Nickel Phosphide Catalysts as Efficient Systems for CO2 Upgrading via Dry Reforming of Methane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of the Samples
2.2. Surface Characterization of the Samples: XPS Analysis
2.3. Catalytic Activity
2.4. XRD of the Spent Samples
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterisation
3.3. Catalyst Reaction Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A Review of Developments in Carbon Dioxide Storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef] [Green Version]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Boehman, A.L.; Le Corre, O. Combustion of syngas in internal combustion engines. Combust. Sci. Technol. 2008, 180, 1193–1206. [Google Scholar] [CrossRef]
- Dry, M.E. The Fischer-Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of carbon dioxide into methanol-A potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 2014, 31, 221–257. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Grzybek, T.; Galvez, M.E.; Da Costa, P. A short review on the catalytic activity of hydrotalcite-derived materials for dry reforming of methane. Catalysts 2017, 7, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Stroud, T.; Smith, T.J.; Le Saché, E.; Santos, J.L.; Centeno, M.A.; Arellano-Garcia, H.; Odriozola, J.A.; Reina, T.R. Chemical CO2 recycling via dry and bi reforming of methane using Ni-Sn/Al2O3 and Ni-Sn/CeO2-Al2O3 catalysts. Appl. Catal. B Environ. 2018, 224, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A. Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni 2P(001) surface: The importance of ensemble effect. J. Am. Chem. Soc. 2005, 127, 14871–14878. [Google Scholar] [CrossRef]
