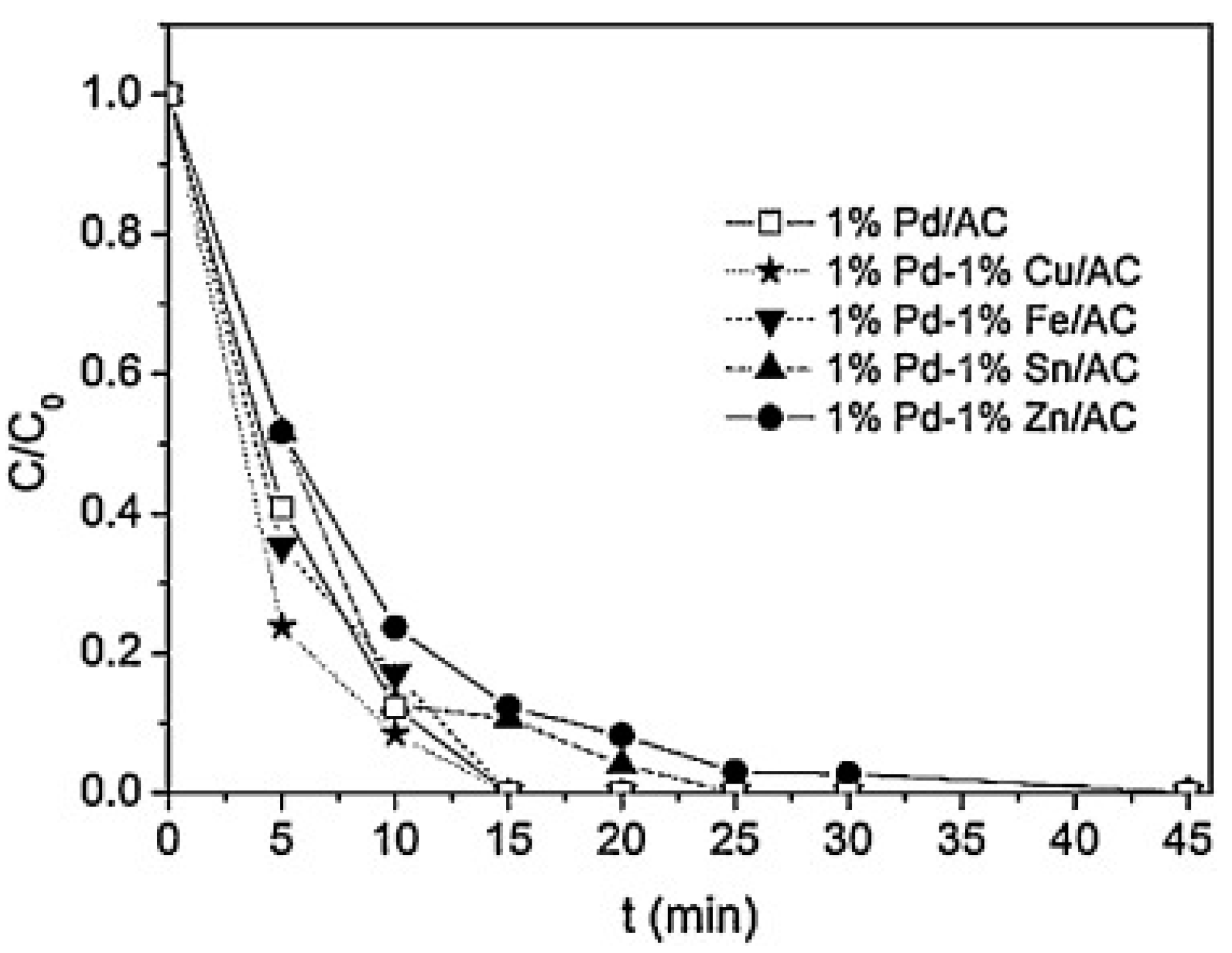
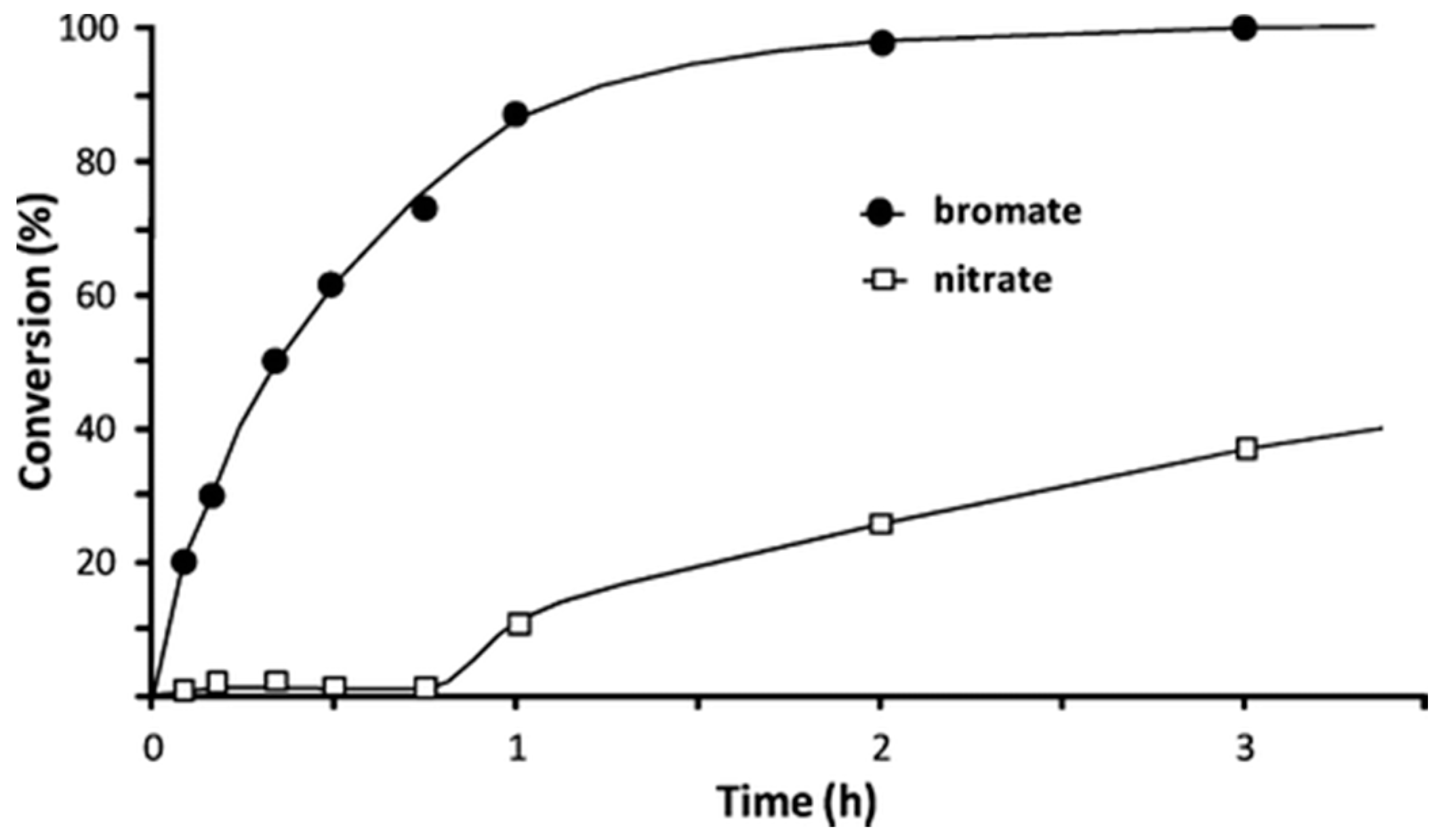
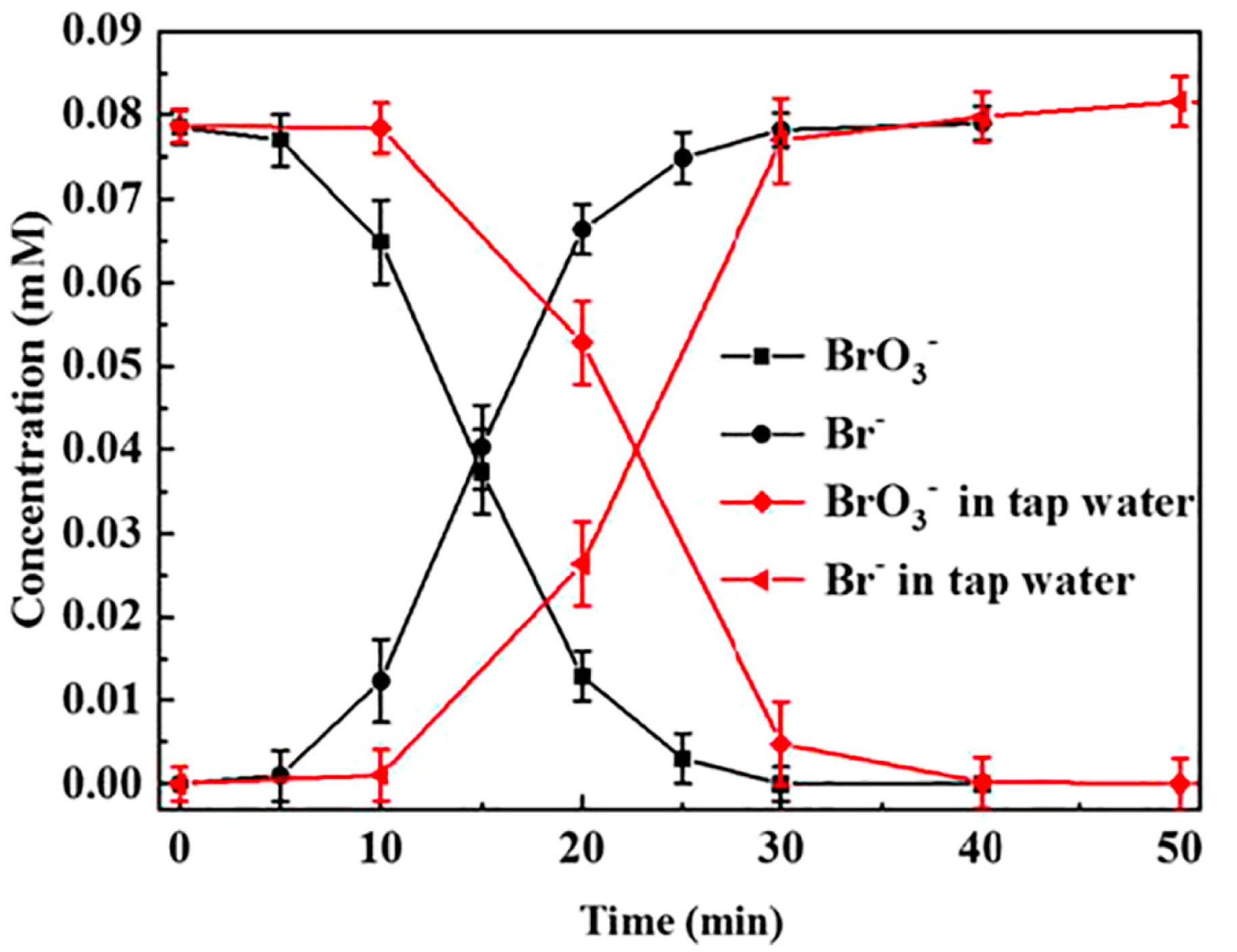

| Support | Metal & Loading | Incorporation Method | BrO3− Initial (mmol·L−1) | Conversion at 5 min (%) | Efficiency at 5 min (mmol BrO3− Conversion g metal−1) | Reference |
|---|---|---|---|---|---|---|
| TiO2 | Pd (3.6%) | Micro-emulsion | 0.078 | 96 | 17.74 | [78] |
| SBA-15 doped with CeO2 | Pd (0.1%) | electrostatic adsorption | 0.6 | 30 | 760.14 | [74] |
| SBA-15 | Pd (3.9%) | adsorption | 0.78 | 45 | 30.07 | [62] |
| Pt (3.9%) | adsorption | 0.78 | 65 | 43.22 | ||
| SBA-15 doped with -NH2 | Pt (4.0%) | adsorption | 0,78 | 63 | 47.89 | [84] |
| Al2O3 | Pd (0.9%) | spraying | 0.4 | 47 | 427.28 | [72] |
| Activated Carbon | Pd (5.0%) | commercial | 1 | 15 | 3.19 | [63] |
| Rh (5.0%) | commercial | 1 | 100 | 21.28 | ||
| Ru (5.0%) | commercial | 1 | 2 | 0.43 | ||
| Pt (5.0%) | commercial | 1 | 5 | 1.06 | ||
| Ir (1.0%) | commercial | 1 | 10 | 10.64 | ||
| Al2O3 | Pd (5.0%) | commercial | 1 | 10 | 2.13 | |
| Rh (5.0%) | commercial | 1 | 50 | 10.64 | ||
| Ru (5.0%) | commercial | 1 | 7 | 1.49 | ||
| Pt (5.0%) | commercial | 1 | 9 | 1.92 | ||
| Al2O3 | Pd (1.0%) | impregnation | 0.39 | 12 | 56 | [77] |
| Activated Carbon | Pd (1.0%) | impregnation | 0.39 | 7 | 3.23 | |
