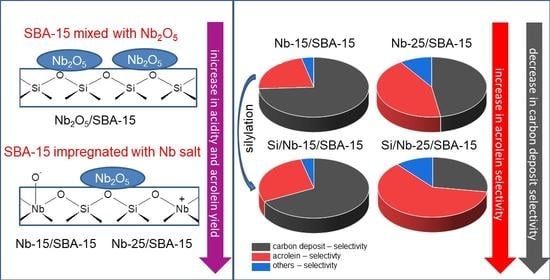
Insight into Active Centers and Anti-Coke Behavior of Niobium-Containing SBA-15 for Glycerol Dehydration
Abstract
1. Introduction
2. Results and Discussion
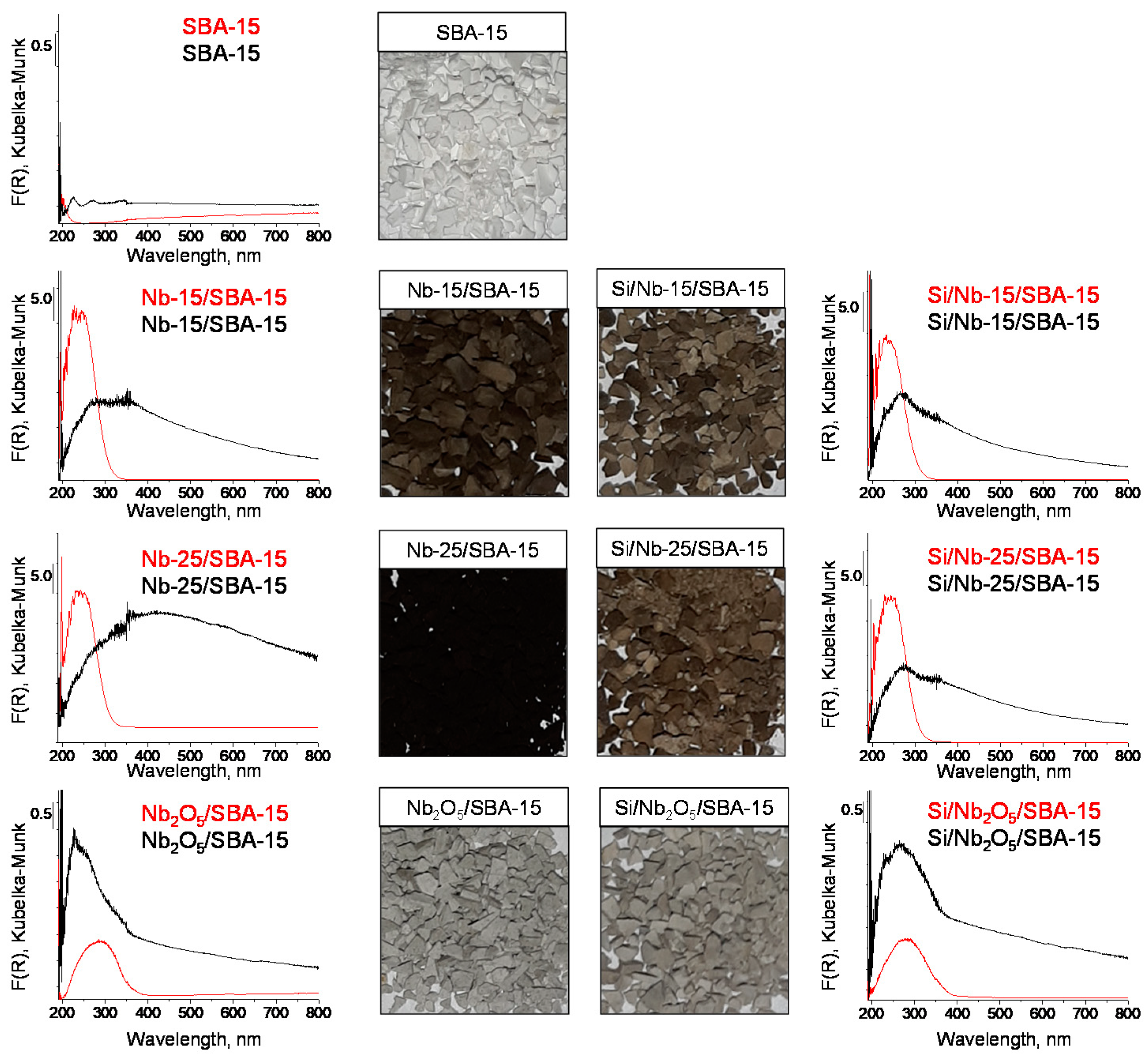
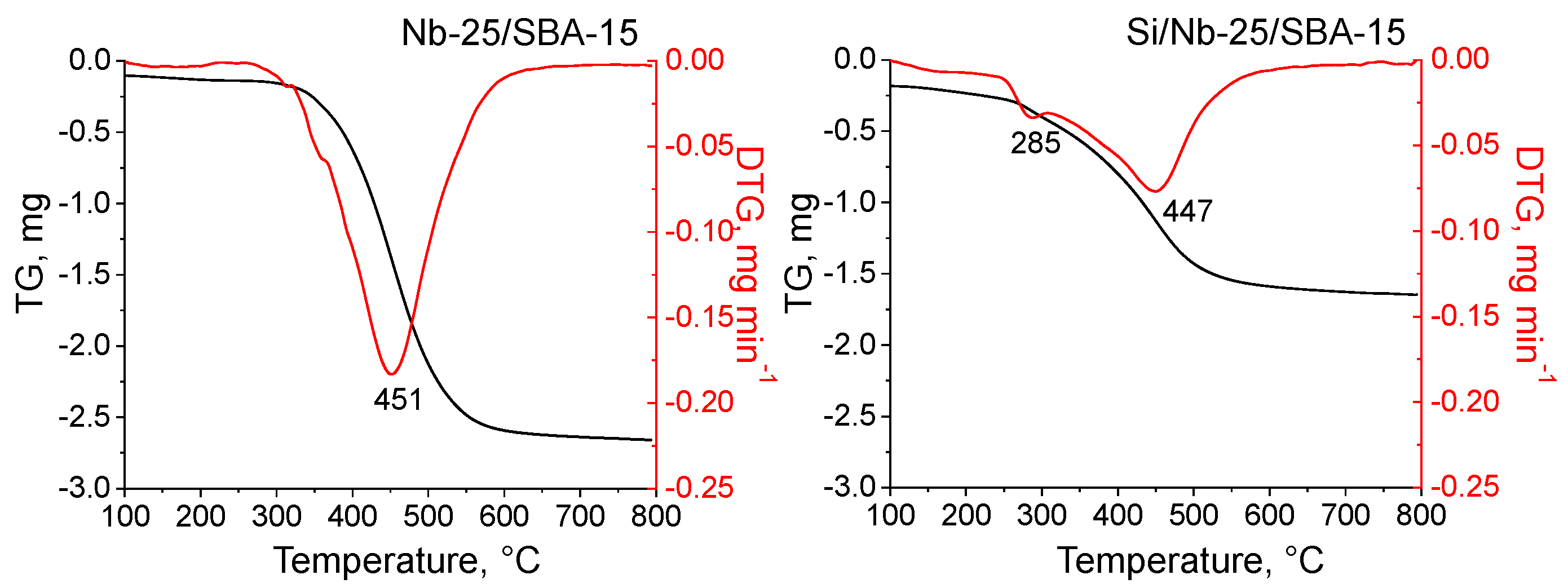
2.1. Texture/Structure Characterization
2.2. Surface Properties
2.2.1. Hydroxyls on the Catalysts Surface
2.2.2. Acid-Base Properties
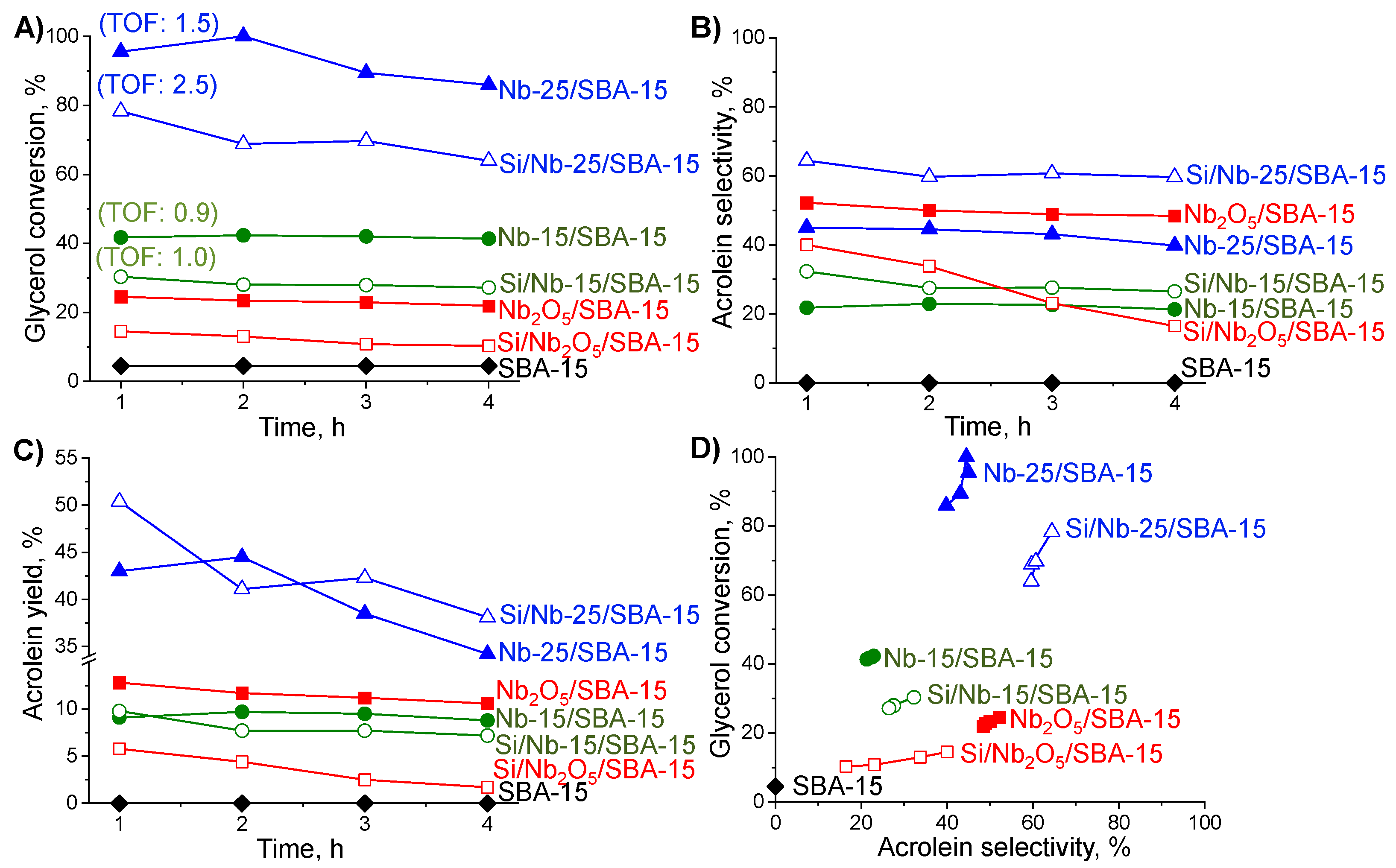
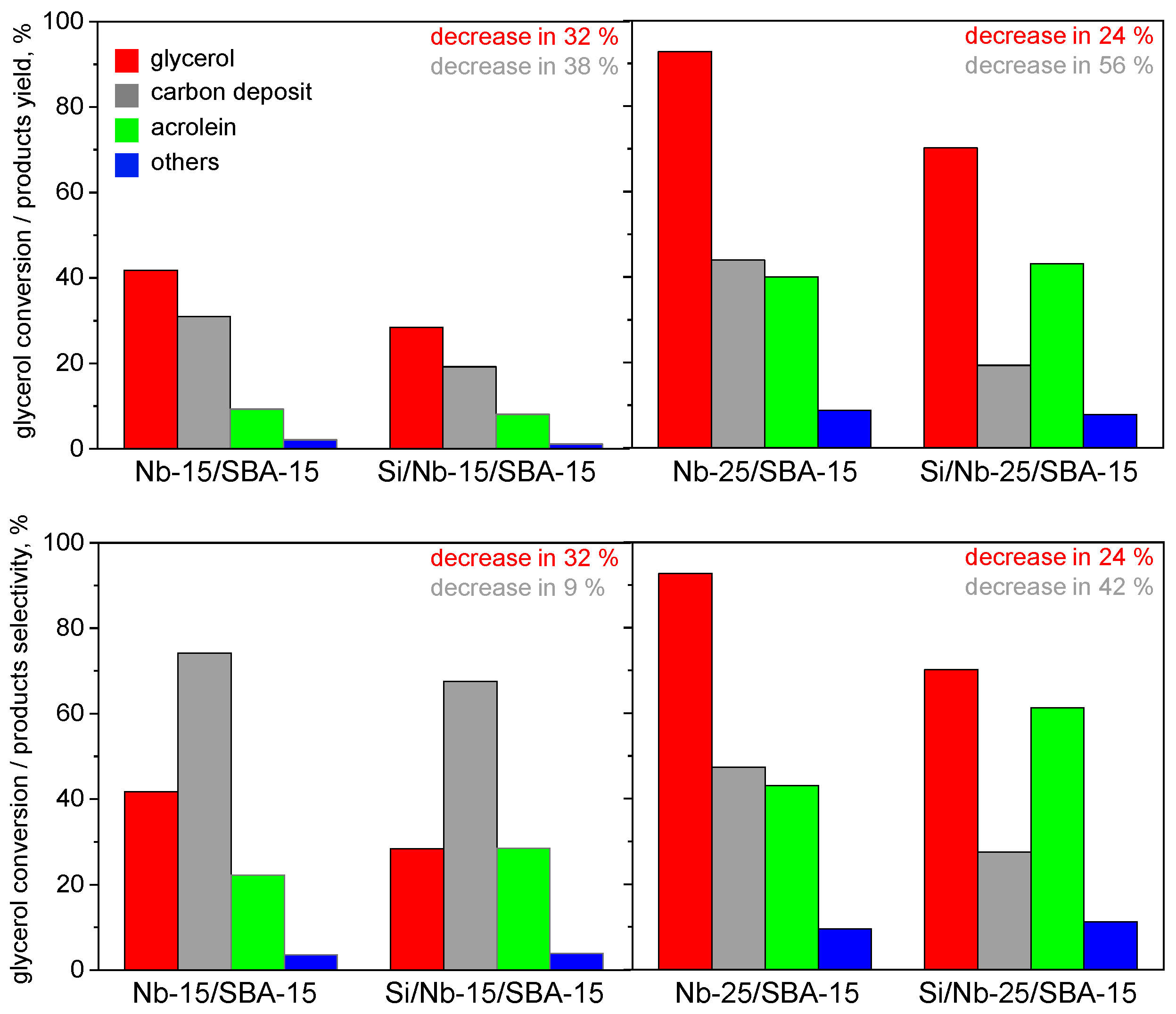
2.3. Glycerol Dehydration
3. Materials and Methods
3.1. Materials
- Pluronic P123 (Aldrich, St. Louis, MO, USA);
- HCl (Stanlab, Lublin, Poland);
- Tetraethoxysilane (TEOS) (Aldrich, St. Louis, MO, USA);
- Ammonium niobate(V) oxalate (Aldrich, St. Louis, MO, USA);
- Nb2O5 (CBMM, Araxá, Minas Gerais, Brazil);
- (3-aminopropyl)trimethoxysilane (APTMS) (Aldrich, St. Louis, MO, USA);
- Toluene (Stanlab, Lublin, Poland);
- Acetonitrile (Aldrich, St. Louis, MO, USA);
- Pyridine (Aldrich, St. Louis, MO, USA);
- Glycerol (Aldrich, St. Louis, MO, USA);
- Hydroquinone (Aldrich, St. Louis, MO, USA).
3.2. Catalysts Preparation
3.2.1. Synthesis of SBA-15
3.2.2. SBA-15 Modification with Niobium Precursor
3.2.3. Silylation of Niobiosilicas
3.3. Samples Characterization
