Ni-N-Doped Carbon-Modified Reduced Graphene Oxide Catalysts for Electrochemical CO2 Reduction Reaction
Abstract
1. Introduction
2. Results
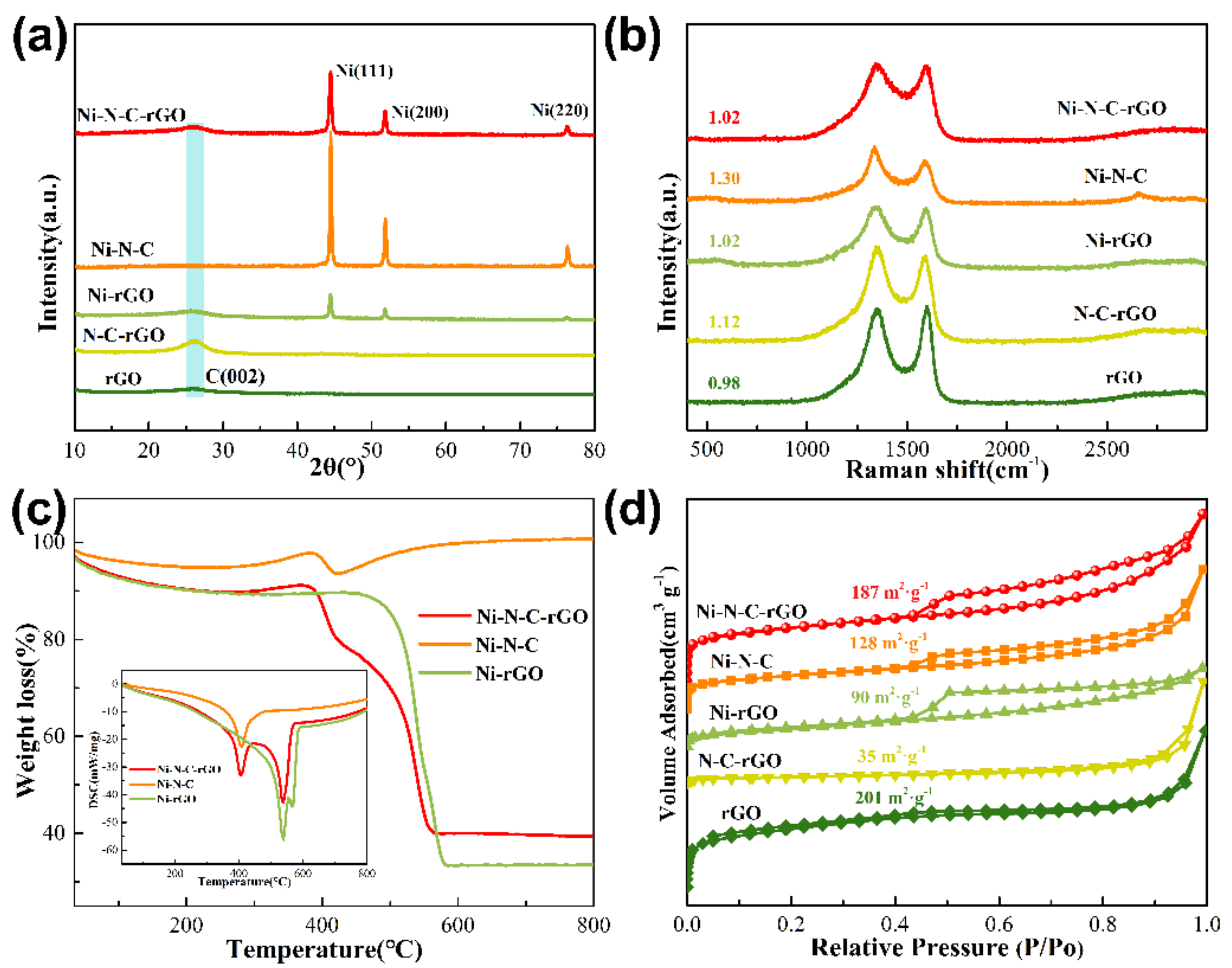
2.1. Textural Properties of Ni–N–C–rGO Materials
2.2. Chemical Bonds of Ni–N–C–rGO Materials
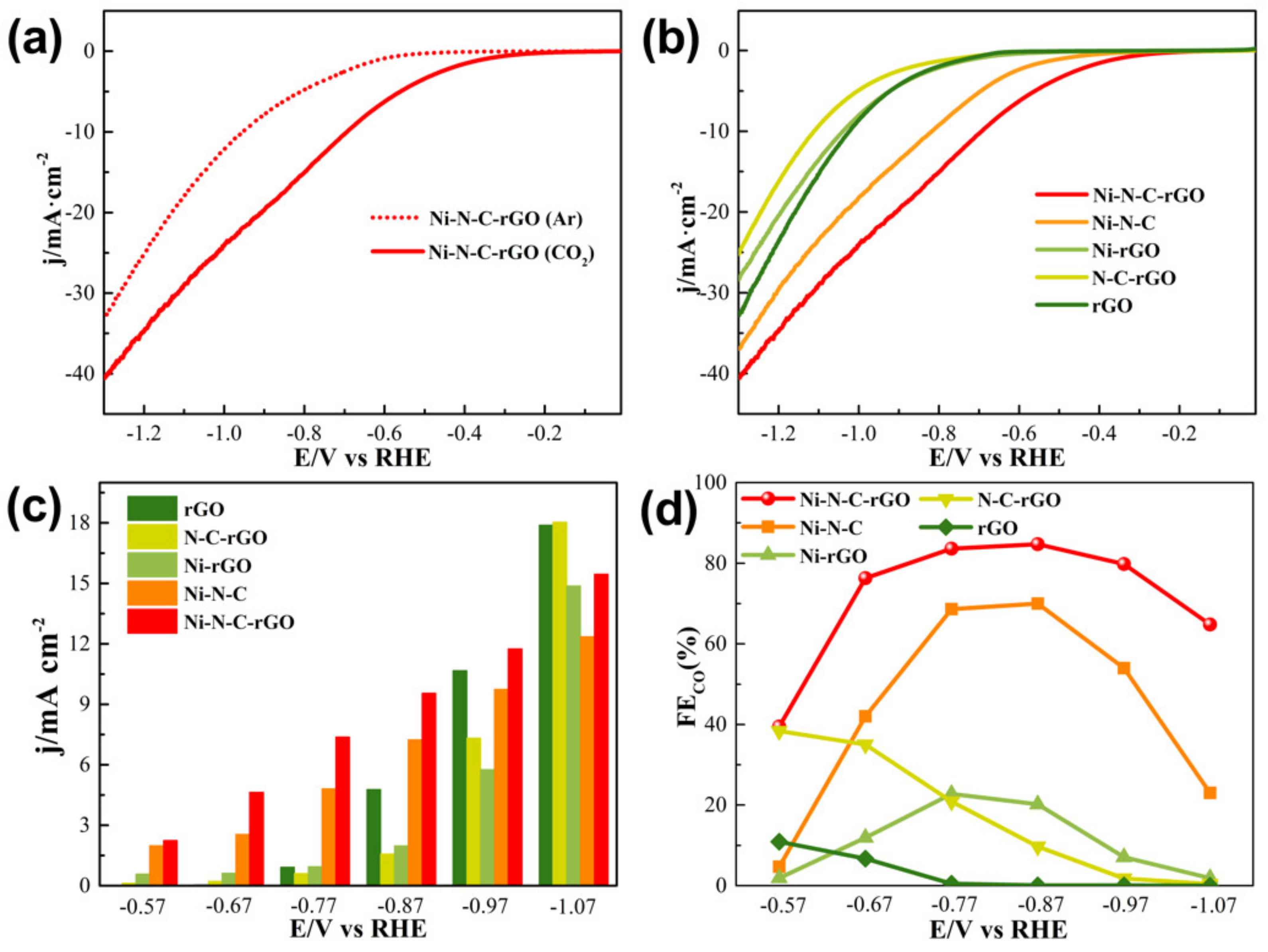
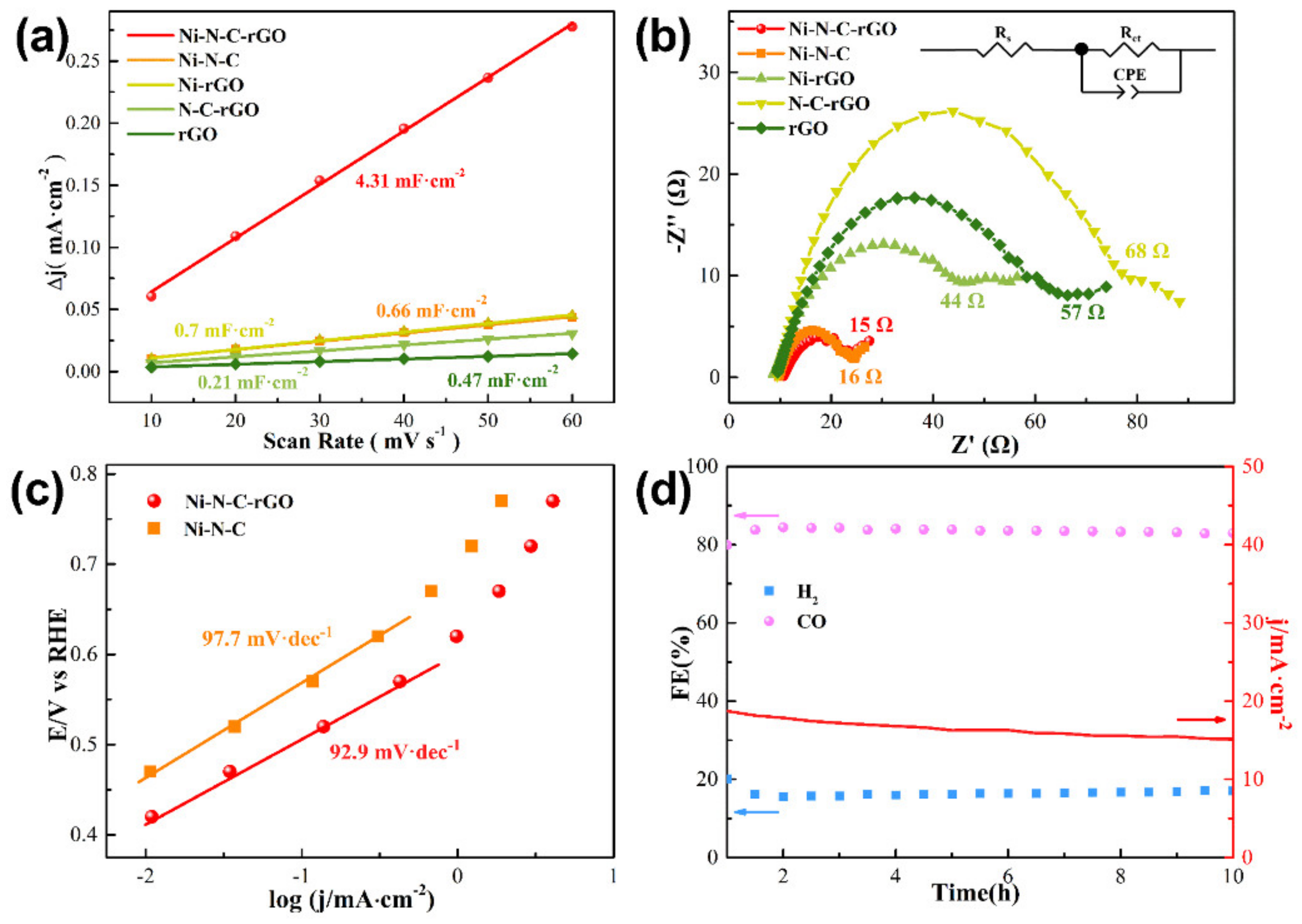
2.3. Catalytic Performance of Ni–N–C–rGO Materials
3. Materials and Methods
3.1. Materials
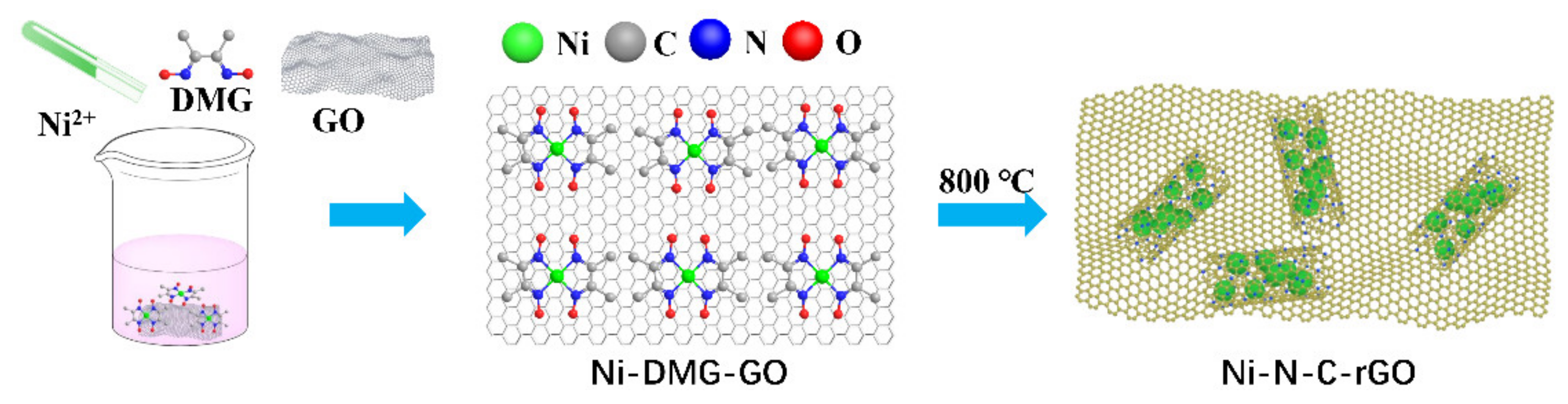
3.2. Synthesis of Materials
3.2.1. Synthesis of Graphene Oxide (GO)
3.2.2. Preparation of Different Precursors Ni–DMG–GO
3.2.3. Preparation of Ni–N–C–rGO Materials
3.3. Materials Characterization
3.4. Preparation of Working Electrodes
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, Y.; Zhang, X.; Xie, K.; Wang, G.; Bao, X. High-Temperature CO2 Electrolysis in Solid Oxide Electrolysis Cells: Developments, Challenges, and Prospects. Adv. Mater. 2019, 31, 1902033. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, W.; Zhang, D.; Du, Y.; Amal, R.; Qiao, S.; Wu, J.; Yin, Z. Surface strategies for catalytic CO2 reduction: From two-dimensional materials to nanoclusters to single atoms. Chem. Soc. Rev. 2019, 48, 5310–5349. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Dokania, A.; Ramirez, A.; Gascon, J. Advances in the Design of Heterogeneous Catalysts and Thermocatalytic Processes for CO2 Utilization. ACS Catal. 2020, 10, 14147–14185. [Google Scholar] [CrossRef]
- Lang, X.-D.; He, L.-N. Integration of CO2 Reduction with Subsequent Carbonylation: Towards Extending Chemical Utilization of CO2. ChemSusChem 2018, 11, 2062–2067. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.-F. Essential Role of the Support for Nickel-Based CO2 Methanation Catalysts. ACS Catal. 2020, 10, 14581–14591. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Luo, W.; Sherrell, P.C.; Chen, J.; Yang, J. Heterogeneous Single-Atom Catalysts for Electrochemical CO2 Reduction Reaction. Adv. Mater. 