Synthesis of an Ag3PO4/Nb2O5 Photocatalyst for the Degradation of Dye
Abstract
:1. Introduction
2. Results
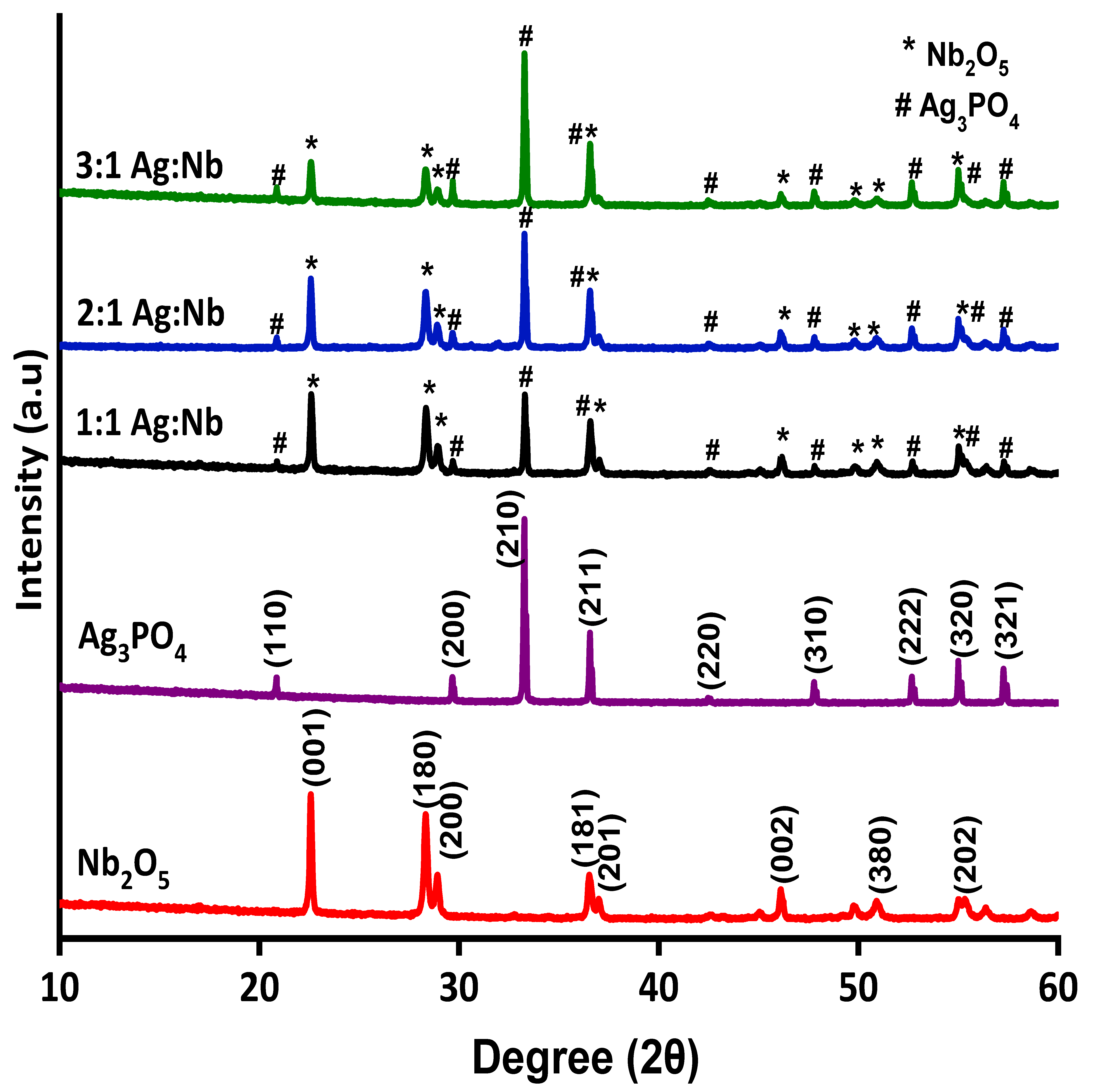
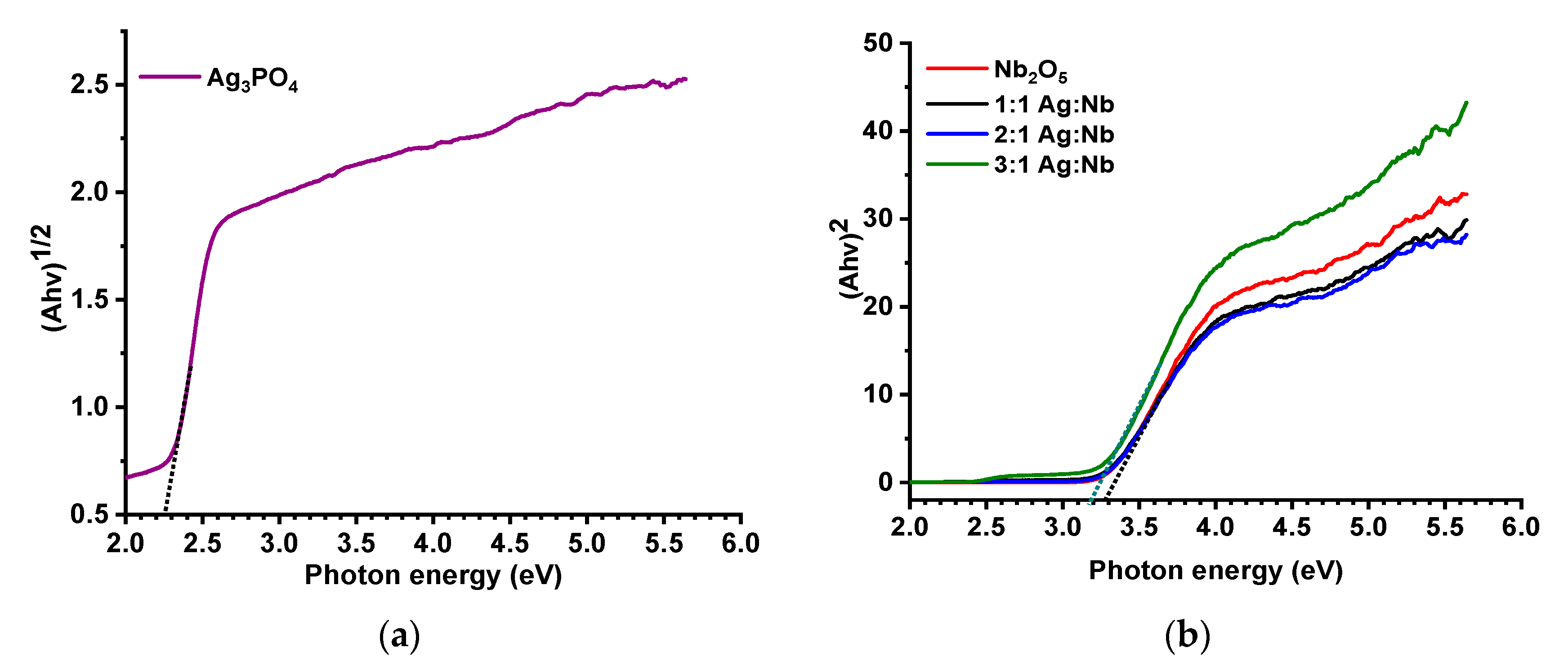
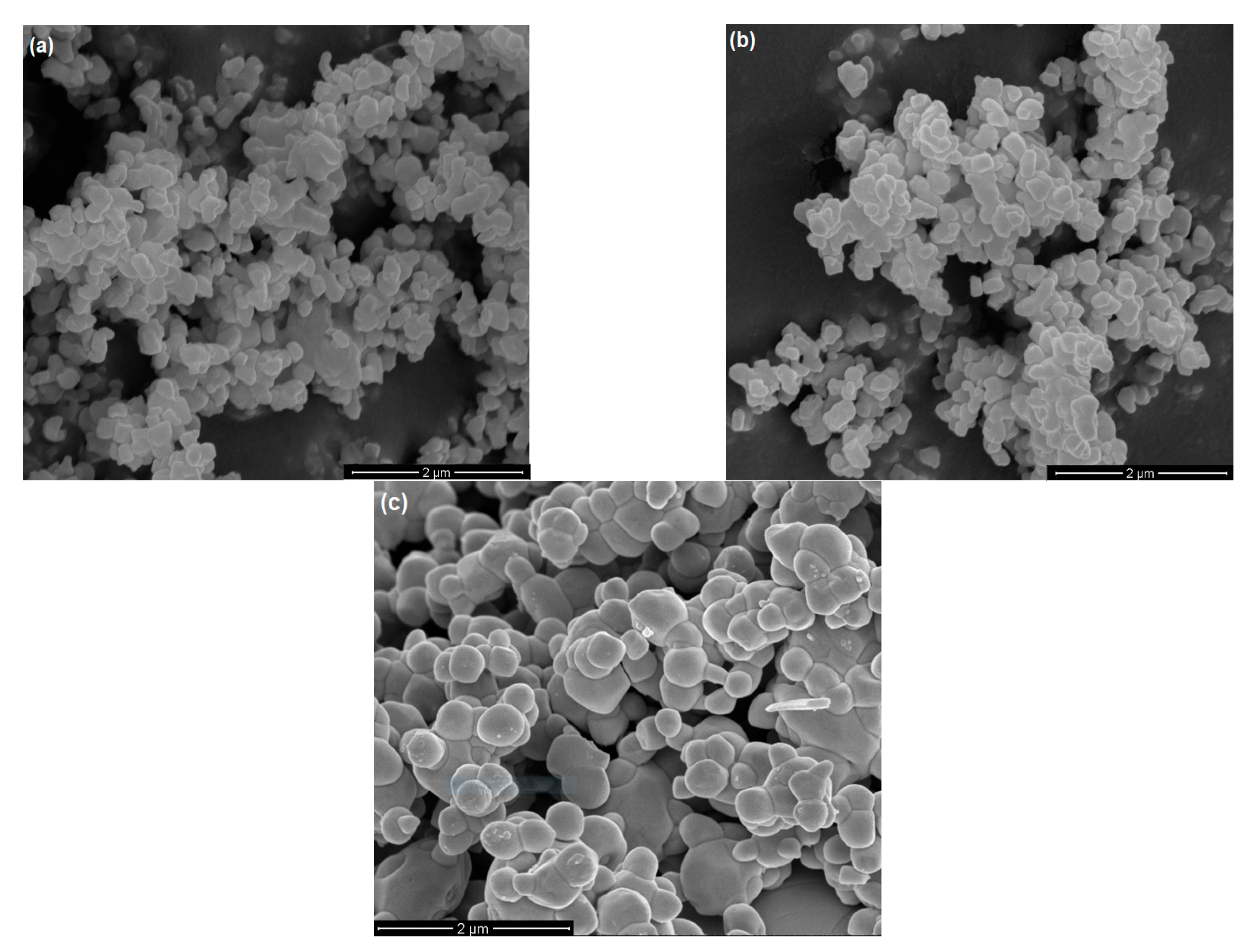
2.1. Characteristics of the Photocatalysts
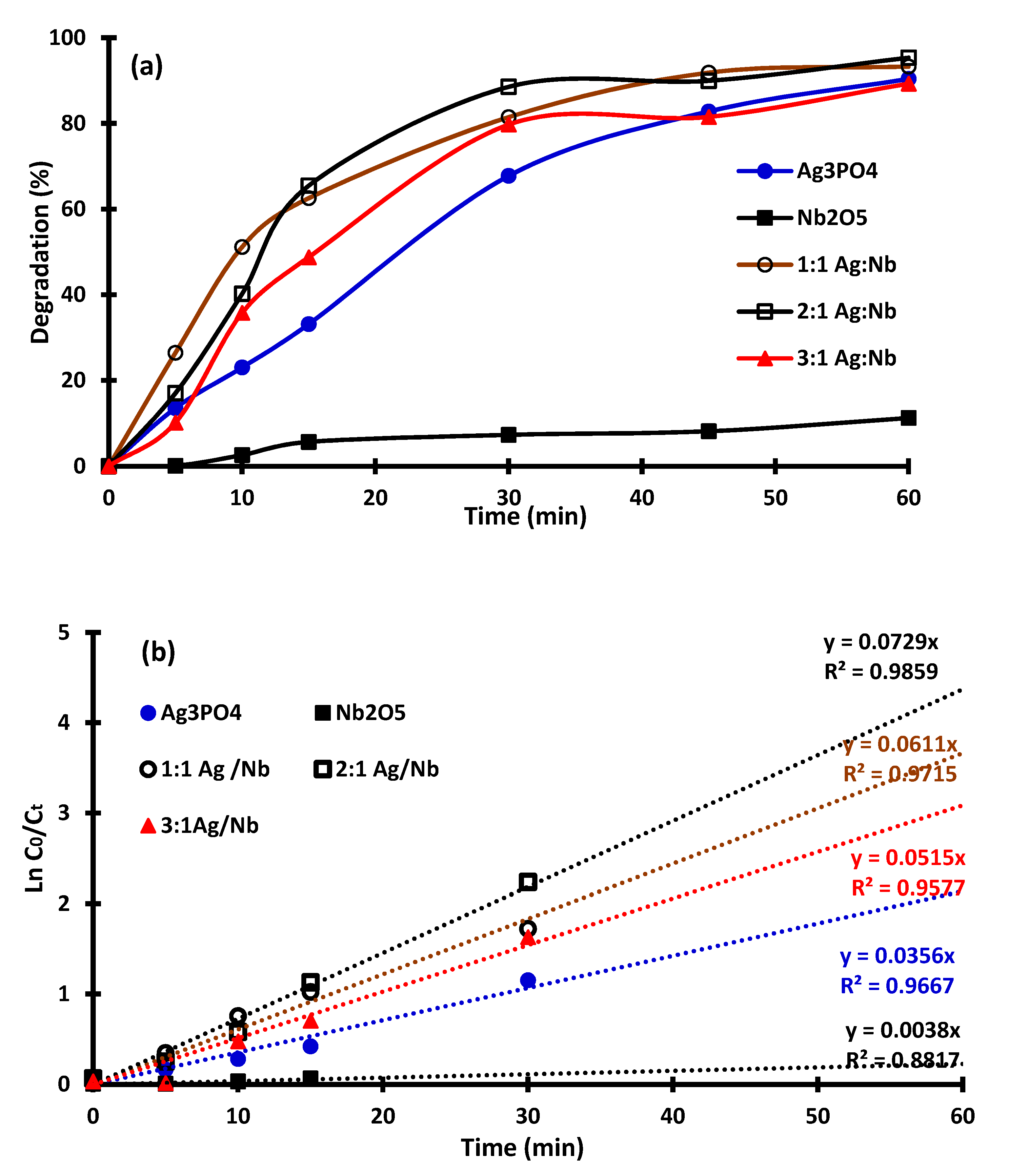
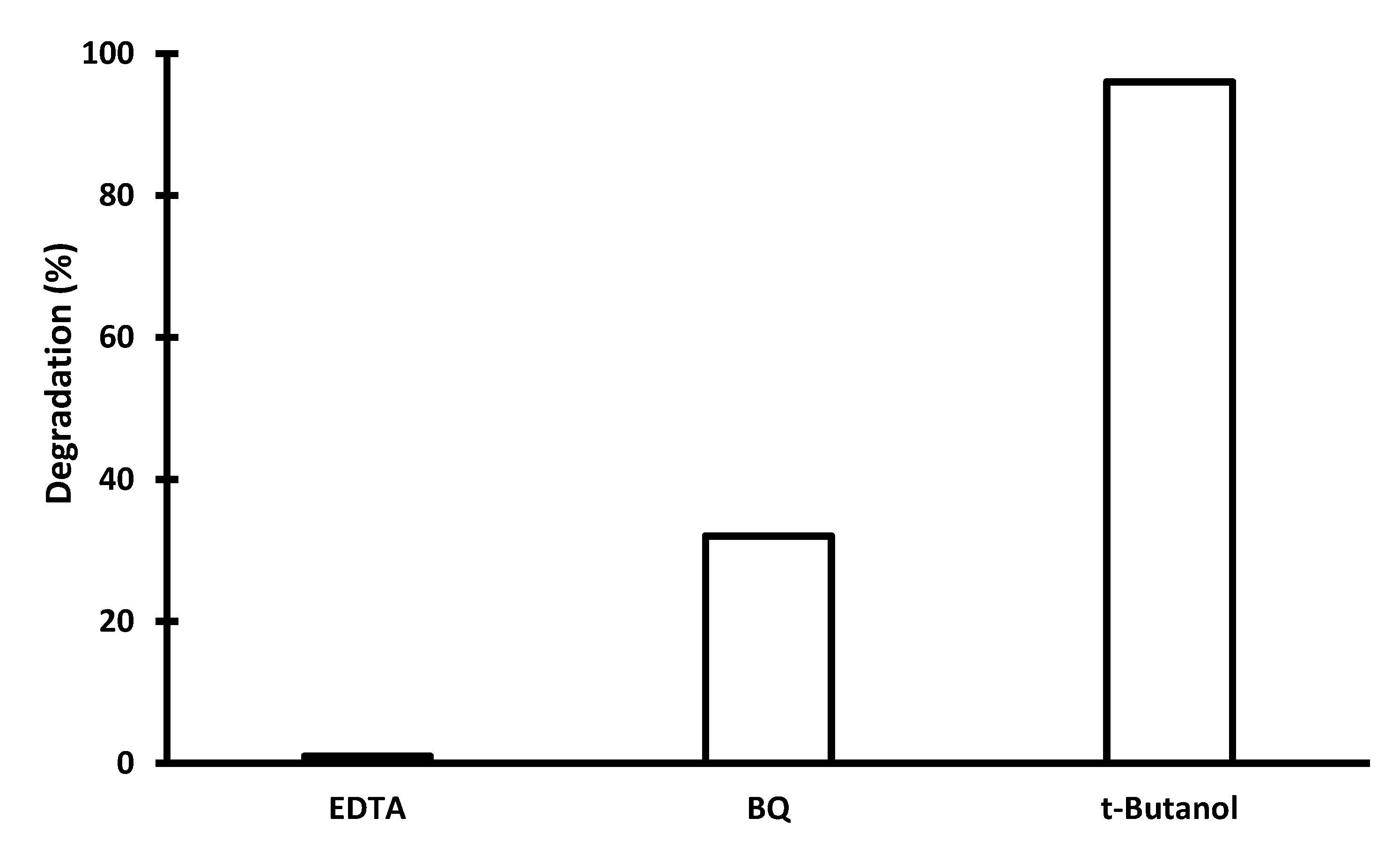
2.2. Photocatalytic Activity
2.3. Reusability of the Ag3PO4/Nb2O5 Photocatalyst
3. Materials and Methods
3.1. The Synthesis of the Ag3PO4/Nb2O5 Photocatalyst
3.2. Characterization of the Catalyst
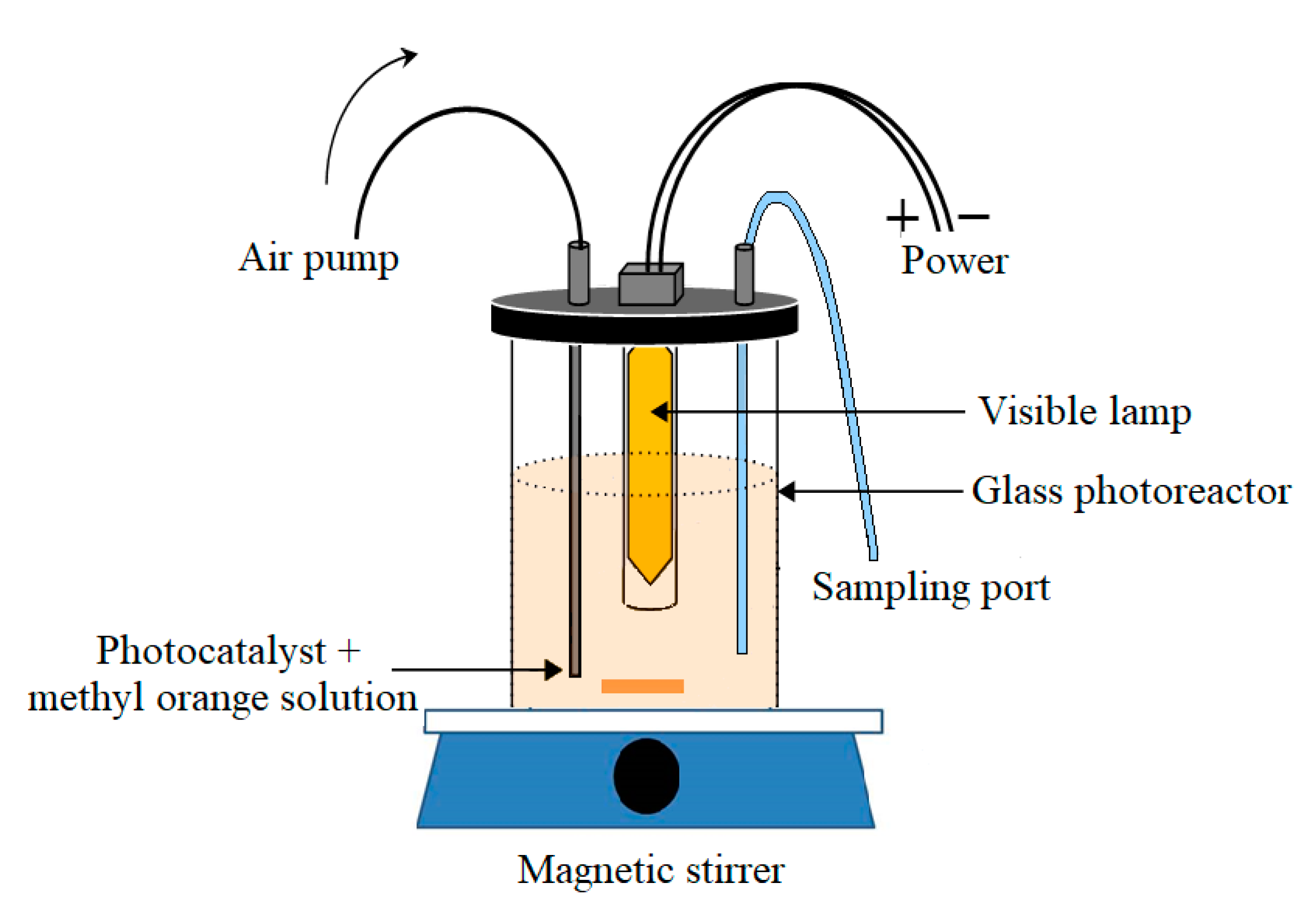
3.3. Photoactivity of the Ag3PO4/Nb2O5 Photocatalyst
3.4. Reusability Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandal, S.; Natarajan, S.; Tamilselvi, A.; Mayadevi, S. Photocatalytic and antimicrobial activities of zinc ferrite nanoparticles synthesized through soft chemical route: A magnetically recyclable catalyst for water/wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 2706–2712. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Zeng, X.; Cao, Z.; Dong, H.; Wang, L.; Wang, F.; Liu, J.; Li, Z.; Liang, Q. A novel Ag3PO4/Nb2O5 fiber composite with enhanced photocatalytic performance and stability. RSC Adv. 2015, 5, 102101–102107. [Google Scholar] [CrossRef]
- Cai, L.; Long, Q.; Yin, C. Synthesis and characterization of high photocatalytic activity and stable Ag3PO4/TiO2 fibers for photocatalytic degradation of black liquor. Appl. Surf. Sci. 2014, 319, 60–67. [Google Scholar] [CrossRef]
- Zhao, F.M.; Pan, L.; Wang, S.; Deng, Q.; Zou, J.J.; Wang, L.; Zhang, X. Ag3PO4/TiO2 composite for efficient photodegradation of organic pollutants under visible light. Appl. Surf. Sci. 2014, 317, 833–838. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, B.; Huang, C.; Ma, C.; Song, X.; Xu, Q. Synthesis and characterization of high efficiency and stable Ag3PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions. J. Mater. Chem. 2012, 22, 4050–4055. [Google Scholar] [CrossRef]
- Dong, C.; Wu, K.L.; Li, M.R.; Liu, L.; Wei, X.W. Synthesis of Ag3PO4-ZnO nanorod composites with high visible-light photocatalytic activity. Catal. Commun. 2014, 46, 32–35. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, C.; Zhai, W.; Wang, Z.; Sun, Y.; Chi, F.; Ran, S.; Liu, X.; Lv, Y. Novel Ag3PO4/CeO2 p-n Hierarchical Heterojunction with Enhanced Photocatalytic Performance. Mater. Res. 2016, 19, 673–679. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Huang, G.; Zhang, Z.; Han, L.; Song, W.; Li, M.; Zhang, Y. Facile synthesis of urchin-like hierarchical Nb2O5 nanospheres with enhanced visible light photocatalytic activity. J. Alloys Compd. 2017, 9, 19–28. [Google Scholar] [CrossRef]