| Hydrotalcite (Mg/Al) | Pd (1.0%) | impregnation | 0.39 | 2 | 1.04 | |
| Al2O3 | Pd (2.0%) | impregnation | 0.4 | 18 | 73.84 | [61] |
| Pd (5.0%) | impregnation | 0.4 | 33 | 53.33 | ||
| Pt (2.0%) | impregnation | 0.4 | 10 | 40 | ||
| SiO2 | Pd (2.0%) | impregnation | 0.4 | 3 | 12 | |
| Activated Carbon | Pd (2.0%) | impregnation | 0.4 | 6 | 24 | |
| Activated carbon fibers coating sintered metal fibers (CNF/SMFs) | Pd (0.3%) | impregnation | 0.39 | 60 | 49.66 | [70,81] |
| Activated carbon fibers coating cordierite monoliths | Pd (0.5%) | adsorption | 0.39 | 35 | 57.47 | [85] |
| Activated carbon | Pt (1.0%) | impregnation | 0.078 | 30 | 1.06 | [64] |
| Pd (1.0%) | impregnation | 0.078 | 60 | 2.13 | ||
| Sn (1.0%) | impregnation | 0.078 | 69 | 3.19 | ||
| Rh (1.0%) | impregnation | 0.078 | 50 | 2.13 | ||
| Ru (1.0%) | impregnation | 0.078 | 67 | 3.19 | ||
| Pd/Cu (1.0/1.0%) | impregnation | 0.078 | 78 | 3.19 | [67] | |
| Pd/Fe (1.0/1.0%) | impregnation | 0.078 | 65 | 3.19 | ||
| Pd/Sn (1.0/1.0%) | impregnation | 0.078 | 50 | 2.13 | ||
| Pd/Zn (1.0/1.0%) | impregnation | 0.078 | 50 | 2.13 | ||
| Cu (1.0%) | impregnation | 0.078 | 57 | 2.13 | ||
| Ir/Cu (1.0/1.0%) | impregnation | 0.078 | 50 | 2.13 | ||
| Pt/Cu (1.0/1.0%) | impregnation | 0.078 | 65 | 3.19 | ||
| Ru/Cu (1.0/1.0%) | impregnation | 0.078 | 48 | 2.13 | ||
