Enzymatic Synthesis, Structural Analysis, and Evaluation of Antibacterial Activity and α-Glucosidase Inhibition of Hesperidin Glycosides
Abstract
1. Introduction
2. Results
2.1. Production and Purification of Hesperidin Glycoside
2.2. Structural Analysis of Hesperidin Glycosides
2.2.1. Digestion with Amylolytic Enzyme
2.2.2. LC-MS/MS Analysis
2.2.3. NMR Analysis
2.3. Antibacterial Activity
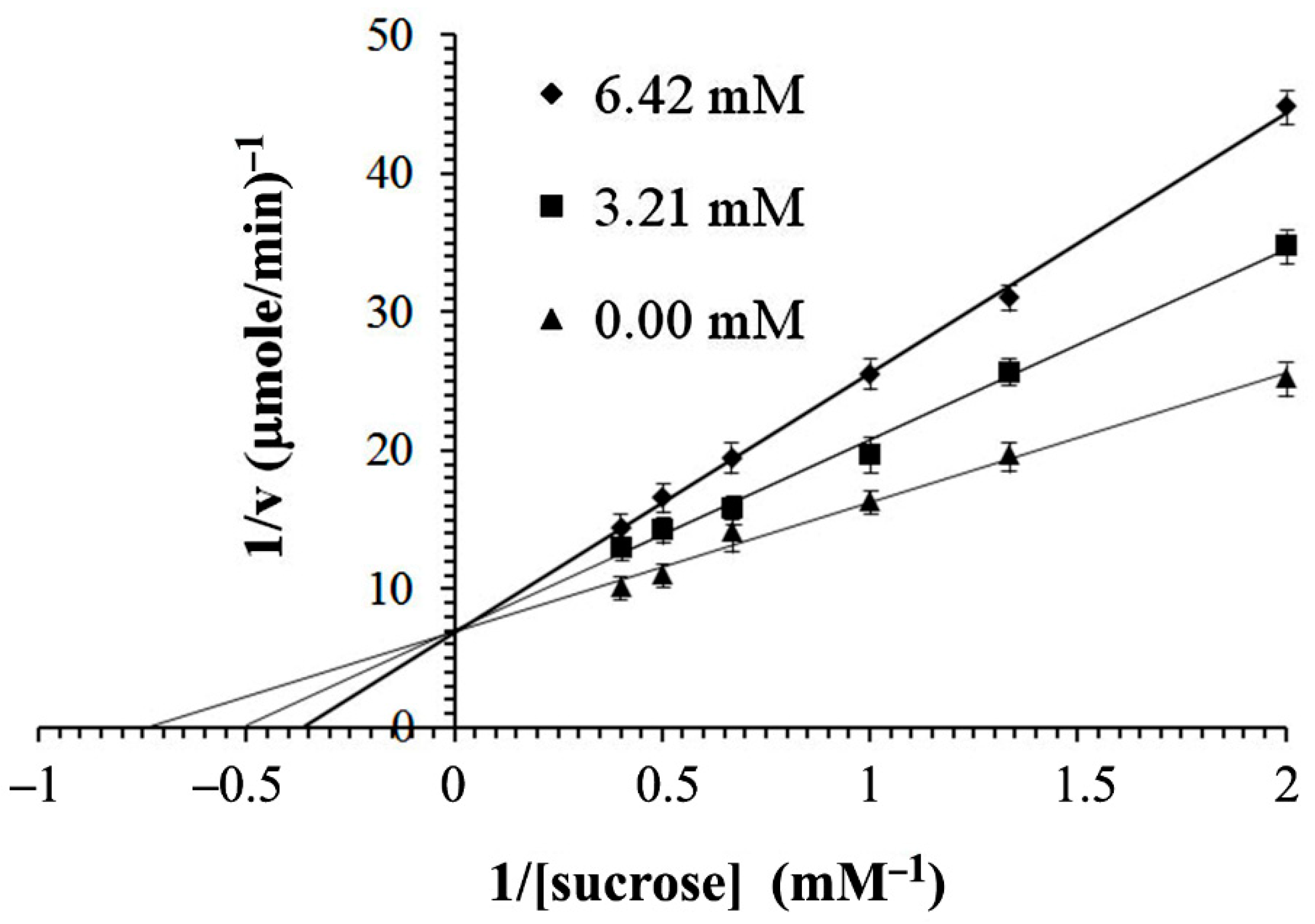
2.4. In Vitro α-Glucosidase Inhibitory Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis and Detection of Hesperidin Glycosides
4.3. Production and Product Purification
4.4. Structural Analysis of Hesperidin Glycosides
4.4.1. Digestion with Amylolytic Enzyme
4.4.2. Liquid Chromatography Mass Spectrometer (LC-MS/MS) Analysis
4.4.3. NMR Analysis
4.5. Antibacterial Activity
4.5.1. Minimal Inhibitory Concentration (MIC)
4.5.2. Minimal Bactericidal Concentration (MBC)
4.6. In Vitro α-Glucosidase Inhibitory Activity
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Villiers, A.; Venter, P.; Pasch, H. Recent advances and trends in the liquid-chromatography-mass spectrometry analysis of flavonoids. J. Chromatogr. A 2016, 1430, 16–78. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Biochem. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Kuntic, V.; Brboric, J.; Holclajtner-Antunović, I.; Uskoković-Marković, S. Evaluating the bioactive effects of flavonoid hesperidin: A new literature data survey. Vojnosanit. Pregl. 2014, 71, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Kometani, T.; Terada, Y.; Nishimura, T.; Nakae, T.; Takii, H.; Okada, S. Acceptor specificity of cyclodextrin glucanotransferase from an alkalophilic Bacillus species and synthesis of glucosyl rhamnose. Biosci. Biotechnol. Biochem. 