- Souza, R.P.; Freitas, T.K.F.S.; Domingues, F.S.; Pezoti, O.; Ambrosio, E.; Ferrari-Lima, A.M.; Garcia, J.C. Photocatalytic activity of TiO2, ZnO and Nb2O5 applied to degradation of textile wastewater. J. Photochem. Photobiol. A Chem. 2016, 329, 9–17. [Google Scholar] [CrossRef]
- Prado, A.G.S.; Bolzon, L.B.; Pedroso, C.P.; Moura, A.O.; Costa, L.L. Nb2O5 as efficient and recyclable photocatalyst for indigo carmine degradation. Appl. Catal. B Environ. 2008, 82, 219–224. [Google Scholar] [CrossRef]
- Morais, L.A.; Adán, C.; Araujo, A.S.; Guedes, A.P.M.A.; Marugán, J. Synthesis, Characterization, and Photonic Efficiency of Novel Photocatalytic Niobium Oxide Materials. Glob. Chall. 2017, 1, 1700066. [Google Scholar] [CrossRef]
- He, J.; Hu, Y.; Wang, Z.; Lu, W.; Yang, S.; Wu, G.; Wang, Y.; Wang, S.; Gu, H.; Wang, J. Hydrothermal growth and optical properties of Nb2O5 nanorod arrays. J. Mater. Chem. C 2014, 2, 8185–8190. [Google Scholar] [CrossRef]
