Traceless Directing Groups in Sustainable Metal-Catalyzed C–H Activation
Abstract
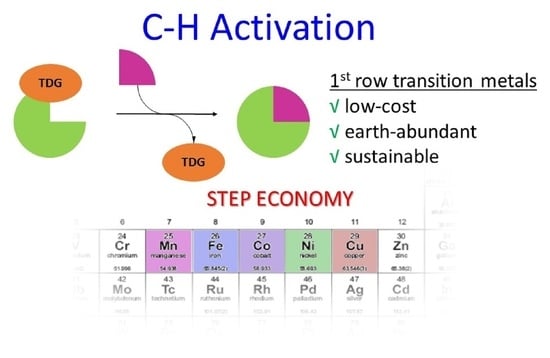
1. Introduction
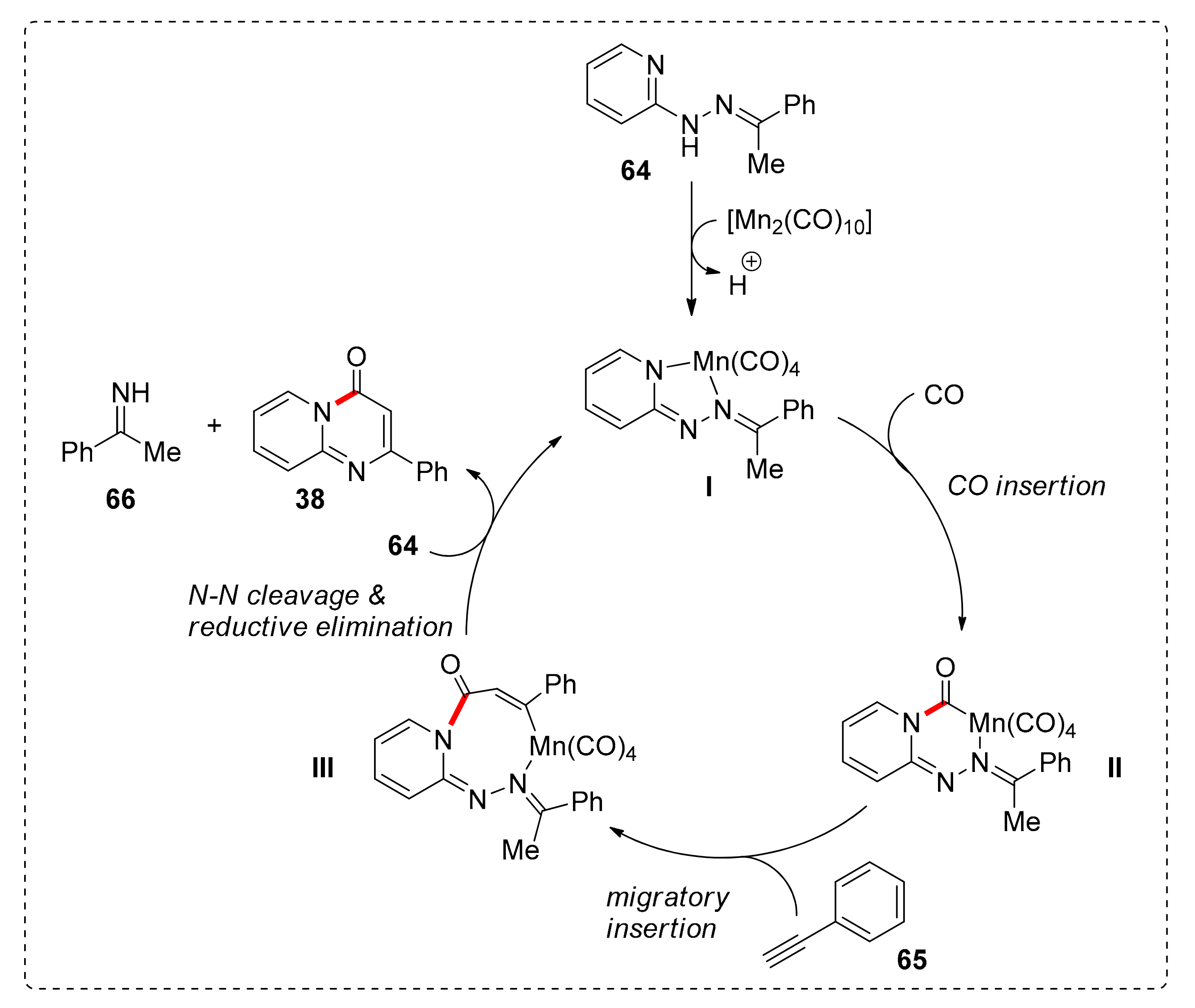
2. Manganese
Annulations via C(sp2)–H Bond Activation
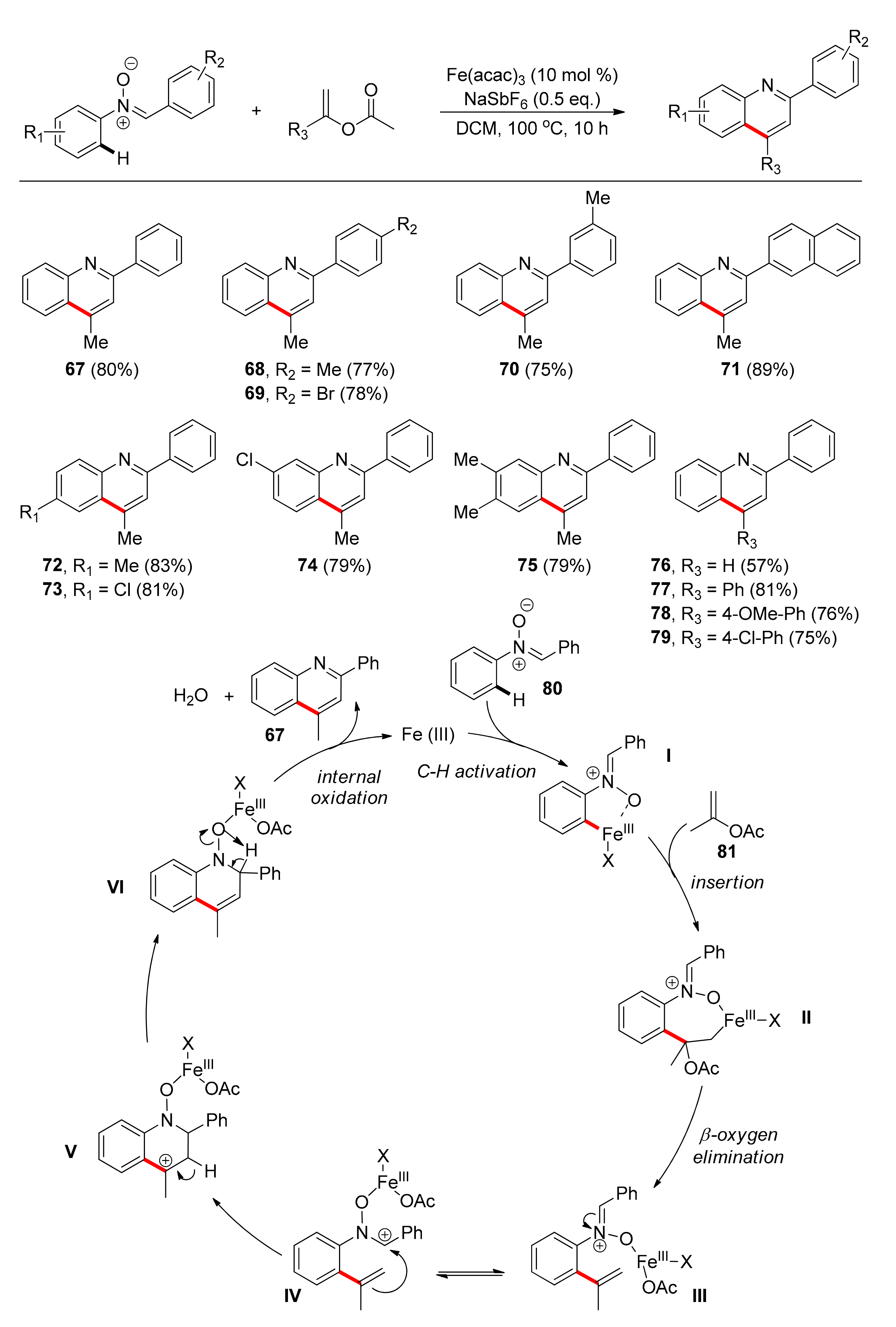
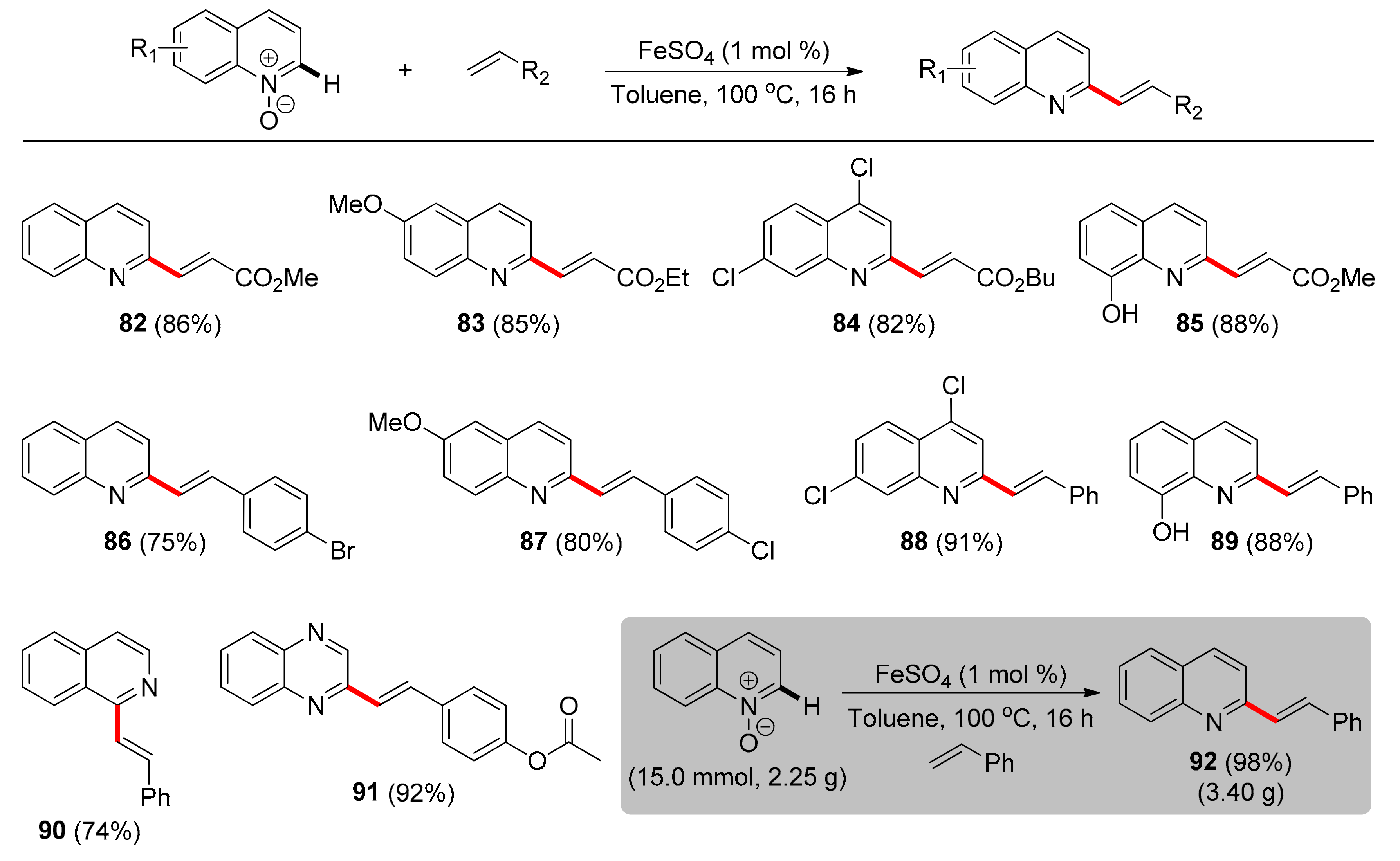
3. Iron
Transformations with N-oxide as TDG
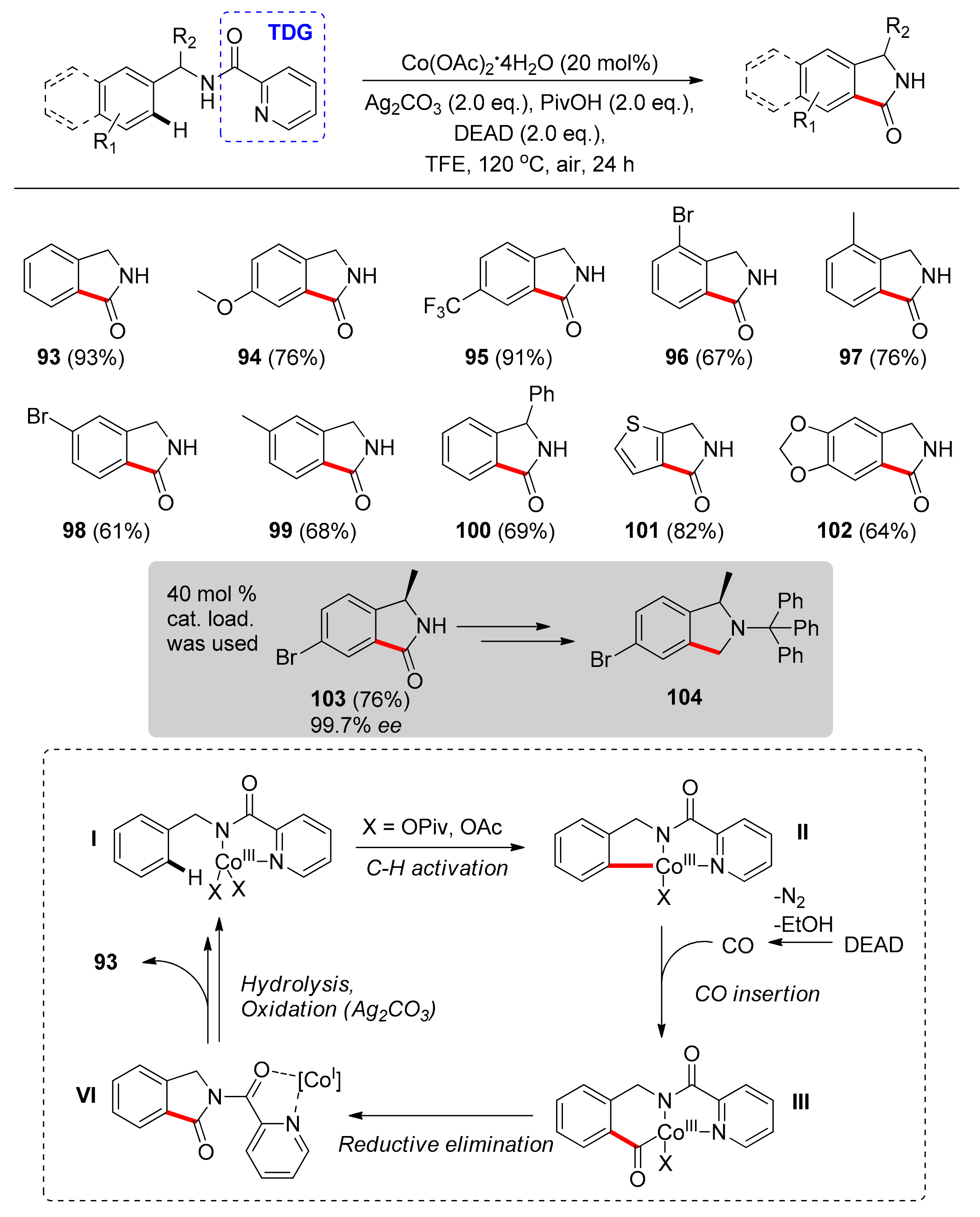
4. Cobalt
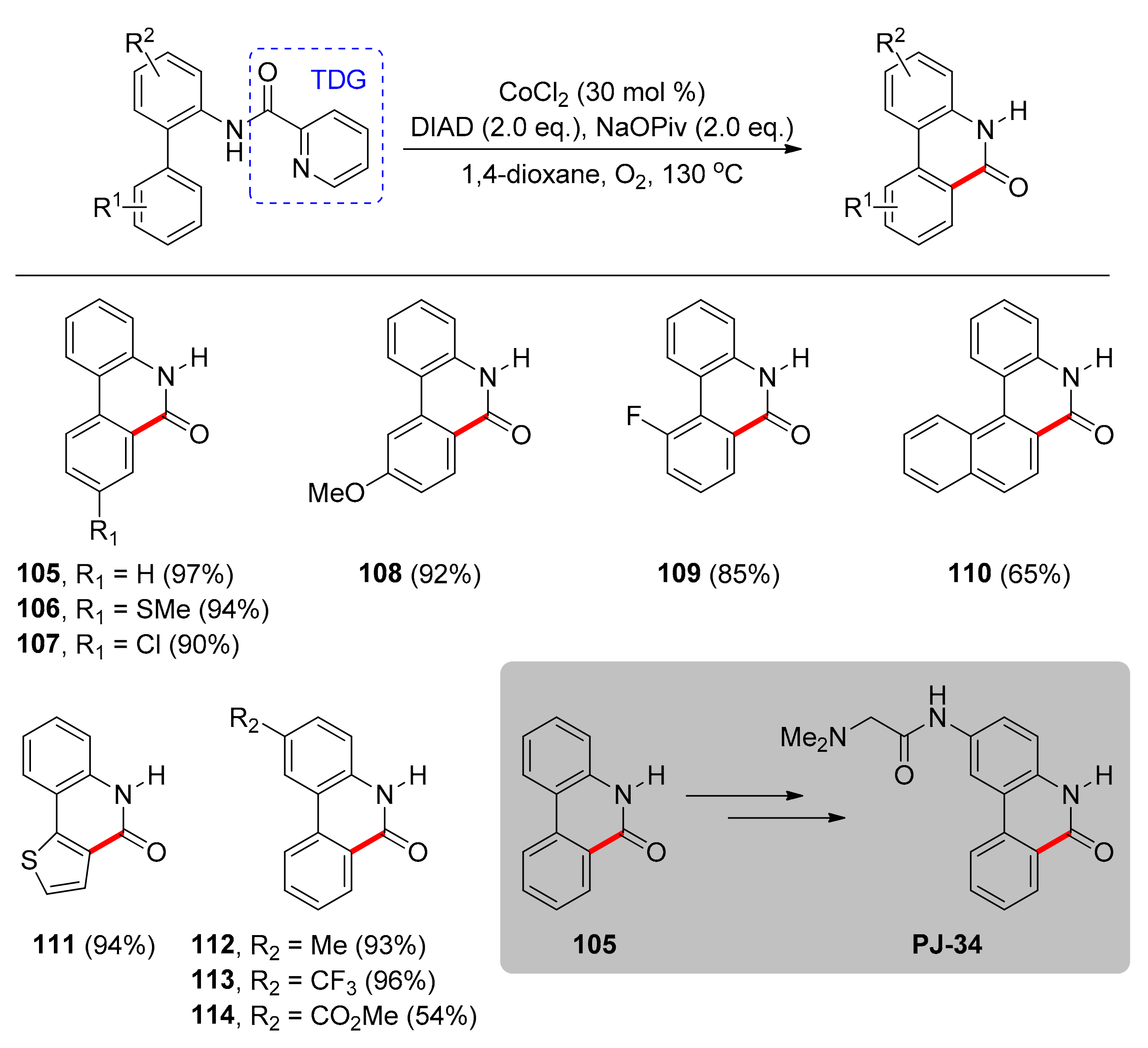
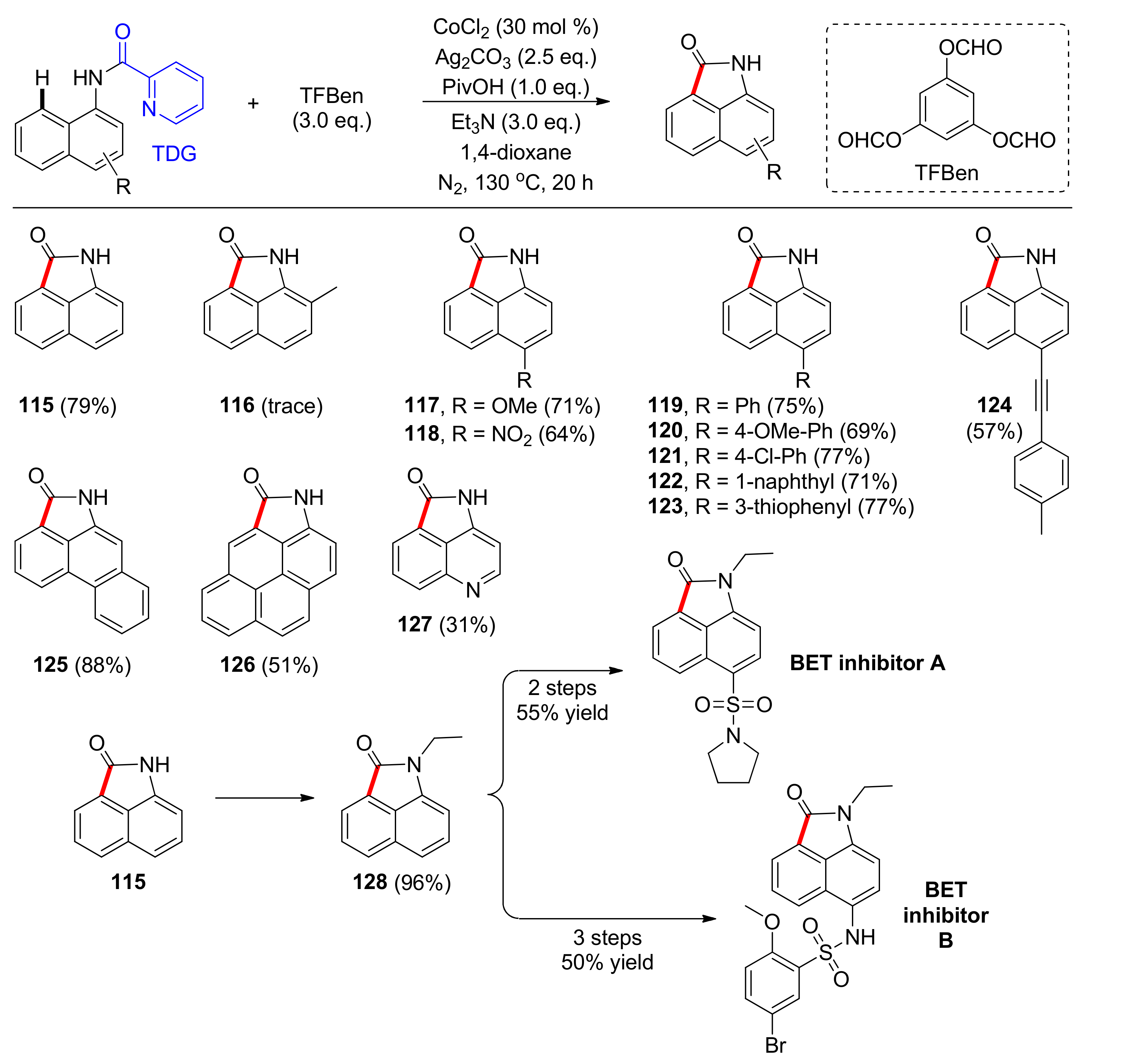
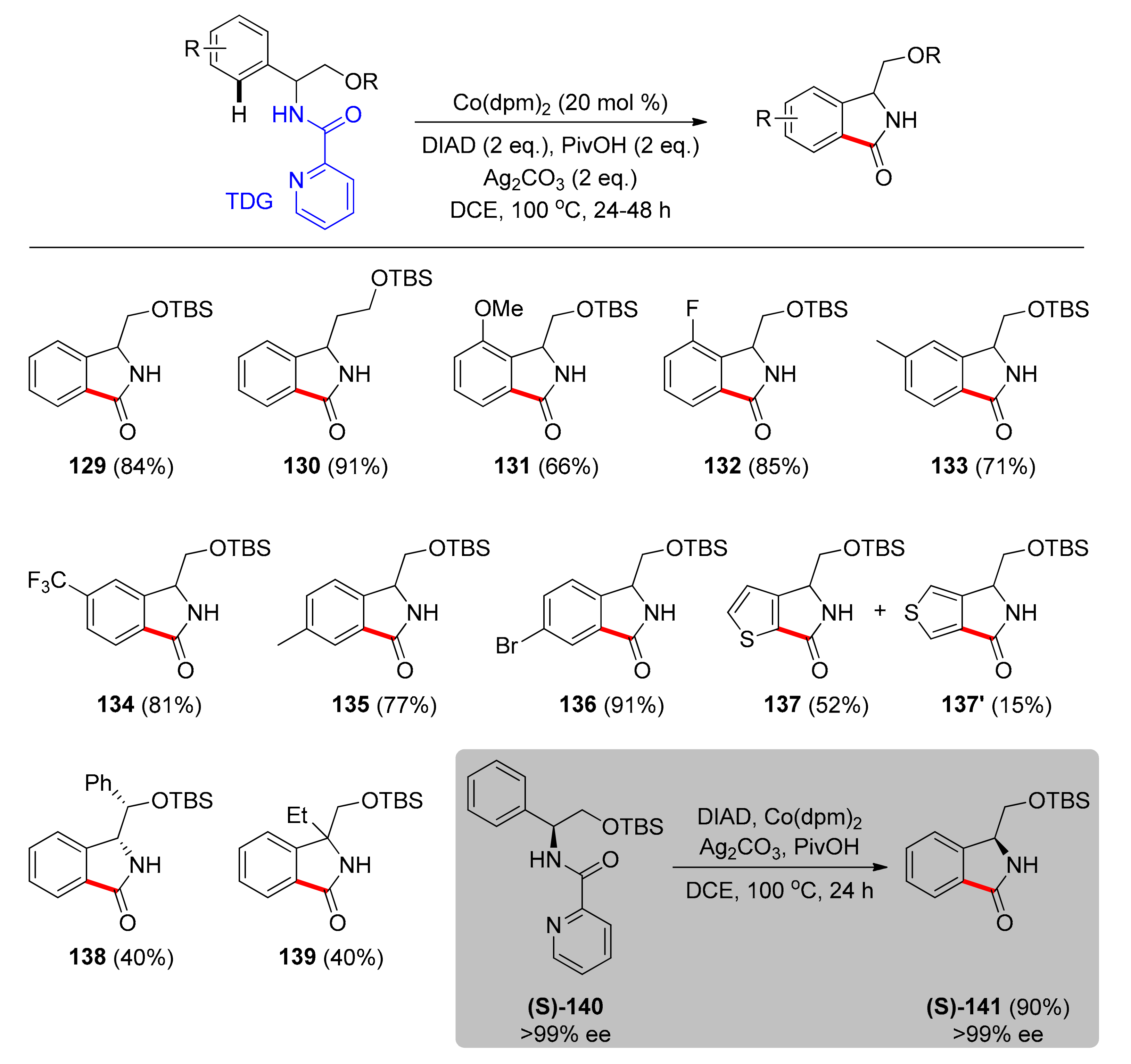
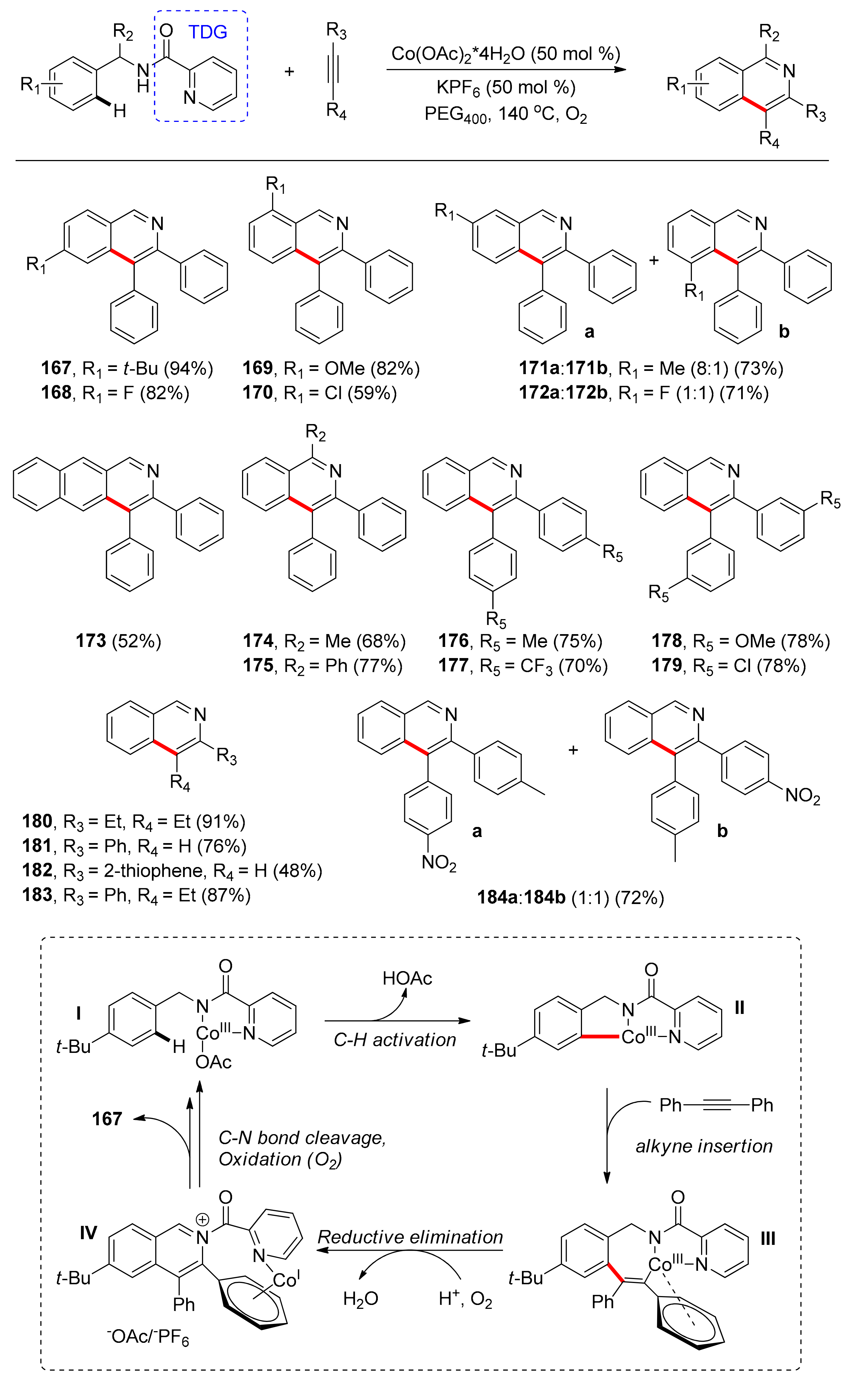
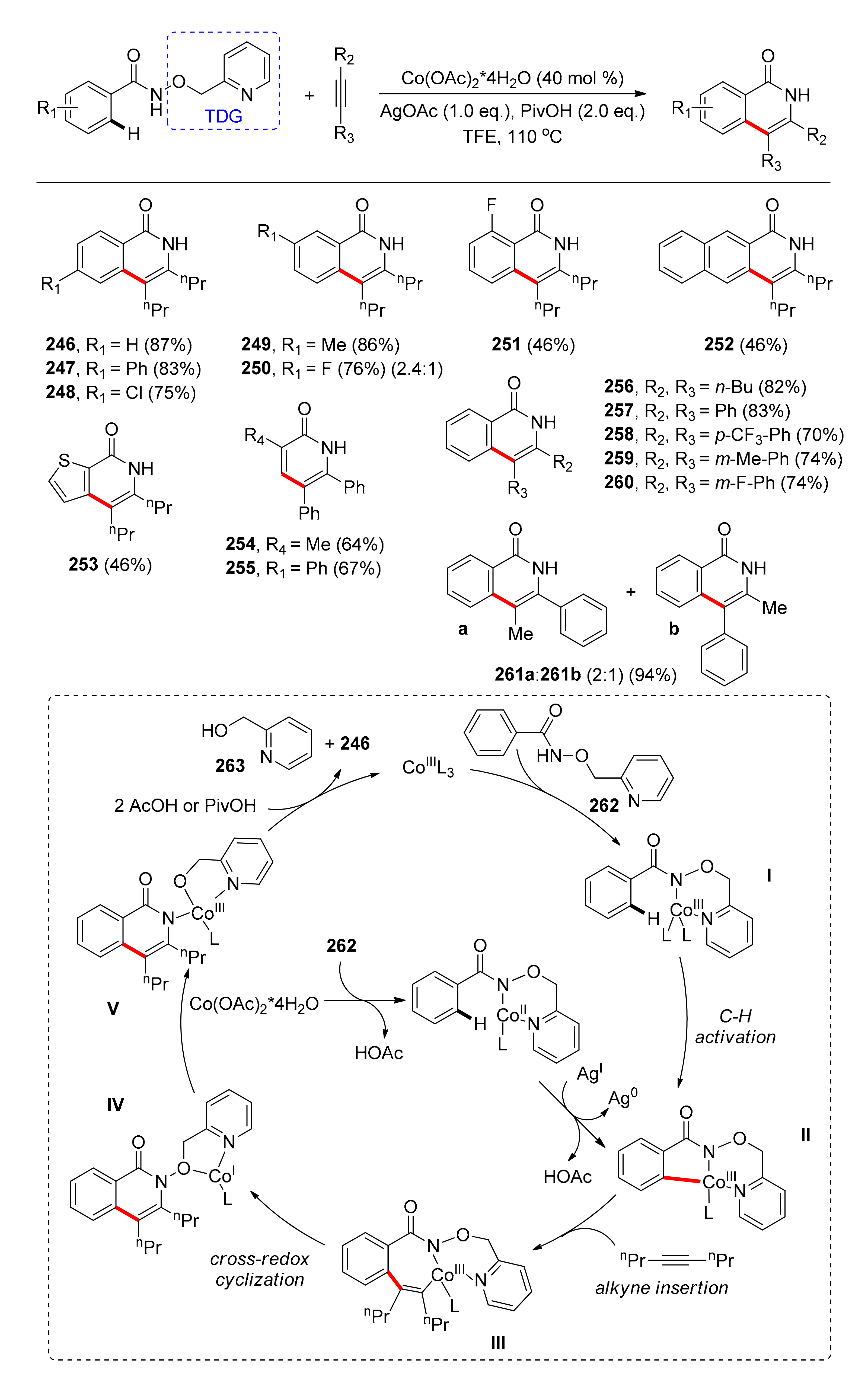
4.1. C–H Carbonylation with TDGs
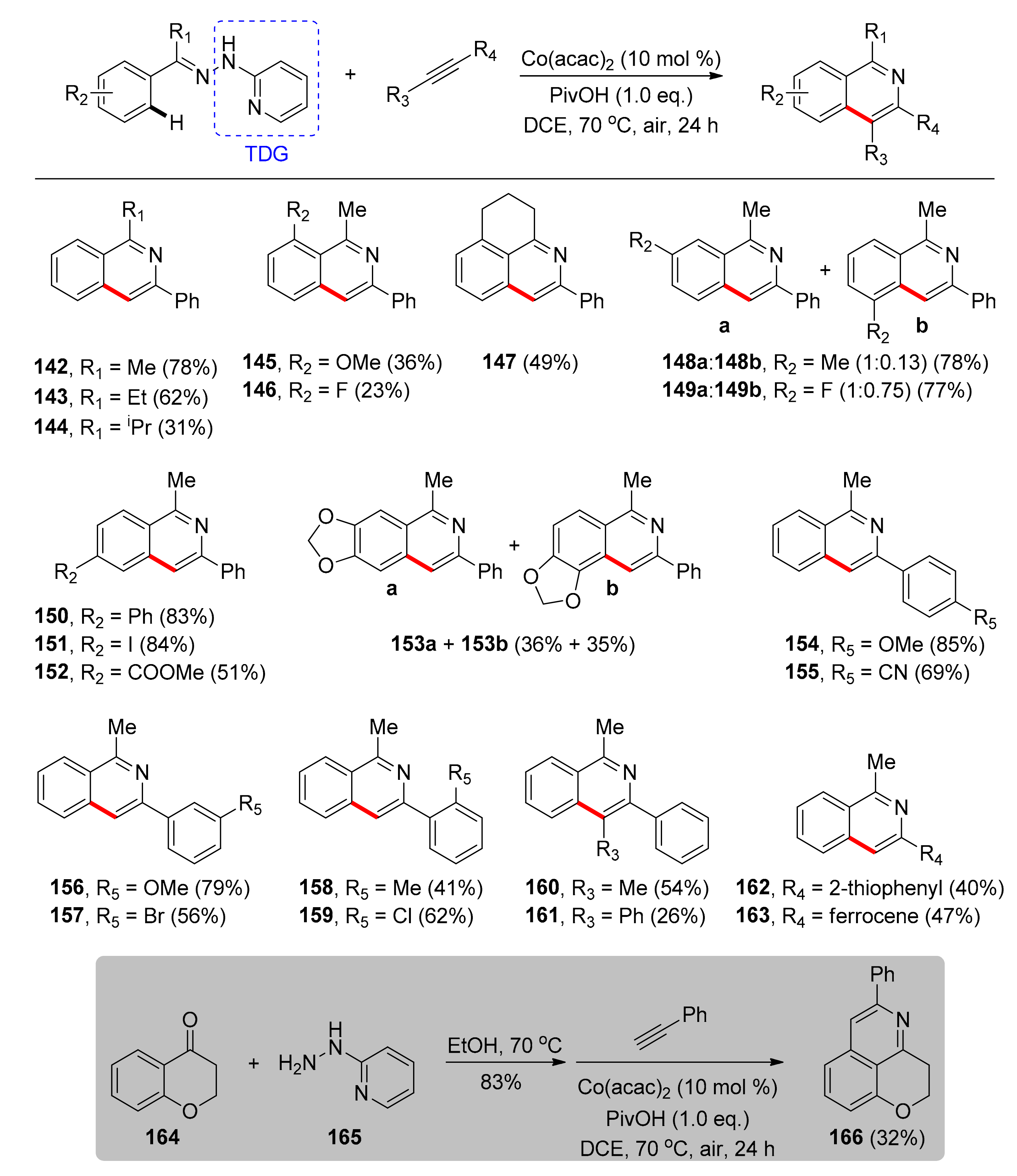
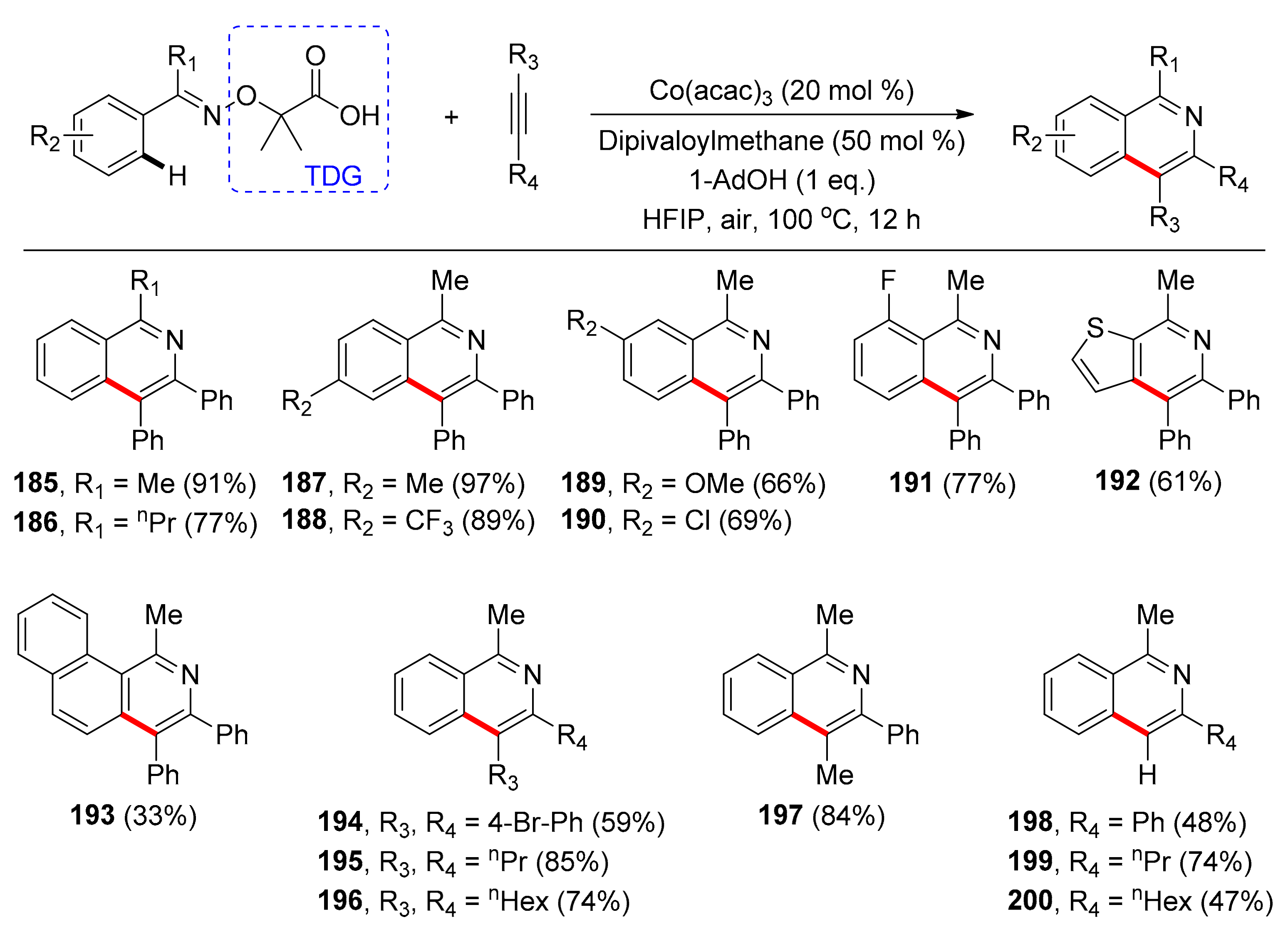
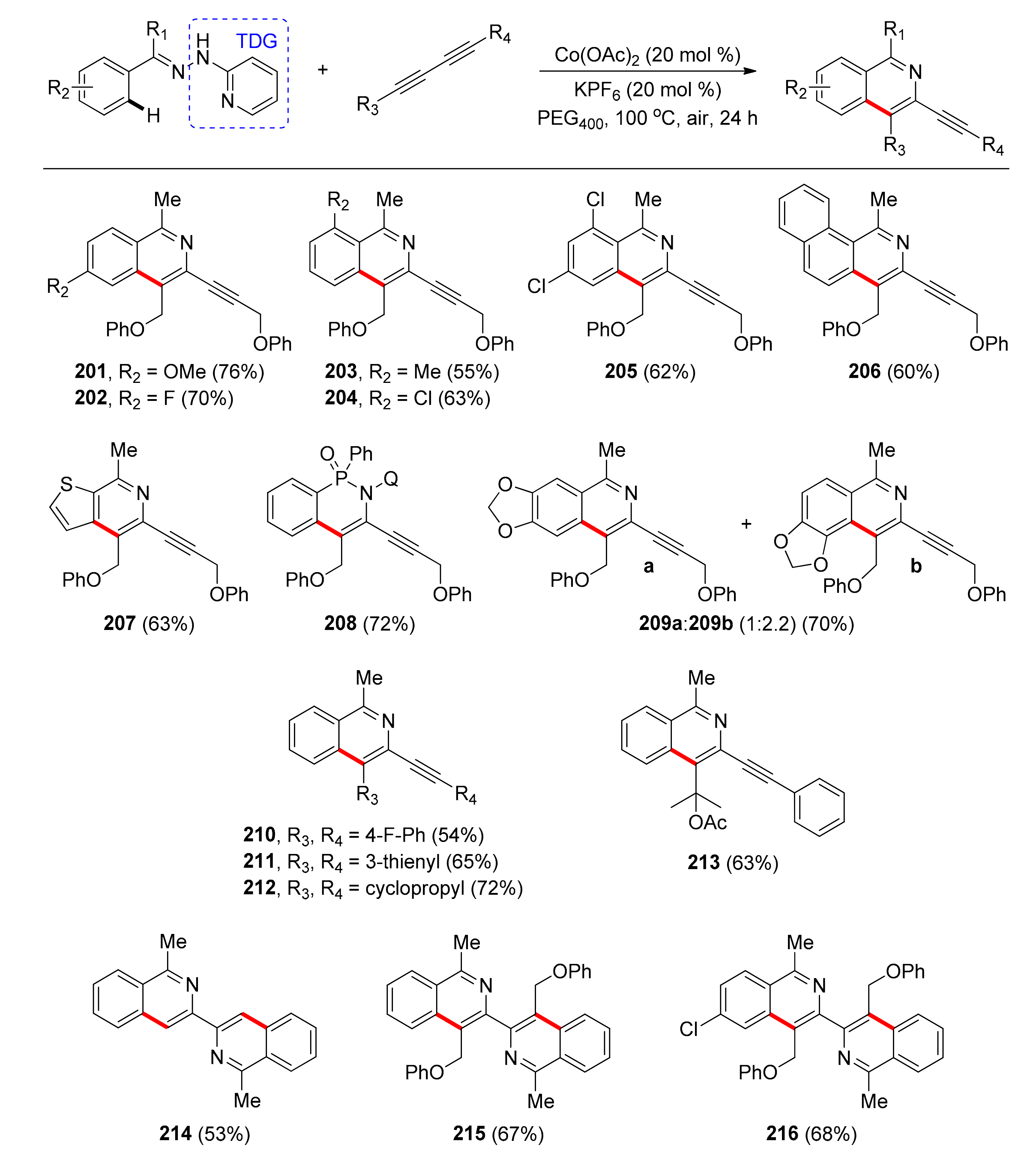
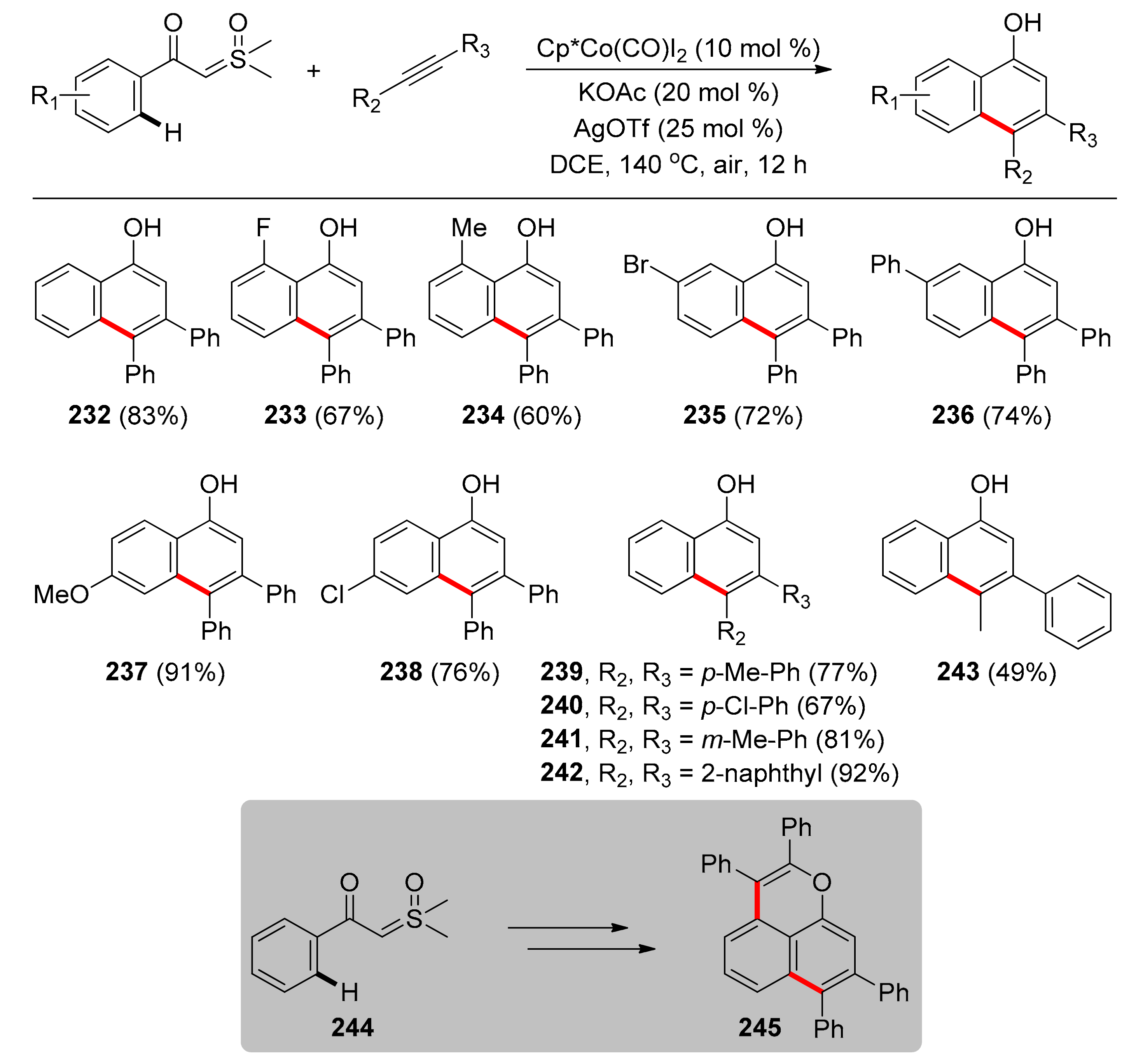
4.2. Synthesis of Isoquinolines with TDGs
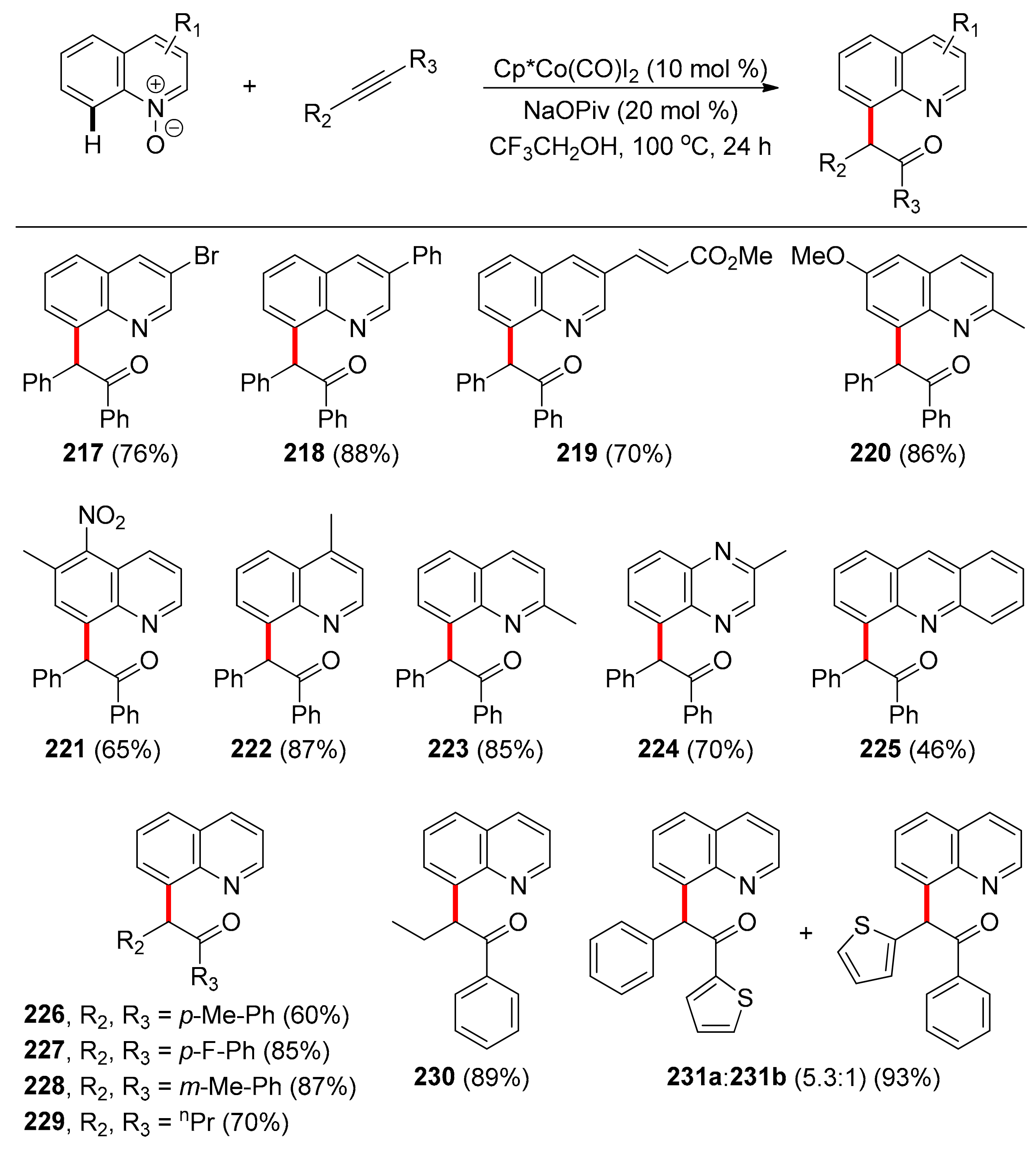
4.3. Other TDG-Assisted Transformations
5. Nickel
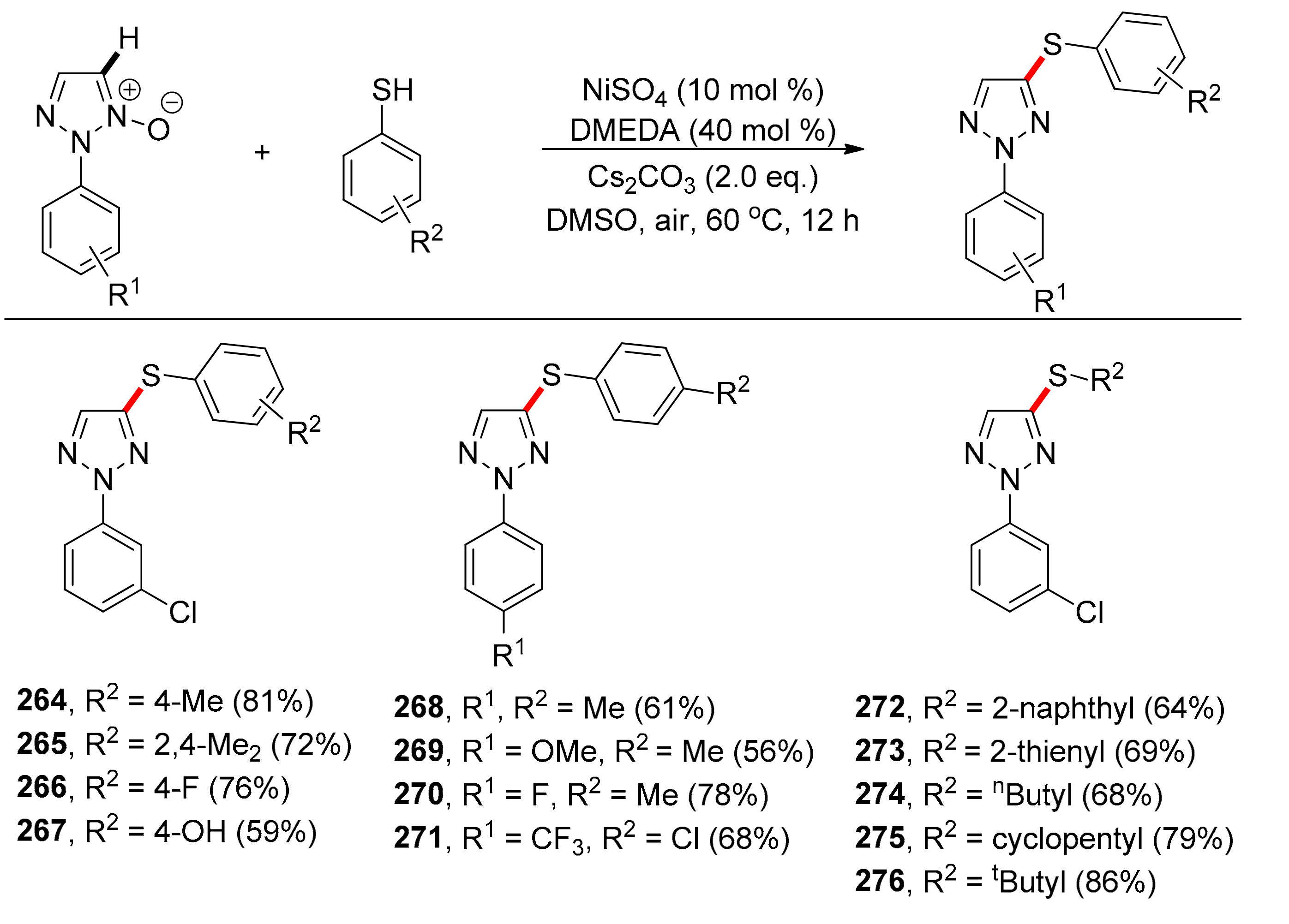
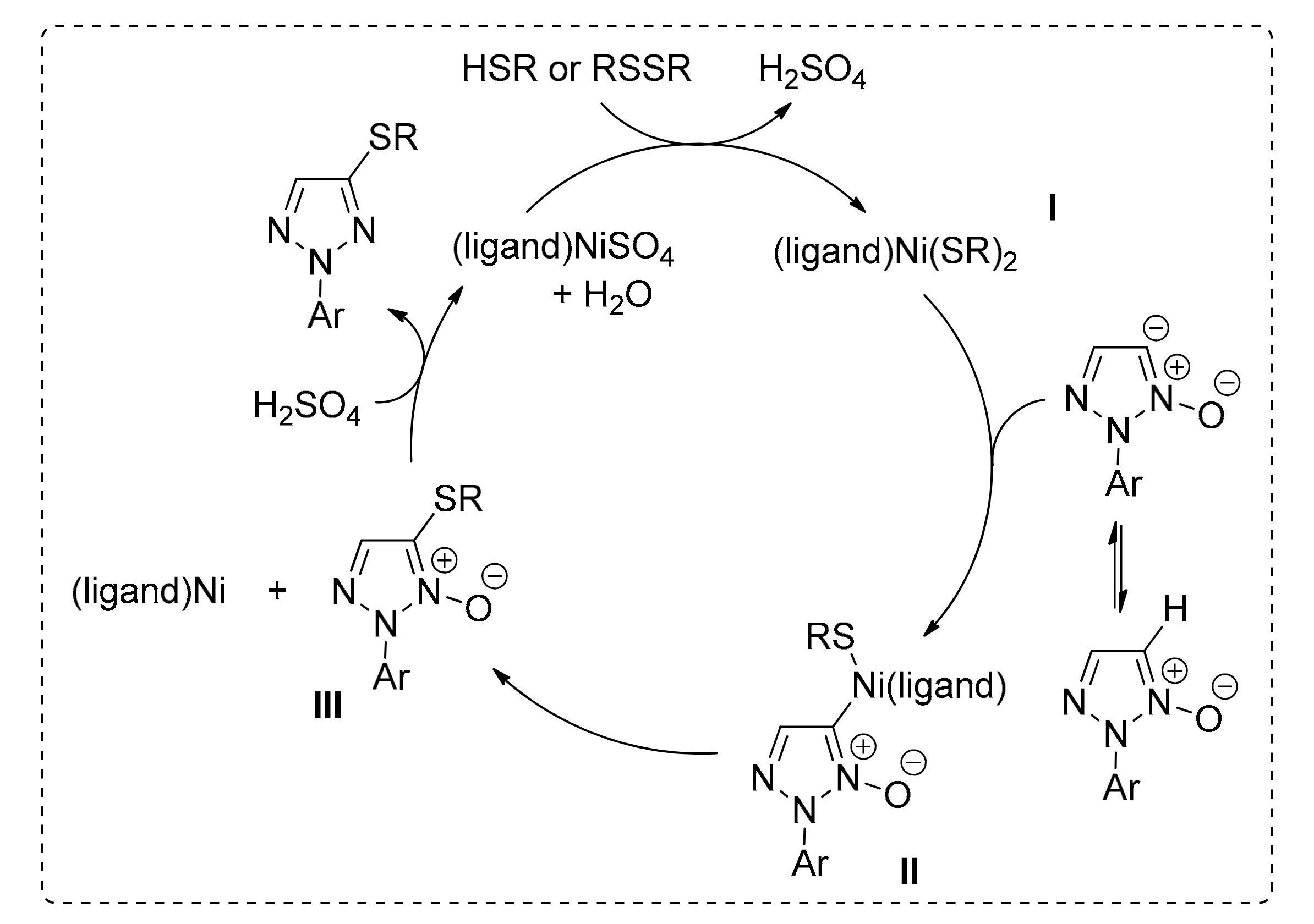
Thiolation via C(sp2)–H Bond Activation
6. Copper
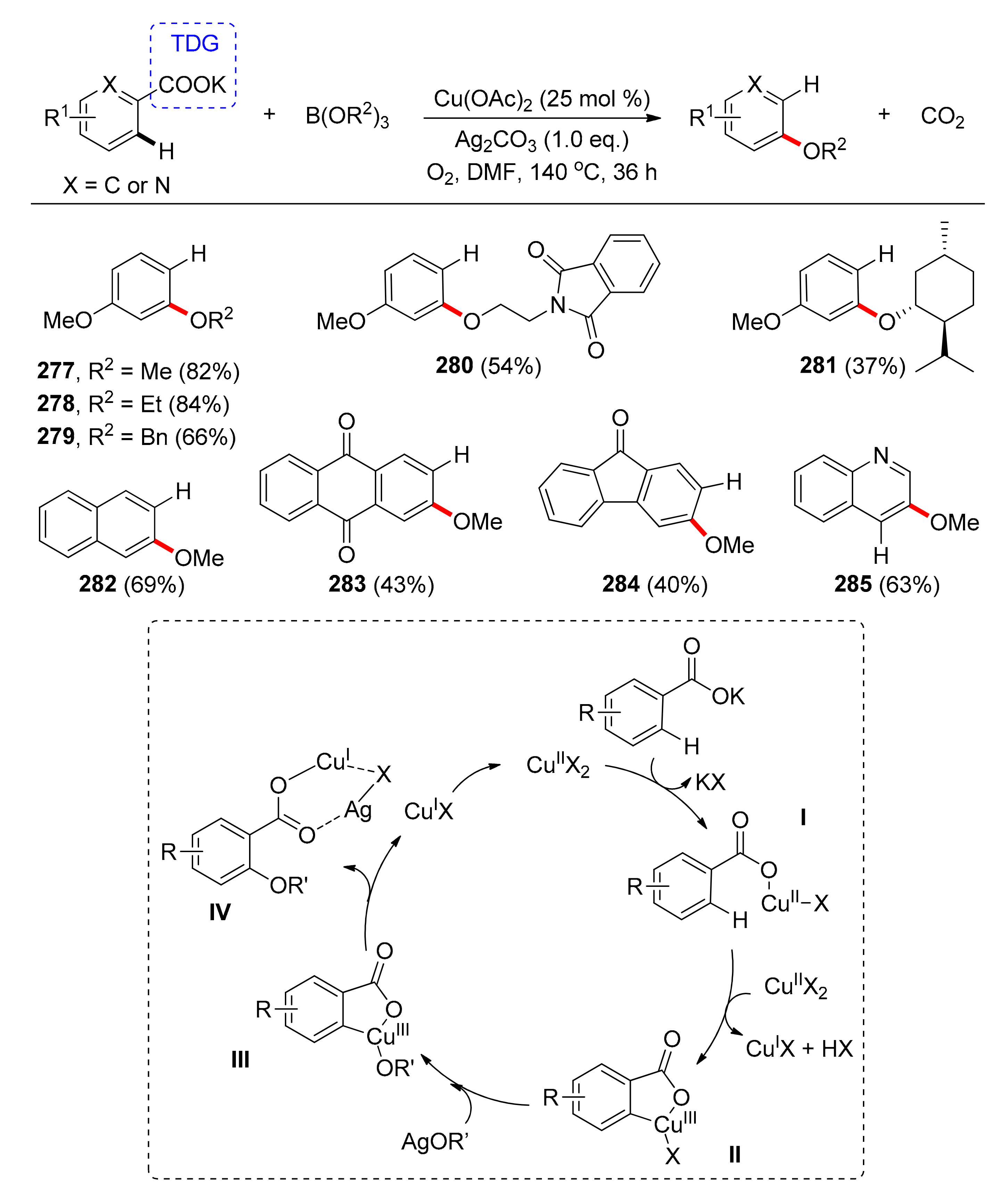
6.1. Etheration via C(sp2)–H Bond Activation
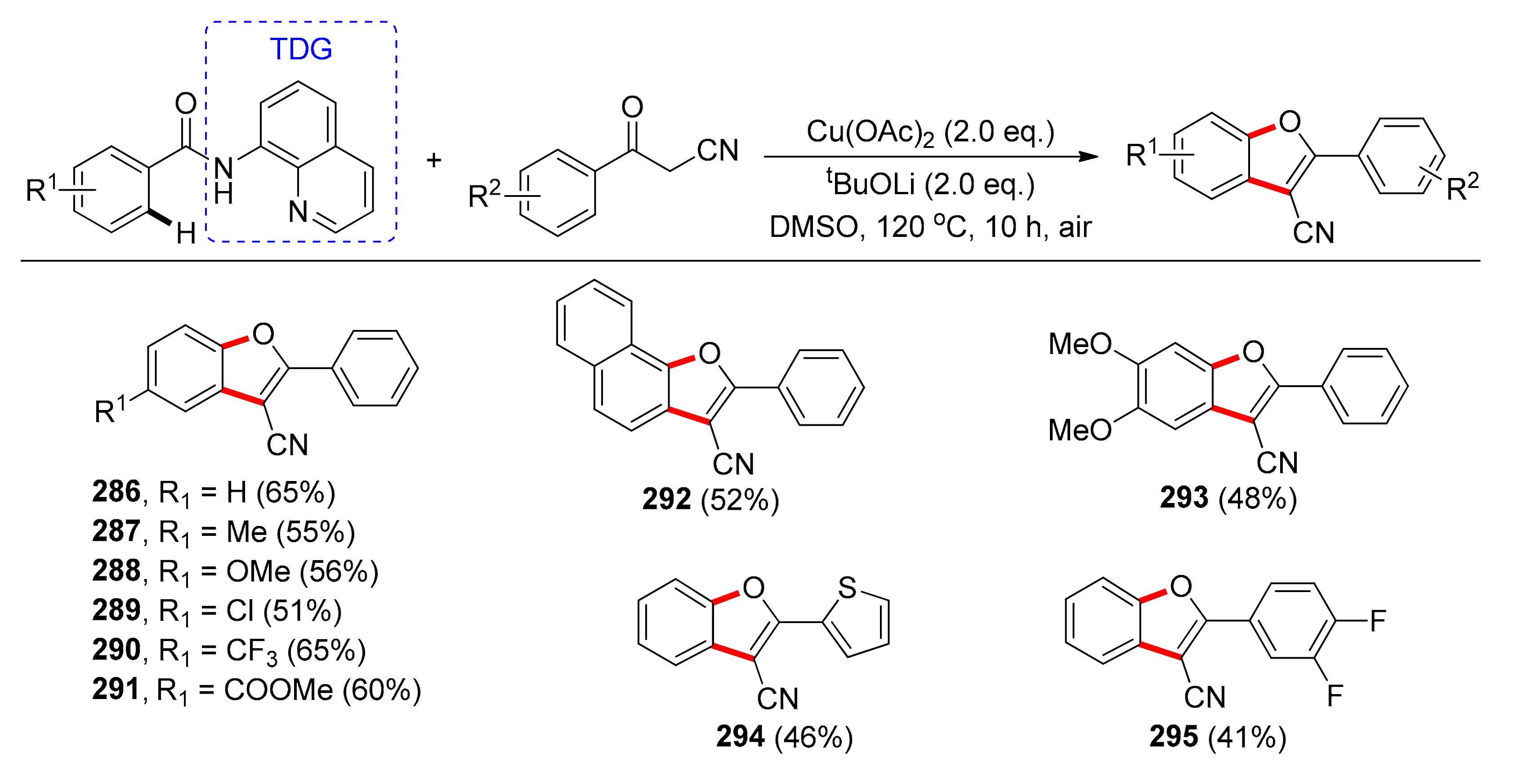
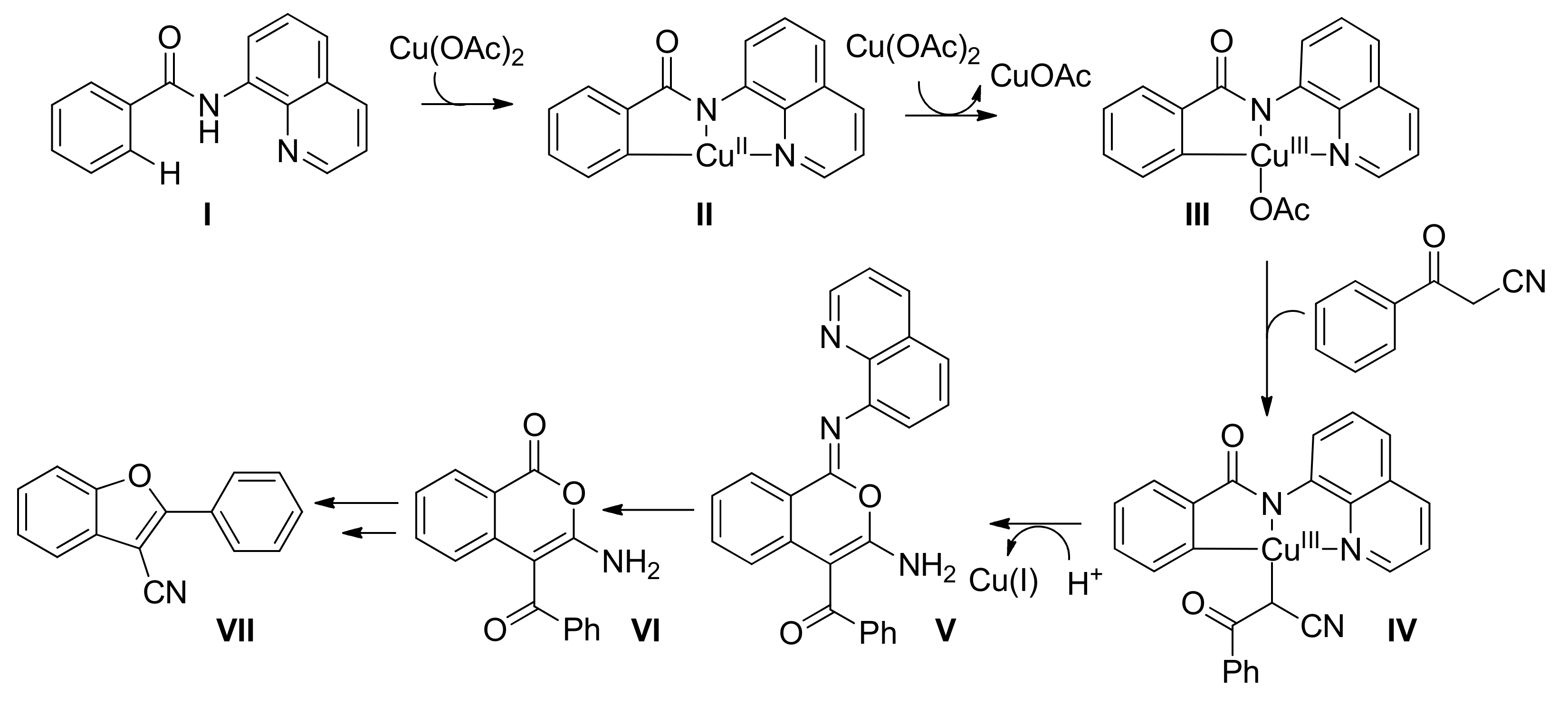
6.2. Benzofuran Synthesis via C(sp2)–H Bond Activation
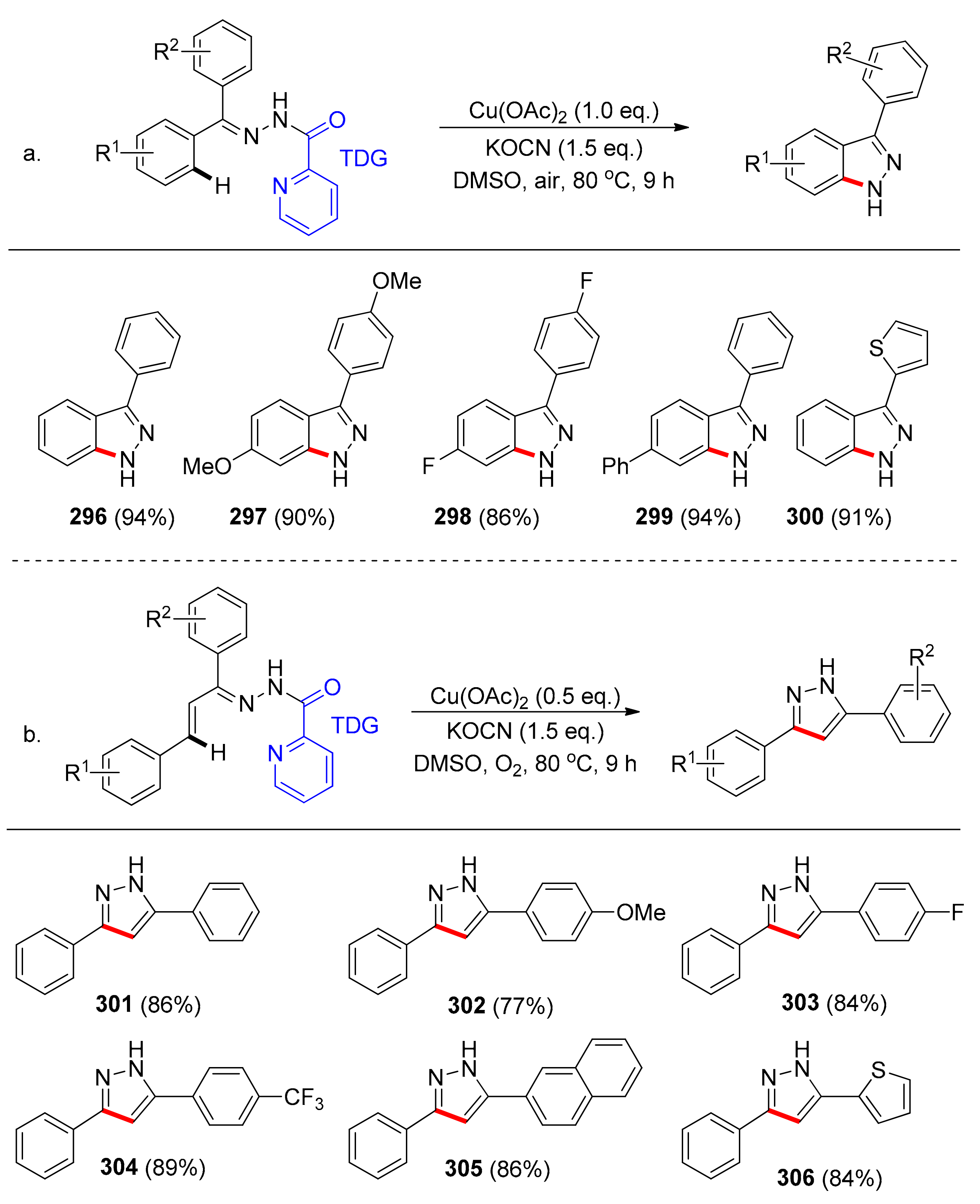
6.3. Amination via C(sp2)–H Bond Activation
7. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jenck, J.F.; Agterberg, F.; Droescher, M.J. Products and Processes for a Sustainable Chemical Industry: A Review of Achievements and Prospects. Green Chem. 2004, 6, 544–556. [Google Scholar] [CrossRef]
- Poliakoff, M.; Fitzpatrick, J.M.; Farren, T.R.; Anastas, P.T. Green Chemistry: Science and Politics of Change. Science 2002, 297, 807–810. [Google Scholar] [CrossRef]
- Armor, J.N. A History of Industrial Catalysis. Catal. Today 2011, 163, 3–9. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Zimmerman, J.B. Design through the 12 Principles of Green Engineering. Environ. Sci. Technol. 2003, 37, 94–101. [Google Scholar] [CrossRef]
- Turner, J.A. A Realizable Renewable Energy Future. Science 1999, 285, 687. [Google Scholar] [CrossRef] [PubMed]
- Styring, S. Artificial Photosynthesis for Solar Fuels. Faraday Discuss. 2012, 155, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Saeedmanesh, A.; Mac Kinnon, M.A.; Brouwer, J. Hydrogen Is Essential for Sustainability. Curr. Opin. Electrochem. 2018, 12, 166–181. [Google Scholar] [CrossRef]
- Frontana-Uribe, B.A.; Little, R.D.; Ibanez, J.G.; Palma, A.; Vasquez-Medrano, R. Organic Electrosynthesis: A Promising Green Methodology in Organic Chemistry. Green Chem. 2010, 12, 2099–2119. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the Planet: Chemical Challenges in Solar Energy Utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729. [Google Scholar] [CrossRef]
- Behr, A.; Neubert, P. Applied Homogeneous Catalysis; John Wiley & Sons: Weinheim, Germany, 2012; ISBN 3-527-32641-3. [Google Scholar]
- Lersch, M.; Tilset, M. Mechanistic Aspects of C−H Activation by Pt Complexes. Chem. Rev. 2005, 105, 2471–2526. [Google Scholar] [CrossRef] [PubMed]
- Mkhalid, I.A.I.; Barnard, J.H.; Marder, T.B.; Murphy, J.M.; Hartwig, J.F. C-H Activation for the Construction of C-B Bonds. Chem. Rev. 2010, 110, 890–931. [Google Scholar] [CrossRef]
