Enhanced C2 and C3 Product Selectivity in Electrochemical CO2 Reduction on Carbon-Doped Copper Oxide Catalysts Prepared by Deep Eutectic Solvent Calcination
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Synthesis and Material Characterization
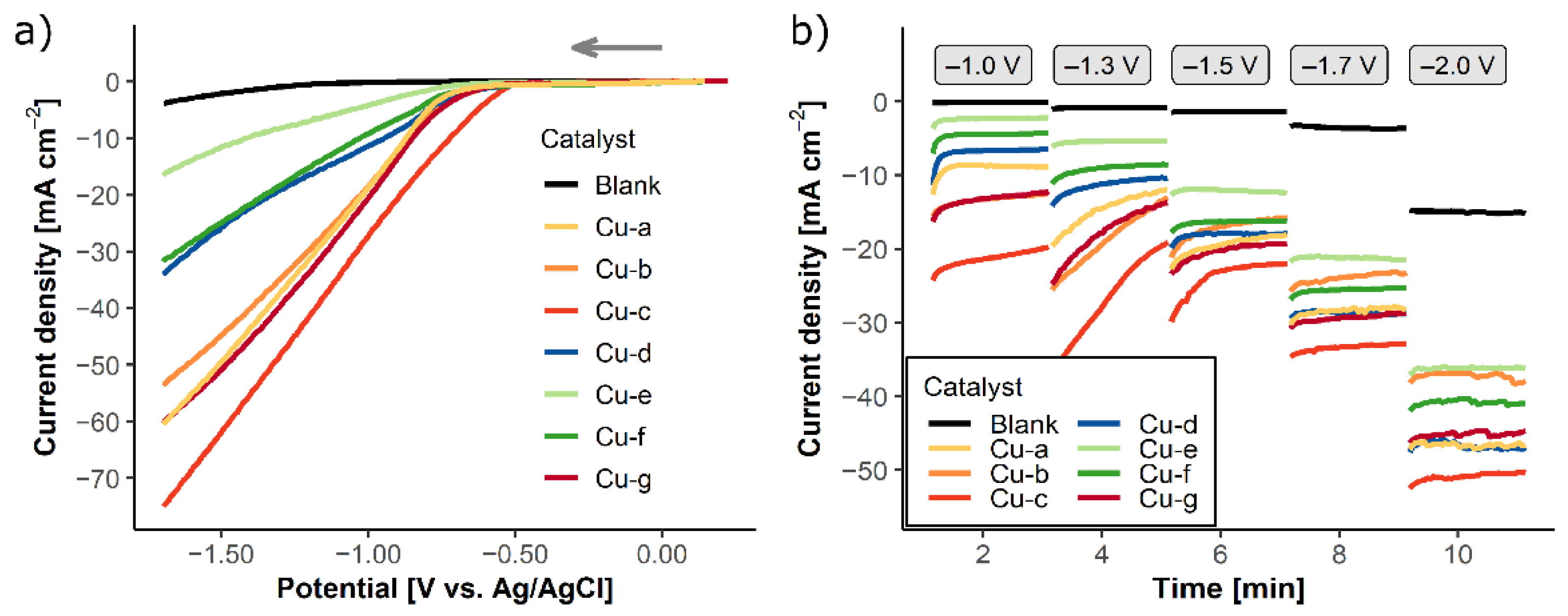
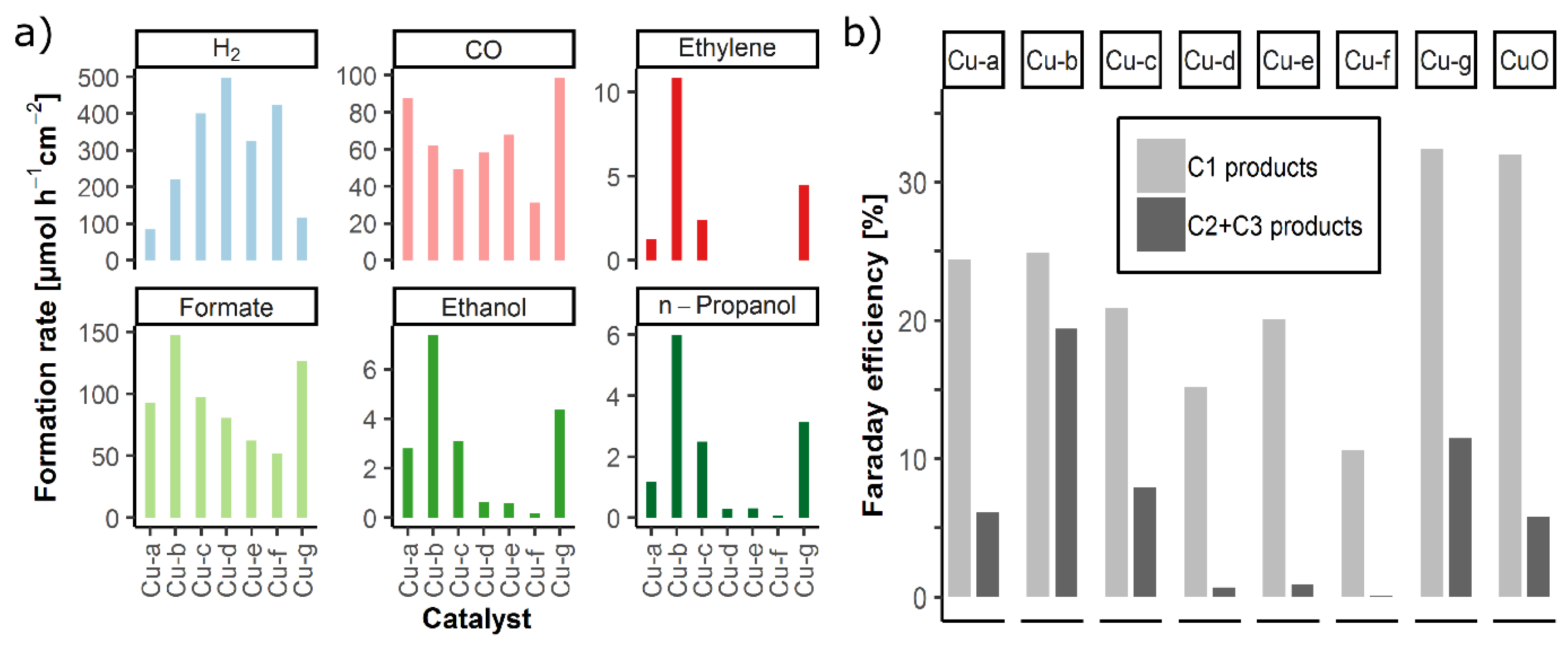
2.2. Electrochemical CO2 Reduction
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Materials Characterization
3.2.1. Scanning Electron Microscopy (SEM)
3.2.2. Powder Diffractometry (XRD)
3.3. Electrochemical Measurements
3.3.1. Electrode Preparation
3.3.2. Electrochemical Cells
3.4. Product Analysis
3.4.1. Gas Chromatography with Thermal Conductivity Detector (GC-TCD)
3.4.2. Gas Chromatography with Flame Ionization Detector (GC-FID)
3.4.3. High-Pressure Liquid Chromatography (HPLC)
3.4.4. NMR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Indermühle, A.; Monnin, E.; Stauffer, B.; Stocker, T.F.; Wahlen, M. Atmospheric CO2 concentration from 60 to 20 kyr BP from the Taylor Dome Ice Core, Antarctica. Geophys. Res. Lett. 2000, 27, 735–738. [Google Scholar] [CrossRef]
- Benson, S.M.; Orr, F.M. Carbon Dioxide Capture and Storage. MRS Bull. 2008, 33, 303–305. [Google Scholar] [CrossRef]
- Rahman, F.A.; Aziz, M.A.; Saidur, R.; Abu Bakar, W.A.W.; Hainin, M.; Putrajaya, R.; Hassan, N.A. Pollution to solution: Capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future. Renew. Sustain. Energy Rev. 2017, 71, 112–126. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- “Molly” Jhong, H.-R.; Ma, S.; Kenis, P.J. Electrochemical conversion of CO2 to useful chemicals: Current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2013, 2, 191–199. [Google Scholar] [CrossRef]
- Kumar, B.; Brian, J.P.; Atla, V.; Kumari, S.; Bertram, K.A.; White, R.T.; Spurgeon, J.M. New trends in the development of heterogeneous catalysts for electrochemical CO2 reduction. Catal. Today 2016, 270, 19–30. [Google Scholar] [CrossRef]
- Whipple, D.T.; Kenis, P.J.A. Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458. [Google Scholar] [CrossRef]
- Azuma, M.; Hashimoto, K.; Hiramoto, M.; Watanabe, M.; Sakata, T. Carbon dioxide reduction at low temperature on various metal electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1989, 260, 441–445. [Google Scholar] [CrossRef]
