Encapsulated NOLA™ Fit 5500 Lactase—An Economically Beneficial Way to Obtain Lactose-Free Milk at Low Temperature
Abstract
:1. Introduction
- C—concentration (g/L)
- Ki—inhibition constant (g/L)
- Km—Michaelis–Menten constant (g/L)
- k3—kinetic constant (1/min)
- r—reaction rate (g/(L∙min))
- rmax—maximum reaction rate (g/(L∙min))
2. Results
2.1. Kinetic Characteristic of NOLA in the Native Form
- C—concentration (g/L)
- Ka—activation constant (g/L)
- Ki—inhibition constant (g/L)
- Km—Michaelis–Menten constant (g/L)
- k3—kinetic constant (1/min)
- r—reaction rate (g/(L∙min))
2.2. Encapsulated NOLA Characteristics
- A—enzyme activity expressed by the initial reaction rate [g/L∙h]
- kinact.—inactivation constant [1/h]
- t—time [h]
- Ds—substrate diffusion coefficient (m2/h)
- k—kinetic constant (1/h)
- R—capsules diameter (m)
- —Thiele modulus
2.3. Kinetic of Encapsulated NOLA
- mglucose—glucose (product) mass (g)
- r—reaction rate (g/(L∙min))
- t—time (min)
- Vbuffer—volume of buffer with substrate (L)
- Vcapsules—volume of capsules (L)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Kinetic Characteristic of NOLA in Native Form
4.2.2. The Effect of Glucose and Galactose on NOLA Activity
4.2.3. Kinetic Measurements
4.2.4. NOLA Encapsulation
4.2.5. The Activity of Encapsulated NOLA
4.2.6. NOLA Stability at the Presence of Calcium Ions
4.2.7. NOLA Reuse
4.2.8. Diffusion Restrictions
4.2.9. Determination of Encapsulated NOLA Kinetic Parameters
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McCain, H.; Kaliappan, S.; Drake, M. Invited review: Sugar reduction in dairy products. J. Dairy Sci. 2018, 101, 8619–8640. [Google Scholar] [CrossRef] [Green Version]
- Saqib, S.; Akram, A.; Halim, S.A.; Tassaduq, R. Sources of β-galactosidase and its applications in food industry. 3 Biotech 2017, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Velázquez, A.L.; Vidal, L.; Alcaire, F.; Varela, P.; Ares, G. Significant sugar-reduction in dairy products targeted at children is possible without affecting hedonic perception. Int. Dairy J. 2020, 114, 104937. [Google Scholar] [CrossRef]