1996, 60, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Chaisin, T.; Kaulpiboon, J.; Poomipark, N. Acceptor specificity of recombinant cyclodextrin glycosyltransferase from Bacillus circulans A11. STA 2018, 23, 86–97. [Google Scholar]
- Poomipark, N.; Chaisin, T.; Kaulpiboon, J. Synthesis and evaluation of antioxidant and β-glucuronidase inhibitory activity of hesperidin glycosides. Agric. Nat. Resour. 2020, 54, 165–172. [Google Scholar]
- Elavarasan, J.; Velusamy, P.; Ganesan, T.; Ramakrishnan, S.; Rajasekaran, D.; Kalaiselvi, P. Hesperidin-mediated expression of Nrf2 and upregulation of antioxidant status in senescent rat heart. J. Pharm. Pharmacol. 2012, 64, 1472–1482. [Google Scholar] [CrossRef]
- Kang, S.; Park, K.; Park, H.S.; Lee, D.H.; Kim, J.; Nagappan, A.; Kim, E.H.; Lee, W.; Shin, S.; Park, M.; et al. Anti-inflammatory effect of flavonoids isolated from Korea Citrus aurantium L. on lipopolysaccharide-induced mouse macrophage RAW 264.7 cells by blocking of nuclear factor-kappa B (NF-kB) and mitogen-activated protein kinase (MAPK) signalling pathways. Food Chem. 2011, 129, 1721–1728. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Cincin, Z.; Kiran, B.; Baran, Y.; Cakmakoglu, B. Hesperidin promotes programmed cell death by downregulation of nongenomic estrogen receptor signalling pathway in endometrial cancer cells. Biomed. Pharmacother. 2018, 103, 336–345. [Google Scholar] [CrossRef]
- Cincin, Z.; Unlu, M.; Kiran, B.; Sinem Bireller, E.; Baran, Y.; Cakmakoglu, B. Anti-proliferative, apoptotic and signal transduction effects of hesperidin in non-small cell lung cancer cells. Cell Oncol. 2015, 38, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Zhang, J.; Gao, J.; Ge, X.; Lou, G. Hesperidin inhibits HeLa cell proliferation through apoptosis mediated by endoplasmic reticulum stress pathways and cell cycle arrest. BMC Cancer 2015, 15, 682. [Google Scholar] [CrossRef]
