Hydrogen Evolution on Reduced Graphene Oxide-Supported PdAu Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Graphene/GC
2.2. AFM and XPS Characterization of Au/rGO and PdAu/rGO
2.3. Electrochemical Measurements
2.3.1. Cyclic Voltammetry of PdAu/rGO
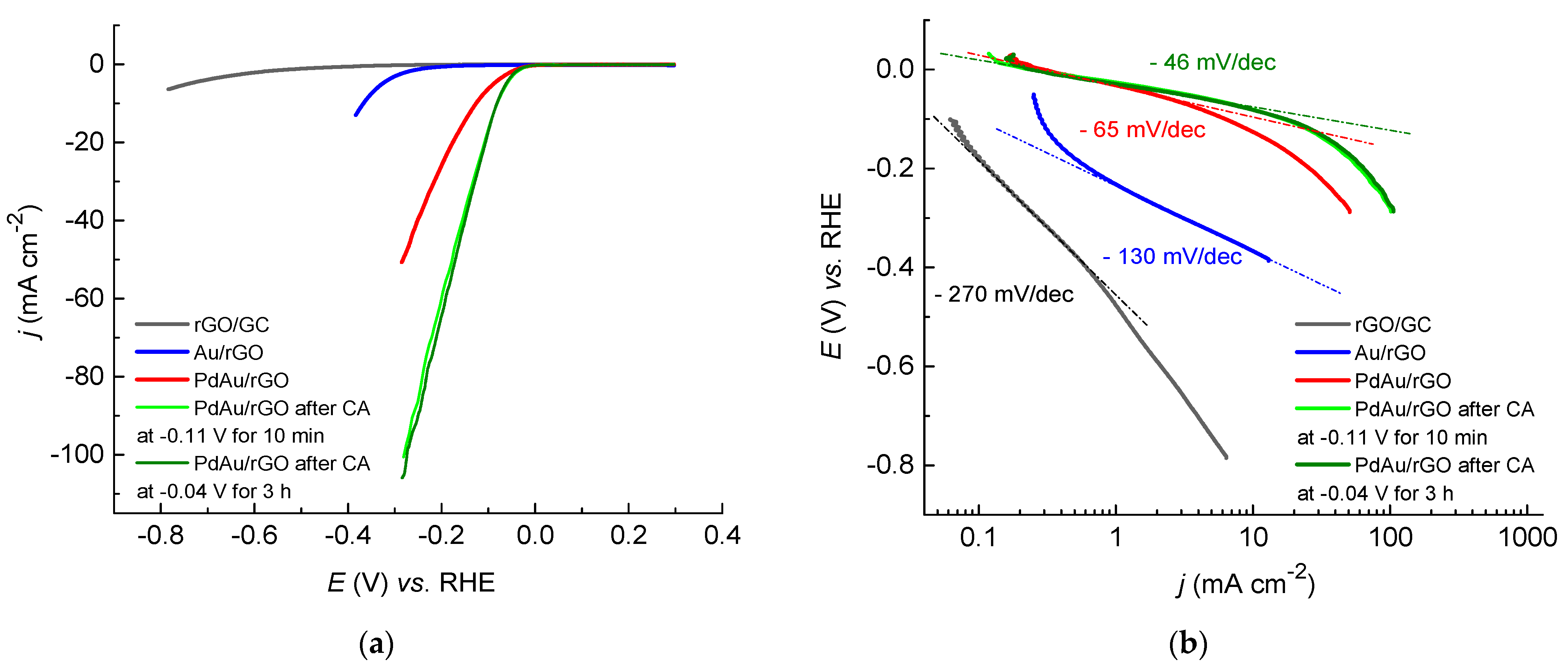
2.3.2. Hydrogen Evolution on Au/rGO and PdAu/rGO Electrodes
2.3.3. Stability and Durability Test for HER on PdAu/rGO Electrode
3. Materials and Methods
3.1. Materials Preparation
3.1.1. Graphene Synthesis
3.1.2. Preparation of Au/rGO and PdAu/rGO Electrodes
3.2. Materials Characterization
3.3. Electrochemical Measurements
3.4. Chemicals
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hou, J.; Yang, M.; Ke, C.; Wei, G.; Priest, C.; Qiao, Z.; Wu, G.; Zhang, J. Platinum-group-metal catalysts for proton exchange membrane fuel cells: From catalyst design to electrode structure optimization. Energy Chem. 2020, 2, 100023. [Google Scholar] [CrossRef]
- Zheng, J.; Sheng, W.; Zhuang, Z.; Xu, B.; Yan, Y. Universal dependence of hydrogen oxidation and evolution reaction activity of platinum-group metals on pH and hydrogen binding energy. Sci. Adv. 2016, 2, e1501602. [Google Scholar] [CrossRef] [Green Version]
- Martín, A.; Escarpa, A. Graphene: The cutting–edge interaction between chemistry and electrochemistry. Trends Analyt. Chem. 2014, 56, 13–26. [Google Scholar] [CrossRef]
- Garlyyev, B.; Fichtner, J.; Piqué, O.; Schneider, O.; Bandarenka, A.S.; Calle-Vallejo, F. Revealing the nature of active sites in electrocatalysis. Chem. Sci. 2019, 10, 8060–8075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23–J26. [Google Scholar] [CrossRef] [Green Version]
- Kibler, L.A. Hydrogen electrocatalysis. ChemPhysChem 2006, 7, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Pandelov, S.; Stimming, U. Reactivity of monolayers and nanoislands of palladium on Au(111) with respect to proton reduction. Electrochim. Acta 2007, 52, 5548–5555. [Google Scholar] [CrossRef]
- Smiljanić, M.; Srejić, I.; Grgur, B.; Rakočević, Z.; Štrbac, S. Catalysis of hydrogen evolution on Au(111) modified by spontaneously deposited Pd islands. Electrocatalysis 2012, 3, 369–375. [Google Scholar] [CrossRef]
- Darabdhara, G.; Amin, M.A.; Mersal, G.A.M.; Ahmed, E.M.; Das, M.R.; Zakaria, M.B.; Malgras, V.; Alshehri, S.M.; Yamauchi, Y.; Szunerits, S.; et al. Reduced graphene oxide nanosheets decorated with Au, Pd and Au-Pd bimetallic nanoparticles as highly efficient catalysts for electrochemical hydrogen generation. J. Mater. Chem. A 2015, 3, 20254–20266. [Google Scholar] [CrossRef]
- Feng, J.-J.; Chen, L.-X.; Song, P.; Wu, X.-l.; Wang, A.-J.; Yuan, J. Bimetallic AuPd nanoclusters supported on graphitic carbon nitride: One-pot synthesis and enhanced electrocatalysis for oxygen reduction and hydrogen evolution. Int. J. Hydrog. Energy 2016, 41, 8839–8846. [Google Scholar] [CrossRef]