- Dekker, P.J.T.; Koenders, D.; Bruins, M.J. Lactose-Free Dairy Products: Market Developments, Production, Nutrition and Health Benefits. Nutrients 2019, 11, 551. [Google Scholar] [CrossRef] [Green Version]
- Harju, M.; Kallioinen, H.; Tossavainen, O. Lactose hydrolysis and other conversions in dairy products: Technological aspects. Int. Dairy J. 2012, 22, 104–109. [Google Scholar] [CrossRef]
- Pawlos, M.; Znamirowska, A.; Kluz, M.; Szajnar, K.; Kowalczyk, M. Low-lactose fermented goat milks with bifidobacterium animalis SSP. lactis BB-12. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 751–755. [Google Scholar] [CrossRef]
- Zagorska, J.; Ciprovica, I.; Straumite, E.; Majore, K. Acceptance of low-sugar yoghurt among Latvian teenagers. Agron. Res. 2020, 18, 1897–1905. [Google Scholar] [CrossRef]
- Luzzi, G.; Steffens, M.; Clawin-Rädecker, I.; Hoffmann, W.; Franz, C.M.A.P.; Fritsche, J.; Lorenzen, P.C. Enhancing the sweetening power of lactose by enzymatic modification in the reformulation of dairy products. Int. J. Dairy Technol. 2020, 73, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Trusek, A.; Dworakowska, D.; Czyzewska, K. 3D enzymatic preparations with graphene oxide flakes and hydrogel to obtain lactose-free products. Food Bioprod. Process. 2020, 121, 224–229. [Google Scholar] [CrossRef]
- Botvynko, A.; Bednářová, A.; Henke, S.; Shakhno, N.; Čurda, L. Production of galactooligosaccharides using various combinations of the commercial β-galactosidases. Biochem. Biophys. Res. Commun. 2019, 517, 762–766. [Google Scholar] [CrossRef]
- Pulicherla, K.; Ghosh, M.; Rao, K.R.S.S.; Kumar, P.S. Psychrozymes—The Next Generation Industrial Enzymes. J. Mar. Sci. Res. Dev. 2011, 1, 1. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kumari, S.; Panesar, R. Potential Applications of Immobilized β-Galactosidase in Food Processing Industries. Enzym. Res. 2010, 2010, 473137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [Green Version]
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef] [PubMed]
