Dry Reforming of Methane over Carbon Fibre-Supported CeZrO2, Ni-CeZrO2, Pt-CeZrO2 and Pt-Ni-CeZrO2 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
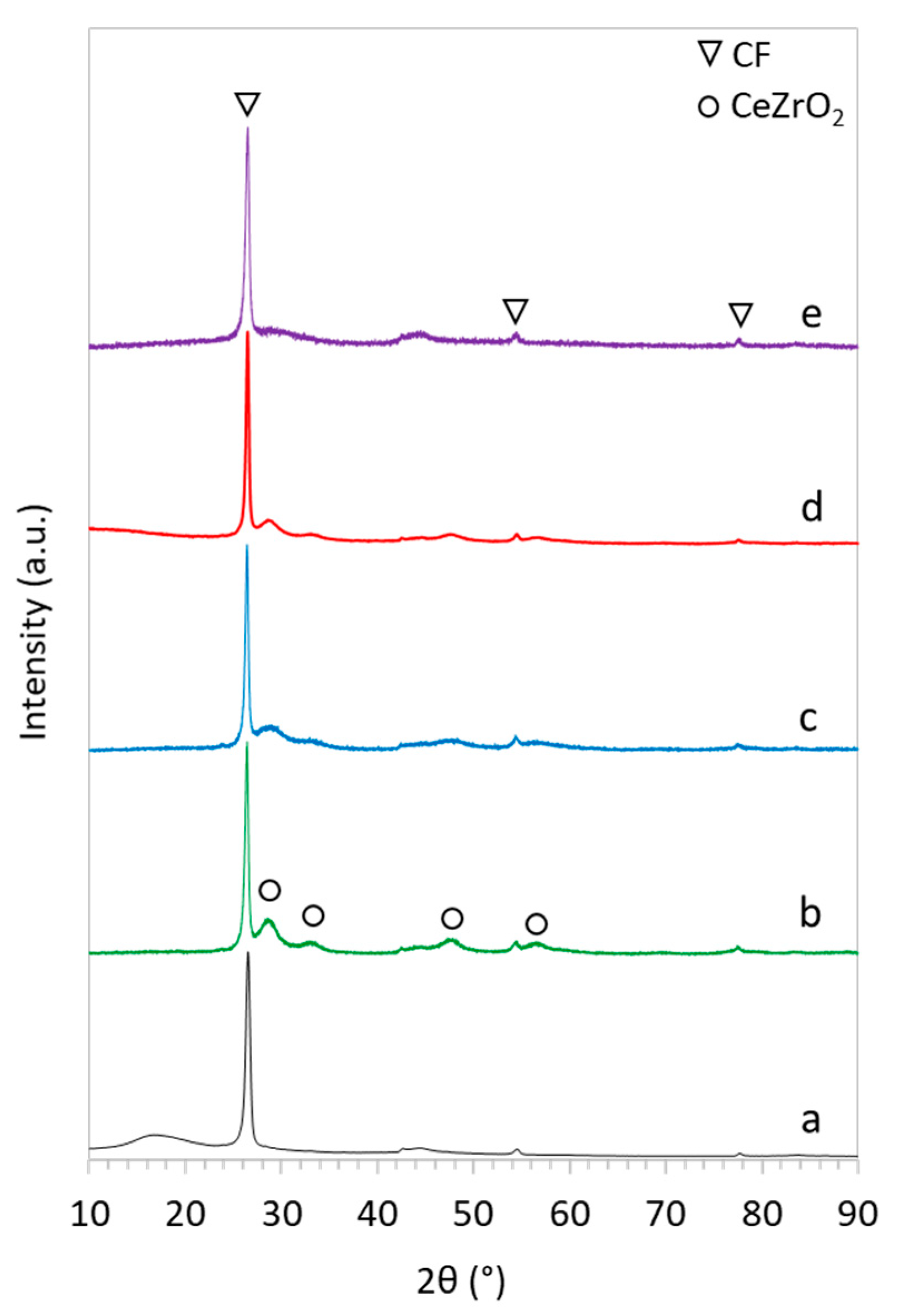
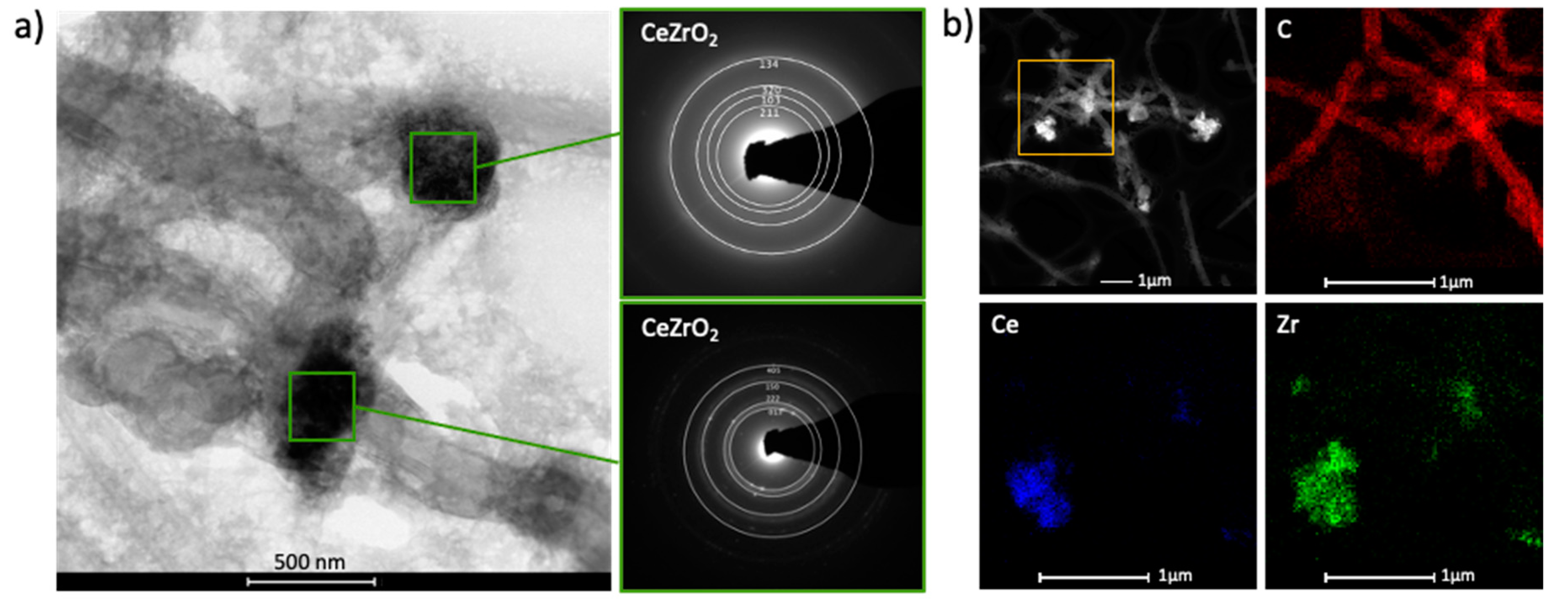
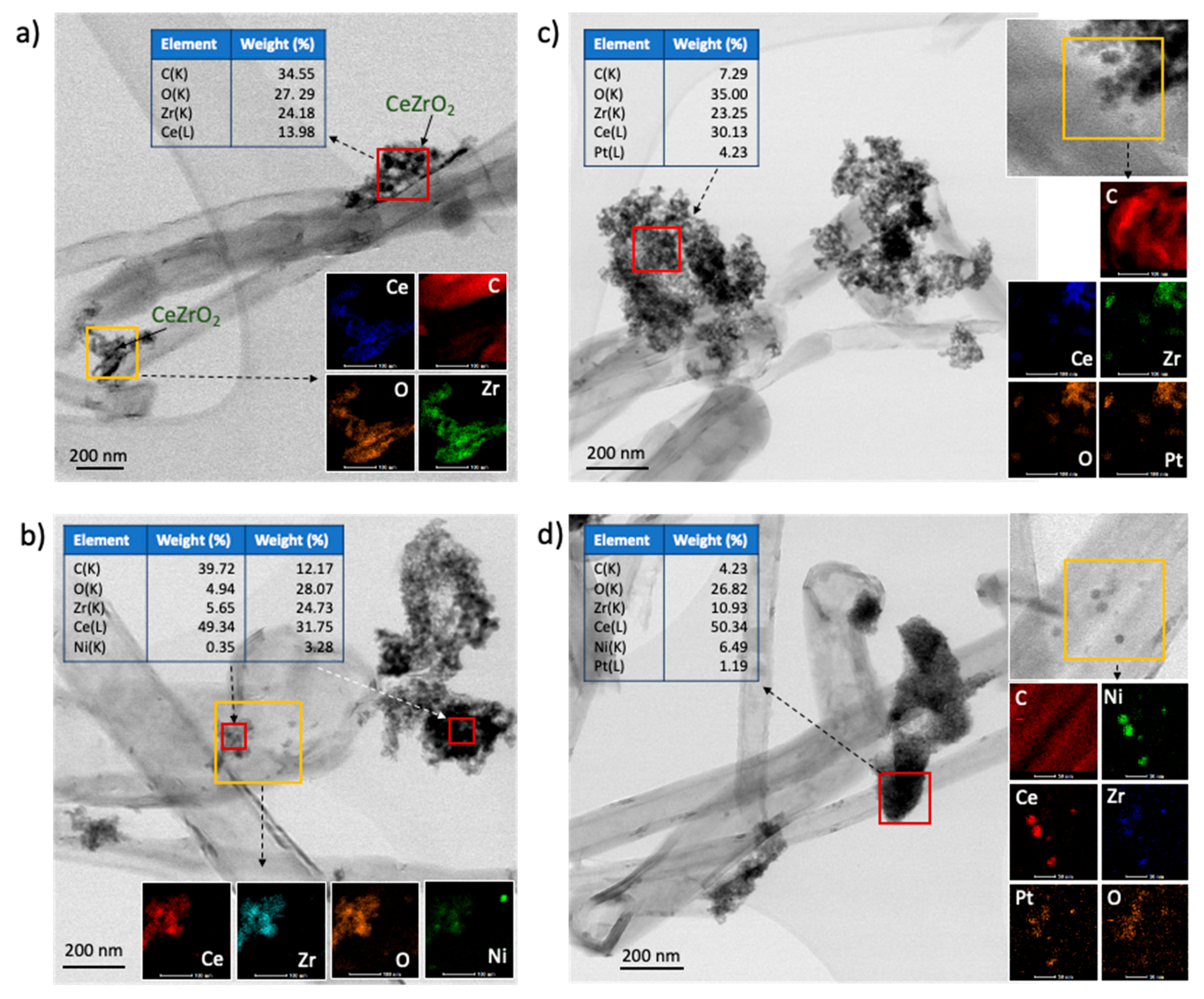
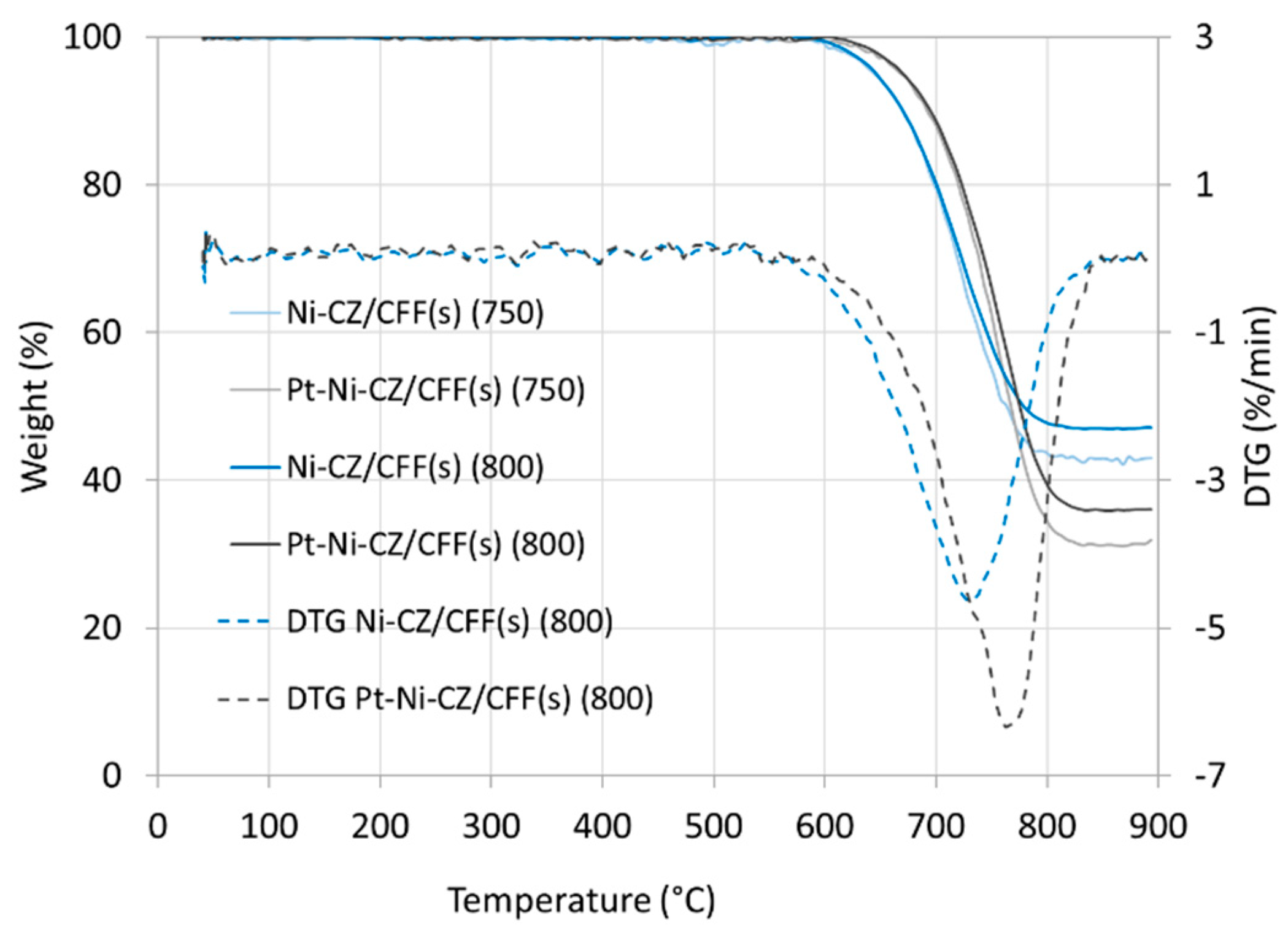
2.1. Catalyst Characterization
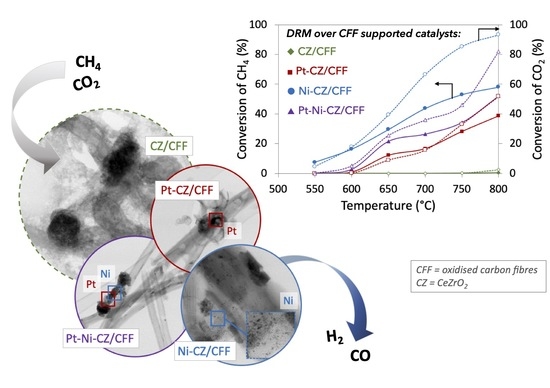
