Mechanochemical Synthesis of Nickel-Modified Metal–Organic Frameworks for Reduction Reactions
Abstract
:1. Introduction
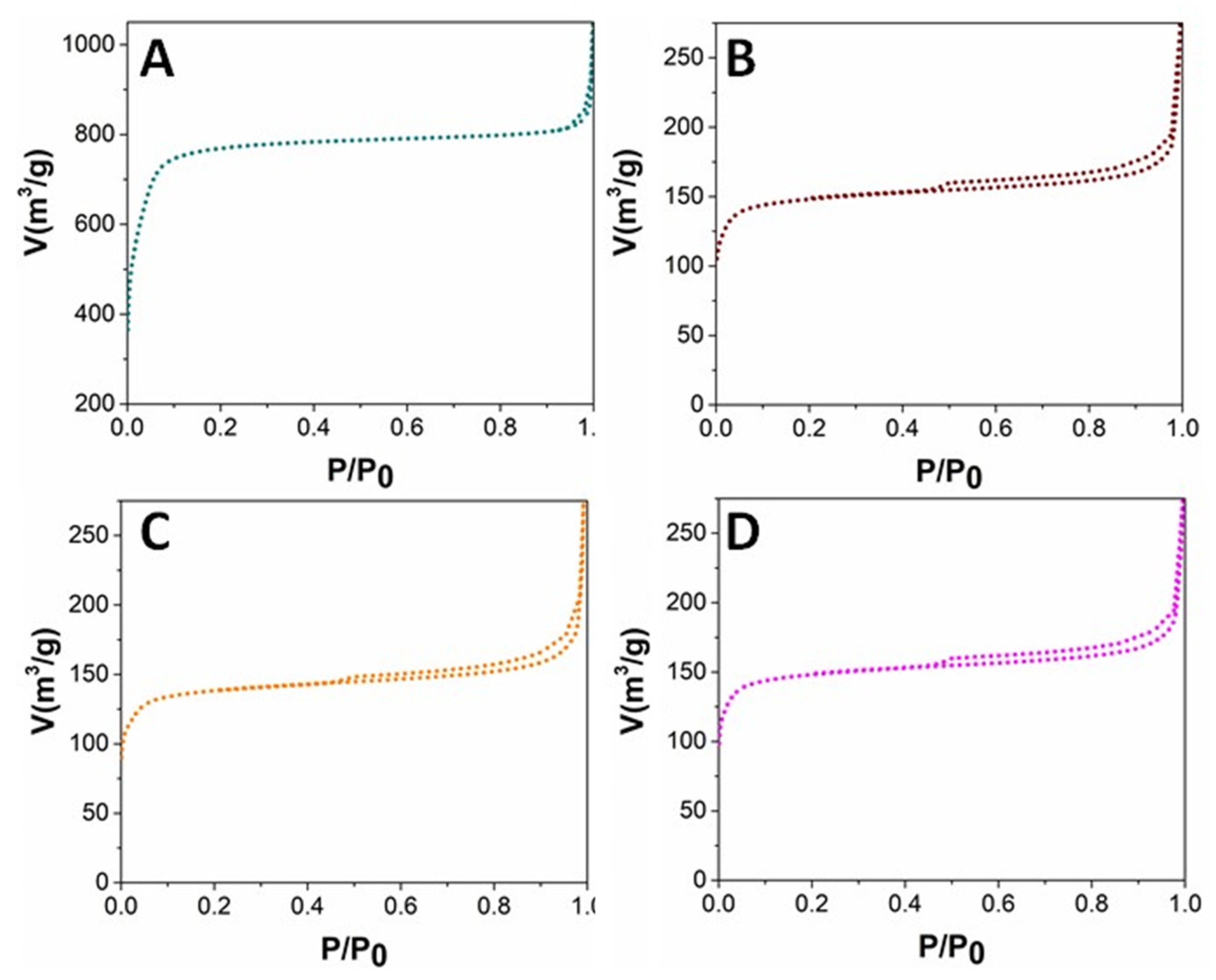
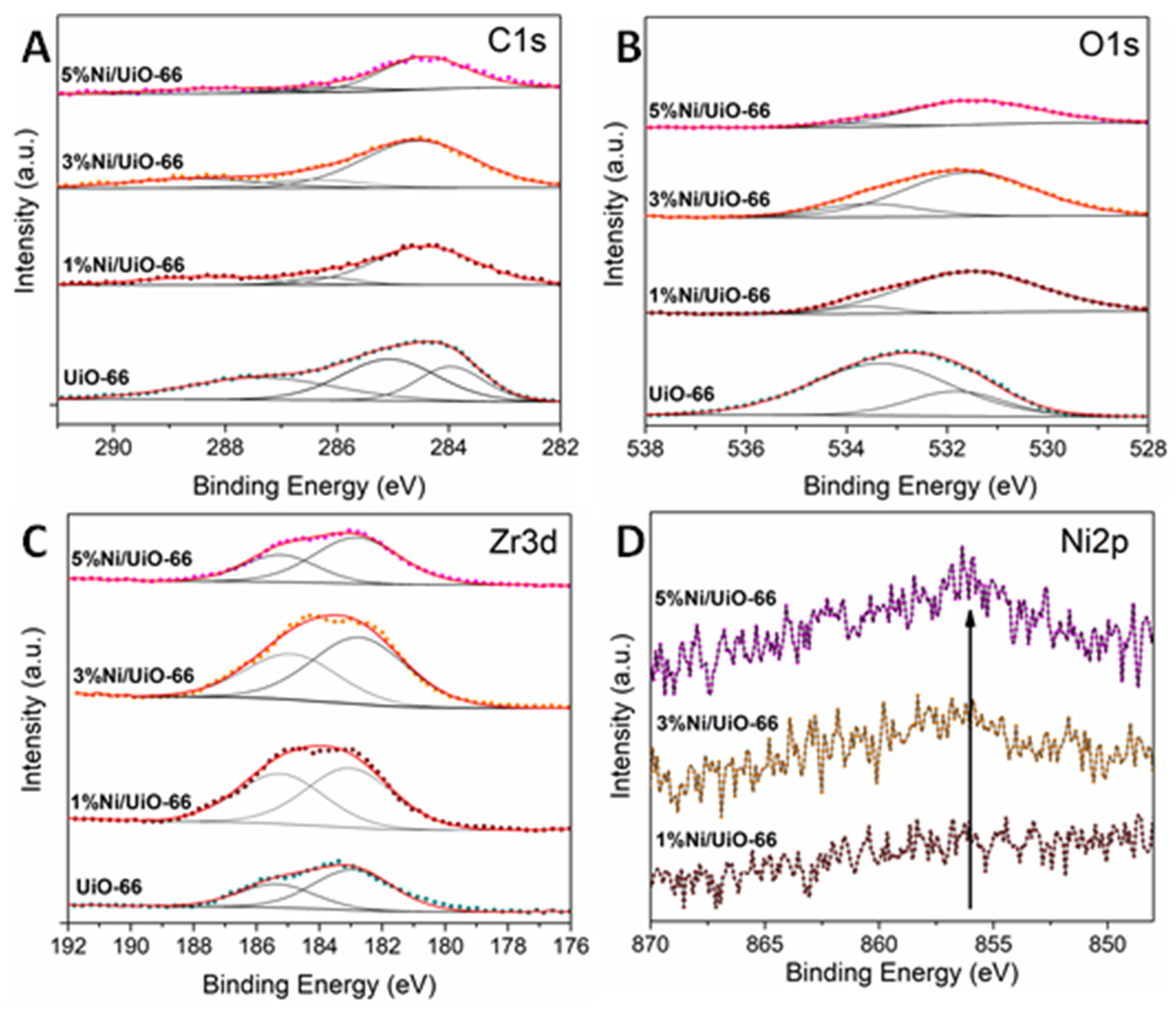
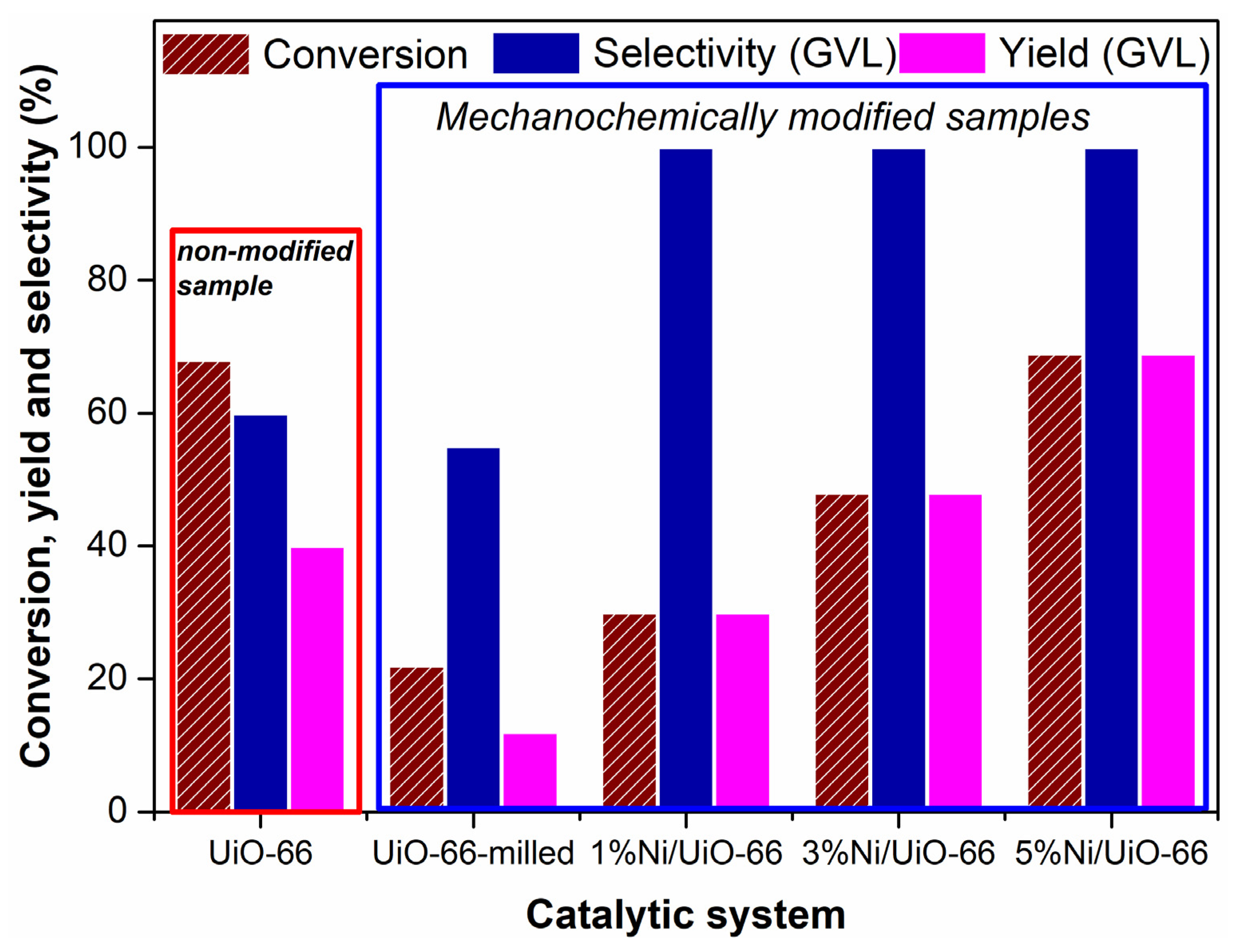
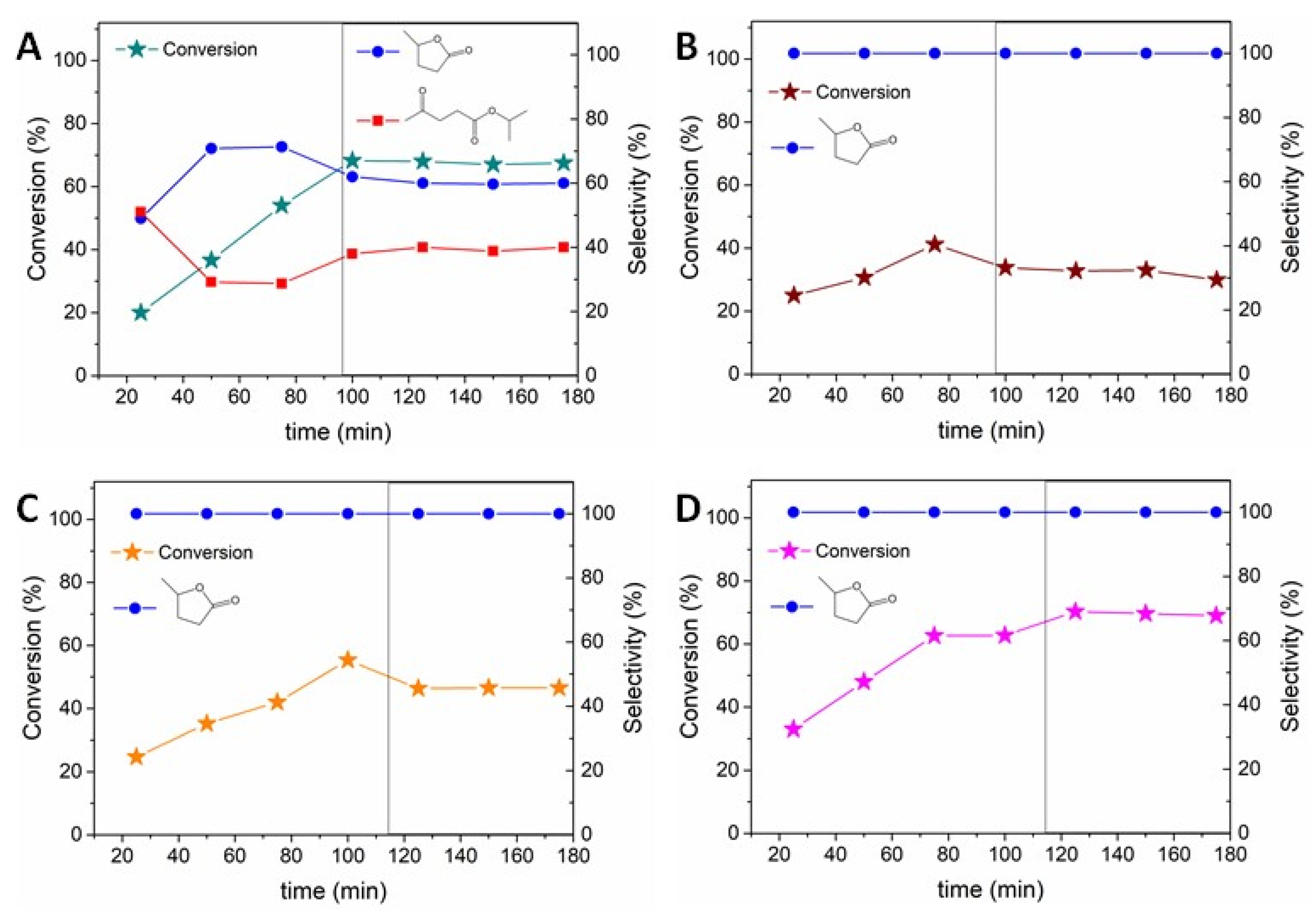
2. Results and Discussion
3. Experimental
3.1. Preparation of Ni/UiO-66 MOFs
3.2. Catalysts Characterization
3.3. Catalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I Results of Screening for Potential Candidates from Sugars and Synthesis Gas. NRLE Naracoorte Reg. Livest. Exch. 2004, 94, 340–362. [Google Scholar]
- Huang, Y.B.; Yang, T.; Lin, Y.T.; Zhu, Y.Z.; Li, L.C.; Pan, H. Facile and high-yield synthesis of methyl levulinate from cellulose. Green Chem. 2018, 20, 1323–1334. [Google Scholar] [CrossRef]
- Wright, W.R.; Palkovits, R. Development of heterogeneous catalysts for the conversion of levulinic acid to γ-valerolactone. ChemSusChem 2012, 5, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Iris, K.M.; Tsang, D.C.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, X.; Xiong, J.; Ji, N. Transformation of levulinic acid to valeric biofuels: A review on heterogeneous bifunctional catalytic systems. ChemSusChem 2019, 17, 3915–3930. [Google Scholar] [CrossRef]
