Removal of VOCs by Ozone: n-Alkane Oxidation under Mild Conditions
Abstract
:1. Introduction
2. Results
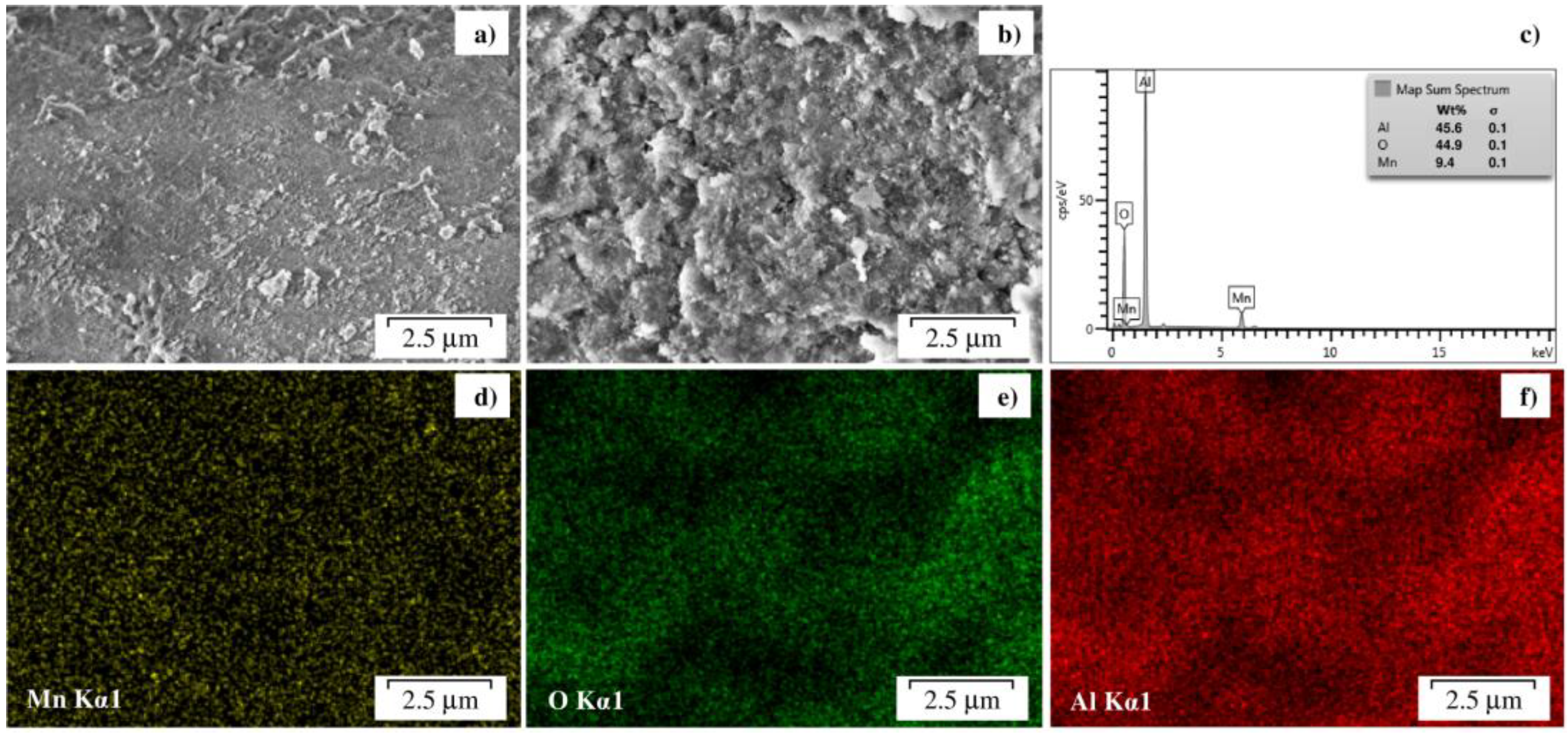
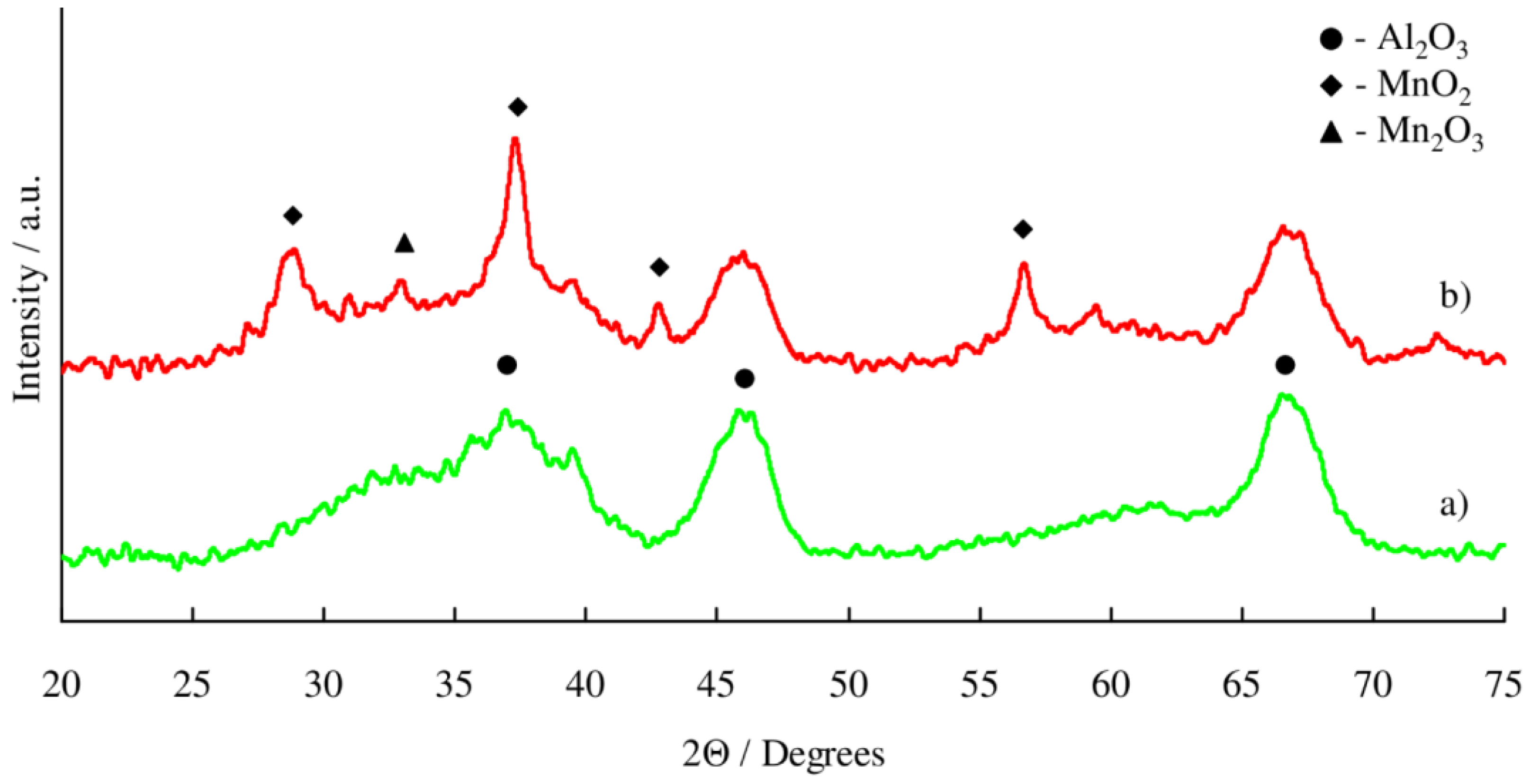
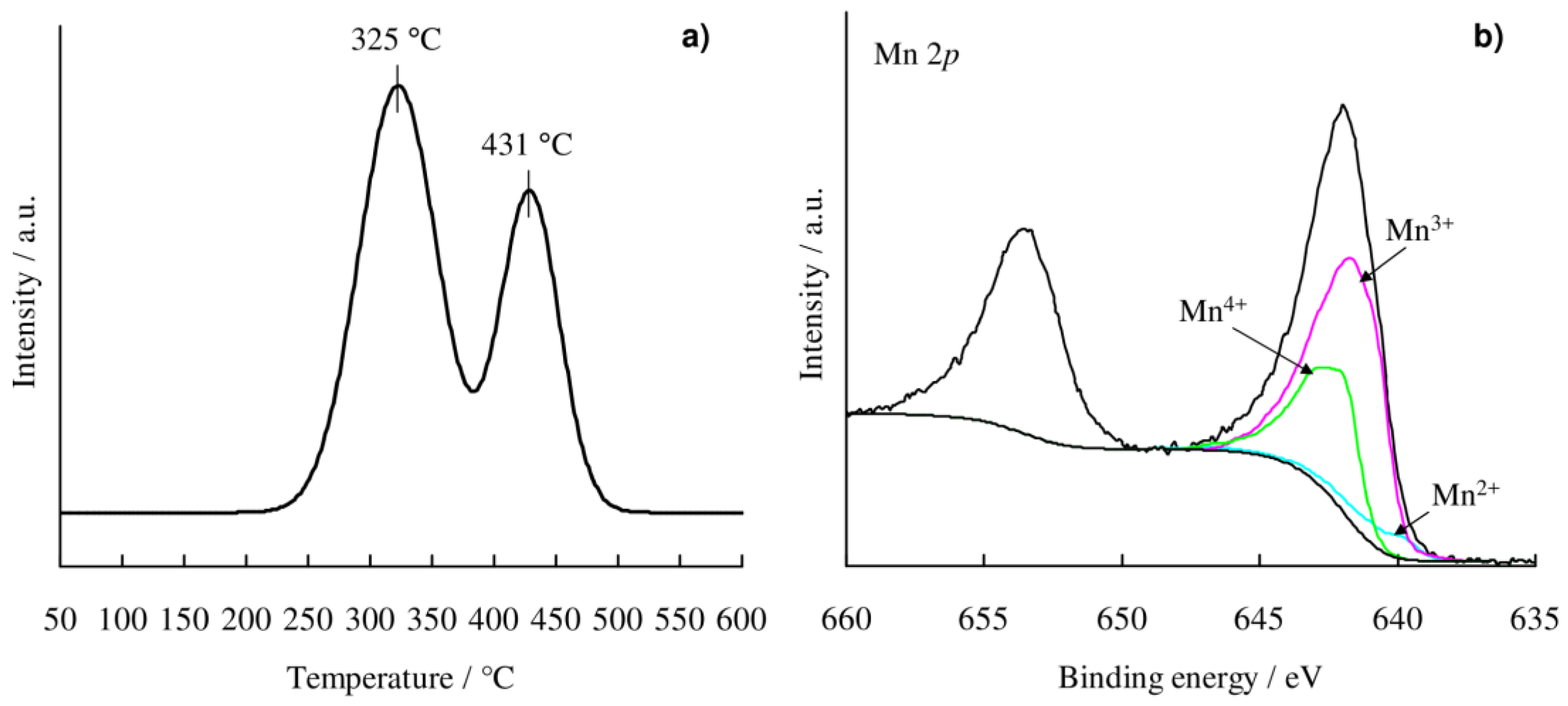
2.1. Catalyst Characterization
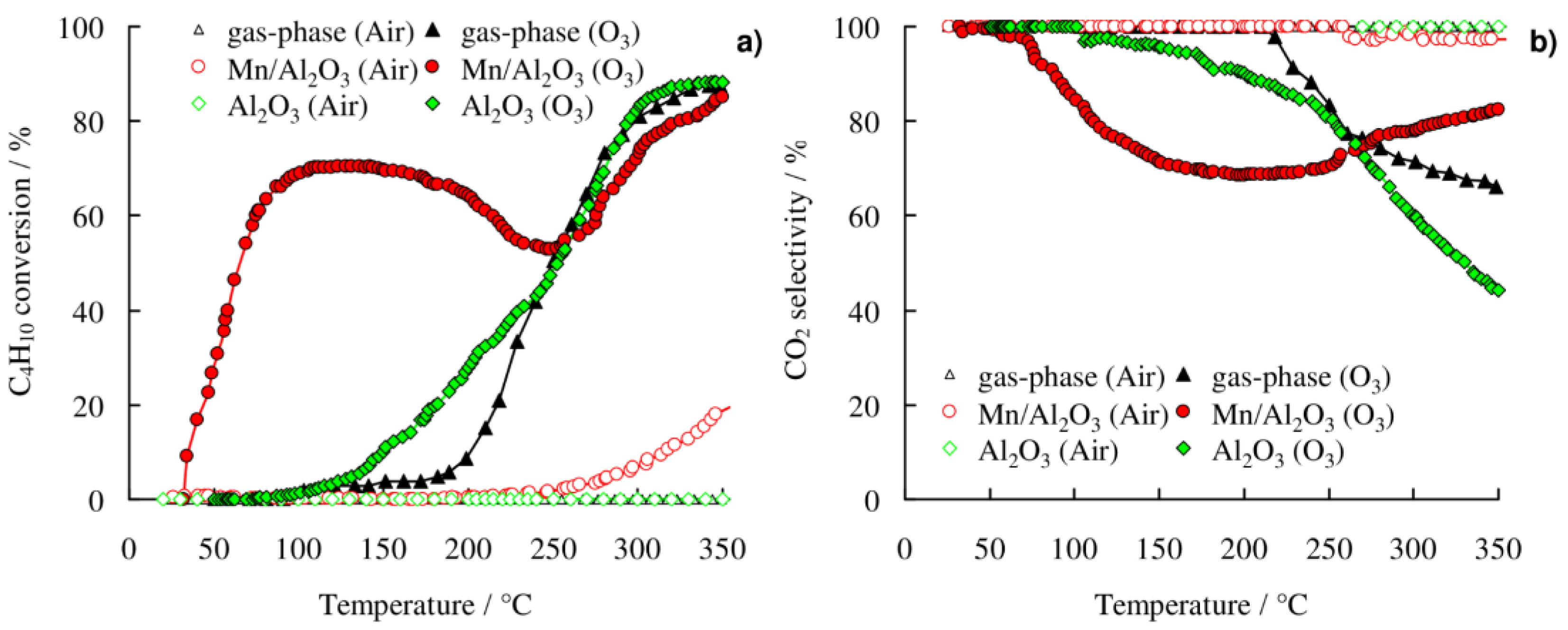
2.2. Catalytic Activity
2.2.1. n-C4H10 Oxidation
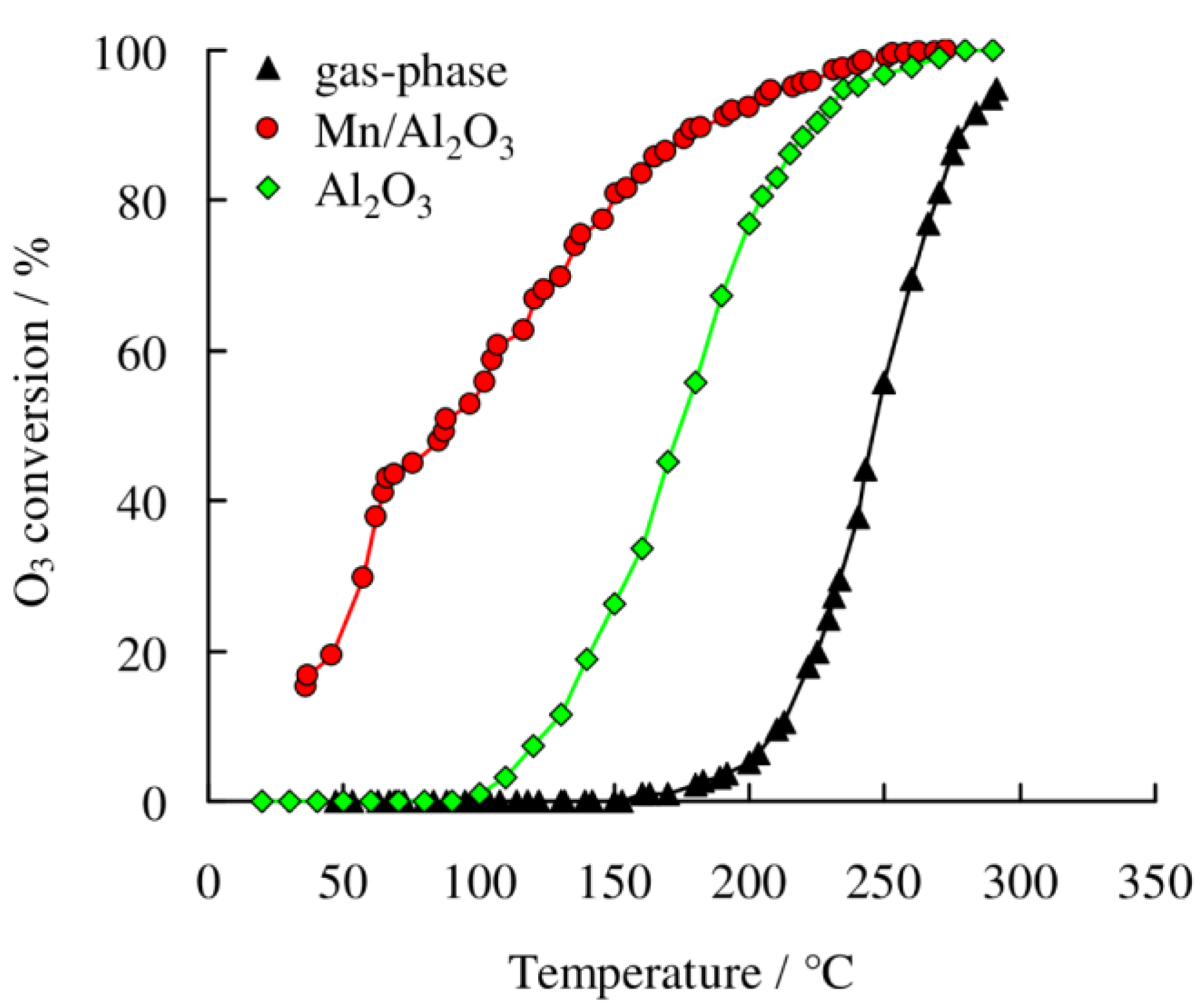
2.2.2. O3 Decomposition
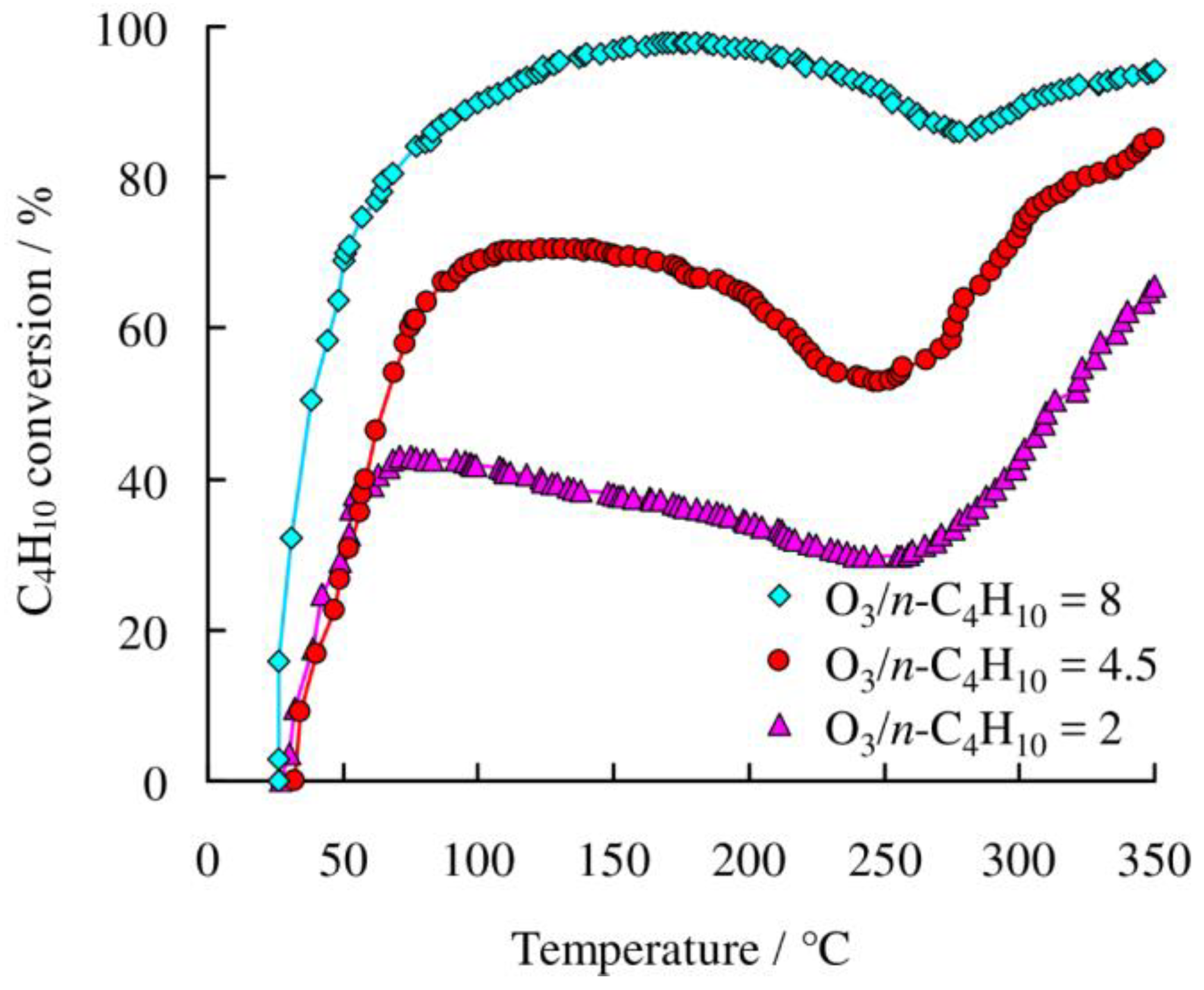
2.2.3. The Effect of O3/n-C4H10 Ratio on the Oxidation Efficiency
3. Discussion
3.1. Structure of Mn/Al2O3 Catalyst
3.2. Catalytic Activity
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalytic Tests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Berezina, E.; Moiseenko, K.; Skorokhod, A.; Pankratova, N.V.; Belikov, I.; Belousov, V.; Elansky, N.F. Impact of VOCs and NOx on ozone formation in Moscow. Atmosphere 2020, 11, 1262. [Google Scholar] [CrossRef]
- Juráň, S.; Grace, J.; Urban, O. Temporal changes in ozone concentrations and their impact on vegetation. Atmosphere 2021, 12, 82. [Google Scholar] [CrossRef]
- Juráň, S.; Edwards-Jonášová, M.; Cudlín, P.; Zapletal, M.; Šigut, L.; Grace, J.; Urban, O. Prediction of ozone effects on net ecosystem production of Norway spruce forest. iForest-Biogeosci. For. 2021, 11, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, Q.; Gao, Y.; Zhou, M.; Jing, S.; Qiao, L.; Yuan, B.; Huang, D.; Huang, C.; Lou, S.; et al. Estimation of secondary organic aerosol formation during a photochemical smog episode in Shanghai, China. JGR Atmos. 2020, 125, e2019JD032033. [Google Scholar] [CrossRef]