- Hori, Y.; Murata, A.; Takahashi, R.; Suzuki, S. Enhanced formation of ethylene and alcohols at ambient temperature and pressure in electrochemical reduction of carbon dioxide at a copper electrode. J. Chem. Soc. Chem. Commun. 1988, 1, 17–19. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Roberts, F.S.; Kuhl, K.P.; Nilsson, A. High Selectivity for Ethylene from Carbon Dioxide Reduction over Copper Nanocube Electrocatalysts. Angew. Chem. Int. Ed. 2015, 54, 5179–5182. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Loiudice, A.; Lobaccaro, P.; Kamali, E.A.; Thao, T.; Huang, B.H.; Ager, J.W.; Buonsanti, R. Tailoring Copper Nanocrystals towards C2 Products in Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2016, 55, 5789–5792. [Google Scholar] [CrossRef]
- Kim, D.; Kley, C.S.; Li, Y.; Yang, P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products. Proc. Natl. Acad. Sci. USA 2017, 114, 10560–10565. [Google Scholar] [CrossRef]
- Li, C.W.; Kanan, M.W. CO2 Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu2O Films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; pp. 89–189. [Google Scholar]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef] [PubMed]
- Iwanow, M.; Finkelmeyer, J.; Söldner, A.; Kaiser, M.; Gärtner, T.; Sieber, V.; König, B. Preparation of Supported Palladium Catalysts using Deep Eutectic Solvents. Chem. A Eur. J. 2017, 23, 12467–12470. [Google Scholar] [CrossRef] [PubMed]
- Iwanow, M.; Vieira, L.; Rud, I.; Seidler, J.; Kaiser, M.; Van Opdenbosch, D.; Zollfrank, C.; Richter, M.; Gärtner, T.; König, B.; et al. Pyrolysis of Deep Eutectic Solvents for the Preparation of Supported Copper Electrocatalysts. Chemestry 2020, 5, 11714–11720. [Google Scholar] [CrossRef]
- Muniz, F.T.L.; Miranda, M.A.R.; Dos Santos, C.M.; Sasaki, J.M. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallogr. Sect. A Found. Adv. 2016, 72, 385–390. [Google Scholar] [CrossRef]
- Wagle, D.V.; Zhao, H.; Baker, G.A. Deep Eutectic Solvents: Sustainable Media for Nanoscale and Functional Materials. Accounts Chem. Res. 2014, 47, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Hori, Y.; Takahashi, R.; Yoshinami, A.Y.; Murata, A. Electrochemical Reduction of CO at a Copper Electrode. J. Phys. Chem. B 1997, 101, 7075–7081. [Google Scholar] [CrossRef]