2020, 32, 2001848. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, T.; Fenwick, A.Q.; Baroud, T.N.; Rosas-Hernández, A.; Ko, J.H.; Gan, Q.; Iii, W.A.G.; Grubbs, R.H. Selective CO2 Electrochemical Reduction Enabled by a Tricomponent Copolymer Modifier on a Copper Surface. J. Am. Chem. Soc. 2021, 143, 2857–2865. [Google Scholar] [CrossRef]
- Wang, G.; Chen, J.; Ding, Y.; Cai, P.; Yi, L.; Li, Y.; Tu, C.; Hou, Y.; Wen, Z.; Dai, L. Electrocatalysis for CO2 conversion: From fundamentals to value-added products. Chem. Soc. Rev. 2021. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sharifi, T.; Gao, Y.; Zhang, T.; Ajayan, P.M. Emerging Carbon-Based Heterogeneous Catalysts for Electrochemical Reduction of Carbon Dioxide into Value-Added Chemicals. Adv. Mater. 2019, 31, 1804257. [Google Scholar] [CrossRef]
- He, R.; Zhang, A.; Ding, Y.; Kong, T.; Xiao, Q.; Li, H.; Liu, Y.; Zeng, J. Achieving the Widest Range of Syngas Proportions at High Current Density over Cadmium Sulfoselenide Nanorods in CO2 Electroreduction. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Xing, Y.; Kong, X.; Guo, X.; Liu, Y.; Li, Q.; Zhang, Y.; Sheng, Y.; Yang, X.; Geng, Z.; Zeng, J. Bi@Sn Core–Shell Structure with Compressive Strain Boosts the Electroreduction of CO2 into Formic Acid. Adv. Sci. 2020, 7, 1902989. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Song, S.; Liu, M.; Yao, S.; Liang, Z.; Cheng, H.; Ren, Z.; Liu, W.; Lin, R.; Qi, G.; et al. Stable and Efficient Single-Atom Zn Catalyst for CO2 Reduction to CH4. J. Am. Chem. Soc. 2020, 142, 12563–12567. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Yin, Z.; Wei, X.; Peng, H.; Lyu, K.; Wei, F.; Xiao, L.; Wang, G.; Abruña, H.D.; et al. Interface-Enhanced Catalytic Selectivity on the C2 Products of CO2 Electroreduction. ACS Catal. 2021, 11, 2473–2482. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Q.; Fu, Y.; Lei, C.; Yang, B.; Li, Z.; Lei, L.; Wu, G.; Hou, Y. Carbon-Rich Nonprecious Metal Single Atom Electrocatalysts for CO2 Reduction and Hydrogen Evolution. Small Methods 2019, 3, 1900210. [Google Scholar] [CrossRef]