3.4. Glycerol Dehydration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Chen, X.; Shen, Y. Commodity chemicals derived from glycerol, an important biorefinery feedstock. Chem. Rev. 2008, 108, 5253–5277. [Google Scholar] [CrossRef]
- Zhou, C.H.; Beltramini, J.N.; Fan, Y.-X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García-Sancho, C.; Jiménez-Gómez, C.P.; Moreno-Tost, R.; Maireles-Torres, P. Porous silicon-based catalysts for the dehydration of glycerol to high value-added products. Materials 2018, 11, 1569. [Google Scholar] [CrossRef]
- Wu, S.T.; She, Q.M.; Tesser, R.; Di Serio, M.; Zhou, C.H. Catalytic glycerol dehydration-oxidation to acrylic acid. Catal. Rev. Sci. Eng. 2020, 62, 481–523. [Google Scholar] [CrossRef]
- Avalos, A.S.; Hakkarainen, M.; Odelius, K. Superiorly plasticized PVC/PBSA blends through crotonic and acrylic acid functionalization of PVC. Polymers 2017, 9, 84. [Google Scholar] [CrossRef]
- Cabana, S.; Lecona-Vargas, C.S.; Meléndez-Ortiz, H.I.; Contreras-García, A.; Barbosa, S.; Taboada, P.; Magariños, B.; Bucio, E.; Concheiro, A.; Alvarez-Lorenzo, C. Silicone rubber films functionalized with poly(acrylic acid) nanobrushes for immobilization of gold nanoparticles and photothermal therapy. J. Drug Deliv. Sci. Technol. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Riccardi, C.M.; Kasi, R.M.; Kumar, C.V. Nanoarmoring of Enzymes by Interlocking in Cellulose Fibers with Poly(Acrylic Acid), 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 590. [Google Scholar]