- Okal, J.; Zawadzki, M. Catalytic combustion of butane on Ru/γ-Al2O3 catalysts. Appl. Catal. B Environ. 2009, 89, 22–32. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef]
- Ozturk, B.; Yilmez, D. Absorptive removal of volatile organic compounds from flue gas streams. Process Saf. Environ. Prot. 2006, 84, 391–398. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashimoto, S.; Katsuura, K.; Yamamoto, M.; Takahashi, M. Photocatalytic activity for decomposition of volatile organic compound on Pt-WO3 enhanced by simple physical mixing with TiO2. Catal. Commun. 2019, 133, 105831. [Google Scholar] [CrossRef]
- Sansotera, M.; Geran Malek Kheyli, S.; Baggioli, A.; Bianchi, C.L.; Pedeferri, M.P.; Diamanti, M.V.; Navarrini, V. Absorption and photocatalytic degradation of VOCs by perfluorinated ionomeric coating with TiO2 nanopowders for air purification. Chem. Eng. J. 2019, 361, 885–896. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; You, J.; Kennes, C.; Cheng, Z.; Ye, J.; Chen, D.; Chen, J.; Wang, L. Current advances of VOCs degradation by bioelectrochemical systems: A review. Chem. Eng. J. 2018, 334, 2625–2637. [Google Scholar] [CrossRef]
- Cools, P.; De Geyter, N.; Morent, R. Plasma-catalytic removal of VOCs. In Plasma Catalysis. Springer Series on Atomic, Optical, and Plasma Physics; Tu, X., Whitehead, J., Nozaki, T., Eds.; Springer: Cham, Switzerland, 2019; Volume 106, pp. 145–180. [Google Scholar] [CrossRef]