- Iranshahi, M.; Rezaee, R.; Parhiz, H.; Roohbakhsh, A.; Soltani, F. Protective effects of flavonoids against microbes and toxins: The cases of hesperidin and hesperetin. Life Sci. 2015, 137, 125–132. [Google Scholar] [CrossRef]
- Yi, Z.; Yu, Y.; Liang, Y.; Zeng, B. In vitro antioxidant and antimicrobial activities of the extract of pericarpium citri reticulatae of a new citrus cultivar and its main flavonoids. Food Sci. Technol. 2008, 41, 597–603. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Mahmoud, A.M.; Abdel-Moneim, A.; Ashour, M.B. Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol. Croat. 2012, 41, 53–67. [Google Scholar]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, R.N.; Carvalho, F.; Carvalho, M.; Fernandes, E.; Soares, E.; Bastos, M.D.L.; Pereira, M.D.L. Protective activity of hesperidin and lipoic acid against sodium arsenite acute toxicity in mice. Toxicol. Pathol. 2004, 32, 527–535. [Google Scholar] [CrossRef]
- Cvetnić, Z.; Vladimir-Knezević, S. Antimicrobial activity of grapefruit seed and pulp ethanolic extract. Acta Pharm. 2004, 54, 243–250. [Google Scholar]
- Taskinen, M.R. Diabetic dyslipidemia. Atheroscl. Suppl. 2002, 3, 47–51. [Google Scholar] [CrossRef]
- Od-ek, P.; Phoungpetchara, I.; Hanchang, W.; Tunsophon, S. Inhibitory effect of hesperidin on glucosidase activity and small intestinal morphology in diabetic rats. Naresuan Univ. J. Sci. Technol. 2016, 24, 88–99. [Google Scholar]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef]