- Foo, G.S.; Wei, D.; Sholl, D.S.; Sievers, C. Role of Lewis and Brønsted acid sites in the dehydration of glycerol over niobia. ACS Catal. 2014, 4, 3180–3192. [Google Scholar] [CrossRef]
- Copeland, J.R.; Santillan, I.A.; Schimming, S.M.N.; Ewbank, J.L.; Sievers, C. Surface interactions of glycerol with acidic and basic metal oxides. J. Phys. Chem. C 2013, 117, 21413–21425. [Google Scholar] [CrossRef]
- Nimlos, M.R.; Blanksby, S.J.; Qian, X.; Himmel, M.E.; Johnson, D.K. Mechanisms of glycerol dehydration. J. Phys. Chem. A 2006, 110, 6145–6156. [Google Scholar] [CrossRef]
- Park, H.; Yun, Y.S.; Kim, T.Y.; Lee, K.R.; Baek, J.; Yi, J. Kinetics of the dehydration of glycerol over acid catalysts with an investigation of deactivation mechanism by coke. Appl. Catal. B Environ. 2015, 176–177, 1–10. [Google Scholar] [CrossRef]
- Tsukuda, E.; Sato, S.; Takahashi, R.; Sodesawa, T. Production of acrolein from glycerol over silica-supported heteropoly acids. Catal. Commun. 2007, 8, 1349–1353. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Moon, D.J.; Kim, J.H.; Park, N.C.; Kim, Y.C. Studies on the dehydration of glycerol over niobium catalysts. J. Nanosci. Nanotechnol. 2011, 11, 7128–7131. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.H.; Wang, H.P.; Liang, Y.; Xu, B.Q. Sustainable production of acrolein: Gas-phase dehydration of glycerol over Nb2O5 catalyst. J. Catal. 2007, 250, 342–349. [Google Scholar] [CrossRef]