- Wang, B.; Xu, X.; Xu, W.; Wang, N.; Xiao, H.; Sun, Y.; Huang, H.; Yu, L.; Fu, M.; Wu, J.; et al. The Mechanism of non-thermal plasma catalysis on volatile organic compounds removal. Catal. Surv. Asia 2018, 22, 73–94. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Lee, J.E.; Ok, Y.S.; Tsang, D.C.W.; Song, J.; Jung, S.-C.; Park, Y.-K. Recent advances in volatile organic compounds abatement by catalysis and catalytic hybrid processes: A critical review. Sci. Total Environ. 2020, 719, 137405. [Google Scholar] [CrossRef]
- Shu, Y.; Xu, Y.; Huang, H.; Ji, J.; Liang, S.; Wu, M.; Leung, D.Y.C. Catalytic oxidation of VOCs over Mn/TiO2/activated carbon under 185 nm VUV irradiation. Chemosphere 2018, 208, 550–558. [Google Scholar] [CrossRef]
- Aghbolaghy, M.; Ghavami, M.; Soltan, J.; Chen, N. Effect of active metal loading on catalyst structure and performance in room temperature oxidation of acetone by ozone. J. Ind. Eng. Chem. 2019, 77, 118–127. [Google Scholar] [CrossRef]
- Fang, R.; Huang, H.; Huang, W.; Ji, J.; Feng, Q.; Shu, Y.; Zhan, Y.; Liu, G.; Xie, R. Influence of peracetic acid modification on the physicochemical properties of activated carbon and its performance in the ozone-catalytic oxidation of gaseous benzene. Appl. Surf. Sci. 2017, 420, 905–910. [Google Scholar] [CrossRef]
- Sun, W.; Gao, X.; Wu, B.; Ombrello, T. The effect of ozone addition on combustion: Kinetics and dynamics. Prog. Energy Combust. Sci. 2019, 73, 1–25. [Google Scholar] [CrossRef]