- Colson, P.; King, R.R. The 13C-n.m.r. spectra of disaccharides of d-glucose, d-galactose, and l-rhamnose as models for immununological polysaccharides. Carbohydr. Res. 1976, 47, 1–13. [Google Scholar] [CrossRef]
- Kitty, S.C.T.; Izabela, K.; Jian, Z. Identification and quantification of phenolics in Australian native mint (Mentha australis R. Br.). Food Chem. 2016, 192, 698–705. [Google Scholar]
- Charoensapyanan, R.; Ito, K.; Rudeekulthamrong, P.; Kaulpiboon, J. Enzymatic synthesis of propyl-α-glycosides and their application as emulsifying and antibacterial agents. Biotechnol. Bioprocess Eng. 2016, 21, 389–401. [Google Scholar] [CrossRef]
- Aramsangtienchai, P.; Chavasiri, W.; Ito, K.; Pongsawasdi, P. Synthesis of epicatechin glucosides by a β-cyclodextrin glycosyltransferase. J. Mol. Catal. B-Enzym. 2011, 73, 27–34. [Google Scholar] [CrossRef]
- Khummanee, N.; Rudeekulthamrong, R.; Kaulpiboon, J. Cyclodextrin glycosyltransferase-catalyzed synthesis of pinoresinol-α-D-glucoside having antioxidant and anti-inflammatory activities. Appl. Biochem. Microbiol. 2019, 55, 360–370. [Google Scholar] [CrossRef]
- Kaulpiboon, J.; Rudeekulthamrong, P. Biosynthesis of methyl glucoside and its antibacterial activity against Staphylococcus aureus and Escherichia coli. Bioact. Carbohydr. Diet. Fibre 2019, 20, 100197. [Google Scholar] [CrossRef]
- Kometani, T.; Terada, Y.; Nishimura, T.; Takii, H.; Okada, S. Transglycosylation to hesperidin by cyclodextrin glucanotransferase from an alkalophilic Bacillus species in alkaline pH and properties of hesperidin glycosides. Biosci. Biotechnol. Biochem. 1994, 58, 1990–1994. [Google Scholar] [CrossRef]