- Satoh, T.; Miura, M. Oxidative Coupling of Aromatic Substrates with Alkynes and Alkenes under Rhodium Catalysis. Chem. Eur. J. 2010, 16, 11212–11222. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C-H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef]
- Kuninobu, Y.; Takai, K. Organic Reactions Catalyzed by Rhenium Carbonyl Complexes. Chem. Rev. 2011, 111, 1938–1953. [Google Scholar] [CrossRef]
- Arockiam, P.B.; Bruneau, C.; Dixneuf, P.H. Ruthenium(II)-Catalyzed C-H Bond Activation and Functionalization. Chem. Rev. 2012, 112, 5879–5918. [Google Scholar] [CrossRef]
- Rao, Y.; Shan, G.; Yang, X. Some Recent Advances in Transition-Metal-Catalyzed Ortho SP2 C-H Functionalization Using Ru, Rh, and Pd. Sci. China Chem. 2014, 57, 930–944. [Google Scholar] [CrossRef]
- Motevalli, S.; Sokeirik, Y.; Ghanem, A. Rhodium-Catalysed Enantioselective C-H Functionalization in Asymmetric Synthesis. Eur. J. Org. Chem. 2016, 2016, 1459–1475. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef]
- Bullock, R.M. Catalysis without Precious Metals; Wiley: Weinheim, Germany, 2011; ISBN 978-3-527-63240-4. [Google Scholar]
- Clavier, H.; Grela, K.; Kirschning, A.; Mauduit, M.; Nolan, S.P. Sustainable Concepts in Olefin Metathesis. Angew. Chem. Int. Ed. 2007, 46, 6786–6801. [Google Scholar] [CrossRef] [PubMed]
- Vougioukalakis, G.C. Removing Ruthenium Residues from Olefin Metathesis Reaction Products. Chem. Eur. J. 2012, 18, 8868–8880. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, T.; Baran, P.S.; Hoffmann, R.W. The Economies of Synthesis. Chem. Soc. Rev. 2009, 38, 3010–3021. [Google Scholar] [CrossRef]
- Gaich, T.; Baran, P.S. Aiming for the Ideal Synthesis. J. Org. Chem. 2010, 75, 4657–4673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cue, B.W. Green Techniques for Organic Synthesis and Medicinal Chemistry, 2nd ed.; Wiley: Hoboken, NJ, USA, 2018; ISBN 978-1-119-28817-6. [Google Scholar]
- Caro-Diaz, E.J.E.; Urbano, M.; Buzard, D.J.; Jones, R.M. C–H Activation Reactions as Useful Tools for Medicinal Chemists. Bioorg. Med. Chem. Lett. 2016, 26, 5378–5383. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, T.; Kang, D.; Zhang, J.; Song, Y.; Namasivayam, V.; Kongsted, J.; Pannecouque, C.; De Clercq, E.; Poongavanam, V.; et al. Overview of Recent Strategic Advances in Medicinal Chemistry. J. Med. Chem. 2019, 62, 9375–9414. [Google Scholar] [CrossRef]
- Abrams, D.J.; Provencher, P.A.; Sorensen, E.J. Recent Applications of C–H Functionalization in Complex Natural Product Synthesis. Chem. Soc. Rev. 2018, 47, 8925–8967. [Google Scholar] [CrossRef]
- Choi, J.; Wang, D.Y.; Kundu, S.; Choliy, Y.; Emge, T.J.; Krogh-Jespersen, K.; Goldman, A.S. Net Oxidative Addition of C(sp3)-F Bonds to Iridium via Initial C-H Bond Activation. Science 2011, 332, 1545–1548. [Google Scholar] [CrossRef]
- Wu, X.F. Transition Metal.-Catalyzed Heterocycle Synthesis via C-H Activation; Wiley: Weinheim, Germany, 2016; ISBN 978-3-527-33888-7. [Google Scholar]
- Prendergast, A.M.; McGlacken, G.P. Transition Metal Mediated C–H Activation of 2-Pyrones, 2-Pyridones, 2-Coumarins and 2-Quinolones. Eur. J. Org. Chem. 2018, 2018, 6068–6082. [Google Scholar] [CrossRef]
- Cano, R.; Mackey, K.; McGlacken, G.P. Recent Advances in Manganese-Catalysed C-H Activation: Scope and Mechanism. Catal. Sci. Technol. 2018, 8, 1251–1266. [Google Scholar] [CrossRef]