- Rezaei, E.; Soltan, J.; Chen, N. Catalytic oxidation of toluene by ozone over alumina supported manganese oxides: Effect of catalyst loading. Appl. Catal. B Environ. 2013, 136–137, 239–247. [Google Scholar] [CrossRef]
- Zhu, B.; Li, X.-S.; Sun, P.; Liu, J.-L.; Ma, X.-Y.; Zhu, X.; Zhu, A.-M. A novel process of ozone catalytic oxidation for low concentration formaldehyde removal. Chin. J. Catal. 2017, 38, 1759–1769. [Google Scholar] [CrossRef]
- Xi, Y.; Reed, C.; Lee, Y.-K.; Oyama, S.T. Acetone oxidation using ozone on manganese oxide catalysts. J. Phys. Chem. B 2005, 109, 17587–17596. [Google Scholar] [CrossRef] [PubMed]
- Einaga, H.; Futamura, S. Comparative study on the catalytic activities of alumina-supported metal oxides for oxidation of benzene and cyclohexane with ozone. React. Kinet. Catal. Lett. 2004, 81, 121–128. [Google Scholar] [CrossRef]
- Einaga, H.; Maeda, N.; Nagai, Y. Comparison of catalytic properties of supported metal oxides for benzene oxidation using ozone. Catal. Sci. Technol. 2015, 5, 3147–3158. [Google Scholar] [CrossRef]
- Huang, H.; Ye, X.; Huang, W.; Chen, J.; Xu, Y.; Wu, M.; Shao, Q.; Peng, Z.; Ou, G.; Shi, J.; et al. Ozone-catalytic oxidation of gaseous benzene over MnO2/ZSM-5 at ambient temperature: Catalytic deactivation and its suppression. Chem. Eng. J. 2015, 264, 24–31. [Google Scholar] [CrossRef]