- Labus, K.; Drozd, A.; Trusek-Holownia, A. Preparation and characterisation of gelatine hydrogels predisposed to use as matrices for effective immobilisation of biocatalysts. Chem. Pap. 2015, 5, 523–530. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef] [Green Version]
- Labus, K.; Wolanin, K.; Radosiński, Ł. Comparative Study on Enzyme Immobilization Using Natural Hydrogel Matrices—Experimental Studies Supported by Molecular Models Analysis. Catalysts 2020, 10, 489. [Google Scholar] [CrossRef]
- Jurado, E.; Camacho, F.; Luzón, G.; Vicaria, J. A new kinetic model proposed for enzymatic hydrolysis of lactose by a β-galactosidase from Kluyveromyces fragilis. Enzym. Microb. Technol. 2002, 31, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Illanes, A.; Wilson, L.; Tomasello, G. Effect of modulation of enzyme inactivation on temperature optimization for reactor operation with chitin-immobilized lactase. J. Mol. Catal. B Enzym. 2001, 11, 531–540. [Google Scholar] [CrossRef]
- Ladero, M.; Santos, A.; García-Ochoa, F. Kinetic modeling of lactose hydrolysis with an immobilized β-galactosidase from Kluyveromyces fragilis. Enzym. Microb. Technol. 2000, 27, 583–592. [Google Scholar] [CrossRef]
- Chen, K.-C.; Houng, J.-Y.; Ling, A.C. Product inhibition of the enzymatic hydrolysis of lactose. Enzym. Microb. Technol. 1985, 7, 510–514. [Google Scholar] [CrossRef]
- Boyacı, I.H.; Baş, D.; Dudak, F.C.; Topçu, A.; Saldamlı, I.; Şeker, U.; Özgür, Ş.; Tamerler, C.; Boyaci, I.H.; Saldamli, I. Statistical Modeling of β-galactosidase Inhibition During Lactose Hydrolysis. Food Biotechnol. 2006, 20, 79–91. [Google Scholar] [CrossRef]
- Suri, S.; Kumar, V.; Prasad, R.; Tanwar, B.; Goyal, A.; Kaur, S.; Gat, Y.; Kumar, A.; Kaur, J.; Singh, D. Considerations for development of lactose-free food. J. Nutr. Intermed. Metab. 2019, 15, 27–34. [Google Scholar] [CrossRef]