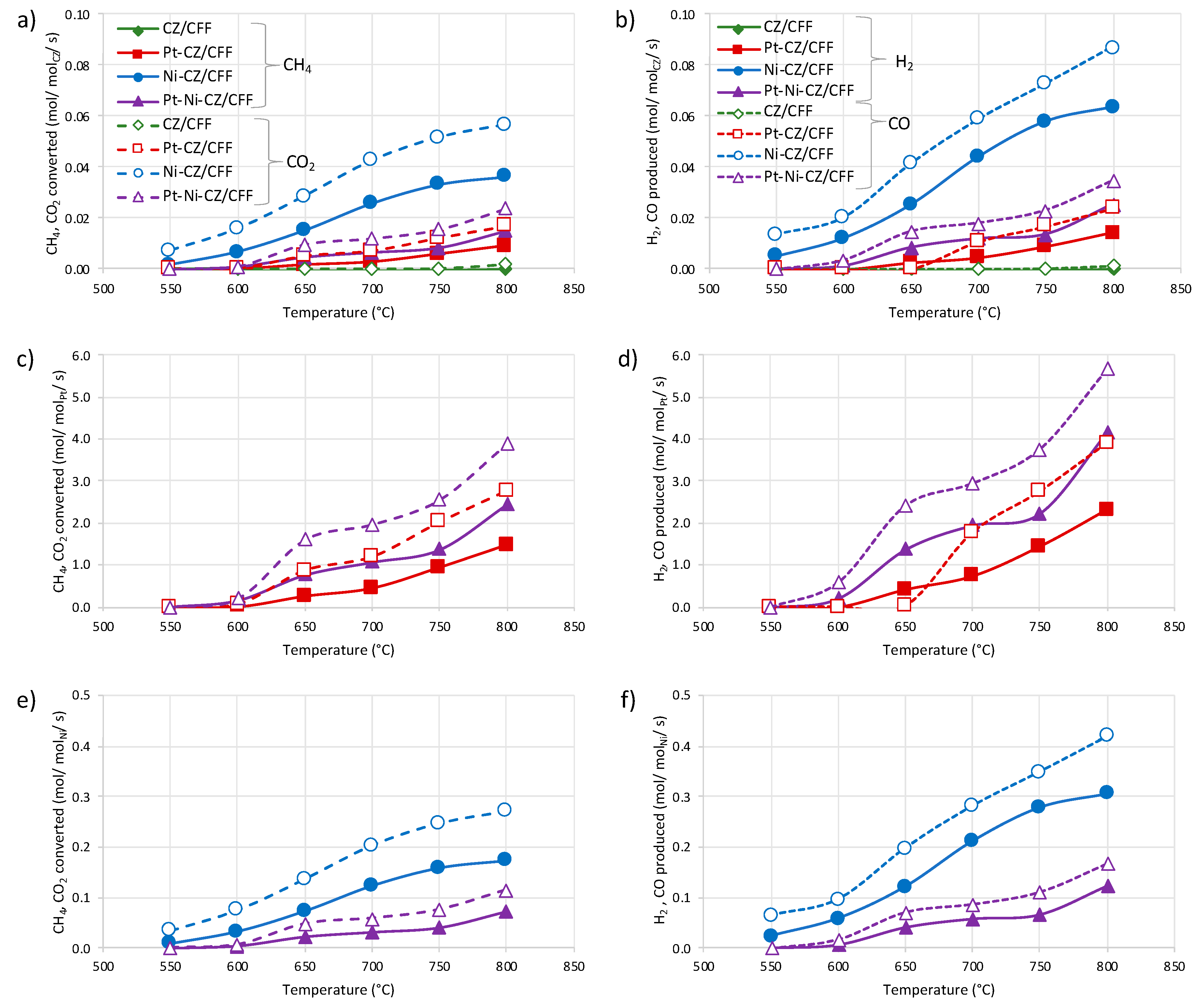
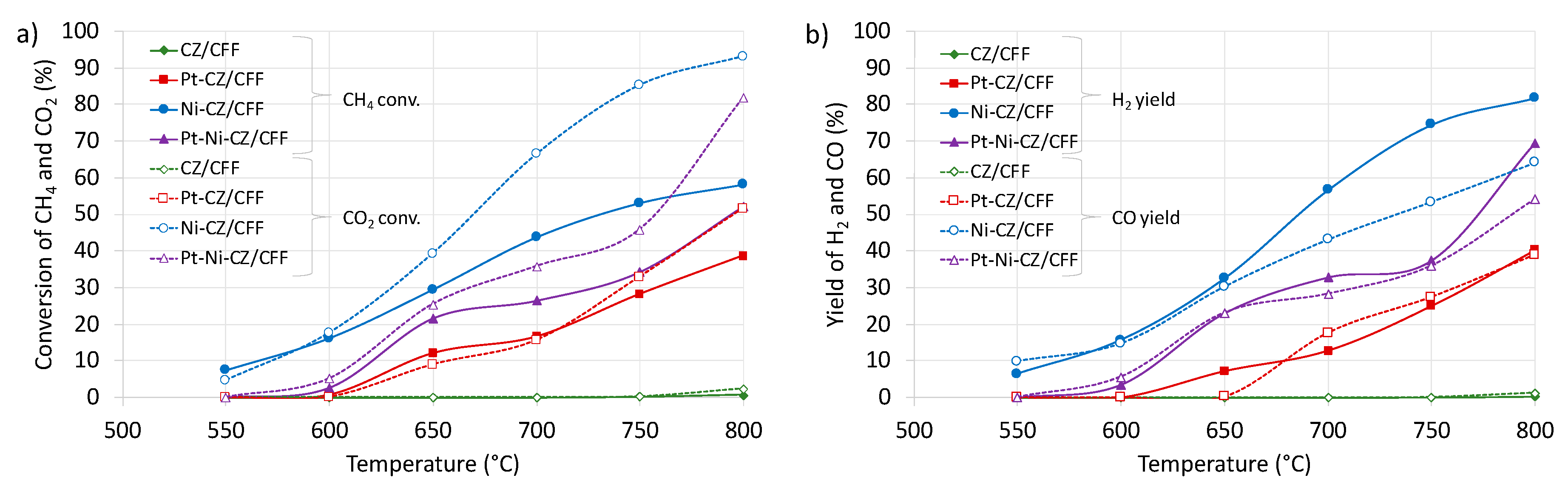
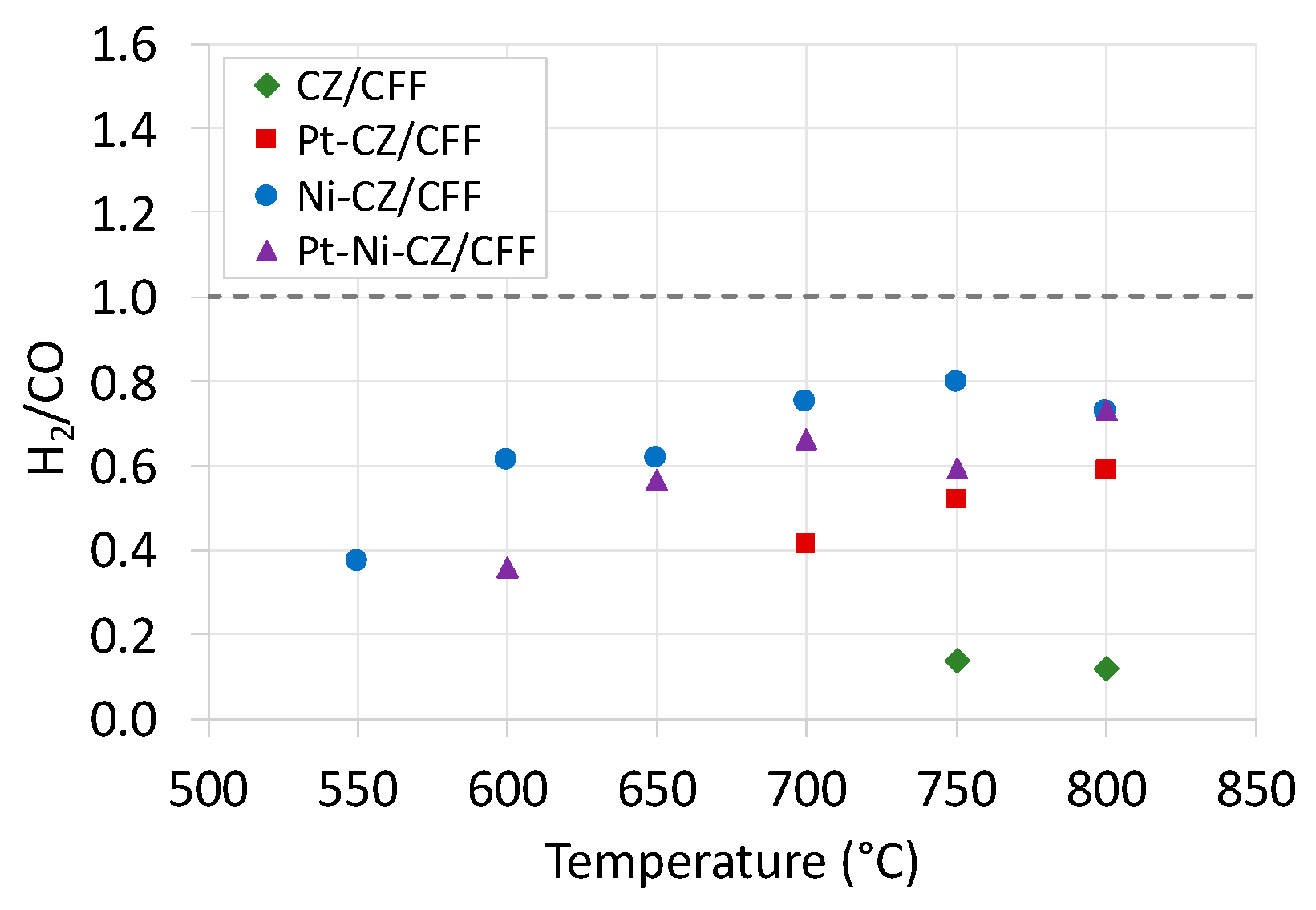
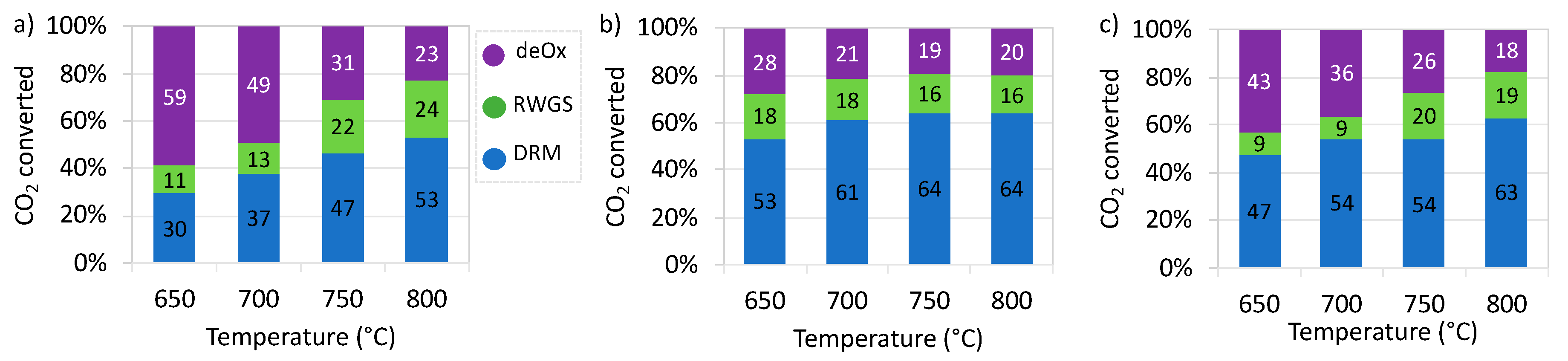
2.2. Catalytic Tests of Dry Reforming of Methane (DRM)
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterisation
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.-J.; Da, Y.-B. The decomposition of energy-related carbon emission and its decoupling with economic growth in China. Renew. Sustain. Energy Rev. 2015, 41, 1255–1266. [Google Scholar] [CrossRef]
- Davis, S.J.; Caldeira, K.; Matthews, H.D. Future CO2 Emissions and Climate Change from Existing Energy Infrastructure. Science 2010, 329, 1330–1333. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Guo, X.; Xiao, B. What causes growth of global greenhouse gas emissions? Evidence from 40 countries. Sci. Total Environ. 2019, 661, 750–766. [Google Scholar] [CrossRef]
- Royal Society (Great Britain); Royal Academy of Engineering (Great Britain). Greenhouse Gas Removal. 2018. Available online: https://www.raeng.org.uk/publications/reports/greenhouse-gas-removal (accessed on 28 April 2021).