- Shiju, N.R.; Brown, D.R.; Wilson, K.; Rothenberg, G. Glycerol valorization: Dehydration to acrolein over silica-supported niobia catalysts. Top. Catal. 2010, 53, 1217–1223. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Marzo, M. Dispersed NbOx catalytic phases in silica matrixes: Influence of niobium concentration and preparative route. J. Phys. Chem. C 2008, 112, 14064–14074. [Google Scholar] [CrossRef]
- Li, W.; Xu, K.; Xu, L.; Hu, J.; Ma, F.; Guo, Y. Preparation of highly ordered mesoporous AlSBA-15-SO3H hybrid material for the catalytic synthesis of chalcone under solvent-free condition. Appl. Surf. Sci. 2010, 256, 3183–3190. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Deng, J.; Dai, H.; He, H.; Au, C.T. Preparation, characterization, and catalytic activity of chromia supported on SBA-15 for the oxidative dehydrogenation of isobutane. Appl. Catal. A Gen. 2009, 355, 192–201. [Google Scholar] [CrossRef]
- Selvaraj, M.; Kawi, S.; Park, D.W.; Ha, C.S. A merit synthesis of well-ordered two-dimensional mesoporous niobium silicate materials with enhanced hydrothermal stability and catalytic activity. J. Phys. Chem. C 2009, 113, 7743–7749. [Google Scholar] [CrossRef]