- Güven, R.G.; Kaplan, A.; Güven, K.; Matpan, F.; Dogru, M. Effects of various inhibitors on β-galactosidase purified from the thermoacidophilic Alicyclobacillus acidocaldarius subsp. Rittmannii isolated from Antarctica. Biotechnol. Bioprocess Eng. 2011, 16, 114–119. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, F.; Song, L.; Sun, N.; Guan, W.; Liu, B.; Tian, J.; Zhang, Y.; Zhang, W. Site-directed mutation of β-galactosidase from Aspergillus candidus to reduce galactose inhibition in lactose hydrolysis. 3 Biotech 2018, 8, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czyzewska, K.; Trusek, A. Encapsulated catalase from Serratia genus for H2O2 decomposition in food applications. Pol. J. Chem. Technol. 2018, 20, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Trusek-Holownia, A.; Noworyta, A. Efficient utilisation of hydrogel preparations with encapsulated enzymes—A case study on catalase and hydrogen peroxide degradation. Biotechnol. Rep. 2015, 6, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Samadov, R.; Ciprovica, I.; Zolnere, K.; Cinkmanis, I. The optimization of acid whey permeate hydrolysis for glucose-galactose syrup production. In Proceedings of the 13th Baltic Conference on Food Science and Technology, Jelgava, Latvia, 2–3 May 2019; pp. 254–257. [Google Scholar] [CrossRef]
- Elnashar, M.M.; Awad, G.E.; Hassan, M.E.; Eldin, M.S.M.; Haroun, B.M.; El-Diwany, A.I. Optimal Immobilization ofβ-Galactosidase ontoκ-Carrageenan Gel Beads Using Response Surface Methodology and Its Applications. Sci. World J. 2014, 2014, 571682. [Google Scholar] [CrossRef] [Green Version]
- Harsa, S.T. β-Galactosidase Immobilization on Chitosan-Hydroxyapatite Complex: Effects of Immobilization Conditions. J. Nutr. Health Food Eng. 2014, 1, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Tanriseven, A.; Doğan, Ş. A novel method for the immobilization of β-galactosidase. Process. Biochem. 2002, 38, 27–30. [Google Scholar] [CrossRef]
- Ansari, S.A.; Satar, R.; Zaidi, S.K.; Ahmad, A. Immobilization of Aspergillus oryzae β-Galactosidase on Cellulose Acetate-Polymethylmethacrylate Membrane and Its Application in Hydrolysis of Lactose from Milk and Whey. Int. Sch. Res. Not. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Abbaspourrad, A. Production of galacto-oligosaccharides from whey permeate using β-galactosidase immobilized on functionalized glass beads. Food Chem. 2018, 251, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. The influence of pH and divalent/monovalent cations on the internal electron transfer (IET), enzymatic activity, and structure of fructose dehydrogenase. Anal. Bioanal. Chem. 2018, 410, 3253–3264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshpande, S.S.; Cheryan, M. Effects of Phytic Acid, Divalent Cations, and Their Interactions on α-Amylase Activity. J. Food Sci. 1984, 49, 516–519. [Google Scholar] [CrossRef]
- Zolnere, K.; Liepins, J.; Ciprovica, I. The impact of calcium ions on commercially available β-galactosidase. In Proceedings of the 11th Baltic Conference on Food Science and Technology, Jelgava, Latvia, 27–28 April 2017; pp. 27–30. [Google Scholar]
- Coker, J.A.; Brenchley, J.E. Protein engineering of a cold-active β-galactosidase from Arthrobacter sp. SB to increase lactose hydrolysis reveals new sites affecting low temperature activity. Extremophiles 2006, 10, 515–524. [Google Scholar] [CrossRef]
- Freitas, F.F.; Marquez, L.D.S.; Ribeiro, G.P.; Brandão, G.C.; Cardoso, V.L.; Ribeiro, E.J. Optimization of the immobilization process of β-galatosidade by combined entrapment-cross-linking and the kinetics of lactose hydrolysis. Braz. J. Chem. Eng. 2012, 29, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Al-Muftah, A.E.; Abu-Reesh, I.M. Effects of internal mass transfer and product inhibition on a simulated immobilized enzyme-catalyzed reactor for lactose hydrolysis. Biochem. Eng. J. 2005, 23, 139–153. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]








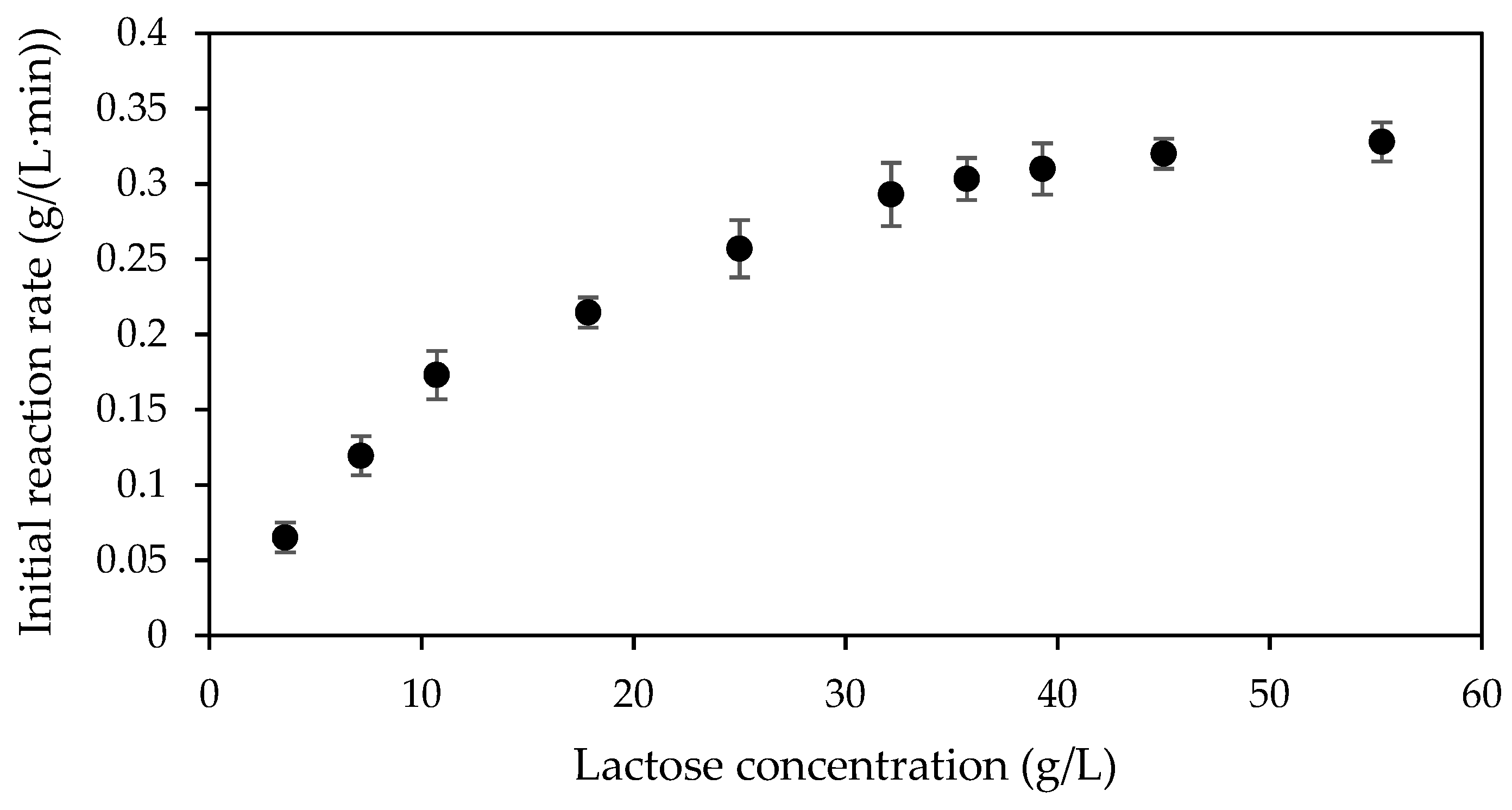
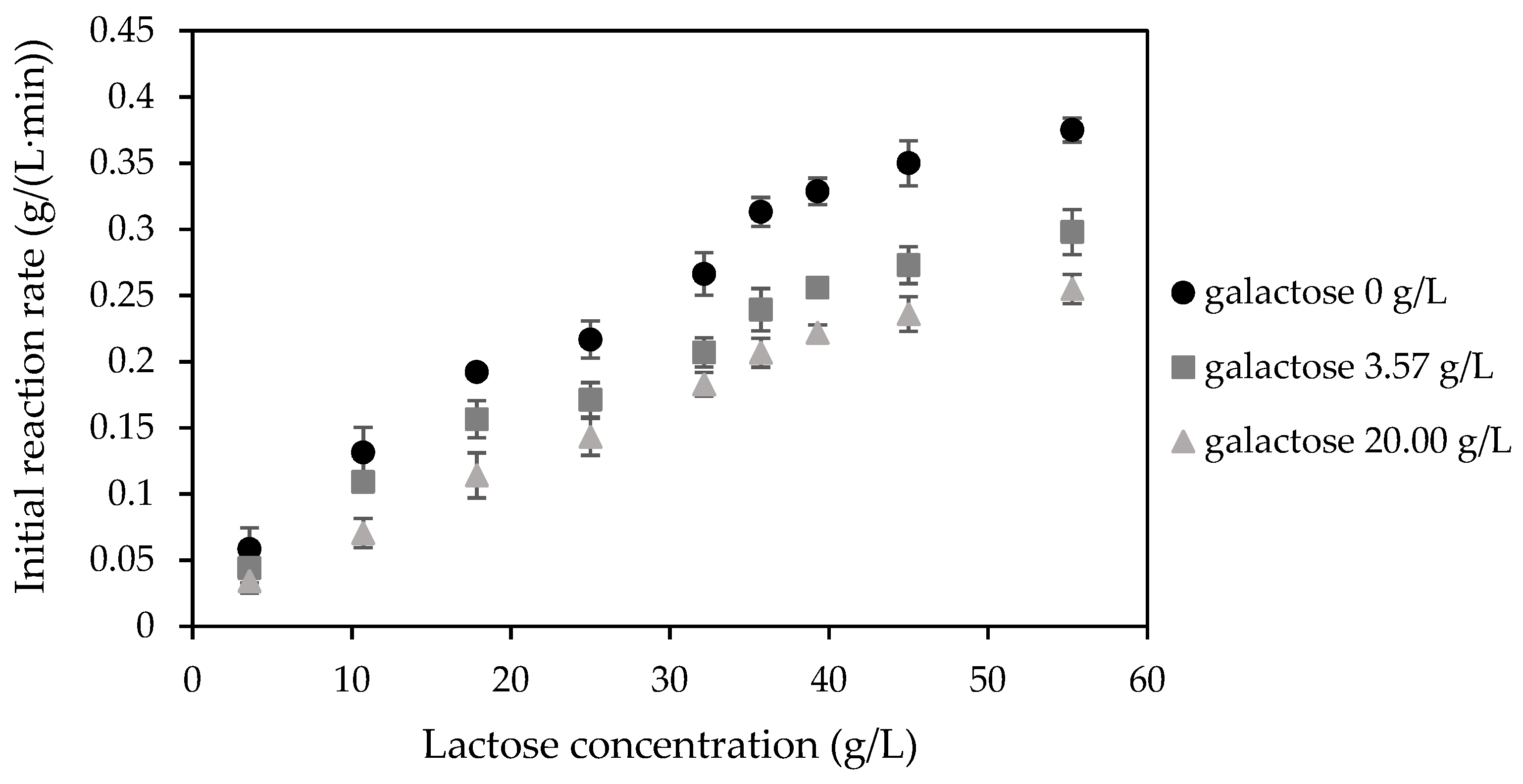

| Galactose (g/L) | Ki (g/L) |
|---|---|
| 5 | 24.49 ± 2.93 |
| 10 | 29.78 ± 4.68 |
| 20 | 29.46 ± 3.64 |
| 28 | 21.39 ± 0.72 |
| Lactose (g/L) | Glucose 5 g/L | Glucose 10 g/L | Glucose 20 g/L | Glucose 28 g/L | ||||
|---|---|---|---|---|---|---|---|---|
| Exper. | Calcul. | Exper. | Calcul. | Exper. | Calcul. | Exper. | Calcul. | |