- Zhao, Y.; Kang, Y.; Li, H.; Li, H. CO2 conversion to synthesis gas via DRM on the durable Al2O3/Ni/Al2O3 sandwich catalyst with high activity and stability. Green Chem. 2018, 20, 2781–2787. [Google Scholar] [CrossRef]
- Shagdar, E.; Lougou, B.G.; Shuai, Y.; Ganbold, E.; Chinonso, O.P.; Tan, H. Process analysis of solar steam reforming of methane for producing low-carbon hydrogen. RSC Adv. 2020, 10, 12582–12597. [Google Scholar] [CrossRef]
- Kikuchi, E.; Chen, Y. Syngas Formation by Partial Oxidation of Methane in Palladium Membrane Reactor. In Studies in Surface Science and Catalysis; Parmaliana, A., Sanfilippo, D., Frusteri, F., Vaccari, A., Arena, F., Eds.; Natural Gas Conversion V; Elsevier: Amsterdam, The Netherlands, 1998; Volume 119, pp. 441–446. [Google Scholar]
- Castro-Luna, A.E.; Iriarte, M.E. Carbon dioxide reforming of methane over a metal modified Ni-Al2O3 catalyst. Appl. Catal. Gen. 2008, 343, 10–15. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Annesini, M.C.; Piemonte, V.; Turchetti, L. Carbon formation in the steam reforming process: A thermodynamic analysis based on the elemental compositions. Chem. Eng. Trans. 2007, 11, 21–26. [Google Scholar]
- García-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Malpartida, I.; Alemany, L.J. Transient study of the dry reforming of methane over Pt supported on different γ-Al2O3. Catal. Today 2010, 149, 380–387. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Yan, Z.-F. Syngas Production by Methane Reforming with Carbon Dioxide on Noble Metal Catalysts. J. Nat. Gas Chem. 2006, 15, 327–334. [Google Scholar] [CrossRef]
- Gronchi, P.; Mazzocchia, C.; del Rosso, R. Carbon dioxide reaction with methane on La2O3 supported Rh catalysts. Energy Convers. Manag. 1995, 36, 605–608. [Google Scholar] [CrossRef]
- Xu, Y.; Du, X.; Li, J.; Wang, P.; Zhu, J.; Ge, F.; Zhou, J.; Song, M.; Zhu, W. A comparison of Al2O3 and SiO2 supported Ni-based catalysts in their performance for the dry reforming of methane. J. Fuel Chem. Technol. 2019, 47, 199–208. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Pham, G.H.; Ran, R.; Vagnoni, R.; Pareek, V.; Liu, S. Dry reforming of methane over Co–Mo/Al2O3 catalyst under low microwave power irradiation. Catal. Sci. Technol. 2018, 8, 5315–5324. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Z.; Liu, S.; Wang, Z.; Liu, C.; Huang, W.; Huang, J.; Liu, P. Dry Reforming of Methane on Single-Site Ni/MgO Catalysts: Importance of Site Confinement. ACS Catal. 2018, 8, 9821–9835. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Wu, Y.; Pan, J.; Zhang, Q.; Tan, Y.; Han, Y. Insight into the effects of the oxygen species over Ni/ZrO2 catalyst surface on methane reforming with carbon dioxide. Appl. Catal. B Environ. 2019, 244, 427–437. [Google Scholar] [CrossRef]
- Alotaibi, R.; Alenazey, F.; Alotaibi, F.; Wei, N.; Al-Fatesh, A.; Fakeeha, A. Ni catalysts with different promoters supported on zeolite for dry reforming of methane. Appl. Petrochem. Res. 2015, 5, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Najfach, A.J.; Almquist, C.B.; Edelmann, R.E. Effect of Manganese and zeolite composition on zeolite-supported Ni-catalysts for dry reforming of methane. Catal. Today 2020. [Google Scholar] [CrossRef]
- Łamacz, A.; Jagódka, P.; Stawowy, M.; Matus, K. Dry Reforming of Methane over CNT-Supported CeZrO2, Ni and Ni-CeZrO2 Catalysts. Catalysts 2020, 10, 741. [Google Scholar] [CrossRef]
- Figueira, C.E.; Moreira, P.F.; Giudici, R.; Alves, R.M.B.; Schmal, M. Nanoparticles of Ce, Sr, Co in and out the multi-walled carbon nanotubes applied for dry reforming of methane. Appl. Catal. Gen. 2018, 550, 297–307. [Google Scholar] [CrossRef]
- Otsuka, K.; Ogihara, H.; Takenaka, S. Decomposition of methane over Ni catalysts supported on carbon fibers formed from different hydrocarbons. Carbon 2003, 41, 223–233. [Google Scholar] [CrossRef]
- Wang, H.; Han, J.; Bo, Z.; Qin, L.; Wang, Y.; Yu, F. Non-thermal plasma enhanced dry reforming of CH4 with CO2 over activated carbon supported Ni catalysts. Mol. Catal. 2019, 475, 110486. [Google Scholar] [CrossRef]
- Cai, X.; Hu, Y.H. Advances in catalytic conversion of methane and carbon dioxide to highly valuable products. Energy Sci. Eng. 2019, 7, 4–29. [Google Scholar] [CrossRef] [Green Version]
- Boldrin, P.; Ruiz-Trejo, E.; Mermelstein, J.; Menéndez, J.M.B.; Reina, T.R.; Brandon, N.P. Strategies for carbon and sulfur tolerant solid oxide fuel cell materials, incorporating lessons from heterogeneous catalysis. Chem. Rev. 2016, 116, 13633–13684. [Google Scholar] [CrossRef] [PubMed]