- Pascanu, V.; González Miera, G.; Inge, A.K.; Martín-Matute, B. Metal–organic frameworks as catalysts for organic synthesis: A critical perspective. J. Am. Chem. Soc. 2019, 18, 7223–7234. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Fang, R.; Luque, R.; Chen, L.; Li, Y. Functional metal–organic frameworks for catalytic applications. Coord. Chem. Rev. 2019, 388, 268–292. [Google Scholar] [CrossRef]
- Yang, D.; Gates, B.C. Catalysis by Metal Organic Frameworks: Perspective and Suggestions for Future Research. ACS Catal. 2019, 3, 1779–1798. [Google Scholar] [CrossRef]
- Ouyang, W.; Zhao, D.; Wang, Y.; Balu, A.M.; Len, C.; Luque, R. Continuous Flow Conversion of Biomass-Derived Methyl Levulinate into γ-Valerolactone Using Functional Metal Organic Frameworks. ACS Sustain. Chem. Eng. 2018, 5, 6746–6752. [Google Scholar] [CrossRef]
- Feng, J.; Zhong, Y.; Xie, M.; Li, M.; Jiang, S. Using MOF-808 as a Promising Support to Immobilize Ru for Selective Hydrogenation of Levulinic Acid to γ-Valerolactone. Catal. Lett. 2020, 151, 86–94. [Google Scholar] [CrossRef]
- Feng, J.; Li, M.; Zhong, Y.; Xu, Y.; Meng, X.; Zhao, Z.; Feng, C. Hydrogenation of levulinic acid to γ-valerolactone over Pd@ UiO-66-NH2 with high metal dispersion and excellent reusability. Micropor. Mesopor. Mat. 2020, 294, 109858. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Corma, A.; Llabres Xamena, F.X. Zirconium-containing metal organic frameworks as solid acid catalysts for the esterification of free fatty acids: Synthesis of biodiesel and other compounds of interest. Catal. Today 2015, 257, 213–220. [Google Scholar] [CrossRef]