- Korányi, T.I.; Vít, Z.; Poduval, D.G.; Ryoo, R.; Kim, H.S.; Hensen, E.J.M. SBA-15-supported nickel phosphide hydrotreating catalysts. J. Catal. 2008, 253, 119–131. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yang, X.; Boscoboinik, J.A.; Chen, J.G. Molybdenum carbide as alternative catalysts to precious metals for highly selective reduction of CO2 to CO. Angew. Chemie Int. Ed. 2014, 53, 6705–6709. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A. Water-gas-shift reaction on molybdenum carbide surfaces: Essential role of the oxycarbide. J. Phys. Chem. B 2006, 110, 19418–19425. [Google Scholar] [CrossRef]
- Yao, Z.; Luan, F.; Sun, Y.; Jiang, B.; Song, J.; Wang, H. Molybdenum phosphide as a novel and stable catalyst for dry reforming of methane. Catal. Sci. Technol. 2016, 6, 7996–8004. [Google Scholar] [CrossRef]
- Guharoy, U.; Ramirez Reina, T.; Gu, S.; Cai, Q. Mechanistic insights into selective CO2 Conversion via RWGS on Transition Metal Phosphides: A DFT Study. J. Phys. Chem. C 2019, 123, 22918–22931. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A.; Takahashi, Y.; Nakamura, K. Water-gas-shift reaction on a Ni2P(001) catalyst: Formation of oxy-phosphides and highly active reaction sites. J. Catal. 2009, 262, 294–303. [Google Scholar] [CrossRef]
- Sawhill, S.J.; Phillips, D.C.; Bussell, M.E. Thiophene hydrodesulfurization over supported nickel phosphide catalysts. J. Catal. 2003, 215, 208–219. [Google Scholar] [CrossRef]
- Guharoy, U.; Ramirez Reina, T.; Olsson, E.; Gu, S.; Cai, Q. Theoretical Insights of Ni2P (0001) Surface toward Its Potential Applicability in CO2 Conversion via Dry Reforming of Methane. ACS Catal. 2019, 9, 3487–3497. [Google Scholar] [CrossRef]
- Zhang, Q.; Pastor-Pérez, L.; Jin, W.; Gu, S.; Reina, T.R. Understanding the promoter effect of Cu and Cs over highly effective Β-Mo2C catalysts for the reverse water-gas shift reaction. Appl. Catal. B Environ. 2019, 244, 889–898. [Google Scholar] [CrossRef]