- Vásquez-Céspedes, S.; Wang, X.; Glorius, F. Plausible Rh(V) Intermediates in Catalytic C-H Activation Reactions. ACS Catal. 2018, 8, 242–257. [Google Scholar] [CrossRef]
- Gensch, T.; James, M.J.; Dalton, T.; Glorius, F. Increasing Catalyst Efficiency in C−H Activation Catalysis. Angew. Chem. Int. Ed. 2018, 57, 2296–2306. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Pombeiro, A.J.L. Homo- and Heterometallic Polynuclear Transition Metal Catalysts for Alkane C–H Bonds Oxidative Functionalization: Recent Advances. Coord. Chem. Rev. 2018, 355, 199–222. [Google Scholar] [CrossRef]
- Sauermann, N.; Meyer, T.H.; Qiu, Y.; Ackermann, L. Electrocatalytic C–H Activation. ACS Catal. 2018, 8, 7086–7103. [Google Scholar] [CrossRef]
- Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C-H Activation. Chem. Rev. 2019, 119, 2192–2452. [Google Scholar] [CrossRef] [PubMed]
- Tzouras, N.V.; Stamatopoulos, I.K.; Papastavrou, A.T.; Liori, A.A.; Vougioukalakis, G.C. Sustainable Metal Catalysis in CH Activation. Coord. Chem. Rev. 2017, 343, 25–138. [Google Scholar] [CrossRef]
- Trowbridge, A.; Walton, S.M.; Gaunt, M.J. New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev. 2020, 120, 2613–2692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Poisson, T.; Pannecoucke, X.; Besset, T. The Transient Directing Group Strategy: A New Trend in Transition-Metal-Catalyzed C–H Bond Functionalization. Synthesis 2017, 49, 4808–4826. [Google Scholar]
- Chen, Z.; Wang, B.; Zhang, J.; Yu, W.; Liu, Z.; Zhang, Y. Transition Metal-Catalyzed C–H Bond Functionalizations by the Use of Diverse Directing Groups. Org. Chem. Front. 2015, 2, 1107–1295. [Google Scholar] [CrossRef]
- Ujwaldev, S.M.; Harry, N.A.; Divakar, M.A.; Anilkumar, G. Cobalt-Catalyzed C–H Activation: Recent Progress in Heterocyclic Chemistry. Catal. Sci. Technol. 2018, 8, 5983–6018. [Google Scholar] [CrossRef]
- Prakash, S.; Kuppusamy, R.; Cheng, C.-H. Cobalt-Catalyzed Annulation Reactions via C−H Bond Activation. ChemCatChem 2018, 10, 683–705. [Google Scholar] [CrossRef]
- Liu, M.; Niu, J.-L.; Yang, D.; Song, M.-P. Development of a Traceless Directing Group: Cp*-Free Cobalt-Catalyzed C–H Activation/Annulations to Access Isoquinolinones. J. Org. Chem. 2020, 85, 4067–4078. [Google Scholar] [CrossRef]
- Kommagalla, Y.; Chatani, N. Cobalt(II)-Catalyzed CH Functionalization Using an N,N′-Bidentate Directing Group. Coord. Chem. Rev. 2017, 350, 117–135. [Google Scholar] [CrossRef]
- Dutta, C.; Choudhury, J. C–H Activation-Annulation on the N-Heterocyclic Carbene Platform. RSC Adv. 2018, 8, 27881–27891. [Google Scholar] [CrossRef]
- Martínez de Salinas, S.; Sanjosé-Orduna, J.; Odena, C.; Barranco, S.; Benet-Buchholz, J.; Pérez-Temprano, M.H. Weakly Coordinated Cobaltacycles: Trapping Catalytically Competent Intermediates in Cp*CoIII Catalysis. Angew. Chem. Int. Ed. 2020, 59, 6239–6243. [Google Scholar] [CrossRef] [PubMed]
- Sanjosé-Orduna, J.; Benet-Buchholz, J.; Pérez-Temprano, M.H. Unravelling Molecular Aspects of the Migratory Insertion Step in Cp*CoIII Metallacyclic Systems. Inorg. Chem. 2019, 58, 10569–10577. [Google Scholar] [CrossRef]
- Sanjosé-Orduna, J.; Mudarra, Á.L.; Martínez de Salinas, S.; Pérez-Temprano, M.H. Sustainable Knowledge-Driven Approaches in Transition-Metal-Catalyzed Transformations. ChemSusChem 2019, 12, 2882–2897. [Google Scholar] [CrossRef] [PubMed]