- Gopi, T.; Swetha, G.; Chandra Shekar, S.; Krishna, R.; Ramakrishna, C.; Saini, B.; Rao, P.V.L. Ozone catalytic oxidation of toluene over 13X zeolite supported metal oxides and the effect of moisture on the catalytic process. Arab. J. Chem. 2019, 12, 4502–4513. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wang, Z.; Lin, F.; Zhang, Z.; Yu, H.; Yan, B. Comparative investigation on catalytic ozonation of VOCs in different types over supported MnOx catalysts. J. Hazard. Mater. 2020, 391, 122218. [Google Scholar] [CrossRef]
- Li, W.; Gibbs, G.V.; Oyama, S.T. Mechanism of ozone decomposition on a manganese oxide catalyst. 1. In situ Raman spectroscopy and Ab initio molecular orbital calculations. J. Am. Chem. Soc. 1998, 120, 9041–9046. [Google Scholar] [CrossRef]
- Li, W.; Oyama, S.T. Mechanism of ozone decomposition on a manganese oxide catalyst. 2. Steady-state and transient kinetic studies. J. Am. Chem. Soc. 1998, 120, 9047–9052. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Shekar, S.C.; Gupta, A.K.; Saini, B.; Krishna, R.; Swetha, G.; Gopi, T. Degradation of diethyl sulfide vapors with manganese oxide catalysts supported on zeolite-13X: The influence of process parameters and mechanism in presence of ozone. J. Environ. Chem. Eng. 2017, 5, 1484–1493. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Kurita, Y.; Sankoda, K.; Namiki, N.; Yasui, F.; Tamura, H. Ozone catalytic oxidation of gaseous toluene over MnO2-based ozone decomposition catalysts immobilized on a nonwoven fabric. Aerosol Air Qual. Res. 2017, 17, 2110–2118. [Google Scholar] [CrossRef] [Green Version]