- Ling, S.; Slater, B. Dynamic acidity in defective UiO-66. Chem. Sci. 2016, 7, 4706–4712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiz, U.; Vanolli, F.; Sanchez, A.; Schneider, W.D. Catalytic Oxidation of Carbon Monoxide on Monodispersed Platinum Clusters: Each Atom Counts. J. Am. Chem. Soc. 1998, 120, 9668–9671. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Wang, L.; Li, D.; Wang, K.; Wang, Y.; Zhu, H.; Yang, D.; Wang, R. Nickel-Mediated Asymmetric Allylic Alkylation between Nitroallylic Acetates and Acyl Imidazoles. Org. Lett. 2017, 19, 4826–4829. [Google Scholar] [CrossRef] [PubMed]
- Ngamnithiporn, A.; Jette, C.I.; Bachman, S.; Virgil, S.C.; Stoltz, B.M. Nickel-catalyzed enantioselective allylic alkylation of lactones and lactams with unactivated allylic alcohol. Chem. Sci. 2018, 9, 2547–2551. [Google Scholar] [CrossRef] [Green Version]
- Sha, S.-C.; Jiang, H.; Mao, J.; Bellomo, A.; Jeong, S.A.; Walsh, P.J. Nickel-Catalyzed Allylic Alkylation with Diarylmethane Pronucleophiles: Reaction Development and Mechanistic Insights. Angew. Chem. 2016, 55, 1070–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquez-Medina, M.D.; Mhadmhan, S.; Balu, A.M.; Romero, A.A.; Luque, R. Post-synthetic mechanochemical incorporation of Al-species into the framework of porous materials: Toward more sustainable redox chemistries. ACS Sustain. Chem. Eng. 2019, 10, 9537–9543. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 31, 6698–6713. [Google Scholar] [CrossRef]