- Kryszak, D.; Stawicka, K.; Calvino-Casilda, V.; Martin-Aranda, R.; Ziolek, M. Imidazole immobilization in nanopores of silicas and niobiosilicates SBA-15 and MCF—A new concept towards creation of basicity. Appl. Catal. A Gen. 2017, 531, 139–150. [Google Scholar] [CrossRef]
- Tranca, D.C.; Wojtaszek-Gurdak, A.; Ziolek, M.; Tielens, F. Supported and inserted monomeric niobium oxide species on/in silica: A molecular picture. Phys. Chem. Chem. Phys. 2015, 17, 22402–22411. [Google Scholar] [CrossRef] [PubMed]
- Tiozzo, C.; Bisio, C.; Carniato, F.; Gallo, A.; Scott, S.L.; Psaro, R.; Guidotti, M. Niobium–silica catalysts for the selective epoxidation of cyclic alkenes: The generation of the active site by grafting niobocene dichloride. Phys. Chem. Chem. Phys. 2013, 15, 13354–13362. [Google Scholar] [CrossRef]
- Van Der Voort, P.; Vansant, E.F. Silylation of the silica surface A review. J. Liq. Chromatogr. Relat. Technol. 1996, 19, 2723–2752. [Google Scholar] [CrossRef]
- Capel-Sanchez, M.C.; Barrio, L.; Campos-Martin, J.M.; Fierro, J.L. Silylation and surface properties of chemically grafted hydrophobic silica. J. Colloid Interface Sci. 2004, 277, 146–153. [Google Scholar] [CrossRef]
- Khabtou, S.; Chevreau, T.; Lavalley, J.C. Quantitative infrared study of the distinct acidic hydroxyl groups contained in modiifed Y zeolites. Microporous Mater. 1994, 3, 133–148. [Google Scholar] [CrossRef]