- Wan, M.P.; Hui, K.S.; Chao, C.Y.H.; Kwong, C.W. Catalytic combustion of methane with ozone using Pd-exchanged zeolite X: Experimental investigation and kinetics model. Combust. Sci. Technol. 2010, 182, 1429–1445. [Google Scholar] [CrossRef]
- Hui, K.S.; Kwong, C.W.; Chao, C.Y.H. Methane emission abatement by Pd-ion-exchanged zeolite 13X with ozone. Energy Environ. Sci. 2010, 3, 1092–1098. [Google Scholar] [CrossRef]
- Chen, C.-L.; Fang, H.Y.; Shu, C.-M. Source location and characterization of volatile organic compound emissions at a petrochemical plant in Kaohsiung, Taiwan. J. Air Waste Manag. Assoc. 2005, 55, 1487–1497. [Google Scholar] [CrossRef]
- Ragothaman, A.; Anderson, W.A. Air quality impacts of petroleum refining and petrochemical industries. Environments 2017, 4, 66. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Jin, Y.; Zhang, M.; Liu, X.; Yu, Z. Emission characteristics of VOCs from on-road vehicles in an urban tunnel in Eastern China and predictions for 2017–2026. Aerosol Air Qual. Res. 2018, 18, 3025–3034. [Google Scholar] [CrossRef]
- Ryu, H.W.; Song, M.Y.; Park, J.S.; Kim, J.M.; Jung, S.-C.; Song, J.-H.; Kim, B.-J.; Park, Y.-K. Removal of toluene using ozone at room temperature over mesoporous Mn/Al2O3 catalysts. Environ. Res. 2019, 172, 649–657. [Google Scholar] [CrossRef]
- Langell, M.A.; Hutchings, C.W.; Carson, G.A.; Nassir, M.H. High resolution electron energy loss spectroscopy of MnO(100) and oxidized MnO(100). J. Vac. Sci. Technol. A 1996, 14, 1656–1661. [Google Scholar] [CrossRef] [Green Version]