- Djinović, P.; Batista, J.; Pintar, A. Efficient catalytic abatement of greenhouse gases: Methane reforming with CO2 using a novel and thermally stable Rh–CeO2 catalyst. Int. J. Hydrog. Energy 2012, 37, 2699–2707. [Google Scholar] [CrossRef]
- Rostrupnielsen, J.R.; Hansen, J.H.B. CO2-Reforming of Methane over Transition Metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Singh, S.A.; Madras, G. Sonochemical synthesis of Pt, Ru doped TiO2 for methane reforming. Appl. Catal. Gen. 2016, 518, 102–114. [Google Scholar] [CrossRef]
- Li, D.; Nakagawa, Y.; Tomishige, K. Methane reforming to synthesis gas over Ni catalysts modified with noble metals. Appl. Catal. Gen. 2011, 408, 1–24. [Google Scholar] [CrossRef]
- Crisafulli, C.; Scirè, S.; Maggiore, R.; Minicò, S.; Galvagno, S. CO2 reforming of methane over Ni–Ru and Ni–Pd bimetallic catalysts. Catal. Lett. 1999, 59, 21–26. [Google Scholar] [CrossRef]
- Choi, J.-S.; Moon, K.-I.; Kim, Y.G.; Lee, J.S.; Kim, C.-H.; Trimm, D.L. Stable carbon dioxide reforming of methane over modified Ni/Al2O3 catalysts. Catal. Lett. 1998, 52, 43–47. [Google Scholar] [CrossRef]
- Yokota, S.; Okumura, K.; Niwa, M. Support Effect of Metal Oxide on Rh Catalysts in the CH4-CO2 Reforming Reaction. Catal. Lett. 2002, 84, 131–134. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Comparative study at low and medium reaction temperatures of syngas production by methane reforming with carbon dioxide over silica and alumina supported catalysts. Appl. Catal. Gen. 1998, 170, 177–187. [Google Scholar] [CrossRef]
- Soria, M.A.; Mateos-Pedrero, C.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Thermodynamic and experimental study of combined dry and steam reforming of methane on Ru/ ZrO-LaO catalyst at low temperature. Int. J. Hydrogen. Energy 2011, 36, 15212–15220. [Google Scholar] [CrossRef]
- Kim, J.-H.; Suh, D.J.; Park, T.-J.; Kim, K.-L. Effect of metal particle size on coking during CO2 reforming of CH4 over Ni–alumina aerogel catalysts. Appl. Catal. A Gen. 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, C.; Park, J.S.; Lee, H. Highly Coke-Resistant Ni Nanoparticle Catalysts with Minimal Sintering in Dry Reforming of Methane. ChemSusChem 2014, 7, 451–456. [Google Scholar] [CrossRef]
- Han, J.W.; Park, J.S.; Choi, M.S.; Lee, H. Uncoupling the size and support effects of Ni catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 203, 625–632. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; Shi, Y.; Zhao, B.; Wang, M.; Liu, Q.; Wang, J.; Long, K.; Duan, Y.; Ning, P. A sintering and carbon-resistant Ni-SBA-15 catalyst prepared by solid-state grinding method for dry reforming of methane. J. CO2 Util. 2017, 17, 10–19. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Zeaiter, J.; Kwapinski, W.; Ahmad, M.N. Thermodynamic analysis of methane dry reforming: Effect of the catalyst particle size on carbon formation. Energy Convers. Manag. 2017, 150, 614–622. [Google Scholar] [CrossRef]
- Seo, H.O. Recent Scientific Progress on Developing Supported Ni Catalysts for Dry (CO2) Reforming of Methane. Catalysts 2018, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Abd Ghani, N.A.; Azapour, A.; Syed Muhammad, S.A.F.; Ramli, N.M.; Vo, D.-V.N.; Abdullah, B. Dry reforming of methane for syngas production over Ni–Co-supported Al2O3–MgO catalysts. Appl. Petrochem. Res. 2018, 8, 263–270. [Google Scholar] [CrossRef]
- Hu, Y.H.; Ruckenstein, E. Catalytic conversion of methane to synthesis gas by partial oxidation and CO2, reforming. ChemInform 2004, 35, 297–345. [Google Scholar] [CrossRef]
- Chein, R.-Y.; Fung, W.-Y. Syngas production via dry reforming of methane over CeO2 modified Ni/Al2O3 catalysts. Int. J. Hydrogen Energy 2019, 44, 14303–14315. [Google Scholar] [CrossRef]
- Liotta, L.F.; Macaluso, A.; Longo, A.; Pantaleo, G.; Martorana, A.; Deganello, G. Effects of redox treatments on the structural composition of a ceria–zirconia oxide for application in the three-way catalysis. Appl. Catal. Gen. 2003, 240, 295–307. [Google Scholar] [CrossRef]
- Kim, J.-R.; Lee, K.-Y.; Suh, M.-J.; Ihm, S.-K. Ceria–zirconia mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as catalyst support. Catal. Today 2012, 185, 25–34. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic dry reforming of methane over high surface area ceria. Appl. Catal. B Environ. 2005, 60, 107–116. [Google Scholar] [CrossRef]
- Gutiérrez-Ortiz, J.I.; de Rivas, B.; López-Fonseca, R.; González-Velasco, J.R. Characterization of the catalytic properties of ceria-zirconia mixed oxides by temperature-programmed techniques. J. Therm. Anal. Calorim. 2005, 80, 225–228. [Google Scholar] [CrossRef]