- Stawicka, K.; Decyk, P.; Wojtaszek-Gurdak, A.; Ziolek, M. Comparative study of acid-basic properties of MCF impregnated with niobium and cerium species. Catal. Today 2019, 325, 2–10. [Google Scholar] [CrossRef]
- Busca, G. Spectroscopic characterization of the acid properties of metal oxide catalysts. Catal. Today 1998, 41, 191–206. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Ziolek, M.; Sobczak, I.; Lewandowska, A.; Nowak, I.; Decyk, P.; Renn, M.; Jankowska, B. Oxidative properties of niobium-containing mesoporous silica catalysts. Catal. Today 2001, 70, 169–181. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I. Identification of neutral and charged NxOy surface species by IR spectroscopy. Catal. Rev. Sci. Eng. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Ziolek, M.; Decyk, P.; Sobczak, I.; Trejda, M.; Florek, J.; Klimas, W.; Golinska, H.; Wojtaszek, A. Catalytic performance of niobium species in crystalline and amorphous solids—Gas and liquid phase oxidation. Appl. Catal. A Gen. 2011, 391, 194–204. [Google Scholar] [CrossRef]
- Wojtaszek, A.; Sobczak, I.; Ziolek, M. NO adsorption combined with FTIR spectroscopy as a useful tool for characterization of niobium species in crystalline and amorphous molecular sieves. Catal. Today 2012, 192, 149–153. [Google Scholar] [CrossRef]