- Stranick, M.A. MnO2 by XPS. Surf. Sci. Spectra 1999, 6, 31–38. [Google Scholar] [CrossRef]
- Stranick, M.A. Mn2O3 by XPS. Surf. Sci. Spectra 1999, 6, 39–46. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Oyama, S.T.; Chen, J.G.; Asakura, K. Electron transfer effects in ozone decomposition on supported manganese oxide. J. Phys. Chem. B 2001, 105, 4245–4253. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Yang, L.; He, G.; Zhang, C.; Zhang, R.; He, H. Oxygen vacancies induced by transition metal doping in γ-MnO2 for highly efficient ozone decomposition. Environ. Sci. Technol. 2018, 52, 12685–12696. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhu, J.; Jiang, W.; Zhang, Z.; Wang, J.; Zhu, Y.; Zhang, Q. Surface oxygen vacancy induced α-MnO2 nanofiber for highly efficient ozone elimination. Appl. Catal. B Environ. 2017, 209, 729–737. [Google Scholar] [CrossRef]
- Peng, B.; Bao, W.; Wei, L.; Zhang, R.; Wang, Z.; Wang, Z.; Wie, Y. Highly active OMS-2 for catalytic ozone decomposition under humid conditions. Pet. Sci. 2019, 16, 912–919. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.H.; Zhang, X.L.; Chen, L.; Wang, X.R.; Zhang, N.; Liu, Y.F.; Fang, Y.Z. Oxygen vacancies enabled enhancement of catalytic property of Al reduced anatase TiO2 in the decomposition of high concentration ozone. J. Solid State Chem. 2017, 250, 121–127. [Google Scholar] [CrossRef]
- Li, J.; Na, H.; Zeng, X.; Zhu, T.; Liu, Z. In situ DRIFTS investigation for the oxidation of toluene by ozone overMn/HZSM-5, Ag/HZSM-5 and Mn–Ag/HZSM-5. Appl. Surf. Sci. 2014, 311, 690–696. [Google Scholar] [CrossRef]