- Wojtowicz, J.A. The carbonate system in swimming pool water. J. Swim. Pool Spa Ind. 2001, 4, 54–59. [Google Scholar]
- Lum, Y.; Yue, B.; Lobaccaro, P.; Bell, A.T.; Ager, J.W. Optimizing C–C Coupling on Oxide-Derived Copper Catalysts for Electrochemical CO2 Reduction. J. Phys. Chem. C 2017, 121, 14191–14203. [Google Scholar] [CrossRef]
- Lum, Y.; Ager, J.W. Stability of Residual Oxides in Oxide-Derived Copper Catalysts for Electrochemical CO2 Reduction Investigated with 18 O Labeling. Angew. Chem. Int. Ed. 2018, 57, 551–554. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [PubMed]
- Handoko, A.D.; Chan, K.W.; Yeo, B.S. –CH3 Mediated Pathway for the Electroreduction of CO2 to Ethane and Ethanol on Thick Oxide-Derived Copper Catalysts at Low Overpotentials. ACS Energy Lett. 2017, 2, 2103–2109. [Google Scholar] [CrossRef]
- Zheng, Y.; Vasileff, A.; Zhou, X.; Jiao, Y.; Jaroniec, M.; Qiao, S.-Z. Understanding the Roadmap for Electrochemical Reduction of CO2 to Multi-Carbon Oxygenates and Hydrocarbons on Copper-Based Catalysts. J. Am. Chem. Soc. 2019, 141, 7646–7659. [Google Scholar] [CrossRef]
- Pander, J.E.; Ren, D.; Huang, Y.; Loo, N.W.X.; Hong, S.H.L.; Yeo, B.S. Understanding the Heterogeneous Electrocatalytic Reduction of Carbon Dioxide on Oxide-Derived Catalysts. ChemElectroChem 2018, 5, 219–237. [Google Scholar] [CrossRef]
- Kas, R.; Kortlever, R.; Milbrat, A.; Koper, M.T.M.; Mul, G.; Baltrusaitis, J. Electrochemical CO2 reduction on Cu2O-derived copper nanoparticles: Controlling the catalytic selectivity of hydrocarbons. Phys. Chem. Chem. Phys. 2014, 16, 12194–12201. [Google Scholar] [CrossRef]
- Albo, J.; Vallejo, D.; Beobide, G.; Castillo, O.; Castaño, P.; Irabien, A. Copper-Based Metal-Organic Porous Materials for CO2 Electrocatalytic Reduction to Alcohols. ChemSusChem 2017, 10, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Handoko, A.D.; Wan, J.H.; Ma, L.; Ren, D.; Yeo, B.S. Stable and selective electrochemical reduction of carbon dioxide to ethylene on copper mesocrystals. Catal. Sci. Technol. 2014, 5, 161–168. [Google Scholar] [CrossRef]