- Zhao, C.; Dai, X.; Yao, T.; Chen, W.; Wang, X.; Wang, J.; Yang, J.; Wei, S.; Wu, Y.; Li, Y. Ionic Exchange of Metal–Organic Frameworks to Access Single Nickel Sites for Efficient Electroreduction of CO2. J. Am. Chem. Soc. 2017, 139, 8078–8081. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Z.; Zuo, Z.; Pan, W.; Zhang, J. The ensemble effect of nitrogen doping and ultrasmall SnO2 nanocrystals on graphene sheets for efficient electroreduction of carbon dioxide. Appl. Catal. B Environ. 2018, 239, 441–449. [Google Scholar] [CrossRef]
- Cao, C.; Wen, Z. Cu nanoparticles decorating rGO nanohybrids as electrocatalyst toward CO2 reduction. J. CO2 Util. 2017, 22, 231–237. [Google Scholar] [CrossRef]
- Zhang, Z.; Ahmad, F.; Zhao, W.; Yan, W.; Zhang, W.; Huang, H.; Ma, C.; Zeng, J. Enhanced Electrocatalytic Reduction of CO2 via Chemical Coupling between Indium Oxide and Reduced Graphene Oxide. Nano Lett. 2019, 19, 4029–4034. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Xi, S.; Lee, J.M.; Wang, C.; Du, Y.; Wang, X. Linkage Effect in the Heterogenization of Cobalt Complexes by Doped Graphene for Electrocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2019, 58, 13532–13539. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Liu, Y.; Macfarlane, D.R.; Wallace, G.G. Engineering Surface Amine Modifiers of Ultrasmall Gold Nanoparticles Supported on Reduced Graphene Oxide for Improved Electrochemical CO2 Reduction. Adv. Energy Mater. 2018, 8, 1801400. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Vo, T.-T.T.; Do, H.H.; Le, V.T.; Nguyen, T.D.; Vo, T.K.; Nguyen, B.-S.; Nguyen, T.T.; Phung, T.K.; Tran, V.A. Ag@ZnO porous nanoparticle wrapped by rGO for the effective CO2 electrochemical reduction. Chem. Eng. Sci. 2021, 232, 116381. [Google Scholar] [CrossRef]