- Botelho, G.; Andres, J.; Gracia, L.; Matos, L.S.; Longo, E. Photoluminescence and Photocatalytic Properties of Ag3PO4 Microcrystals: An Experimental and Theoretical Investigation. ChemPlusChem 2016, 81, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zheng, Y.F.; Zhou, H.; Yin, H.Y.; Song, X.C. The effect of synthesis temperature on the morphologies and visible light photocatalytic performance of Ag3PO4. J. Taiwan Inst. Chem. Eng. 2016, 60, 328–334. [Google Scholar] [CrossRef]
- Katsumata, H.; Sakai, T.; Suzuki, T.; Kaneco, S. Highly Efficient Photocatalytic Activity of g-C3N4/Ag3PO4 Hybrid Photocatalysts through Z-Scheme Photocatalytic Mechanism under Visible Light. Ind. Eng. Chem. Res. 2014, 53, 8018–8025. [Google Scholar] [CrossRef]
- Li, Y.; Yu, L.; Li, N.; Yan, W.; Li, X. Heterostructures of Ag3PO4/TiO2 mesoporous spheres with highly efficient visible light photocatalytic activity. J. Colloid Interface Sci. 2015, 450, 246–253. [Google Scholar] [CrossRef]
- Qamar, M.; Abdalwadoud, M.; Ahmed, M.I.; Azad, A.M.; Merzougui, B.; Bukola, S.; Yamani, Z.H.; Siddiqui, M.N. Single-Pot Synthesis of 001-Faceted N-Doped Nb2O5/Reduced Graphene Oxide Nanocomposite for Efficient Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2015, 7, 17954–17962. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wang, R.; Zhang, Z.; Qiu, S. Facile preparation of C, N co-modified Nb2O5 nanoneedles with enhanced visible light photocatalytic activity. Dalton Trans. 2016, 45, 16519–16525. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, C.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Nanoarchitectured Nb2O5 hollow, Nb2O5@carbon and NbO2@carbon Core-Shell Microspheres for Ultrahigh-Rate Intercalation Pseudocapacitors. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Song, X.; Huang, Y.; Fang, Y.; Jia, M.; Ma, W. Visible-light photocatalytic degradation of azo dyes in water by Ag3PO4: An unusual dependency between adsorption and the degradation rate on pH value. J. Mol. Catal. A Chem. 2016, 421, 57–65. [Google Scholar] [CrossRef]