| TiO2 | Ru (1.0%) | impregnation | 0.078 | 53 | 16.50 | [79] |
| Rh (1.0%) | impregnation | 0.078 | 99 | 30.80 | ||
| Pd (1.0%) | impregnation | 0.078 | 100 | 31.22 | ||
| Pt (1.0%) | impregnation | 0.078 | 100 | 31.22 | ||
| Carbon nanotube (MWCNT) | Ru (1.0%) | impregnation | 0.078 | 40 | 12.48 | |
| Rh (1.0%) | impregnation | 0.078 | 52 | 16.22 | ||
| Pd (1.0%) | impregnation | 0.078 | 55 | 16.85 | ||
| Pt (1.0%) | impregnation | 0.078 | 58 | 18.10 | ||
| Activated carbon fibers (ACF) | Pd (1.0%) | impregnation | 0.39 | 15 | 9.32 | [73] |
| Carbon nanofibers over carbon felt (CNF/CF) | Pd (0.3%) | impregnation | 0.39 | 30 | 46.80 | [15] |
| Carbon nanofibers over metal fibers (CNF/SMF) | Pd (0.3%) | impregnation | 0.39 | 12 | 18.76 | |
| Zeolite (FAU) | Pd (1.0%) | Ion-exchange | 0.078 | 18 | 3.47 | [68] |
| Cu (0.6%) | Ion-exchange | 0.078 | 10 | 3.55 | ||
| Rh (0.2%) | Ion-exchange | 0.078 | 5 | 7.60 | ||
| Th (0.2%) | Ion-exchange | 0.078 | 5 | 6.26 | ||
| Pd/Cu (1.6/1.0%) | Ion-exchange | 0.078 | 75 | 7.98 | ||
| Cu/Pd (0.7/1.8%) | Ion-exchange | 0.078 | 85 | 7.69 | ||
| Rh/Cu (0.1/0.6%) | Ion-exchange | 0.078 | 7 | - | ||
| Th/Cu (0.1/2.3%) | Ion-exchange | 0.078 | 15 | - | ||
| Zeolite (ZSM-5) | Pd (1.5%) | Ion-exchange | 0.078 | 20 | 3.19 | [69] |
| Cu (0.9%) | Ion-exchange | 0.078 | 12 | - | ||