- Ren, D.; Wong, N.T.; Handoko, A.D.; Huang, Y.; Yeo, B.S. Mechanistic Insights into the Enhanced Activity and Stability of Agglomerated Cu Nanocrystals for the Electrochemical Reduction of Carbon Dioxide to n-Propanol. J. Phys. Chem. Lett. 2016, 7, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Merino-Garcia, I.; Albo, J.; Solla-Gullón, J.; Montiel, V.; Irabien, A. Cu oxide/ZnO-based surfaces for a selective ethylene production from gas-phase CO2 electroconversion. J. CO2 Util. 2019, 31, 135–142. [Google Scholar] [CrossRef]


| Chemical Equations | Potential V vs. SHE |
|---|---|
| −0.43 V | |
| −0.52 V | |
| −0.34 V | |
| −0.33 V | |
| −0.32 V |
| Precursor | Pre-Treatment | Calcination | ||||||
|---|---|---|---|---|---|---|---|---|
| DES | Metal | t [h] | T [°C] | Atm. | t [min] | T [°C] | Atm. | |
| Cu-a | Glc-U | CuNP | 1.5 | 290 | air | 60 | 500 | air |
| Cu-b | Gal-U | CuNP | 1.5 | 290 | air | 60 | 500 | air |
| Cu-c | Glc-U | CuO | 1.5 | 290 | air | 60 | 500 | air |
| Cu-d | Glc-U | CuNP | 1.5 | 290 | air | 15 | 500 | air |
| Cu-e | Glc-U | CuNP | 1.5 | 290 | N2 | from RT 10 | to 450 500 | N2 air |
| Cu-f | Glc-U | CuNP | 1.5 | 290 | N2 | from RT 10 | to 500 550 | N2 air |
| Cu-g | Glc-U | CuNP | 1.5 | 290 | N2 | 60 | 500 | air |
| Catalyst | Dx [nm] | Dy [nm] | Dz [nm] |
|---|---|---|---|
| Cu-a | 50.5 | 33.4 | 44.5 |
| Cu-b | 58.6 | 35.6 | 89.3 |
| Cu-c | 69.2 | 31.0 | 44.5 |
| Cu-g | 50.8 | 37.2 | 96.0 |
| Formation Rates [µmol h−1 cm−2] | Total FE [%] | J [mA cm−2] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | C2H4 | C2H5OH | C3H7OH | |||||
| Cu-b | 1st run | 210.2 | 59.4 | 141.1 | 10.4 | 7.1 | 5.7 | 70.5 | −43 |
| 2nd run | 373.9 | 72.4 | 124.5 | 13.4 | 7.5 | 5.1 | 65.5 | −61 | |
| Cu-a | 1st batch | 252.5 | 75.3 | 80.3 | 3.3 | 2.5 | 1.3 | 54.9 | −68 |
| 2nd batch | 176.5 | 119.4 | 94.2 | 3.4 | 3.5 | 1.8 | 54.1 | −42 | |
| Electrode | E [V] | Formation Rates [µmol h−1 cm−2] | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| CO | CH4 | C2H4 | C2H5OH | C3H7OH | ||||
| Cu-organic porous materials | −0.90 vs. RHE | - | - | - | - | 0.05 | - | [34] |
| Cu-mesocrystals | −0.99 vs. RHE | 6 | - | - | 17 | - | - | [35] |
| Cu-nanocrystals | −1.05 vs. RHE | 10 | - | - | 12 | 10 | 2 | [36] |
| CuO/ZnO | −2.5 vs. Ag/AgCl | 39.7 | - | 9.7 | 1355 | ? | ? | [37] |
| Cu-b | −1.08 * vs. RHE | 59.4 | 141.1 | 10.4 | 7.1 | 5.7 | this work | |
| Precursor | Pre-Treatment | Calcination | Elemental Composition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DES | Metal | t [h] | T [°C] | Atm. | t [min] | T [°C] | Atm. | Cu [wt%] | O [wt%] | C [wt%] | N [wt%] | |