- Sanjosé-Orduna, J.; Gallego, D.; Garcia-Roca, A.; Martin, E.; Benet-Buchholz, J.; Pérez-Temprano, M.H. Capturing Elusive Cobaltacycle Intermediates: A Real-Time Snapshot of the Cp*CoIII-Catalyzed Oxidative Alkyne Annulation. Angew. Chem. Int. Ed. 2017, 56, 12137–12141. [Google Scholar] [CrossRef] [PubMed]
- Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; et al. A Comprehensive Overview of Directing Groups Applied in Metal-Catalysed C–H Functionalisation Chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [Google Scholar] [CrossRef]
- Rasheed, O.K.; Sun, B. Advances in Development of C–H Activation/Functionalization Using a Catalytic Directing Group. ChemistrySelect 2018, 3, 5689–5708. [Google Scholar] [CrossRef]
- Zhang, F.; Spring, D.R. Arene C–H Functionalisation Using a Removable/Modifiable or a Traceless Directing Group Strategy. Chem. Soc. Rev. 2014, 43, 6906–6919. [Google Scholar] [CrossRef]
- Wei, D.; Zhu, X.; Niu, J.-L.; Song, M.-P. High-Valent-Cobalt-Catalyzed C−H Functionalization Based on Concerted Metalation–Deprotonation and Single-Electron-Transfer Mechanisms. ChemCatChem 2016, 8, 1242–1263. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Gurbanov, A.V.; Guseinov, F.I.; Guedes da Silva, M.F.C. Noncovalent Interactions in Metal Complex Catalysis. Coord. Chem. Rev. 2019, 387, 32–46. [Google Scholar] [CrossRef]
- Rej, S.; Chatani, N. Rhodium-Catalyzed C(sp2)- or C(sp3)−H Bond Functionalization Assisted by Removable Directing Groups. Angew. Chem. Int. Ed. 2019, 58, 8304–8329. [Google Scholar] [CrossRef]
- Gandeepan, P.; Ackermann, L. Transient Directing Groups for Transformative C–H Activation by Synergistic Metal Catalysis. Chem 2018, 4, 199–222. [Google Scholar] [CrossRef]
- De Sarkar, S.; Liu, W.; Kozhushkov, S.I.; Ackermann, L. Weakly Coordinating Directing Groups for Ruthenium(II)-Catalyzed C-H Activation. Adv. Synth. Catal. 2014, 356, 1461–1479. [Google Scholar] [CrossRef]
- Ellman, J.A.; Ackermann, L.; Shi, B.-F. The Breadth and Depth of C–H Functionalization. J. Org. Chem. 2019, 84, 12701–12704. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L. Robust Ruthenium(II)-Catalyzed C-H Arylations: Carboxylate Assistance for the Efficient Synthesis of Angiotensin-II-Receptor Blockers. Org. Process. Res. Dev. 2015, 19, 260–269. [Google Scholar] [CrossRef]
- Ma, W.; Gandeepan, P.; Li, J.; Ackermann, L. Recent Advances in Positional-Selective Alkenylations: Removable Guidance for Twofold C-H Activation. Org. Chem. Front. 2017, 4, 1435–1467. [Google Scholar] [CrossRef]
- Ma, W.; Kaplaneris, N.; Fang, X.; Gu, L.; Mei, R.; Ackermann, L. Chelation-Assisted Transition Metal-Catalysed C–H Chalcogenylations. Org. Chem. Front. 2020, 7, 1022–1060. [Google Scholar] [CrossRef]
- Rej, S.; Ano, Y.; Chatani, N. Bidentate Directing Groups: An Efficient Tool in C–H Bond Functionalization Chemistry for the Expedient Construction of C–C Bonds. Chem. Rev. 2020, 120, 1788–1887. [Google Scholar] [CrossRef]
- Pichette Drapeau, M.; Gooßen, L.J. Carboxylic Acids as Directing Groups for C−H Bond Functionalization. Chem. A Eur. J. 2016, 22, 18654–18677. [Google Scholar] [CrossRef] [PubMed]
- Font, M.; Quibell, J.M.; Perry, G.J.P.; Larrosa, I. The Use of Carboxylic Acids as Traceless Directing Groups for Regioselective C–H Bond Functionalisation. Chem. Commun. 2017, 53, 5584–5597. [Google Scholar] [CrossRef] [PubMed]