- Min, X.; Kanan, M.W. Pd-Catalyzed Electrohydrogenation of Carbon Dioxide to Formate: High Mass Activity at Low Overpotential and Identification of the Deactivation Pathway. J. Am. Chem. Soc. 2015, 137, 4701–4708. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Huang, J.; Chen, J.; Kong, Y.; Yang, B.; Li, Z.; Lei, L.; Chai, G.; Wen, Z.; et al. Boosting Electroreduction Kinetics of Nitrogen to Ammonia via Tuning Electron Distribution of Single-Atomic Iron Sites. Angew. Chem. Int. Ed. 2021. [Google Scholar] [CrossRef]
- Su, P.; Iwase, K.; Harada, T.; Kamiya, K.; Nakanishi, S. Covalent triazine framework modified with coordinatively-unsaturated Co or Ni atoms for CO2 electrochemical reduction. Chem. Sci. 2018, 9, 3941–3947. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Fu, J.; Zhou, Y.; Chang, Y.-C.; Min, Q.; Zhu, J.-J.; Lin, Y.; Zhu, W. Insights on forming N,O-coordinated Cu single-atom catalysts for electrochemical reduction CO2 to methane. Nat. Commun. 2021, 12, 586. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Choi, C.; Wu, T.-S.; Ma, C.; Kang, P.; Tao, H.; Fan, Q.; Hong, S.; Liu, S.; Soo, Y.-L.; et al. Carbon-supported Ni nanoparticles for efficient CO2 electroreduction. Chem. Sci. 2018, 9, 8775–8780. [Google Scholar] [CrossRef]
- Tan, D.; Cui, C.; Shi, J.; Luo, Z.; Zhang, B.; Tan, X.; Han, B.; Zheng, L.; Zhang, J.; Zhang, J. Nitrogen-carbon layer coated nickel nanoparticles for efficient electrocatalytic reduction of carbon dioxide. Nano Res. 2019, 12, 1167–1172. [Google Scholar] [CrossRef]
- Daiyan, R.; Lu, X.; Tan, X.; Zhu, X.; Chen, R.; Smith, S.C.; Amal, R. Antipoisoning Nickel–Carbon Electrocatalyst for Practical Electrochemical CO2 Reduction to CO. ACS Appl. Energy Mater. 2019, 2, 8002–8009. [Google Scholar] [CrossRef]