- Li, D.-N.; Wang, A.-J.; Wei, J.; Zhang, Q.-L.; Feng, J.-J. Facile synthesis of flower-like Au@AuPd nanocrystals with highly electrocatalytic activity for formic acid oxidation and hydrogen evolution reactions. Int. J. Hydrog. Energy 2017, 42, 19894–19902. [Google Scholar] [CrossRef]
- Choi, W.; Hu, G.; Kwak, K.; Kim, M.; Jiang, D.; Choi, J.-P.; Lee, D. Effects of metal-doping on hydrogen evolution reaction catalyzed by MAu24 and M2Au36 nanoclusters (M = Pt, Pd). ACS Appl. Mater. Interfaces 2018, 10, 44645–44653. [Google Scholar] [CrossRef]
- Rakočević, L.; Štrbac, S.; Srejić, I. Hydrogen evolution on Au/GC and PdAu/GC nanostructures in acid solution: AFM, XPS, and electrochemical study. Int. J. Hydrog. Energy 2021, 46, 9052–9063. [Google Scholar] [CrossRef]
- Morar, J.F.; Himpsel, F.J.; Hollinger, G.; Jordan, J.L.; Huges, G.; McFeely, F.R. C 1s excitation studies of diamond (111). I. Surface core levels. Phys. Rev. B 1986, 33, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.Y.; Zhang, L.; Li, D.-W.; Karpuzov, D.; Long, Y.-T. Electrocatalytic oxidation of NADH on graphene oxide and reduced graphene oxide modified screen-printed electrode. Int. J. Electrochem. Sci. 2011, 6, 819–829. [Google Scholar]
- Morimoto, N.; Kubo, T.; Nishina, Y. Tailoring the oxygen content of graphite and reduced graphene oxide for specific applications. Sci. Rep. 2016, 6, 21715. [Google Scholar] [CrossRef] [PubMed]
- Chandrakumara, G.G.; Shang, J.; Qiu, L.; Fang, X.-Y.; Antolasic, F.; Easton, C.D.; Song, J.; Alan, T.; Li, D.; Liu, J.Z. Tuning the oxygen functional groups in reduced graphene oxide papers to enhance the electromechanical actuation. RSC Adv. 2015, 5, 68052–68060. [Google Scholar] [CrossRef]
- Heimann, P.; Van der Veen, J.F.; Eastman, D.E. Structure-dependent surface core level shifts for the Au(111), Au(100), and Au(110) surfaces. Solid State Commun. 1981, 38, 595–598. [Google Scholar] [CrossRef]
- Štrbac, S.; Smiljanić, M.; Rakočević, Z. Spontaneously deposited Rh on Au(111) observed by AFM and XPS: Electrocatalysis of hydrogen evolution. J. Electrochem. Soc. 2016, 163, D3027–D3033. [Google Scholar] [CrossRef]
- Štrbac, S.; Srejić, I.; Rakočević, Z. Catalysis of oxygen reduction on electrochemically activated polycrystalline gold by Pd nanoislands in alkaline solution. J. Electroanal. Chem. 2017, 789, 76–84. [Google Scholar] [CrossRef]
- Turner, N.H.; Single, A.M. Determination of peak positions and areas from wide-scan XPS spectra. Surf. Interface Anal. 1990, 15, 215–222. [Google Scholar] [CrossRef]