- Gonzalez-Castano, M.; Miguel Navarro De, J.C.; Sinha, F.; Wabo, S.G.; Klepel, O.; Arellano-garcia, H. Cu supported Fe-SiO2 nanocomposites for reverse water gas shift reaction. J. CO2 Util. 2021, 46, 101493. [Google Scholar] [CrossRef]
- Omar, F.S.; Numan, A.; Duraisamy, N.; Bashir, S.; Ramesh, K.; Ramesh, S. Ultrahigh capacitance of amorphous nickel phosphate for asymmetric supercapacitor applications. RSC Adv. 2016, 6, 76298–76306. [Google Scholar] [CrossRef]
- Brunauer, B.S.; Emmett, P.H. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Xin, H.; Guo, K.; Li, D.; Yang, H.; Hu, C. Production of high-grade diesel from palmitic acid over activated carbon-supported nickel phosphide catalysts. Appl. Catal. B Environ. 2016, 187, 375–385. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, X.; Guo, X.; Liu, Z.; Asiri, A.M.; Li, B.; Chen, L.; Sun, X. Efficient Hydrogen Evolution Electrocatalysis at Alkaline pH by Interface Engineering of Ni 2 P—CeO2. Inorg. Chem. 2018. [Google Scholar] [CrossRef]
- Suib, S.L.; Winiecki, A.M.; Kostapapas, A. Surface states of aluminophosphate and zeolite molecular sieves. Stud. Surf. Sci. Catal. 1986, 28, 409–414. [Google Scholar] [CrossRef]
- Anh, P.; Cam, N.; Luu, L.; Van Nguyen, T.T.; Nguyen, T.; Cuong, T. Improving the performance of nickel catalyst supported on mesostructured silica nanoparticles in methanation of CO2-rich gas by urea–nitrate combustion. Chem. Pap. 2020, 74, 3925–3935. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. J. Catal. 2004, 224, 370–383. [Google Scholar] [CrossRef]
- Li, S.; Gong, J. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef] [PubMed]
- Kroll, V.C.H.; Swaan, H.M.; Lacombe, S.; Mirodatos, C. Methane reforming reaction with carbon dioxide over Ni/SiO2 catalyst: II. A mechanistic study. J. Catal. 1996, 164, 387–398. [Google Scholar] [CrossRef]