- Naydenov, A.; Mehandjiev, D. Complete oxidation of benzene on manganese dioxide by ozone. Appl. Catal. A Gen. 1993, 97, 17–22. [Google Scholar] [CrossRef]
- Kwong, C.W.; Chao, C.Y.H.; Hui, K.S.; Wan, M.P. Catalytic ozonation of toluene using zeolite and MCM-41 materials. Environ. Sci. Technol. 2008, 42, 8504–8509. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mytareva, A.I.; Mashkovsky, I.S.; Kanaev, S.A.; Bokarev, D.A.; Baeva, G.N.; Kazakov, A.V.; Stakheev, A.Y. Removal of VOCs by Ozone: n-Alkane Oxidation under Mild Conditions. Catalysts 2021, 11, 506. https://doi.org/10.3390/catal11040506
Mytareva AI, Mashkovsky IS, Kanaev SA, Bokarev DA, Baeva GN, Kazakov AV, Stakheev AY. Removal of VOCs by Ozone: n-Alkane Oxidation under Mild Conditions. Catalysts. 2021; 11(4):506. https://doi.org/10.3390/catal11040506
Chicago/Turabian StyleMytareva, Alina I., Igor S. Mashkovsky, Sergey A. Kanaev, Dmitriy A. Bokarev, Galina N. Baeva, Alexander V. Kazakov, and Alexander Yu. Stakheev. 2021. "Removal of VOCs by Ozone: n-Alkane Oxidation under Mild Conditions" Catalysts 11, no. 4: 506. https://doi.org/10.3390/catal11040506
APA StyleMytareva, A. I., Mashkovsky, I. S., Kanaev, S. A., Bokarev, D. A., Baeva, G. N., Kazakov, A. V., & Stakheev, A. Y. (2021). Removal of VOCs by Ozone: n-Alkane Oxidation under Mild Conditions. Catalysts, 11(4), 506. https://doi.org/10.3390/catal11040506