- Militello, M.C.; Simko, S.J. Elemental palladium by XPS. Surf. Sci. Spectra 1994, 3, 387–394. [Google Scholar] [CrossRef]
- Venezia, A.M.; Rossi, A.; Duca, D.; Martorana, A.; Deganello, G. Particle size and metal support interaction effects in pumice supported palladium catalysts. Appl. Catal. A 1995, 125, 113–128. [Google Scholar] [CrossRef]
- Bertolini, J.C.; Delichere, P.; Khanra, B.C.; Massardier, J.; Noupa, C.; Tardy, B. Electronic properties of supported Pd aggregates in relation with their reactivity for 1,3-butadiene hydrogenation. Catal. Lett. 1990, 6, 215–223. [Google Scholar] [CrossRef]
- Kim, K.S.; Grossmann, A.F.; Winograd, N. X-ray photoelectron spectroscopic studies of palladium oxides and the palladium-oxygen electrode. Anal. Chem. 1974, 46, 197–200. [Google Scholar] [CrossRef]
- Militello, M.C.; Simko, S.J. Palladium oxide (PdO) by XPS. Surf. Sci. Spectra 1994, 3, 395–401. [Google Scholar] [CrossRef]
- Moddeman, W.E.; Bowling, W.C.; Carter, D.C.; Grove, D.R. XPS surface and bulk studies of heat treated palladium in the presence of hydrogen at 150 °C. Surf. Interface Anal. 1988, 11, 317–326. [Google Scholar] [CrossRef]
- Marcelina, V.; Syaki, N.; Wyantuti, S.; Hartati, Y.W.; Hidayat, R. Fitrilawati Characteristic of Thermally Reduced Graphene Oxide as Supercapacitors Electrode Materials. IOP Conf. Ser. Mater. Sci. Eng. 2017, 196, 012034. [Google Scholar]
- Karbowska, B.; Rebis, T.; Milczarek, G. Electrode modified by reduced graphene oxide for monitoring of total thallium in grain products. Int. J. Environ. Res. Public Health 2018, 15, 653. [Google Scholar] [CrossRef] [Green Version]
- Juodkazis, K.; Juodkazytė, J.; Šebeka, B.; Stalnionis, G.; Lukinskas, A. Anodic dissolution of palladium in sulfuric acid: An electrochemical quartz crystal microbalance study. Russ. J. Electrochem. 2003, 39, 954–959. [Google Scholar] [CrossRef]
- Grdeń, M.; Łukaszewski, M.; Jerkiewicz, G.; Czerwiński, A. Electrochemical behaviour of palladium electrode: Oxidation, electrodissolution and ionic adsorption. Electrochim. Acta 2008, 53, 7583–7598. [Google Scholar] [CrossRef]
- Suffredini, H.B.; Machado, S.A.S.; Avaca, L.A. The water decomposition reactions on boron-doped diamond electrodes. J. Braz. Chem. Soc. 2004, 15, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.G.M.; Brownson, D.A.C.; Banks, C.E. Investigating the integrity of graphene towards the electrochemical hydrogen evolution reaction (HER). Sci. Rep. 2019, 9, 15961. [Google Scholar] [CrossRef]
- Ghasemi, S.; Hosseini, S.R.; Nabipour, S.; Asen, P. Palladium nanoparticles supported on graphene as an efficient electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 2015, 40, 16184–16191. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High catalytic activity of nitrogen and sulfur co-doped nanoporous graphene in the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2014, 53, 1–7. [Google Scholar]