- Pinaka, A.; Vougioukalakis, G.C. Using Sustainable Metals to Carry out “Green” Transformations: Fe- and Cu-Catalyzed CO2 Monetization. Coord. Chem. Rev. 2015, 288, 69–97. [Google Scholar] [CrossRef]
- Liori, A.A.; Stamatopoulos, I.K.; Papastavrou, A.T.; Pinaka, A.; Vougioukalakis, G.C. A Sustainable, User-Friendly Protocol for the Pd-Free Sonogashira Coupling Reaction. Eur. J. Org. Chem. 2018, 2018, 6134–6139. [Google Scholar] [CrossRef]
- Papastavrou, A.T.; Pauze, M.; Gómez-Bengoa, E.; Vougioukalakis, G.C. Unprecedented Multicomponent Organocatalytic Synthesis of Propargylic Esters via CO2 Activation. ChemCatChem 2019, 11, 5379–5386. [Google Scholar] [CrossRef]
- Voutyritsa, E.; Triandafillidi, I.; Tzouras, N.V.; Nikitas, N.F.; Pefkianakis, E.K.; Vougioukalakis, G.C.; Kokotos, C.G. Photocatalytic Atom Transfer Radical Addition to Olefins Utilizing Novel Photocatalysts. Molecules 2019, 24, 1644. [Google Scholar] [CrossRef] [PubMed]
- Milenković, M.R.; Papastavrou, A.T.; Radanović, D.; Pevec, A.; Jagličić, Z.; Zlatar, M.; Gruden, M.; Vougioukalakis, G.C.; Turel, I.; Anđelković, K.; et al. Highly-Efficient N-Arylation of Imidazole Catalyzed by Cu(II) Complexes with Quaternary Ammonium-Functionalized 2-Acetylpyridine Acylhydrazone. Polyhedron 2019, 165, 22–30. [Google Scholar] [CrossRef]
- Tzouras, N.V.; Neofotistos, S.P.; Vougioukalakis, G.C. Zn-Catalyzed Multicomponent KA2 Coupling: One-Pot Assembly of Propargylamines Bearing Tetrasubstituted Carbon Centers. ACS Omega 2019, 4, 10279–10292. [Google Scholar] [CrossRef]
- Zorba, L.P.; Vougioukalakis, G.C. The Ketone-Amine-Alkyne (KA2) Coupling Reaction: Transition Metal-Catalyzed Synthesis of Quaternary Propargylamines. Coord. Chem. Rev. 2021, 429, 213603. [Google Scholar] [CrossRef]
- Adejumo, T.T.; Tzouras, N.V.; Zorba, L.P.; Radanović, D.; Pevec, A.; Grubišić, S.; Mitić, D.; Anđelković, K.K.; Vougioukalakis, G.C.; Čobeljić, B.; et al. Synthesis, Characterization, Catalytic Activity, and DFT Calculations of Zn(II) Hydrazone Complexes. Molecules 2020, 25, 4043. [Google Scholar] [CrossRef]
- Neofotistos, S.P.; Tzouras, N.V.; Pauze, M.; Gómez-Bengoa, E.; Vougioukalakis, G.C. Manganese-Catalyzed Multicomponent Synthesis of Tetrasubstituted Propargylamines: System Development and Theoretical Study. Adv. Synth. Catal. 2020, 362, 3872–3885. [Google Scholar] [CrossRef]
- Kuninobu, Y.; Nishina, Y.; Takeuchi, T.; Takai, K. Manganese-Catalyzed Insertion of Aldehydes into a C-H Bond. Angew. Chem. Int. Ed. 2007, 46, 6518–6520. [Google Scholar] [CrossRef]
- Kuninobu, Y.; Fujii, Y.; Matsuki, T.; Nishina, Y.; Takai, K. Rhenium-Catalyzed Insertion of Nonpolar and Polar Unsaturated Molecules into an Olefinic C−H Bond. Org. Lett. 2009, 11, 2711–2714. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Z.; Fukuzumi, S.; Nam, W.; Wang, B. Artificial Nonheme Iron and Manganese Oxygenases for Enantioselective Olefin Epoxidation and Alkane Hydroxylation Reactions. Coord. Chem. Rev. 2020, 421, 213443. [Google Scholar] [CrossRef]
- Chandra, P.; Ghosh, T.; Choudhary, N.; Mohammad, A.; Mobin, S.M. Recent Advancement in Oxidation or Acceptorless Dehydrogenation of Alcohols to Valorised Products Using Manganese Based Catalysts. Coord. Chem. Rev. 2020, 411, 213241. [Google Scholar] [CrossRef]
- Liu, W.; Ackermann, L. Manganese-Catalyzed C–H Activation. ACS Catal. 2016, 6, 3743–3752. [Google Scholar] [CrossRef]