- Go, Y.H.; Kim, T.K.; Lee, K.W.; Lee, Y.H. Functional characteristics of cyclodextrin glucanotransferase from alkalophilic Bacillus sp. BL-31 highly specific for intermolecular transglycosylation of bioflavonoids. J. Microbiol. Biotechnol. 2007, 17, 1550–1553. [Google Scholar] [PubMed]
- Chiba, S. Molecular mechanism in α-glucosidase and glucoamylase. Biosci. Biotechnol. Biochem. 1997, 61, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Huh, J.Y.; Nam, S.H.; Moon, S.K.; Lee, S.B. Enzymatic bioconversion of citrus hesperidin by Aspergillus sojae naringinase: Enhanced solubility of hesperetin-7-O-glucoside with in vitro inhibition of human intestinal maltase, HMG-CoA reductase, and growth of Helicobacter pylori. Food Chem. 2012, 135, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Sahnoun, M.; Trabelsi, S.; Bejar, S. Citrus flavonoids collectively dominate the α-amylase and α-glucosidase inhibitions. Biologia 2017, 72, 764–773. [Google Scholar] [CrossRef]
- Gong, Y.; Qin, X.-Y.; Zhai, Y.-Y.; Hao, H.; Lee, J.; Park, Y.-D. Inhibitory effect of hesperetin on α-glucosidase: Molecular dynamics simulation integrating inhibition kinetics. Int. J. Biol. Macromol. 2017, 101, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Specian, R.D.; Oliver, M.G. Functional biology of intestinal goblet cells. Am. J. Physiol. Cell Physiol. 1991, 260, C183–C193. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, S.B.; Lee, H.S.; Lee, S.Y.; Baek, J.S.; Kim, D.; Moon, T.W.; Robyt, J.F.; Park, K.H. Comparative study of the inhibition of alpha-glucosidase, alpha-amylase, and cyclomaltodextrin glucanosyltransferase by acarbose, isoacarbose, and acarviosine-glucose. Arch. Biochem. Biophys. 1999, 371, 277–283. [Google Scholar] [CrossRef]