- Zhang, X.; Song, L.; Bi, F.; Zhang, D.; Wang, Y.; Cui, L. Catalytic oxidation of toluene using a facile synthesized Ag nanoparticle supported on UiO-66 derivative. J. Coll. Int. Sci. 2020, 571, 38–47. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mat. 2010, 24, 6632–6640. [Google Scholar] [CrossRef]
- Rungtaweevoranit, B.; Baek, J.; Araujo, J.R.; Archanjo, B.S.; Choi, K.M.; Yaghi, O.M.G.; Somorjai, A. Copper Nanocrystals Encapsulated in Zr-based Metal–Organic Frameworks for Highly Selective CO2 Hydrogenation to Methanol. Nano Lett. 2016, 12, 7645–7649. [Google Scholar] [CrossRef] [PubMed]
- Bambalaza, S.E.; Langmi, H.W.; Mokaya, R.; Musyoka, N.M.; Khotseng, L. Experimental demonstration of dynamic temperature-dependent behaviour of UiO-66 metal-organic-framework: Compaction of hydroxylated and deydroxylated forms of UiO-66 for high pressure hydrogen storage. ACS Appl. Mater. Interfaces 2020, 22, 24883–24894. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C. Enhanced strength in reduced graphene oxide/nickel composites prepared by molecular-level mixing for structural applications. Appl. Phys. A 2014, 118, 409–416. [Google Scholar] [CrossRef]
- Chen, D.; Sun, H.; Wang, Y.; Quan, H.; Ruan, Z.; Ren, Z.; Luo, X. UiO-66 derived zirconia/porous carbon nanocomposites for efficient removal of carbamazepine and adsorption mechanism. Appl. Surf. Sci. 2019, 507, 145054. [Google Scholar] [CrossRef]