- Wang, C. Manganese-Mediated C–C Bond Formation via C–H Activation: From Stoichiometry to Catalysis. Synlett 2013, 24, 1606–1613. [Google Scholar] [CrossRef]
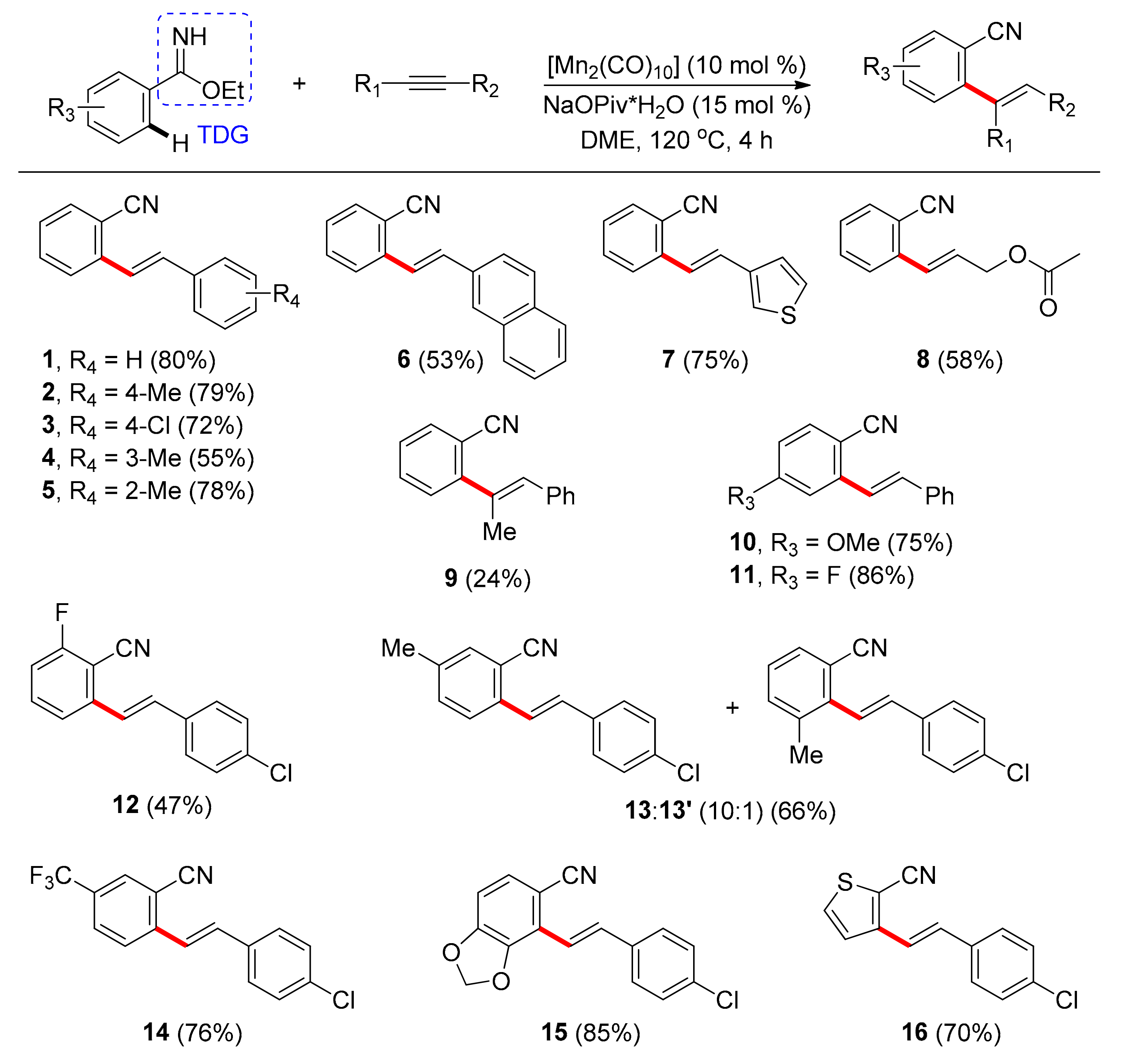
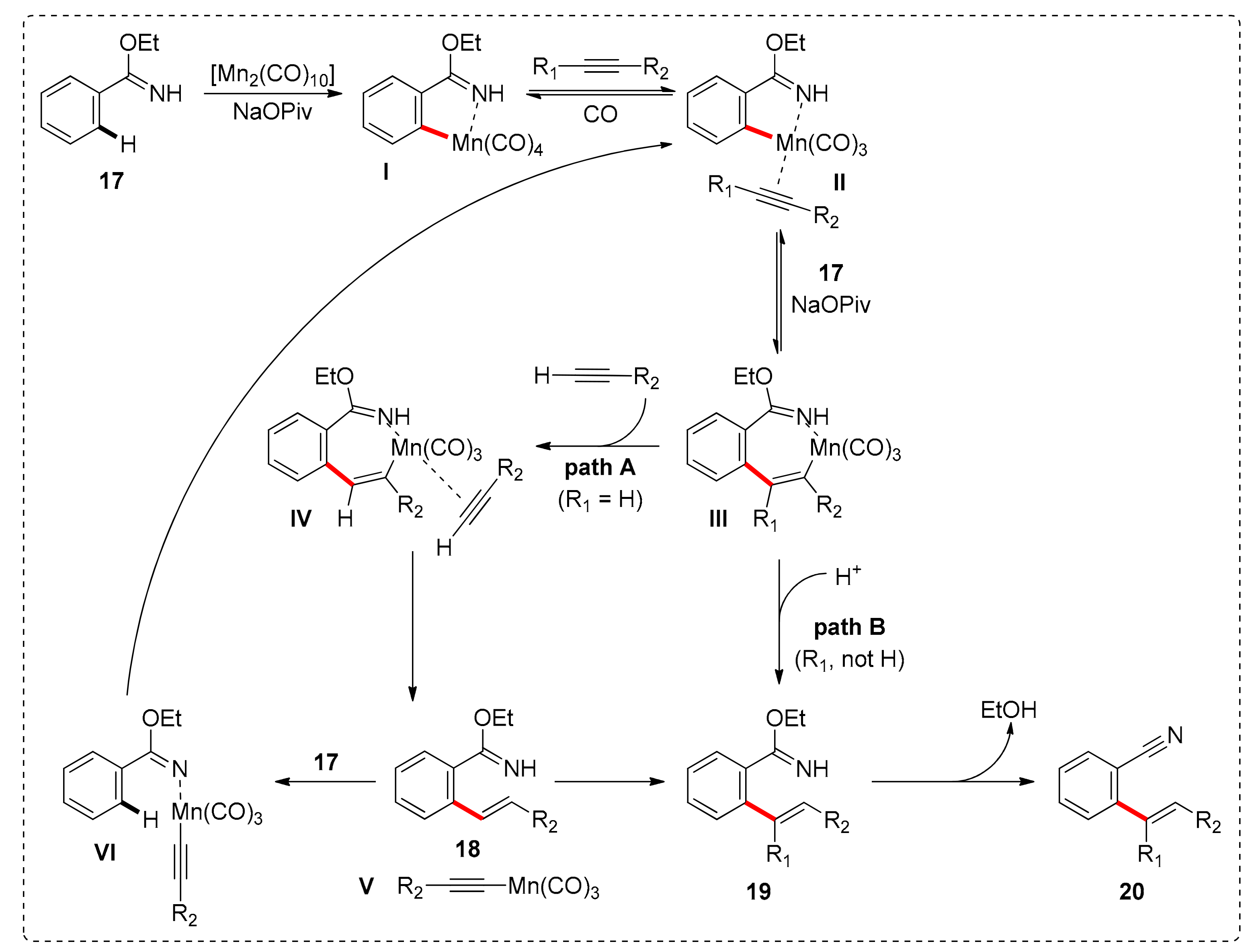
- Yang, X.; Jin, X.; Wang, C. Manganese-Catalyzed Ortho-C−H Alkenylation of Aromatic N−H Imidates with Alkynes: Versatile Access to Mono-Alkenylated Aromatic Nitriles. Adv. Synth. Catal. 2016, 358, 2436–2442. [Google Scholar] [CrossRef]
- Lu, Q.; Greßies, S.; Cembellín, S.; Klauck, F.J.R.; Daniliuc, C.G.; Glorius, F. Redox-Neutral Manganese(I)-Catalyzed C−H Activation: Traceless Directing Group Enabled Regioselective Annulation. Angew. Chem. Int. Ed. 2017, 56, 12778–12782. [Google Scholar] [CrossRef]
- Liang, Y.-F.; Steinbock, R.; Münch, A.; Stalke, D.; Ackermann, L. Manganese-Catalyzed Carbonylative Annulations for Redox-Neutral Late-Stage Diversification. Angew. Chem. Int. Ed. 2018, 57, 5384–5388. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4987-5429-3. [Google Scholar]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Kroneck, P.M.H.; Torres, M.E.S. Metal Ions in Life Sciences. In Sustaining Life on Planet. Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-12415-5. [Google Scholar]
- Battistella, B.; Ray, K. O2 and H2O2 Activations at Dinuclear Mn and Fe Active Sites. Coord. Chem. Rev. 2020, 408, 213176. [Google Scholar] [CrossRef]
- Shang, R.; Ilies, L.; Nakamura, E. Iron-Catalyzed C–H Bond Activation. Chem. Rev. 2017, 117, 9086–9139. [Google Scholar] [CrossRef]
- Viton, F.; Bernardinelli, G.; Kündig, E.P. Iron and Ruthenium Lewis Acid Catalyzed Asymmetric 1,3-Dipolar Cycloaddition Reactions between Nitrones and Enals. J. Am. Chem. Soc. 2002, 124, 4968–4969. [Google Scholar] [CrossRef] [PubMed]
- Bădoiu, A.; Bernardinelli, G.; Mareda, J.; Kündig, E.P.; Viton, F. Iron- and Ruthenium-Lewis Acid Catalyzed Asymmetric 1,3-Dipolar Cycloaddition Reactions between Enals and Diaryl Nitrones. Chem. Asian J. 2008, 3, 1298–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Sun, S.; Cheng, J.; Shao, Y. Iron-Catalyzed Cyclization of Nitrones with Geminal-Substituted Vinyl Acetates: A Direct [4 + 2] Assembly Strategy Leading to 2,4-Disubstituted Quinolines. J. Org. Chem. 2016, 81, 10825–10831. [Google Scholar] [CrossRef]
- Ferlin, F.; Zangarelli, A.; Lilli, S.; Santoro, S.; Vaccaro, L. Waste-Minimized Synthesis of C2 Functionalized Quinolines Exploiting Iron-Catalysed C–H Activation. Green Chem. 2021, 23, 490–495. [Google Scholar] [CrossRef]
- Moselage, M.; Li, J.; Ackermann, L. Cobalt-Catalyzed C–H Activation. ACS Catal. 2016, 6, 498–525. [Google Scholar] [CrossRef]
- Mei, R.; Dhawa, U.; Samanta, R.C.; Ma, W.; Wencel-Delord, J.; Ackermann, L. Cobalt-Catalyzed Oxidative C−H Activation: Strategies and Concepts. ChemSusChem 2020, 13, 3306–3356. [Google Scholar] [CrossRef]
- Murahashi, S. Synthesis of Phthalimidines from Schiff Bases and Carbon Monoxide. J. Am. Chem. Soc. 1955, 77, 6403–6404. [Google Scholar] [CrossRef]
- Lukasevics, L.; Grigorjeva, L. Cobalt-Catalyzed Carbonylation of the C–H Bond. Org. Biomol. Chem. 2020, 18, 7460–7466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Meng, G.; Nolan, S.P.; Szostak, M. N-Heterocyclic Carbene Complexes in C–H Activation Reactions. Chem. Rev. 2020, 120, 1981–2048. [Google Scholar] [CrossRef]