- Li, C.; Tan, P.-J.; Li, X.-D.; Du, Y.-L.; Gao, Z.-H.; Huang, W. Effect of the addition of Ce and Zr on the structure and performances of Ni–Mo/CeZr–MgAl(O) catalysts for CH4–CO2 reforming. Fuel Process. Technol. 2015, 140, 39–45. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane dry reforming over ceria-zirconia supported Ni catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Liu, Z.; Chen, X.; Rui, N.; Betancourt, L.E.; Lin, L.; Xu, W.; Sun, C.-J.; Abeykoon, A.M.M.; Rodriguez, J.A.; et al. The Effects of Zr-doping into Ceria for the Dry Reforming of Methane over Ni/CeZrO2 catalysts: In-situ Studies with XRD, XAFS and AP-XPS. ACS Catal. 2020, 10, 3274–3284. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Yang, J.; Zhang, J.; Zhang, L.; Li, H.; Zhao, Y.; Li, H.X.; Wu, K.; Xu, G.Q.; et al. Enhanced catalytic performance of Ir catalysts supported on ceria-based solid solutions for methane dry reforming reaction. Catal. Today 2017, 281, 295–303. [Google Scholar] [CrossRef]
- Mesrar, F.; Kacimi, M.; Liotta, L.F.; Puleo, F.; Ziyad, M. Syngas production from dry reforming of methane over ni/perlite catalysts: Effect of zirconia and ceria impregnation. Int. J. Hydrog. Energy 2018, 43, 17142–17155. [Google Scholar] [CrossRef]
- Nguyen, T.G.H.; Tran, D.L.; Sakamoto, M.; Uchida, T.; Sasaki, K.; To, T.D.; Doan, D.C.T.; Dang, M.C.; Shiratori, Y. Ni-loaded (Ce,Zr)O2-δ-dispersed paper-structured catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2018, 43, 4951–4960. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, G.; Xue, Q.; Chen, L.; Lu, Y. High carbon-resistance Ni/CeAlO3-Al2O3 catalyst for CH4/CO2 reforming. Appl. Catal. B Eniviron. 2013, 136, 260–268. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Sudarno, U.R.; Zainal, Z. CeO2-SiO2 supported nickel catalysts for dry reforming of methane toward syngas production. Appl. Catal. A Gen. 2013, 468, 359–369. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M. Ce promoting effect on the activity and coke formation of Ni catalysts supported on mesoporous nanocrystalline γ-Al2O3 in autothermal reforming of methane. Int. J. Hydrog. Energy 2017, 42, 11130–11138. [Google Scholar] [CrossRef]
- Smirnova, M.Y.; Bobin, A.S.; Pavlova, S.N.; Ishchenko, A.V.; Selivanova, A.V.; Kaichev, V.V.; Cherepanova, S.V.; Krieger, T.A.; Arapova, M.V.; Roger, A.-C.; et al. Methane dry reforming over Ni catalysts supported on Ce–Zr oxides prepared by a route involving supercritical fluids. Open Chem. 2017, 15, 412–425. [Google Scholar] [CrossRef]
- Le Saché, E.; Pastor-Pérez, L.; Watson, D.; Sepúlveda-Escribano, A.; Reina, T.R. Ni stabilised on inorganic complex structures: Superior catalysts for chemical CO2 recycling via dry reforming of methane. Appl. Catal. B Environ. 2018, 236, 458–465. [Google Scholar] [CrossRef]
- Ross, R.A.; Fairbridge, C.; MacCallum, J.R. Carbon fibres as supports for transition metal catalysts in hydrocarbon oxidation reactions. Carbon 1985, 23, 209–213. [Google Scholar] [CrossRef]
- Shiba, K.; Tagaya, M.; Samitsu, S.; Motozuka, S. Effective Surface Functionalization of Carbon Fibers for Fiber/Polymer Composites with Tailor-Made Interfaces. ChemPlusChem 2014, 79, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Huang, B.; Wang, W.; Wei, Z.; Ye, D. Low-temperature SCR of NO with NH3 over CeO2 supported on modified activated carbon fibers. Catal. Commun. 2011, 12, 394–398. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.; Sun, L.; Zhu, S.; Huang, Z.; Mao, Y.; Lu, W.; Chen, W. Efficient removal of dyes using heterogeneous Fenton catalysts based on activated carbon fibers with enhanced activity. Chem. Eng. Sci. 2013, 101, 424–431. [Google Scholar] [CrossRef]
- Yuan, R.; Guan, R.; Liu, P.; Zheng, J. Photocatalytic treatment of wastewater from paper mill by TiO2 loaded on activated carbon fibers. Colloids Surf. Physicochem. Eng. Asp. 2007, 293, 80–86. [Google Scholar] [CrossRef]
- Lin, C.-L.; Cheng, Y.-H.; Liu, Z.-S.; Chen, J.-Y. Metal catalysts supported on activated carbon fibers for removal of polycyclic aromatic hydrocarbons from incineration flue gas. J. Hazard. Mater. 2011, 197, 254–263. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Y.; Sun, L.; Mao, Y.; Lu, W.; Huang, S.; Chen, W. Rapid removal of dyes under visible irradiation over activated carbon fibers supported Fe(III)–citrate at neutral pH. Sep. Purif. Technol. 2014, 122, 449–455. [Google Scholar] [CrossRef]