| Pd/Cu (1.4/1.0%) | Ion-exchange | 0.078 | 80 | 9.88 | ||
| Cu/Pd (0.6/1.9%) | Ion-exchange | 0.078 | 88 | 7.84 | ||
| Th (0.4%) | Ion-exchange | 0.078 | 5 | 2.66 | ||
| Rh (0.5%) | Ion-exchange | 0.078 | 15 | 4.73 | ||
| Th/Cu (0.4/1.3%) | Ion-exchange | 0.078 | 85 | - | ||
| Rh/Cu (0.4/1.6%) | Ion-exchange | 0.078 | 15 | - | ||
| MCM-41 doped with amino groups | Pd (2.0%) | impregnation | 0.78 | 5 | 41.50 | [76] |
| Deposition—precipitation | 0.78 | 12.5 | 103.76 | |||
| Mesoporous carbon nitride | Pd (2.2%) | impregnation | 0.78 | 12.5 | 157.21 | [80] |
| Mesoporous carbon CMK-3 | Pd (2.1%) | impregnation | 0.78 | 5 | 66.19 | |
| Activated carbon | Pd (2.0%) | impregnation | 0.78 | 3 | 41.30 | |
| Fe3O4 paramagnetic | Pd (1.0%) | Adsorption—impregnation | 0.39 | 35 | 68.43 | [75] |
| Fe3O4 paramagnetic | Pd (3.5%) | Adsorption—sonication | 0.4 | 27 | 61.89 | [83] |
| SiO2 “core-shell” | Pd (3.0%) | impregnation | 0.1 | 33 | 14.54 | [82] |
| SiO2 | Pd (3.0%) | impregnation | 0.1 | 8 | 10.64 | |
| nanoparticles | Pd (3.0%) | 0.1 | 15 | 20.22 | ||
| CoS2 hollow spheres | Co | Hydrothermal process | 0.39 | 12 | - | [71] |
| Support | Metal | Incorpor. Method | Continuous Flow (mL·min−1) | BrO3− Initial (mmol·L−1) | Constant Efficiency | Ref. |
|---|---|---|---|---|---|---|
| Al2O3 | Pd (1.0%) | spraying | 14 mL·min−1 | 0.078 | 100% for 60 h | [72] |