- Alhanash, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Gas-phase dehydration of glycerol to acrolein catalysed by cesium heteropoly salt. Appl. Catal. A Gen. 2010, 378, 11–18. [Google Scholar] [CrossRef]
- Lourenço, J.P.; Macedo, M.I.; Fernandes, A. Sulfonic-functionalized SBA-15 as an active catalyst for the gas-phase dehydration of glycerol. Catal. Commun. 2012, 19, 105–109. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Jiang, Y.; Hunger, M.; Huang, J. Cooperativity of Brønsted and Lewis acid sites on zeolite for glycerol dehydration. ACS Catal. 2014, 4, 1144–1147. [Google Scholar] [CrossRef]
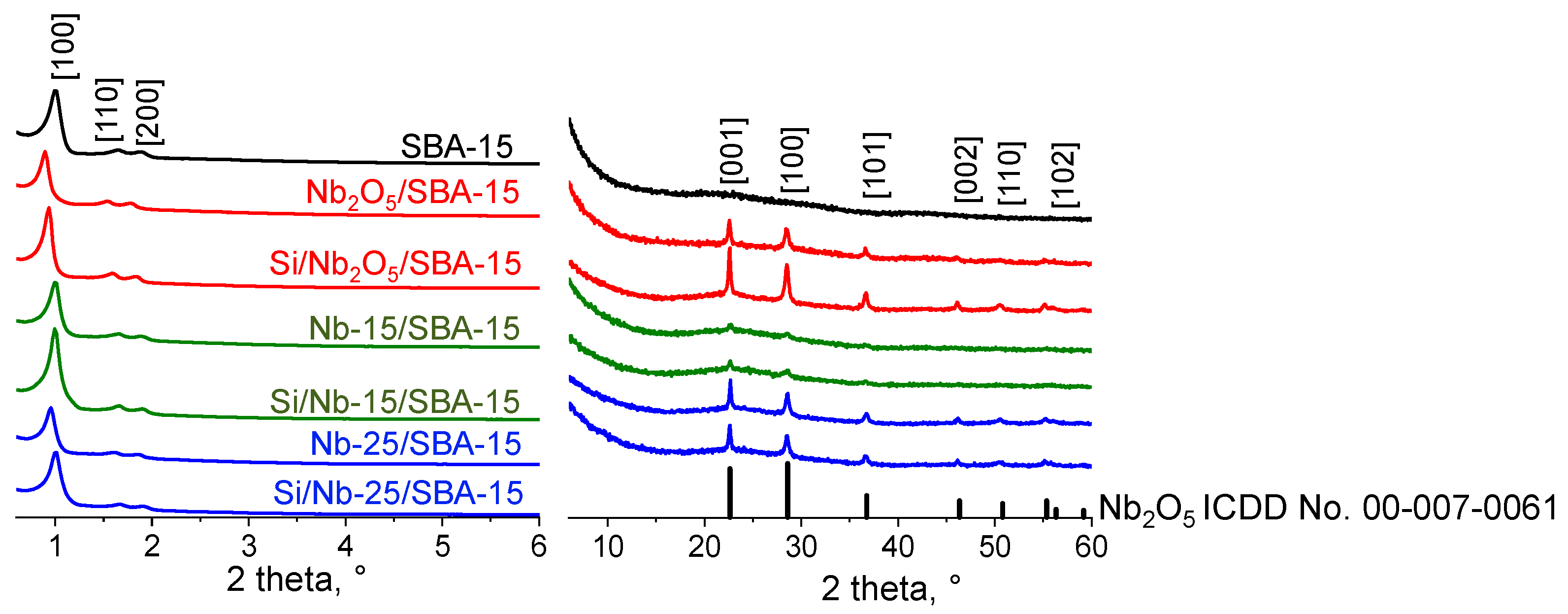
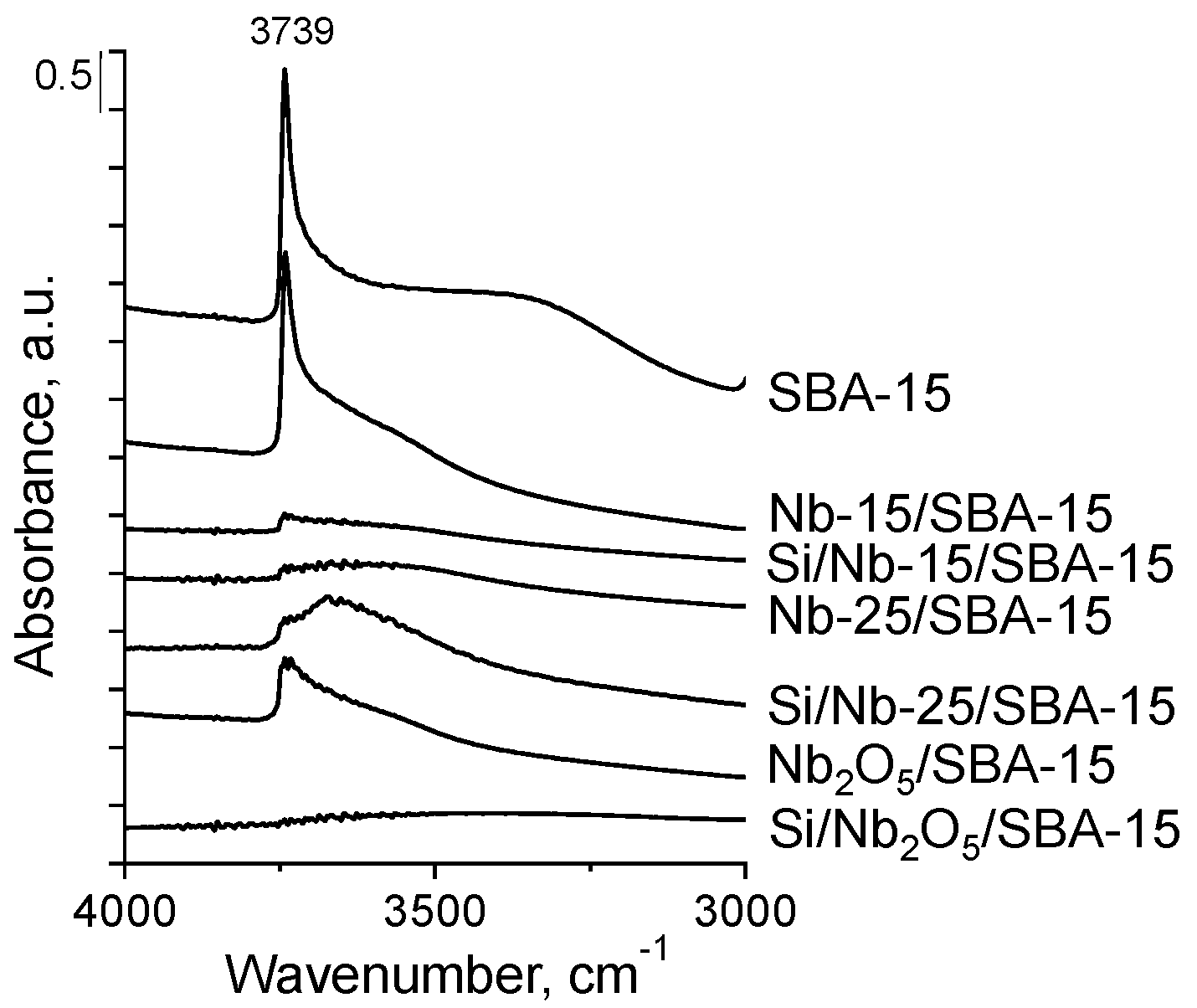
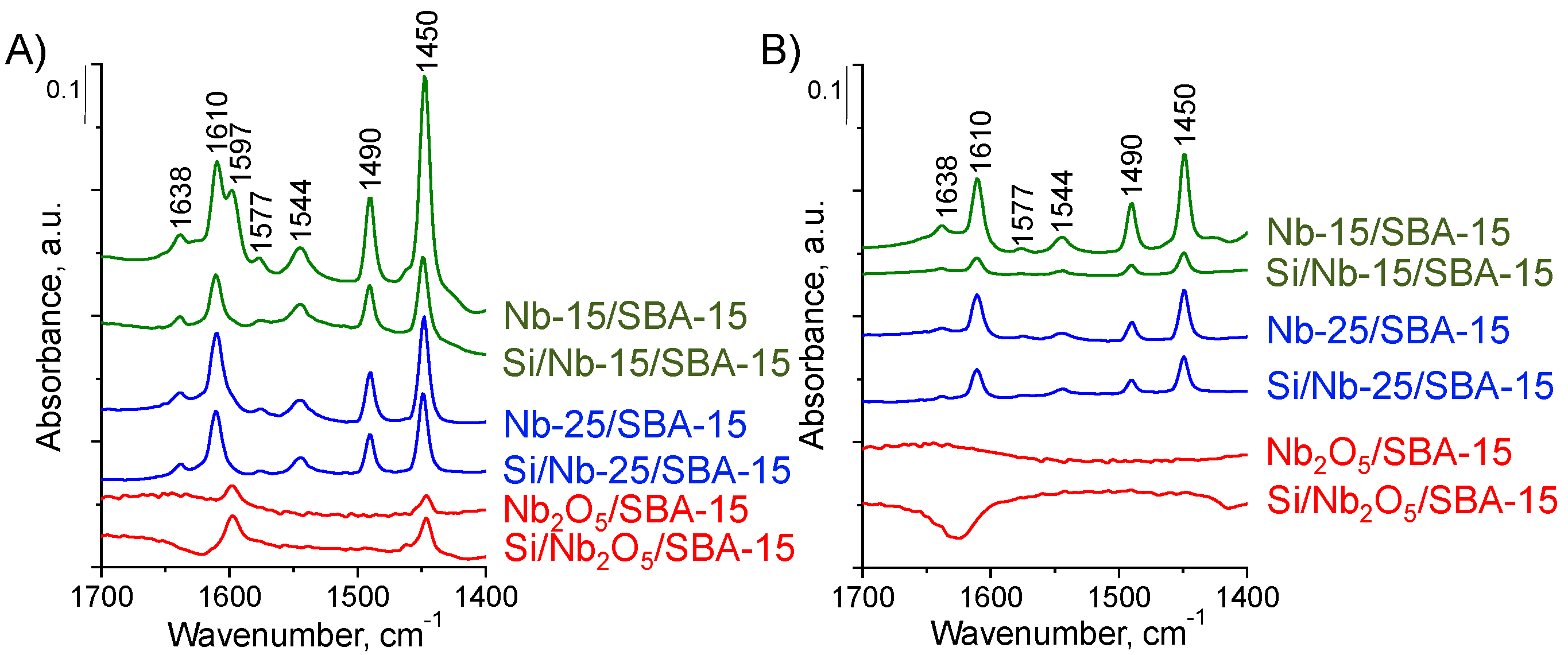


| Catalyst | Surface Area, m2/g | Pore Volume, cm3/g (BJH) | Pore Diameter, nm (DFT) | dwall, nm | Nb2O5 Average Crystal Size *, nm |
|---|---|---|---|---|---|
| SBA-15 | 828 ± 2.0 | 0.64 | 11.8 | 1.74 | - |
| Nb-15/SBA-15 | 533 ± 1.2 | 0.46 | 9.7 | 2.25 | 34 |
| Nb-25/SBA-15 | 441 ± 0.7 | 0.39 | 9.7 | 2.66 | 68 |
| Nb2O5/SBA-15 | 674 ± 1.6 | 0.53 | 10.8 | 2.26 | 55 |
| Si/Nb-15/SBA-15 | 428 ± 0.9 | 0.38 | 9.7 | 2.60 | 68 |
| Si/Nb-25/SBA-15 | 336 ± 0.5 | 0.31 | 9.7 | 2.92 | 91 |
| Si/Nb2O5/SBA-15 | 500 ± 1.2 | 0.42 | 10.1 | 2.58 | 68 |
| Catalyst | LAS, μmol/g | BAS, μmol/g | BAS/LAS | |||||