- Laoufi, H.; Benariba, N.; Adjdir, S.; Djaziri, R. In vitro α-amylase and α-glucosidase inhibitory activity of Ononis angustissima extracts. J. App. Pharm. Sci. 2017, 7, 191–198. [Google Scholar]
- Cuyckens, F.; Ma, Y.L.; Pocsfalvi, G.; Claeysi, M. Tandem mass spectral strategies for the structural characterization of flavonoid glycosides. Analusis 2000, 28, 888–895. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, Supplement M100, 31st ed.; Wayne, P., Ed.; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Awwad, A.; Abdeen, A. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int. J. Ind. Chem. 2013, 4, 29. [Google Scholar] [CrossRef]
- Bhalodia, N.R.; Shukla, V.J. Antibacterial and antifungal activities from leaf extracts of Cassia fistula L.: An ethnomedicinal plant. J. Adv. Pharm. Technol. Res. 2011, 2, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Damsud, T.; Grace, M.; Adisakwattana, S.; Phuwapraisirisan, P. Orthosiphol A from the aerial parts of Orthosiphon aristatus is putatively responsible for hypoglycemic effect via α-glucosidase inhibition. Nat. Prod. Commun. 2014, 9, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T. Enzyme inhibition. In Understanding Enzymes; Acford, R.J., Ed.; Ellis Horwood Limited Publishers: Chichester, UK, 1981; pp. 142–169. [Google Scholar]
- Mechchate, H.; Es-Safi, I.; Louba, A.; Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alharbi, M.S.; Alqahtani, A.; Bari, A.; et al. In vitro alpha-amylase and alpha-glucosidase inhibitory activity and in vivo antidiabetic activity of Withania frutescens L. Foliar extract. Molecules 2021, 26, 293. [Google Scholar] [CrossRef] [PubMed]


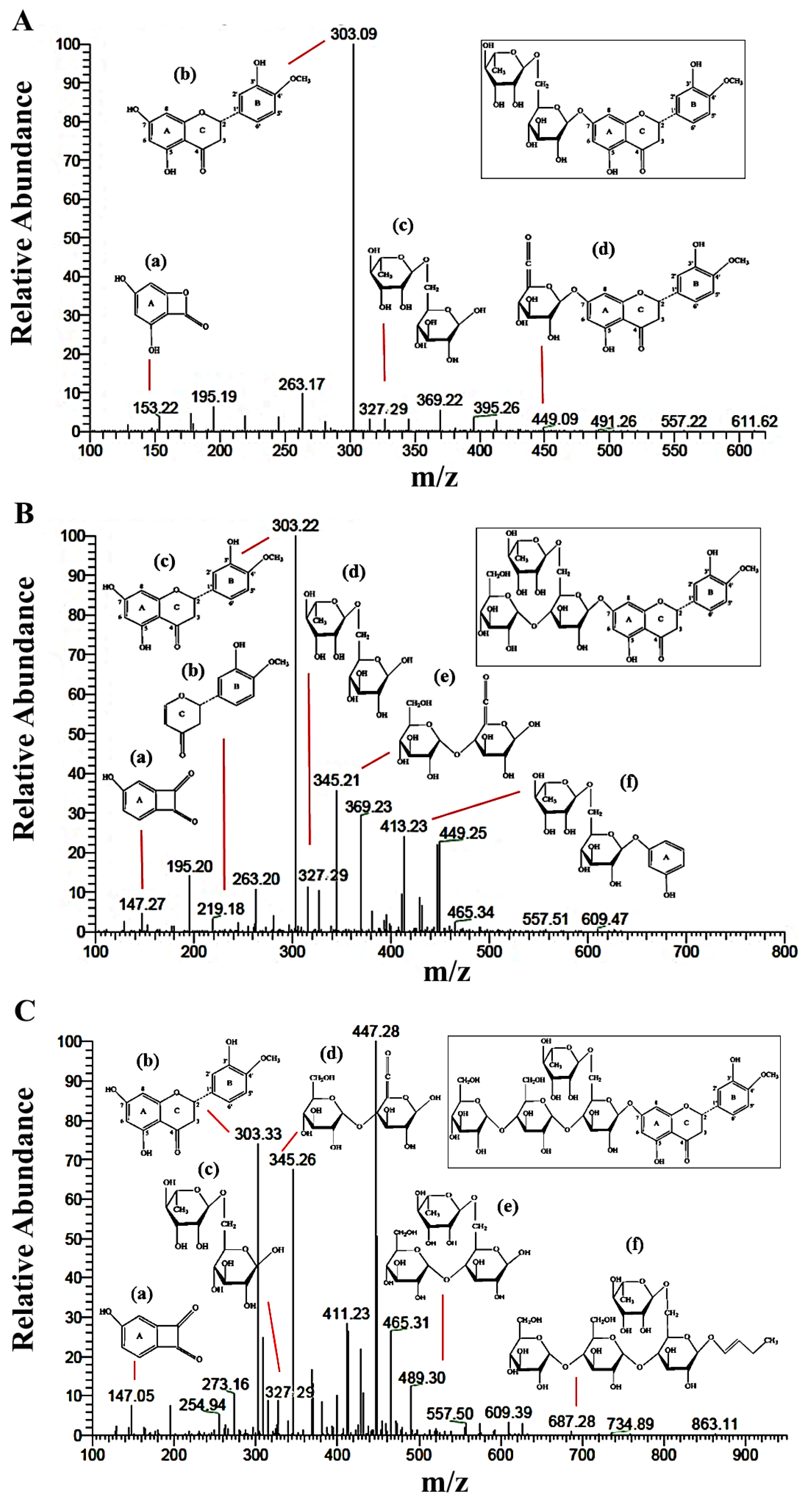

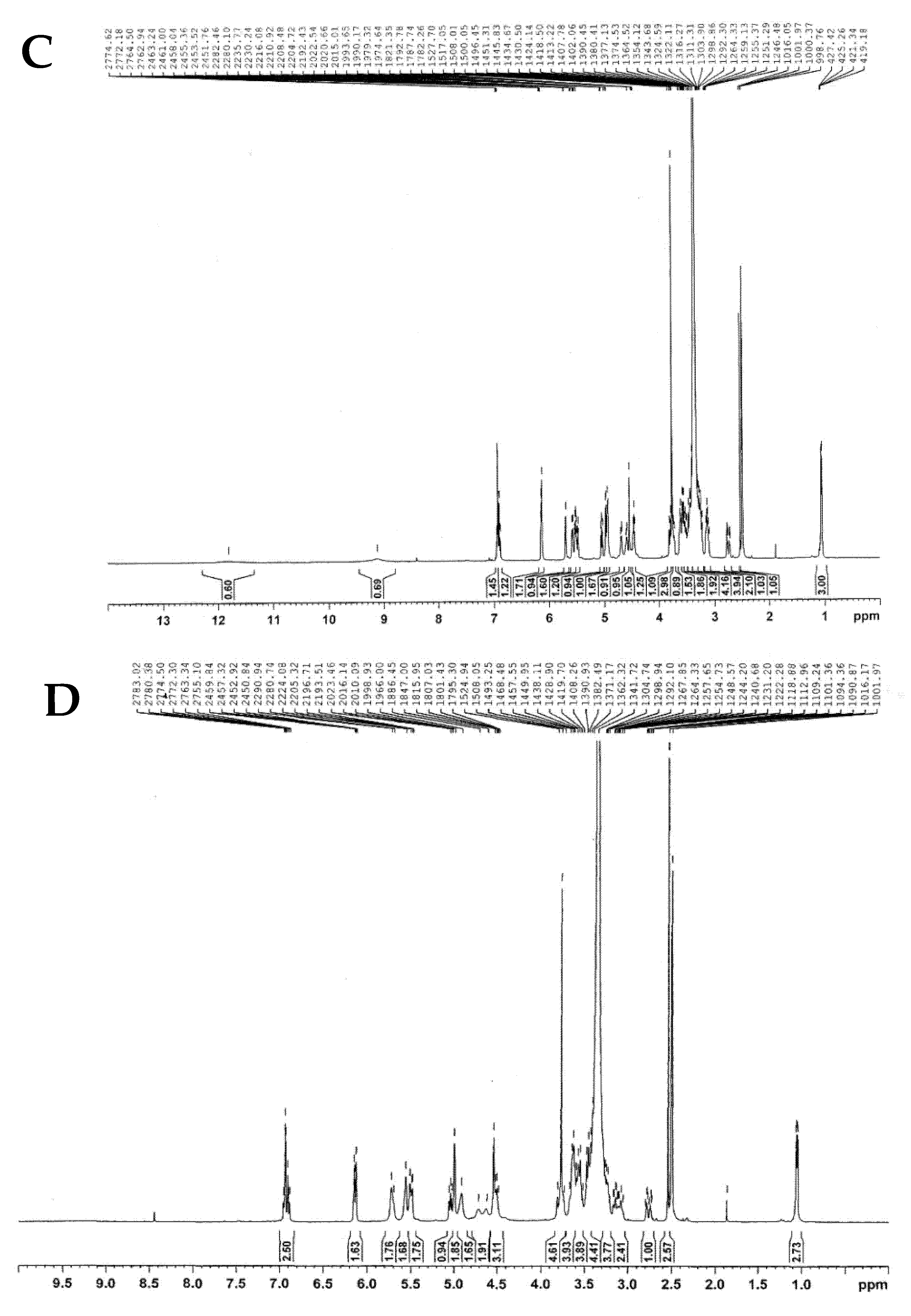
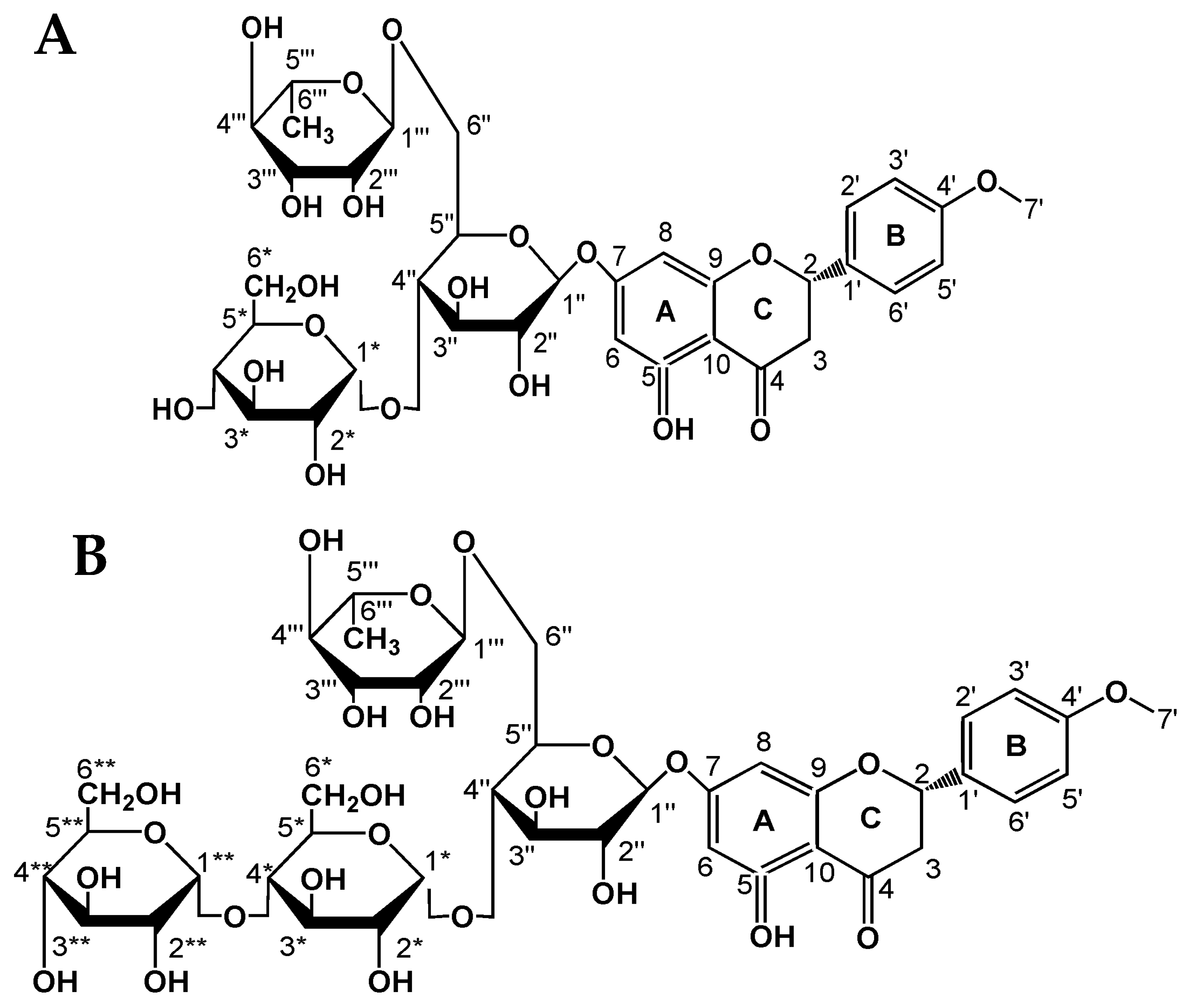

| Carbon Atom | 13C-NMR (δ, ppm) | ||||||