- Oswald, S.; Brückner, W. XPS depth profile analysis of non-stoichiometric NiO films. Surf. Interface Anal. 2004, 36, 17–22. [Google Scholar] [CrossRef]
- Martin-Perales, A.I.; Rodríguez-Padrón, D.; Garcia, A.; Len, C.; de Miguel, G.; Muñoz-Batista, J.M.; Luque, R. Photocatalytic production of vanillin over CeOx and ZrO2 modified biomass-templated titania. Ind. Eng. Chem. Res. 2020, 59, 17085–17093. [Google Scholar] [CrossRef]
- Defilippi, C.; Rodríguez-Padrón, D.; Luque, R.; Giordano, C. Simplifying Levulinic Acid Conversion Towards a Sustainable Biomass Valorisation. Green Chem. 2020, 22, 2929–2934. [Google Scholar] [CrossRef]
- Peck, M.A.; Langell, M.A. Comparison of Nanoscaled and Bulk NiO Structural and Environmental Characteristics by XRD, XAFS, and XPS. Chem. Mater. 2012, 23, 4483–4490. [Google Scholar] [CrossRef]
- Gumina, B.; Mauriello, F.; Pietropaolo, R.; Galvagno, S.; Espro, C. Hydrogenolysis of sorbitol into valuable C3-C2 alcohols at low H2 pressure promoted by the heterogeneous Pd/Fe3O4 catalyst. Mol. Catal. 2018, 446, 152–160. [Google Scholar] [CrossRef]
- Paone, E.; Beneduci, A.; Corrente, G.A.; Malara, A.; Mauriello, F. Hydrogenolysis of aromatic ethers under lignin-first conditions. Mol. Catal. 2020, 497, 111228. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Balu, A.M.; Muñoz-Batista, M.J.; Luque, R. Continuous Flow Synthesis of High Valuable N-Heterocycles via Catalytic Conversion of Levulinic Acid. Front. Chem. 