- Rath, M.; Behera, L.P.; Dash, B.; Sheik, A.R.; Sanjay, K. Recovery of dimethylglyoxime (DMG) from Ni-DMG complexes. Hydrometall 2018, 176, 229–234. [Google Scholar] [CrossRef]
- Gramlich, A.; Moradi, A.B.; Robinson, B.H.; Kaestner, A.; Schulin, R. Dimethylglyoxime (DMG) staining for semi-quantitative mapping of Ni in plant tissue. Environ. Exp. Bot. 2011, 71, 232–240. [Google Scholar] [CrossRef]
- Ning, H.; Mao, Q.; Wang, W.; Yang, Z.; Wang, X.; Zhao, Q.; Song, Y.; Wu, M. N-doped reduced graphene oxide supported Cu2O nanocubes as high active catalyst for CO2 electroreduction to C2H4. J. Alloys Compd. 2019, 785, 7–12. [Google Scholar] [CrossRef]
- Yuan, J.; Zhi, W.-Y.; Liu, L.; Yang, M.-P.; Wang, H.; Lu, J.-X. Electrochemical reduction of CO2 at metal-free N-functionalized graphene oxide electrodes. Electrochim. Acta 2018, 282, 694–701. [Google Scholar] [CrossRef]
- Mao, F.; Liu, P.F.; Yang, P.; Gu, J.; Yang, H.G. One-step coating of commercial Ni nanoparticles with a Ni, N-co-doped carbon shell towards efficient electrocatalysts for CO2 reduction. Chem. Commun. 2020, 56, 7495–7498. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Yu, Y.; Chen, H.; Xu, G.; Miao, L.; Liu, X.; Pan, Z.; Kou, Z.; Wu, Y.; Wang, J.; et al. In situ electrochemical oxidation of electrodeposited Ni-based nanostructure promotes alkaline hydrogen production. Nanotechnology 2019, 30, 474001. [Google Scholar] [CrossRef]
- Miao, Z.; Meng, J.; Liang, M.; Li, Z.; Zhao, Y.; Wang, F.; Xu, L.; Mu, J.; Zhuo, S.; Zhou, J. In-situ CVD synthesis of Ni@N-CNTs/carbon paper electrode for electro-reduction of CO2. Carbon 2021, 172, 324–333. [Google Scholar] [CrossRef]
- Miao, Z.; Liu, W.; Zhao, Y.; Wang, F.; Meng, J.; Liang, M.; Wu, X.; Zhao, J.; Zhuo, S.; Zhou, J. Zn-Modified Co@N–C composites with adjusted Co particle size as catalysts for the efficient electroreduction of CO2. Catal. Sci. Technol. 2020, 10, 967–977. [Google Scholar] [CrossRef]