|---|---|---|---|---|---|---|---|
| Rhamnose a | Glucose b | Hesperetin | Hesperidin | HG1 | HG2 | ||
| C-aromatic ring A | 5 | 163.47 | 163.04 | 163.03 | 163.04 | ||
| 6 | 95.80 | 96.37 | 96.35 | 96.35 | |||
| 7 | 166.65 | 165.13 | 165.03 | 165.01 | |||
| 8 | 94.99 | 95.59 | 95.52 | 95.53 | |||
| 9 | 162.81 | 162.54 | 162.57 | 162.55 | |||
| 10 | 101.80 | 103.33 | 103.38 | 103.39 | |||
| C-aromatic ring C | 2 | 78.23 | 78.45 | 78.45 | 79.23 | ||
| 3 | 42.06 | 42.05 | 40.41 | 40.41 | |||
| 4 | 196.23 | 197.05 | 197.07 | 197.07 | |||
| C-aromatic ring B | 1′ | 131.14 | 130.89 | 130.89 | 130.89 | ||
| 2′ | 114.06 | 114.14 | 114.16 | 114.16 | |||
| 3′ | 146.45 | 146.44 | 146.45 | 146.45 | |||
| 4′ | 147.89 | 147.95 | 147.96 | 147.95 | |||
| 5′ | 111.97 | 112.01 | 112.07 | 112.06 | |||
| 6′ | 117.68 | 117.96 | 117.93 | 117.90 | |||
| 7′ | 55.67 | 55.67 | 55.67 | 55.67 | |||
| C-glucose I | 1″ | 92.77 | 99.42 | 99.10 | 99.10 | ||
| 2″ | 72.15 | 72.98 | 73.59 | 73.29 | |||
| 3″ | 73.43 | 75.50 | 75.80 | 75.73 | |||
| 4″ | 70.32 | 70.27 | 79.72 | 79.82 | |||
| 5″ | 72.10 | 72.98 | 73.54 | 73.06 | |||
| 6″ | 61.27 | 66.08 | 68.33 | 65.53 | |||
| C-rhamnose | 1‴ | 93.70 | 100.61 | 100.44 | 100.97 | ||
| 2‴ | 72.39 | 72.06 | 72.08 | 72.06 | |||
| 3‴ | 71.48 | 70.69 | 70.65 | 70.66 | |||
| 4‴ | 70.41 | 69.58 | 69.50 | 69.79 | |||
| 5‴ | 67.59 | 68.32 | 68.33 | 68.34 | |||
| 6‴ | 17.90 | 17.84 | 17.82 | 17.81 | |||
| C-glucose II | 1 * | 101.25 | 100.91 | ||||
| 2 * | 73.22 | 72.58 | |||||
| 3 * | 75.80 | 73.54 | |||||
| 4 * | 70.32 | 79.23 | |||||
| 5 * | 72.44 | 71.97 | |||||
| 6 * | 60.48 | 60.68 | |||||
| C-glucose III | 1 ** | 100.36 | |||||
| 2 ** | 72.46 | ||||||
| 3 ** | 73.47 | ||||||
| 4 ** | 70.30 | ||||||