- Wang, Y.; Sun, Y.; Liao, H.; Sun, S.; Li, S.; Ager, J.W., III; Xu, Z.J. Activation effect of electrochemical cycling on gold nanoparticles towards the hydrogen evolution reaction in sulfuric acid. Electrochim. Acta 2016, 209, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Shinagava, T.; Garcia-Espanza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.L.; León, C.; Monnier, G.; Ivanova, S.; Centeno, M.Á.; Odriozola, J.A. Bimetallic PdAu catalysts for formic acid dehydrogenation. Int. J. Hydrog. Energy 2020, 45, 23056–23068. [Google Scholar] [CrossRef]
- Al-Odail, F.A.; Anastasopoulos, A.; Hayden, B.E. The hydrogen evolution reaction and hydrogen oxidation reaction on thin film PdAu alloy surfaces. Phys. Chem. Chem. Phys. 2010, 12, 11398–11406. [Google Scholar] [CrossRef]
- Štrbac, S.; Maroun, F.; Magnussen, O.; Behm, R.J. The structure, growth and reactivity of electrodeposited Ru/Au(111) surfaces. Electroanal. Chem. 2001, 500, 479–490. [Google Scholar] [CrossRef]
- Johnston, C.M.; Štrbac, S.; Wieckowski, A. In situ STM study of Au(111)/Os bimetallic surfaces: Spontaneous deposition and electrochemical dissolution. Langmuir 2005, 21, 9610–9617. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, H.; Zhen, S.G.; Poh, C.K.; Chaurasia, A.; Luo, J.; Wu, X.; Lee, E.K.; Sahoo, N.G.; Lin, J.; et al. A green approach to the synthesis of high-quality graphene oxide flakes via electrochemical exfoliation of pencil core. RSC Adv. 2013, 3, 11745–11750. [Google Scholar] [CrossRef]






| Line | Au/rGO Electrode at% | PdAu/rGO Electrode at% |
|---|---|---|
| C 1s | 87.37 | 85.35 |
| O 1s | 11.34 | 13.31 |
| Au 4f7/2 | 1.18 | 0.21 |
| Pd 3d5/2 | 1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakočević, L.; Srejić, I.; Maksić, A.; Golubović, J.; Štrbac, S. Hydrogen Evolution on Reduced Graphene Oxide-Supported PdAu Nanoparticles. Catalysts 2021, 11, 481. https://doi.org/10.3390/catal11040481
Rakočević L, Srejić I, Maksić A, Golubović J, Štrbac S. Hydrogen Evolution on Reduced Graphene Oxide-Supported PdAu Nanoparticles. Catalysts. 2021; 11(4):481. https://doi.org/10.3390/catal11040481
Chicago/Turabian StyleRakočević, Lazar, Irina Srejić, Aleksandar Maksić, Jelena Golubović, and Svetlana Štrbac. 2021. "Hydrogen Evolution on Reduced Graphene Oxide-Supported PdAu Nanoparticles" Catalysts 11, no. 4: 481. https://doi.org/10.3390/catal11040481
APA StyleRakočević, L., Srejić, I., Maksić, A., Golubović, J., & Štrbac, S. (2021). Hydrogen Evolution on Reduced Graphene Oxide-Supported PdAu Nanoparticles. Catalysts, 11(4), 481. https://doi.org/10.3390/catal11040481