2019, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Lázaro, N.; Franco, A.; Ouyang, W.; Balu, A.M.; Romero, A.A.; Luque, R.; Pineda, A. Continuous-Flow Hydrogenation of Methyl Levulinate Promoted by Zr-Based Mesoporous Materials. Catalysts 2019, 2, 142. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-López, P.; Murat, M.; Hidalgo-Herrador, J.M.; Carrillo-Carrión, C.; Balu, A.M.; Luque, R.; Rodríguez-Padrón, D. Mechanochemical Synthesis of Nickel-Modified Metal–Organic Frameworks for Reduction Reactions. Catalysts 2021, 11, 526. https://doi.org/10.3390/catal11050526
Gómez-López P, Murat M, Hidalgo-Herrador JM, Carrillo-Carrión C, Balu AM, Luque R, Rodríguez-Padrón D. Mechanochemical Synthesis of Nickel-Modified Metal–Organic Frameworks for Reduction Reactions. Catalysts. 2021; 11(5):526. https://doi.org/10.3390/catal11050526
Chicago/Turabian StyleGómez-López, Paulette, Martyna Murat, José M. Hidalgo-Herrador, Carolina Carrillo-Carrión, Alina M. Balu, Rafael Luque, and Daily Rodríguez-Padrón. 2021. "Mechanochemical Synthesis of Nickel-Modified Metal–Organic Frameworks for Reduction Reactions" Catalysts 11, no. 5: 526. https://doi.org/10.3390/catal11050526
APA StyleGómez-López, P., Murat, M., Hidalgo-Herrador, J. M., Carrillo-Carrión, C., Balu, A. M., Luque, R., & Rodríguez-Padrón, D. (2021). Mechanochemical Synthesis of Nickel-Modified Metal–Organic Frameworks for Reduction Reactions. Catalysts, 11(5), 526. https://doi.org/10.3390/catal11050526