| 5 ** | 71.80 | ||||||
| 6 ** | 60.01 | ||||||
| Microorganisms | MIC Values a | ||||
|---|---|---|---|---|---|
| Ampicillin (µg/mL) | Hesperidin (mg/mL) | HG1 (mg/mL) | HG2 (mg/mL) | Diosmin (mg/mL) | |
| E. coli ATCC 25922 | 8 ± 0 | 16 ± 0 | 16 ± 0 | 8 ± 0 | 16 ± 0 |
| S. aureus ATCC 25923 | 0.5 ± 0 | 8 ± 0 | 4 ± 0 | 4 ± 0 | 8 ± 0 |
| Microorganisms | MBC Values a | ||||
|---|---|---|---|---|---|
| Ampicillin (µg/mL) | Hesperidin (mg/mL) | HG1 (mg/mL) | HG2 (mg/mL) | Diosmin (mg/mL) | |
| E. coli ATCC 25922 | 8 ± 0 | 32 ± 0 | 32 ± 0 | 32 ± 0 | 32 ± 0 |
| S. aureus ATCC 25923 | 0.5 ± 0 | 16 ± 0 | 8 ± 0 | 8 ± 0 | 16 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaisin, T.; Rudeekulthamrong, P.; Kaulpiboon, J. Enzymatic Synthesis, Structural Analysis, and Evaluation of Antibacterial Activity and α-Glucosidase Inhibition of Hesperidin Glycosides. Catalysts 2021, 11, 532. https://doi.org/10.3390/catal11050532
Chaisin T, Rudeekulthamrong P, Kaulpiboon J. Enzymatic Synthesis, Structural Analysis, and Evaluation of Antibacterial Activity and α-Glucosidase Inhibition of Hesperidin Glycosides. Catalysts. 2021; 11(5):532. https://doi.org/10.3390/catal11050532
Chicago/Turabian StyleChaisin, Titaporn, Prakarn Rudeekulthamrong, and Jarunee Kaulpiboon. 2021. "Enzymatic Synthesis, Structural Analysis, and Evaluation of Antibacterial Activity and α-Glucosidase Inhibition of Hesperidin Glycosides" Catalysts 11, no. 5: 532. https://doi.org/10.3390/catal11050532
APA StyleChaisin, T., Rudeekulthamrong, P., & Kaulpiboon, J. (2021). Enzymatic Synthesis, Structural Analysis, and Evaluation of Antibacterial Activity and α-Glucosidase Inhibition of Hesperidin Glycosides. Catalysts, 11(5), 532. https://doi.org/10.3390/catal11050532