| 5 | 0.856 | 0.756 | 0.897 | 0.803 | 1.066 | 0.880 | 1.005 | 0.930 |
| 15 | 1.824 | 1.793 | 1.853 | 1.929 | 2.455 | 2.164 | 2.546 | 2.322 |
| 25 | 2.409 | 2.469 | 2.929 | 2.682 | 3.534 | 3.056 | 3.330 | 3.315 |
| 35 | 2.943 | 2.946 | 3.277 | 3.221 | 3.712 | 3.712 | 3.400 | 4.059 |
| 45 | 3.166 | 3.300 | 3.213 | 3.625 | 3.312 | 4.215 | 3.642 | 4.637 |
| 50 | 3.187 | 3.445 | 3.497 | 3.792 | 3.769 | 4.425 | 3.978 | 4.881 |
| 55 | 3.363 | 3.573 | 3.672 | 3.940 | 3.794 | 4.612 | 4.162 | 5.100 |
| Galactose (g/L) | Ki (g/L) |
|---|---|
| 3.57 | 7.25 ± 0.22 |
| 7.14 | 7.79 ± 0.51 |
| 14.29 | 6.33 ± 0.44 |
| 20.00 | 8.24 ± 0.13 |
| Lactose (g/L) | Glucose 0.36 g/L | Glucose 1.43 g/L | Glucose 3.57 g/L | |||
|---|---|---|---|---|---|---|
| Exper. | Calcul. | Exper. | Calcul. | Exper. | Calcul. | |
| 5 | 0.048 | 0.067 | 0.053 | 0.067 | 0.058 | 0.053 |
| 15 | 0.111 | 0.157 | 0.132 | 0.158 | 0.131 | 0.137 |
| 25 | 0.157 | 0.217 | 0.186 | 0.217 | 0.192 | 0.200 |
| 35 | 0.183 | 0.258 | 0.211 | 0.258 | 0.217 | 0.249 |
| 45 | 0.216 | 0.289 | 0.244 | 0.289 | 0.266 | 0.288 |
| 50 | 0.243 | 0.302 | 0.300 | 0.302 | 0.313 | 0.305 |
| 55 | 0.282 | 0.313 | 0.308 | 0.313 | 0.329 | 0.320 |
| Native NOLA | Encapsulated NOLA | |
|---|---|---|
| k3 (1/min) | 3.28 | 0.46 |
| Km (g/L) | 30.00 | 21.19 |
| Ka(glucose) (g/L) | 26.28 | 7.40 |
| Ki(glucose) (g/L) | 15.79 | 10.54 |
| Ki(galactose) (g/L) | 0.76 | 4.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czyzewska, K.; Trusek, A. Encapsulated NOLA™ Fit 5500 Lactase—An Economically Beneficial Way to Obtain Lactose-Free Milk at Low Temperature. Catalysts 2021, 11, 527. https://doi.org/10.3390/catal11050527
Czyzewska K, Trusek A. Encapsulated NOLA™ Fit 5500 Lactase—An Economically Beneficial Way to Obtain Lactose-Free Milk at Low Temperature. Catalysts. 2021; 11(5):527. https://doi.org/10.3390/catal11050527
Chicago/Turabian StyleCzyzewska, Katarzyna, and Anna Trusek. 2021. "Encapsulated NOLA™ Fit 5500 Lactase—An Economically Beneficial Way to Obtain Lactose-Free Milk at Low Temperature" Catalysts 11, no. 5: 527. https://doi.org/10.3390/catal11050527