|---|---|---|---|---|---|---|---|---|
| 200 °C | 250 °C | Pyridine Desorbed at 250 °C, % * | 150 °C | 200 °C | 250 °C | Pyridine Desorbed at 250 °C, % * | 200 °C | |
| Nb-15/SBA-15 | 62 | 26 | 58 | 31 | 22 | 3 | 86 | 0.35 |
| Nb-25/SBA-15 | 32 | 16 | 50 | 23 | 9 | 4 | 66 | 0.28 |
| Si/Nb-15/SBA-15 | 14 | 8 | 43 | 16 | 5 | 2 | 60 | 0.36 |
| Si/Nb-25/SBA-15 | 24 | 10 | 58 | 15 | 6 | 2 | 67 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawicka, K.; Trejda, M.; Ziolek, M. Insight into Active Centers and Anti-Coke Behavior of Niobium-Containing SBA-15 for Glycerol Dehydration. Catalysts 2021, 11, 488. https://doi.org/10.3390/catal11040488
Stawicka K, Trejda M, Ziolek M. Insight into Active Centers and Anti-Coke Behavior of Niobium-Containing SBA-15 for Glycerol Dehydration. Catalysts. 2021; 11(4):488. https://doi.org/10.3390/catal11040488
Chicago/Turabian StyleStawicka, Katarzyna, Maciej Trejda, and Maria Ziolek. 2021. "Insight into Active Centers and Anti-Coke Behavior of Niobium-Containing SBA-15 for Glycerol Dehydration" Catalysts 11, no. 4: 488. https://doi.org/10.3390/catal11040488
APA StyleStawicka, K., Trejda, M., & Ziolek, M. (2021). Insight into Active Centers and Anti-Coke Behavior of Niobium-Containing SBA-15 for Glycerol Dehydration. Catalysts, 11(4), 488. https://doi.org/10.3390/catal11040488