| Cu-a | Glc-U | CuNP | 1.5 | 290 | air | 60 | 500 | air | 80.3 | 13.1 | 4.4 | 2.2 |
| Cu-b | Gal-U | CuNP | 1.5 | 290 | air | 60 | 500 | air | 72.8 | 21.6 | 3.2 | 2.5 |
| Cu-c | Glc-U | CuO | 1.5 | 290 | air | 60 | 500 | air | 79.3 | 14.9 | 3.5 | 2.2 |
| Cu-d | Glc-U | CuNP | 1.5 | 290 | air | 15 | 500 | air | 72.9 29.5 | 14.6 7.0 | 9.5 48.0 | 3.2 15.4 |
| Cu-e | Glc-U | CuNP | 1.5 | 290 | N2 | from RT 10 | to 450 500 | N2 air | 62.6 39.2 | 14.3 4.9 | 17.0 43.2 | 6.2 12.7 |
| Cu-f | Glc-U | CuNP | 1.5 | 290 | N2 | from RT 10 | to 500 550 | N2 air | 68.0 49.0 | 22.8 16.8 | 5.7 25.9 | 3.6 8.3 |
| Cu-g | Glc-U | CuNP | 1.5 | 290 | N2 | 60 | 500 | air | 77.1 | 17.7 | 2.2 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwanow, M.; Seidler, J.; Vieira, L.; Kaiser, M.; Van Opdenbosch, D.; Zollfrank, C.; Gärtner, T.; Richter, M.; König, B.; Sieber, V. Enhanced C2 and C3 Product Selectivity in Electrochemical CO2 Reduction on Carbon-Doped Copper Oxide Catalysts Prepared by Deep Eutectic Solvent Calcination. Catalysts 2021, 11, 542. https://doi.org/10.3390/catal11050542
Iwanow M, Seidler J, Vieira L, Kaiser M, Van Opdenbosch D, Zollfrank C, Gärtner T, Richter M, König B, Sieber V. Enhanced C2 and C3 Product Selectivity in Electrochemical CO2 Reduction on Carbon-Doped Copper Oxide Catalysts Prepared by Deep Eutectic Solvent Calcination. Catalysts. 2021; 11(5):542. https://doi.org/10.3390/catal11050542
Chicago/Turabian StyleIwanow, Melanie, Johannes Seidler, Luciana Vieira, Manuela Kaiser, Daniel Van Opdenbosch, Cordt Zollfrank, Tobias Gärtner, Michael Richter, Burkhard König, and Volker Sieber. 2021. "Enhanced C2 and C3 Product Selectivity in Electrochemical CO2 Reduction on Carbon-Doped Copper Oxide Catalysts Prepared by Deep Eutectic Solvent Calcination" Catalysts 11, no. 5: 542. https://doi.org/10.3390/catal11050542
APA StyleIwanow, M., Seidler, J., Vieira, L., Kaiser, M., Van Opdenbosch, D., Zollfrank, C., Gärtner, T., Richter, M., König, B., & Sieber, V. (2021). Enhanced C2 and C3 Product Selectivity in Electrochemical CO2 Reduction on Carbon-Doped Copper Oxide Catalysts Prepared by Deep Eutectic Solvent Calcination. Catalysts, 11(5), 542. https://doi.org/10.3390/catal11050542