| 0.0004 | 100% for 10 days | |||||
| Activated carbon fibers coating sintered metal fibers (CNF/SMFs) | Pd (0.3%) | impregnation | 5 mL·min−1 | 0.39 | 80% for 40 h | [70,81] |
| Activated carbon fibers coating cordierite monoliths | Pd (0.3%) | adsorption | 5 mL·min−1 | 0.39 | 75% for 40 h | [85] |
| Carbon nanofibers over carbon felt (CNF/CF) | Pd (0.3%) | impregnation | 5 mL·min−1 | 0.39 | 60% for 40 h | [15] |
| Carbon nanofibers over metal fibers (CNF/SMF) | Pd (0.3%) | impregnation | 5 mL·min−1 | 0.39 | 80% for 40 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerrillo, J.L.; Palomares, A.E. A Review on the Catalytic Hydrogenation of Bromate in Water Phase. Catalysts 2021, 11, 365. https://doi.org/10.3390/catal11030365
Cerrillo JL, Palomares AE. A Review on the Catalytic Hydrogenation of Bromate in Water Phase. Catalysts. 2021; 11(3):365. https://doi.org/10.3390/catal11030365
Chicago/Turabian StyleCerrillo, Jose Luis, and Antonio Eduardo Palomares. 2021. "A Review on the Catalytic Hydrogenation of Bromate in Water Phase" Catalysts 11, no. 3: 365. https://doi.org/10.3390/catal11030365
APA StyleCerrillo, J. L., & Palomares, A. E. (2021). A Review on the Catalytic Hydrogenation of Bromate in Water Phase. Catalysts, 11(3), 365. https://doi.org/10.3390/catal11030365