- Zhao, J.; Quan, X.; Chen, S.; Liu, Y.; Yu, H. Cobalt Nanoparticles Encapsulated in Porous Carbons Derived from Core–Shell ZIF67@ZIF8 as Efficient Electrocatalysts for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 28685–28694. [Google Scholar] [CrossRef]
- Shi, J.-J.; Hu, X.-M.; Madsen, M.R.; Lamagni, P.; Bjerglund, E.T.; Pedersen, S.U.; Skrydstrup, T.; Daasbjerg, K. Facile Synthesis of Iron- and Nitrogen-Doped Porous Carbon for Selective CO2 Electroreduction. ACS Appl. Nano Mater. 2018, 1, 3608–3615. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, R.; Liu, Y.; Ha, Y.; Guo, Y.; Sun, D.; Liu, M.; Fang, F. Ultrafine Co Nanoparticles Encapsulated in Carbon-Nanotubes-Grafted Graphene Sheets as Advanced Electrocatalysts for the Hydrogen Evolution Reaction. Adv. Mater. 2018, 30, 1802011. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Tang, Z.; Gao, L.; Wang, F.; Zhao, J.; Miao, Z.; Wu, X.; Zhou, J.; Su, Y.; Zhuo, S. Facile and controllable synthesis N-doping porous Graphene for high-performance Supercapacitor. J. Electroanal. Chem. 2020, 871, 114311. [Google Scholar] [CrossRef]
- Yan, C.; Ye, Y.; Lin, L.; Wu, H.; Jiang, Q.; Wang, G.; Bao, X. Improving CO2 electroreduction over ZIF-derived carbon doped with Fe-N sites by an additional ammonia treatment. Catal. Today 2019, 330, 252–258. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, N.; Renab, L.; Garciac, G.C.-; Liua, N.; Liua, Y.; Xua, X.; Haobd, W.; Dou, S.X.; Duab, Y. In-situ grafting of N-doped carbon nanotubes with Ni encapsulation onto MOF-derived hierarchical hybrids for efficient electrocatalytic hydrogen evolution. Carbon 2020, 163, 178–185. [Google Scholar] [CrossRef]
- Zhong, H.-X.; Wang, J.; Zhang, Y.-W.; Xu, W.-L.; Xing, W.; Xu, D.; Zhang, Y.-F.; Zhang, X.-B. ZIF-8 Derived Graphene-Based Nitrogen-Doped Porous Carbon Sheets as Highly Efficient and Durable Oxygen Reduction Electrocatalysts. Angew. Chem. Int. Ed. 2014, 53, 14235–14239. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Huang, T.; Wen, Z.; Mao, S.; Cui, S.; Chen, J. Metal−Organic Framework-Derived Nitrogen-Doped Core-Shell-Structured Porous Fe/Fe3C@C Nanoboxes Supported on Graphene Sheets for Efficient Oxygen Reduction Reactions. Adv. Energy Mater. 2014, 4, 1400337. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Q.; Tian, Z.; Li, B.; Yan, W.; Wang, S.; Jiang, K.; Su, J.; Oloman, C.W.; Gyenge, E.L.; et al. Ammonia Thermal Treatment toward Topological Defects in Porous Carbon for Enhanced Carbon Dioxide Electroreduction. Adv. Mater. 2020, 32, 2001300. [Google Scholar] [CrossRef]
- Pan, F.; Deng, W.; Justiniano, C.; Li, Y. Identification of champion transition metals centers in metal and nitrogen-codoped carbon catalysts for CO2 reduction. Appl. Catal. B Environ. 2018, 226, 463–472. [Google Scholar] [CrossRef]
- Bin Yang, H.; Hung, S.-F.; Liu, S.; Yuan, K.; Miao, S.; Zhang, L.; Huang, X.; Wang, H.-Y.; Cai, W.; Chen, R.; et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy 2018, 3, 140–147. [Google Scholar] [CrossRef]
- Hou, P.; Wang, X.; Wang, Z.; Kang, P. Gas Phase Electrolysis of Carbon Dioxide to Carbon Monoxide Using Nickel Nitride as the Carbon Enrichment Catalyst. ACS Appl. Mater. Interfaces 2018, 10, 38024–38031. [Google Scholar] [CrossRef]
- Zheng, T.; Jiang, K.; Ta, N.; Hu, Y.; Zeng, J.; Liu, J.; Wang, H. Large-Scale and Highly Selective CO2 Electrocatalytic Reduction on Nickel Single-Atom Catalyst. Joule 2019, 3, 265–278. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, S.; Wu, X.; Liang, B.; Zhou, Y.; Zhu, C.; Yang, N.; Jiang, X.; Gao, J.; Bai, L.; et al. A Co3O4-CDots-C3N4 three component electrocatalyst design concept for efficient and tunable CO2 reduction to syngas. Nat. Commun. 2017, 8, 1828. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lu, L.; Zhu, Q.; Wu, C.; Yang, D.; Chen, C.; Han, B. MoP Nanoparticles Supported on Indium-Doped Porous Carbon: Outstanding Catalysts for Highly Efficient CO2 Electroreduction. Angew. Chem. Int. Ed. 2018, 57, 2427–2431. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Liu, Y.; Li, P.; Ke, J.; Liu, Z.; Ahmad, F.; Yan, W.; Li, Z.; Geng, Z.; Zeng, J. Coordinate activation in heterogeneous carbon dioxide reduction on Co-based molecular catalysts. Appl. Catal. B Environ. 2020, 268, 118452. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Zaaba, N.; Foo, K.; Hashim, U.; Tan, S.; Liu, W.-W.; Voon, C. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]



| Sample | XRD | TG | EDS | XPS (at%) | ||||
|---|---|---|---|---|---|---|---|---|
| Size (nm) | Interplanar Space (nm) | Ni (wt%) | Ni (at%) | C | N | O | Ni | |
| rGO | -- | -- | -- | -- | 94.2 | -- | 5.8 | -- |
| N–C–rGO | -- | -- | -- | -- | 89.4 | 6.9 | 3.7 | -- |
| Ni–rGO | 29.6 | 0.205 | 26.4 | 2.4 | 91.0 | -- | 7.3 | 1.7 |
| Ni–N–C | 37.0 | 0.205 | 79.3 | 34.3 | 81.5 | 4.0 | 9.7 | 4.8 |
| Ni–N–C–rGO | 24.7 | 0.205 | 30.8 | 9.2 | 82.8 | 6.2 | 8.0 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liu, Y.; Song, Z.; Miao, Z.; Zhao, J. Ni-N-Doped Carbon-Modified Reduced Graphene Oxide Catalysts for Electrochemical CO2 Reduction Reaction. Catalysts 2021, 11, 561. https://doi.org/10.3390/catal11050561
Wang F, Liu Y, Song Z, Miao Z, Zhao J. Ni-N-Doped Carbon-Modified Reduced Graphene Oxide Catalysts for Electrochemical CO2 Reduction Reaction. Catalysts. 2021; 11(5):561. https://doi.org/10.3390/catal11050561
Chicago/Turabian StyleWang, Fangyuan, Yu Liu, Zhiling Song, Zhichao Miao, and Jinping Zhao. 2021. "Ni-N-Doped Carbon-Modified Reduced Graphene Oxide Catalysts for Electrochemical CO2 Reduction Reaction" Catalysts 11, no. 5: 561. https://doi.org/10.3390/catal11050561
APA StyleWang, F., Liu, Y., Song, Z., Miao, Z., & Zhao, J. (2021). Ni-N-Doped Carbon-Modified Reduced Graphene Oxide Catalysts for Electrochemical CO2 Reduction Reaction. Catalysts, 11(5), 561. https://doi.org/10.3390/catal11050561