- Liang, W.; Yan, H.; Chen, C.; Lin, D.; Tan, K.; Feng, X.; Liu, Y.; Chen, X.; Yang, C.; Shan, H. Revealing the effect of nickel particle size on carbon formation type in the methane decomposition reaction. Catalysts 2020, 10, 890. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ruckenstein, E. Conversions of methane to synthesis gas over Co/γ-Al2O3 by CO2 and/or O2. Catal. Lett. 2001, 75, 13–18. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, A.; Asakura, T. Desulfurization Reactions on Ni2P (001) and α-Mo2C (001) Surfaces: Complex Role of P and C Sites. J. Phys. Chem. B 2005, 109, 4575–4583. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, X.; Zhu, J.; Hu, P. Activity and coke formation of nickel and nickel carbide in dry reforming: A deactivation scheme from density functional theory. J. Catal. 2014, 311, 469–480. [Google Scholar] [CrossRef]
- Kim, D.K.; Stöwe, K.; Müller, F.; Maier, W.F. Mechanistic study of the unusual catalytic properties of a new Ni—Ce mixed oxide for the CO2 reforming of methane. J. Catal. 2007, 247, 101–111. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Z.; Johnston-Peck, A.C.; Senanayake, S.D.; Zhou, G.; Stacchiola, D.; Stach, E.A.; Rodriguez, J.A. Steam reforming of ethanol on Ni/CeO2: Reaction pathway and interaction between Ni and the CeO2 support. ACS Catal. 2013, 3, 975–984. [Google Scholar] [CrossRef]
- Wagner, J.L.; Jones, E.; Sartbaeva, A.; Davis, S.A.; Torrente-Murciano, L.; Chuck, C.J.; Ting, V.P. Zeolite y supported nickel phosphide catalysts for the hydrodenitrogenation of quinoline as a proxy for crude bio-oils from hydrothermal liquefaction of microalgae. Dalt. Trans. 2018, 47, 1189–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, G.; Guan, Q.; Li, W. The synthesis and mechanistic studies of a highly active nickel phosphide catalyst for naphthalene hydrodearomatization. RSC Adv. 2017, 7, 8677–8687. [Google Scholar] [CrossRef] [Green Version]