- Gao, K.; Yoshikai, N. Low-Valent Cobalt Catalysis: New Opportunities for C–H Functionalization. Acc. Chem. Res. 2014, 47, 1208–1219. [Google Scholar] [CrossRef]
- Rani, G.; Luxami, V.; Paul, K. Traceless Directing Groups: A Novel Strategy in Regiodivergent C–H Functionalization. Chem. Commun. 2020, 56, 12479–12521. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Ai, C.; Lv, Y.; Zhong, W. Traceless Directing Group Assisted Cobalt-Catalyzed C−H Carbonylation of Benzylamines. Adv. Synth. Catal. 2017, 359, 3707–3712. [Google Scholar] [CrossRef]
- Ling, F.; Zhang, C.; Ai, C.; Lv, Y.; Zhong, W. Metal-Oxidant-Free Cobalt-Catalyzed C(Sp2)–H Carbonylation of Ortho-Arylanilines: An Approach toward Free (NH)-Phenanthridinones. J. Org. Chem. 2018, 83, 5698–5706. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Fu, L.-Y.; Zhong, G.; Wu, X.-F. Cobalt-Catalyzed Direct Carbonylative Synthesis of Free (NH)-Benzo[Cd]Indol-2(1H)-Ones from Naphthylamides. Org. Lett. 2019, 21, 5694–5698. [Google Scholar] [CrossRef] [PubMed]
- Lukasevics, L.; Cizikovs, A.; Grigorjeva, L. Synthesis of 3-Hydroxymethyl Isoindolinones via Cobalt-Catalyzed C(Sp2)–H Carbonylation of Phenylglycinol Derivatives. Org. Lett. 2020, 22, 2720–2723. [Google Scholar] [CrossRef]
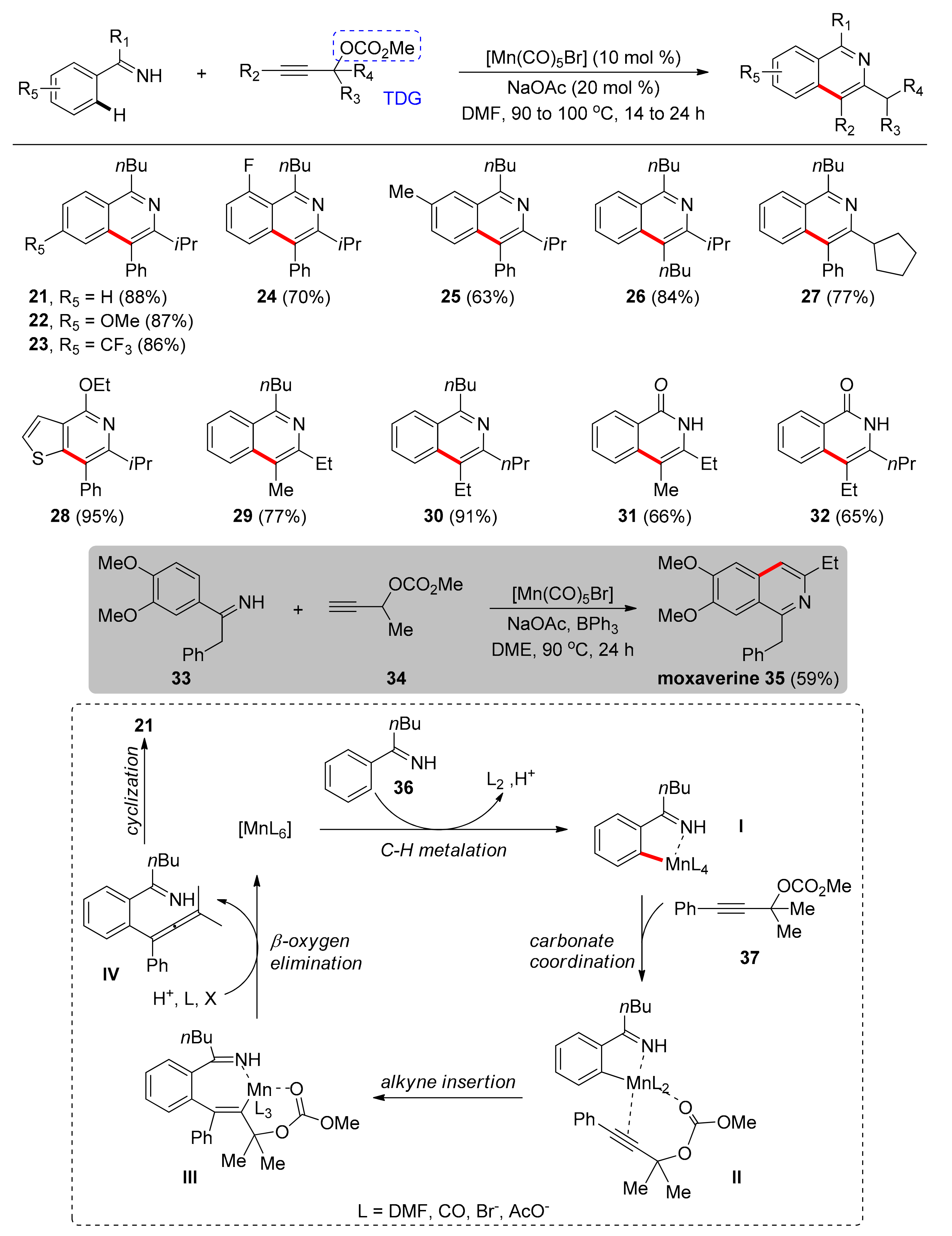
- Zhou, S.; Wang, M.; Wang, L.; Chen, K.; Wang, J.; Song, C.; Zhu, J. Bidentate Directing-Enabled, Traceless Heterocycle Synthesis: Cobalt-Catalyzed Access to Isoquinolines. Org. Lett. 2016, 18, 5632–5635. [Google Scholar] [CrossRef]
- Kuai, C.; Wang, L.; Li, B.; Yang, Z.; Cui, X. Cobalt-Catalyzed Selective Synthesis of Isoquinolines Using Picolinamide as a Traceless Directing Group. Org. Lett. 2017, 19, 2102–2105. [Google Scholar] [CrossRef]
- Li, X.-C.; Du, C.; Zhang, H.; Niu, J.-L.; Song, M.-P. Cp*-Free Cobalt-Catalyzed C–H Activation/Annulations by Traceless N,O-Bidentate Directing Group: Access to Isoquinolines. Org. Lett. 2019, 21, 2863–2866. [Google Scholar] [CrossRef]
- Dey, A.; Volla, C.M.R. Traceless Bidentate Directing Group Assisted Cobalt-Catalyzed sp2-C–H Activation and [4 + 2]-Annulation Reaction with 1,3-Diynes. Org. Lett. 2020, 22, 7480–7485. [Google Scholar] [CrossRef] [PubMed]
- Barsu, N.; Sen, M.; Premkumar, J.R.; Sundararaju, B. Cobalt(III) Catalyzed C-8 Selective C–H and C–O Coupling of Quinoline N-Oxide with Internal Alkynes via C–H Activation and Oxygen Atom Transfer. Chem. Commun. 2016, 52, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, Q.; Liu, D.; Yu, L.; Tan, Z.; Zhu, G. Synthesis of 1-Naphthols via Cp*Co(III)-Catalyzed C–H Activation and Cyclization of Sulfoxonium Ylides with Alkynes. Org. Chem. Front. 2019, 6, 3868–3873. [Google Scholar] [CrossRef]
- Sigel, A.; Sigel, H.; Sigel, R.K.O. Metal Ions in Life Sciences. In Nickel and Its Surprising Impact in Nature; Wiley: Chichester, UK, 2007; ISBN 978-0-470-02812-4. [Google Scholar]
- Brazzolotto, D.; Gennari, M.; Queyriaux, N.; Simmons, T.R.; Pécaut, J.; Demeshko, S.; Meyer, F.; Orio, M.; Artero, V.; Duboc, C. Nickel-Centred Proton Reduction Catalysis in a Model of [NiFe] Hydrogenase. Nat. Chem. 2016, 8, 1054–1060. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Muto, K.; Itami, K. Nickel-Catalyzed Aromatic C–H Functionalization. Top. Curr. Chem. 2016, 374, 55. [Google Scholar] [CrossRef] [PubMed]
- Harry, N.A.; Saranya, S.; Ujwaldev, S.M.; Anilkumar, G. Recent Advances and Prospects in Nickel-Catalyzed C–H Activation. Catal. Sci. Technol. 2019, 9, 1726–1743. [Google Scholar] [CrossRef]