- Gnanaprakasam, A.; Sivakumar, V.M.; Thirumarimurugan, M. Influencing Parameters in the Photocatalytic Degradation of Organic Effluent via Nanometal Oxide Catalyst: A Review. Indian J. Mater. Sci. 2015, 2015, 601827. [Google Scholar] [CrossRef]




| Sample | Elemental Composition (Atom %) | Ag:Nb | |||
|---|---|---|---|---|---|
| 3Ag | 2Nb | P | O | ||
| Ag:Nb (1:1) | 13.37 | 7.83 | 4.14 | 74.66 | 4.4:4.0 |
| Ag:Nb (2:1) | 21.93 | 6.97 | 6.43 | 64.67 | 7.3:3.5 |
| Ag:Nb (3:1) | 30.18 | 6.73 | 8.86 | 54.23 | 10.1:3.3 |
| Sample | Particle Size | BET Surface Area |
|---|---|---|
| (µm) | (m2g−1) | |
| Ag3PO4 | 0.89 ± 0.11 | 1.63 |
| Nb2O5 | 0.68 ± 0.06 | 6.92 |
| Ag:Nb (1:1) | 1.02 ± 0.11 | 1.70 |
| Ag:Nb (2:1) | 1.17 ± 0.09 | 0.89 |
| Ag:Nb (3:1) | 1.31 ± 0.12 | 1.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, N.S.; Sulaiman, S.N.; Muhamad, E.N.; Mukhair, H.; Tan, S.T.; Abdullah, A.H. Synthesis of an Ag3PO4/Nb2O5 Photocatalyst for the Degradation of Dye. Catalysts 2021, 11, 458. https://doi.org/10.3390/catal11040458
Osman NS, Sulaiman SN, Muhamad EN, Mukhair H, Tan ST, Abdullah AH. Synthesis of an Ag3PO4/Nb2O5 Photocatalyst for the Degradation of Dye. Catalysts. 2021; 11(4):458. https://doi.org/10.3390/catal11040458
Chicago/Turabian StyleOsman, Nur Syazwani, Siti Norhasimah Sulaiman, Ernee Noryana Muhamad, Hayati Mukhair, Sin Tee Tan, and Abdul Halim Abdullah. 2021. "Synthesis of an Ag3PO4/Nb2O5 Photocatalyst for the Degradation of Dye" Catalysts 11, no. 4: 458. https://doi.org/10.3390/catal11040458
APA StyleOsman, N. S., Sulaiman, S. N., Muhamad, E. N., Mukhair, H., Tan, S. T., & Abdullah, A. H. (2021). Synthesis of an Ag3PO4/Nb2O5 Photocatalyst for the Degradation of Dye. Catalysts, 11(4), 458. https://doi.org/10.3390/catal11040458