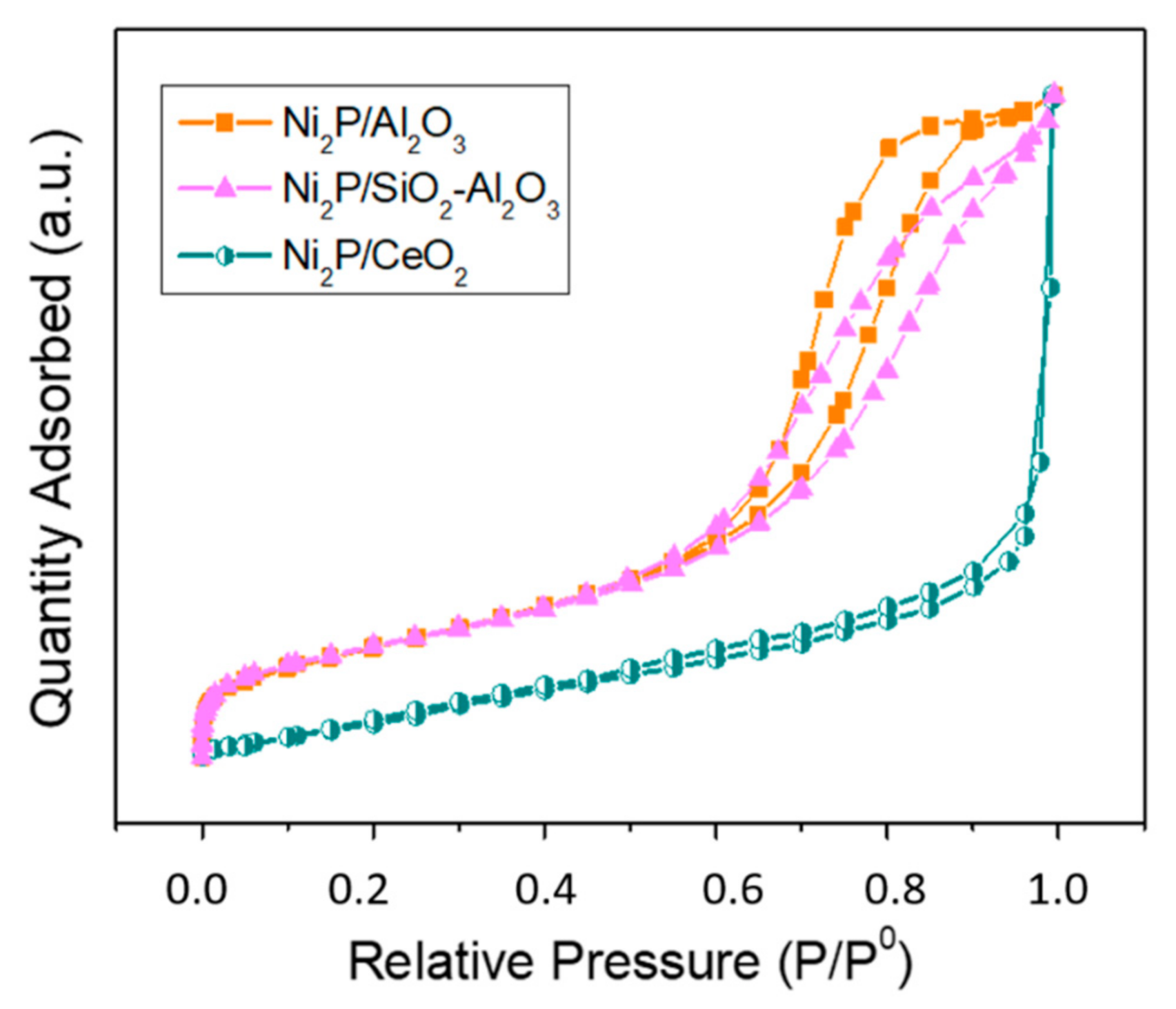
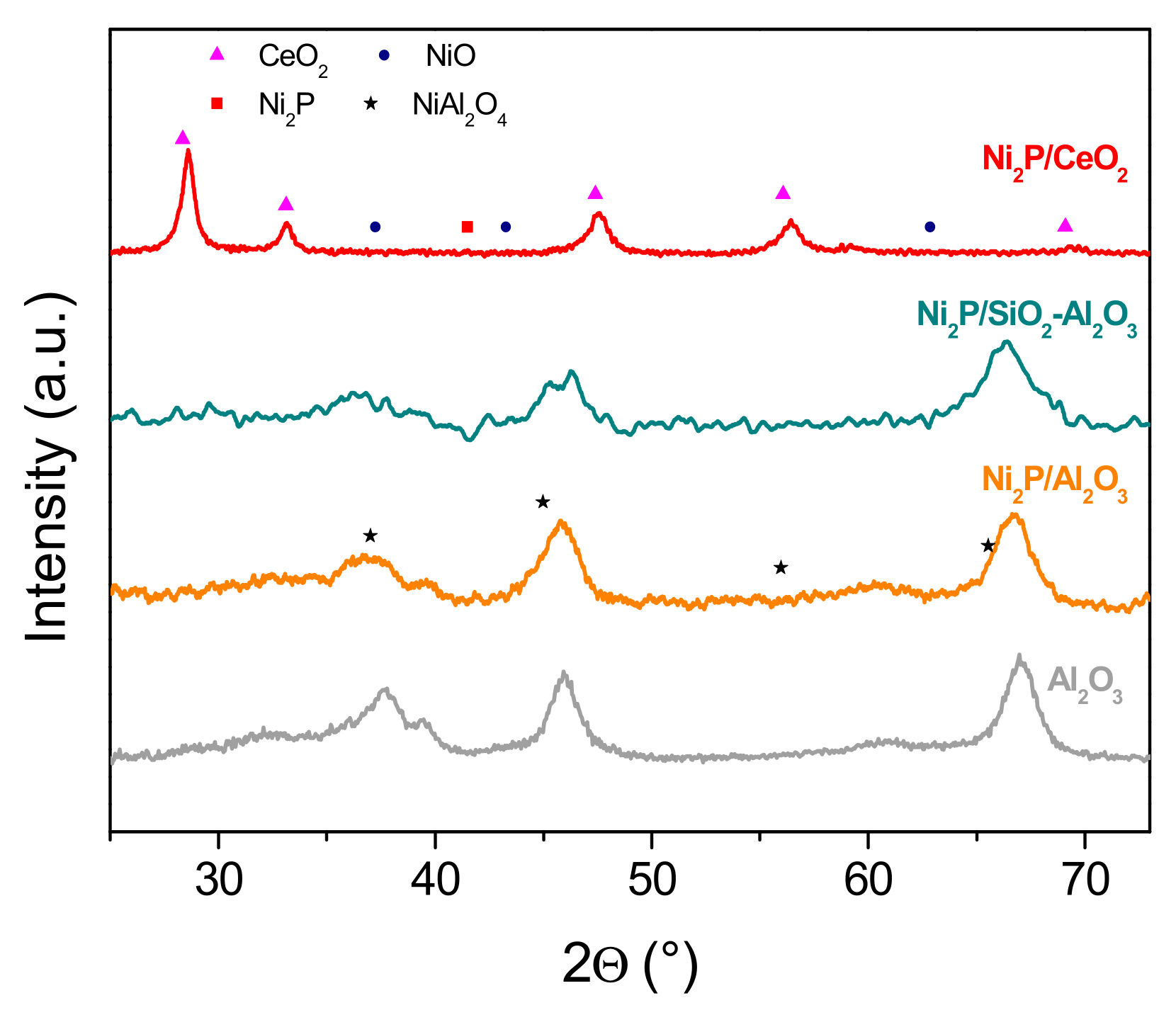


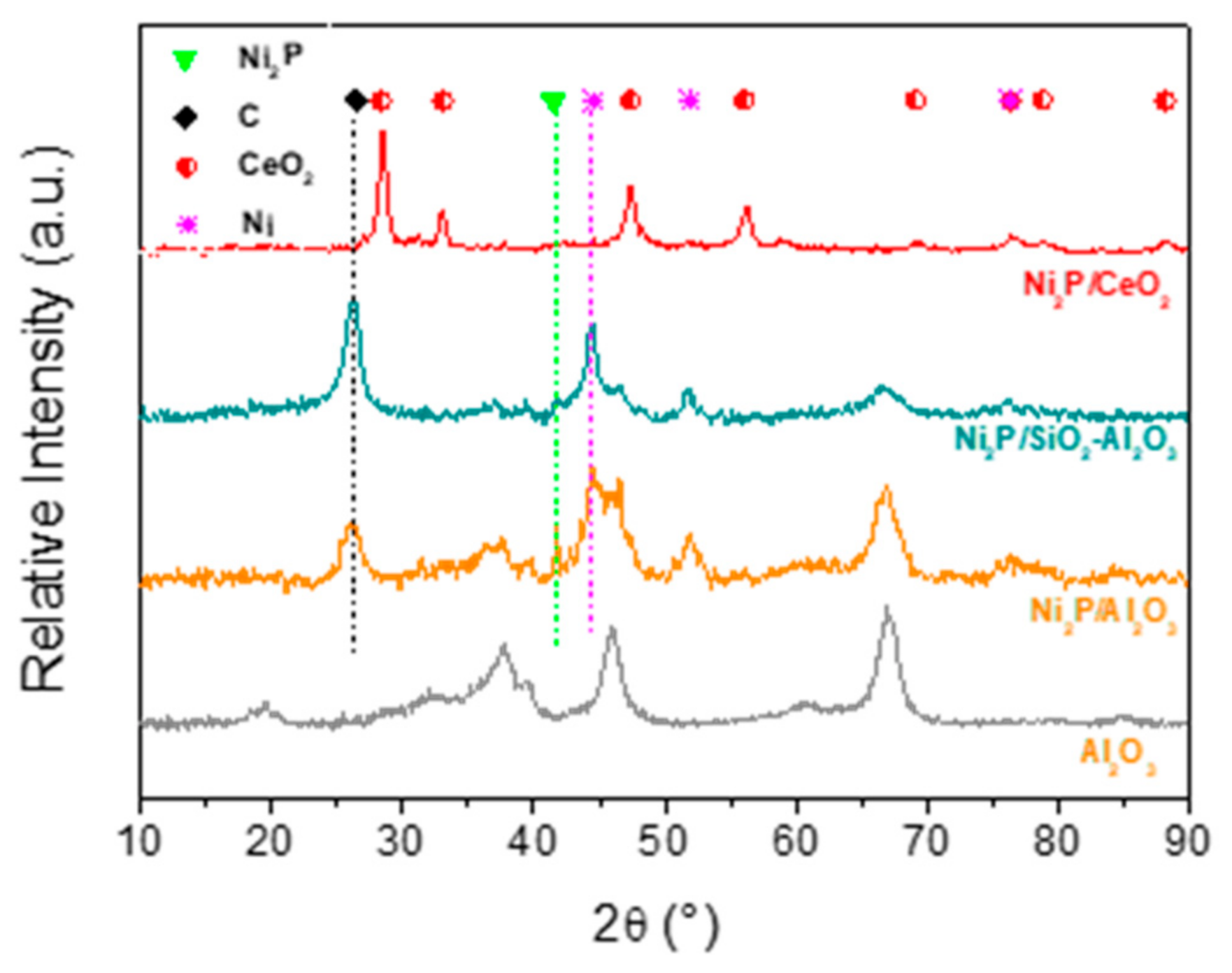
| Support | Surface Area (m2/g) | Dpore (nm) | Vpore (cm3/g) |
|---|---|---|---|
| Al2O3 | 202 | 7.4 | 0.51 |
| SiO2-Al2O3 | 420 | 8.5 | 0.80 |
| Ni2P/Al2O3 | 161 | 6.8 | 0.41 |
| Ni2P/CeO2 | 16 | 2.2 | 0.09 |
| Ni2P/SiO2-Al2O3 | 226 | 6.8 | 0.58 |
| Catalyst | Ni/Support | Ni Coverage (%) | Niδ+/Ni2+ | Pδ−/[P5++P-O] |
|---|---|---|---|---|
| Ni2P/Al2O3 | 0.08 | 1.52 | 0.67 | 0.48 |
| Ni2P/SiO2-Al2O3 | 0.09 | 2.25 | 0.90 | 0.13 |
| Ni2P/CeO2 | 0.28 | 6.24 | 0.78 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Castaño, M.; le Saché, E.; Berry, C.; Pastor-Pérez, L.; Arellano-García, H.; Wang, Q.; Reina, T.R. Nickel Phosphide Catalysts as Efficient Systems for CO2 Upgrading via Dry Reforming of Methane. Catalysts 2021, 11, 446. https://doi.org/10.3390/catal11040446
González-Castaño M, le Saché E, Berry C, Pastor-Pérez L, Arellano-García H, Wang Q, Reina TR. Nickel Phosphide Catalysts as Efficient Systems for CO2 Upgrading via Dry Reforming of Methane. Catalysts. 2021; 11(4):446. https://doi.org/10.3390/catal11040446
Chicago/Turabian StyleGonzález-Castaño, Miriam, Estelle le Saché, Cameron Berry, Laura Pastor-Pérez, Harvey Arellano-García, Qiang Wang, and Tomás R. Reina. 2021. "Nickel Phosphide Catalysts as Efficient Systems for CO2 Upgrading via Dry Reforming of Methane" Catalysts 11, no. 4: 446. https://doi.org/10.3390/catal11040446
APA StyleGonzález-Castaño, M., le Saché, E., Berry, C., Pastor-Pérez, L., Arellano-García, H., Wang, Q., & Reina, T. R. (2021). Nickel Phosphide Catalysts as Efficient Systems for CO2 Upgrading via Dry Reforming of Methane. Catalysts, 11(4), 446. https://doi.org/10.3390/catal11040446