- Khake, S.M.; Chatani, N. Nickel-Catalyzed C−H Functionalization Using A Non-Directed Strategy. Chem 2020, 6, 1056–1081. [Google Scholar] [CrossRef]
- Khake, S.M.; Chatani, N. Chelation-Assisted Nickel-Catalyzed C−H Functionalizations. Trends Chem. 2019, 1, 524–539. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Lin, F.; Wang, B.; Chen, Z.; Liu, L. NiSO4-Catalyzed C–H Activation/C–S Cross-Coupling of 1,2,3-Triazole N-Oxides with Thiols. Org. Biomol. Chem. 2015, 13, 3711–3720. [Google Scholar] [CrossRef]
- Li, Z.-K.; Jia, X.-S.; Yin, L. Recent Advances in Copper(II)-Mediated or -Catalyzed C–H Functionalization. Synthesis 2018, 50, 4165–4188. [Google Scholar] [CrossRef]
- Tonis, E.; Stein, F.; Stamatopoulos, I.K.; Stubbe, J.; Zarkadoulas, A.; Sarkar, B.; Vougioukalakis, G.C. A Palladium-Free Sonogashira Coupling Protocol Employing an In Situ Prepared Copper/Chelating 1,2,3-Triazolylidene System. Synlett 2020. [Google Scholar] [CrossRef]
- DiMucci, I.M.; Lukens, J.T.; Chatterjee, S.; Carsch, K.M.; Titus, C.J.; Lee, S.J.; Nordlund, D.; Betley, T.A.; MacMillan, S.N.; Lancaster, K.M. The Myth of d8 Copper(III). J. Am. Chem. Soc. 2019, 141, 18508–18520. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Liu, S.; Lu, Z.; Lu, C.; Leng, X.; Lan, Y.; Shen, Q. C(sp3)-CF3 Reductive Elimination from a Five-Coordinate Neutral Copper(III) Complex. J. Am. Chem. Soc. 2020, 142, 9785–9791. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Dzik, W.I.; Gooßen, L.J. Synthesis of Aryl Ethers from Benzoates through Carboxylate-Directed C-H-Activating Alkoxylation with Concomitant Protodecarboxylation. Angew. Chem. Int. Ed. 2013, 52, 2959–2962. [Google Scholar] [CrossRef]
- Yu, S.; Lv, N.; Liu, Z.; Zhang, Y. Cu(II)-Mediated C−C/C−O Bond Formation via C−H/C−C Bond Cleavage: Access to Benzofurans Using Amide as a Traceless Directing Group. Adv. Synth. Catal. 2020, 362, 118–125. [Google Scholar] [CrossRef]
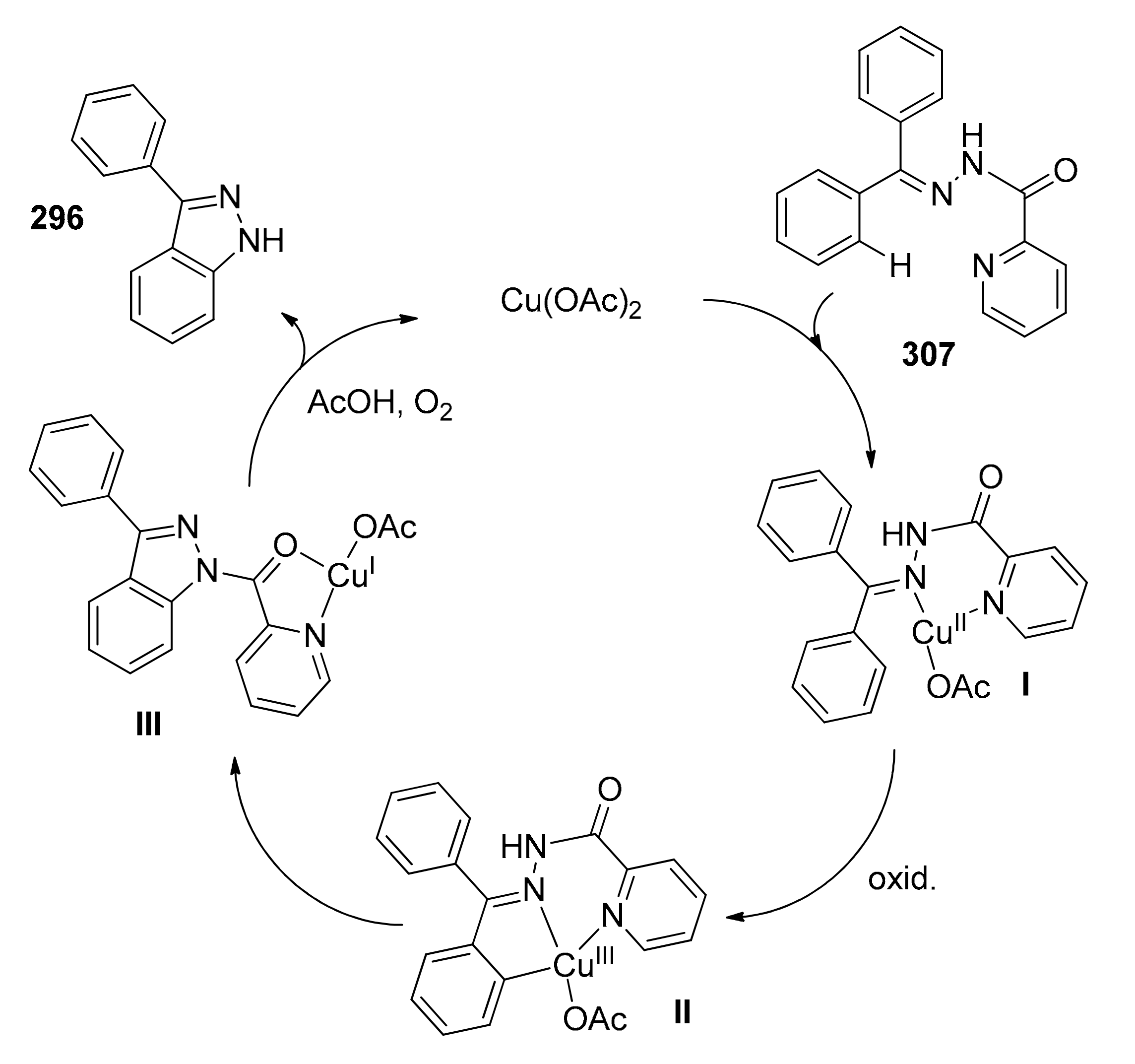
- Zhang, G.; Fan, Q.; Zhao, Y.; Ding, C. Copper-Promoted Oxidative Intramolecular C–H Amination of Hydrazones to Synthesize 1H-Indazoles and 1H-Pyrazoles Using a Cleavable Directing Group. Eur. J. Org. Chem. 2019, 2019, 5801–5806. [Google Scholar] [CrossRef]
- Dalton, T.; Faber, T.; Glorius, F. C–H Activation: Toward Sustainability and Applications. ACS Cent. Sci. 2021, 7, 245–261. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarkadoulas, A.; Zgouleta, I.; Tzouras, N.V.; Vougioukalakis, G.C. Traceless Directing Groups in Sustainable Metal-Catalyzed C–H Activation. Catalysts 2021, 11, 554. https://doi.org/10.3390/catal11050554
Zarkadoulas A, Zgouleta I, Tzouras NV, Vougioukalakis GC. Traceless Directing Groups in Sustainable Metal-Catalyzed C–H Activation. Catalysts. 2021; 11(5):554. https://doi.org/10.3390/catal11050554
Chicago/Turabian StyleZarkadoulas, Athanasios, Ioanna Zgouleta, Nikolaos V. Tzouras, and Georgios C. Vougioukalakis. 2021. "Traceless Directing Groups in Sustainable Metal-Catalyzed C–H Activation" Catalysts 11, no. 5: 554. https://doi.org/10.3390/catal11050554
APA StyleZarkadoulas, A., Zgouleta, I., Tzouras, N. V., & Vougioukalakis, G. C. (2021). Traceless Directing Groups in Sustainable Metal-Catalyzed C–H Activation. Catalysts, 11(5), 554. https://doi.org/10.3390/catal11050554