- Tien, P.D.; Satoh, T.; Miura, M.; Nomura, M. Continuous hydrogen evolution from cyclohexanes over platinum catalysts supported on activated carbon fibers. Fuel Process. Technol. 2008, 89, 415–418. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Yuranov, I.; Suvorova, E.I.; Buffat, P.A.; Kiwi-Minsker, L. Highly dispersed gold on activated carbon fibers for low-temperature CO oxidation. J. Catal. 2004, 224, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Jagdale, P.; Castellino, M.; Rovere, M.; Jehangir, Q.; Mandracci, P.; Rosso, C.; Tagliaferro, A. Innovative functionalized carbon fibers from waste: How to enhance polymer composites properties. Compos. B Part Eng. 2018, 139, 31–39. [Google Scholar] [CrossRef]
- Dobiášová, L.; Starý, V.; Glogar, P.; Valvoda, V. Analysis of carbon fibers and carbon composites by asymmetric X-ray diffraction technique. Carbon 1999, 37, 421–425. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G. Preparation of high oxygen storage capacity and thermally stable ceria–zirconia solid solution. Catal. Sci. Technol. 2016, 6, 897–907. [Google Scholar] [CrossRef] [Green Version]
- Shao, S.; Shi, A.-W.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. Hydrogen production from steam reforming of glycerol over Ni/CeZrO2 catalysts. Fuel Process Technol. 2014, 125, 1–7. [Google Scholar] [CrossRef]
- Matte, L.P.; Kilian, A.S.; Luza, L.; Alves, M.C.; Morais, J.; Baptista, D.L.; Dupont, J.; Bernardi, F. Influence of the CeO2 Support on the Reduction Properties of Cu/CeO2 and Ni/CeO2 Nanoparticles. J. Phys. Chem. C 2015, 119, 26459–26470. [Google Scholar] [CrossRef]
- Thill, A.S.; Kilian, A.S.; Bernardi, F. Key Role Played by Metallic Nanoparticles on the Ceria Reduction. J. Phys. Chem. C 2017, 121, 25323–25332. [Google Scholar] [CrossRef]
- Mao, M.; Zhang, Q.; Yang, Y.; Li, Y.; Huang, H.; Jiang, Z.; Hu, Q.; Zhao, X. Solar-light-driven CO reduction by methane on Pt nanocrystals partially embedded in mesoporous CeO2 nanorods with high light-to-fuel efficiency. Green Chem. 2018, 20, 2857–2869. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, X.; Xu, L.; Wan, C.; Ye, M. Pt-Ni Nanoalloys for H2 Generation from Hydrous Hydrazine. Catalysts 2020, 10, 930. [Google Scholar] [CrossRef]
- Vayssilov, G.N.; Lykhach, Y.; Migani, A.; Staudt, T.; Petrova, G.P.; Tsud, N.; Skála, T.; Matolı’n, V.; Neyman, K.M.; Libuda, J. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 2011, 10, 310–315. [Google Scholar] [CrossRef]
- Mahoney, E.G.; Pusel, J.M.; Stagg-Williams, S.M.; Faraji, S. The effects of Pt addition to supported Ni catalysts on dry (CO2) reforming of methane to syngas. J. CO2 Util. 2014, 6, 40–44. [Google Scholar] [CrossRef]
- Araiza, D.G.; Arcos, D.G.; Gómez-Cortés, A.; Díaz, G. Dry reforming of methane over Pt-Ni/CeO2 catalysts: Effect of the metal composition on the stability. Catal. Today 2021, 360, 46–54. [Google Scholar] [CrossRef]
- Gould, T.D.; Montemore, M.M.; Lubers, A.M.; Ellis, L.D.; Weimer, A.W.; Falconer, J.L.; Medlin, J.W. Enhanced dry reforming of methane on Ni and Ni-Pt catalysts synthesized by atomic layer deposition. Appl. Catal. A Gen. 2015, 492, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Croiset, E.; Ricardez-Sandoval, L. Methane dissociation on Ni (100), Ni (111), and Ni (553): A comparative density functional theory study. J. Mol. Catal. A Chem. 2012, 365, 103–114. [Google Scholar] [CrossRef]
- Liang, W.; Yan, H.; Chen, C.; Lin, D.; Tan, K.; Feng, X.; Liu, Y.; Chen, X.; Yang, C.; Shan, H. Revealing the Effect of Nickel Particle Size on Carbon Formation Type in the Methane Decomposition Reaction. Catalysts 2020, 10, 890. [Google Scholar] [CrossRef]
- Niu, J.; Ran, J.; Du, X.; Qi, W.; Zhang, P.; Yang, L. Effect of Pt addition on resistance to carbon formation of Ni catalysts in methane dehydrogenation over Pt-Ni bimetallic surfaces: A density functional theory study. Mol. Catal. 2017, 434, 206–218. [Google Scholar] [CrossRef]
- Fan, C.; Zhu, Y.-A.; Xu, Y.; Zhou, Y.; Zhou, X.-G.; Chen, D. Origin of synergistic effect over Ni-based bimetallic surfaces: A density functional theory study. J. Chem. Phys. 2012, 137, 014703. [Google Scholar] [CrossRef] [PubMed]
- García-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J. Nanostructured Pt- and Ni-based catalysts for CO2-reforming of methane. J. Catal. 2010, 270, 136–145. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Liland, S.E.; Regli, S.K.; Yang, J.; Rout, K.R.; Luo, J.; Rønning, M.; Ran, J.; Chen, D. Unraveling Enhanced Activity, Selectivity, and Coke Resistance of Pt–Ni Bimetallic Clusters in Dry Reforming. ACS Catal. 2021, 11, 2398–2411. [Google Scholar] [CrossRef]
- Cai, J.; Han, Y.; Chen, S.; Crumlin, E.J.; Yang, B.; Li, Y.; Liu, Z. CO2 Activation on Ni(111) and Ni(100) Surfaces in the Presence of H2O: An Ambient-Pressure X-ray Photoelectron Spectroscopy Study. J. Phys. Chem. C 2019, 123, 12176–12182. [Google Scholar] [CrossRef]
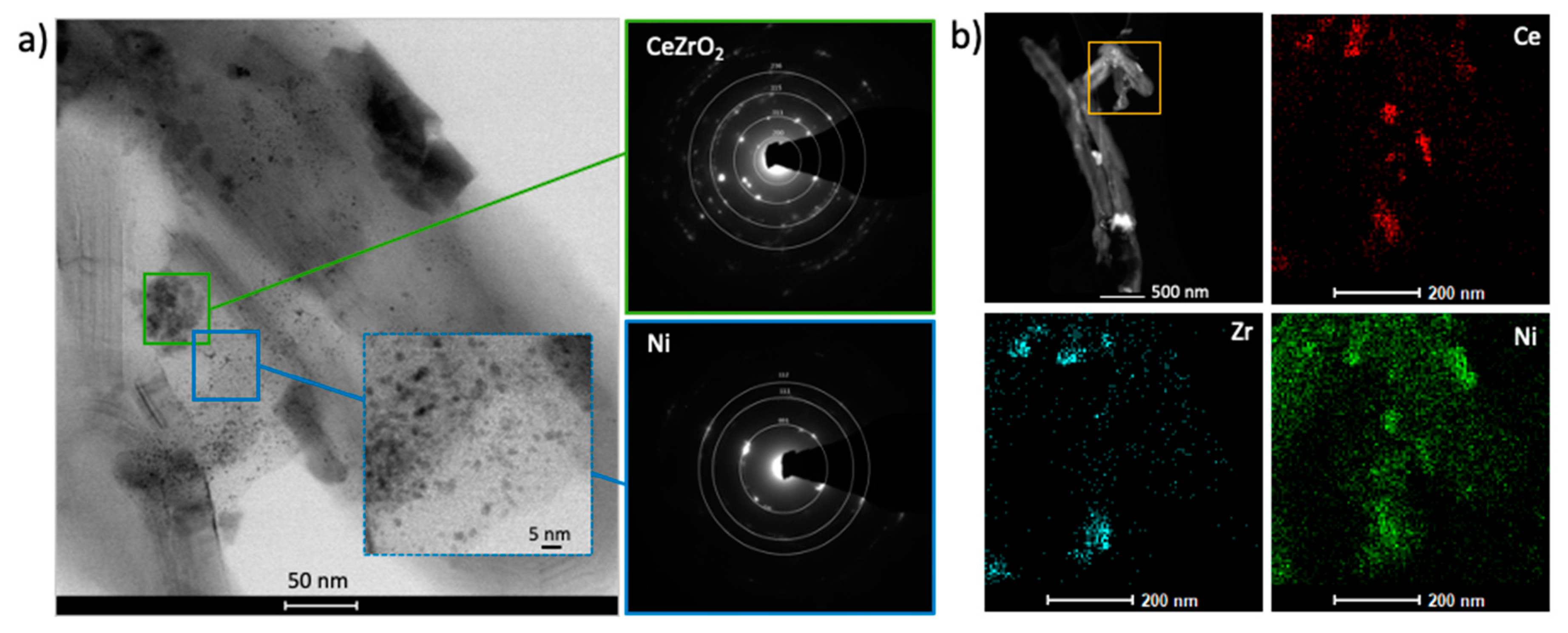


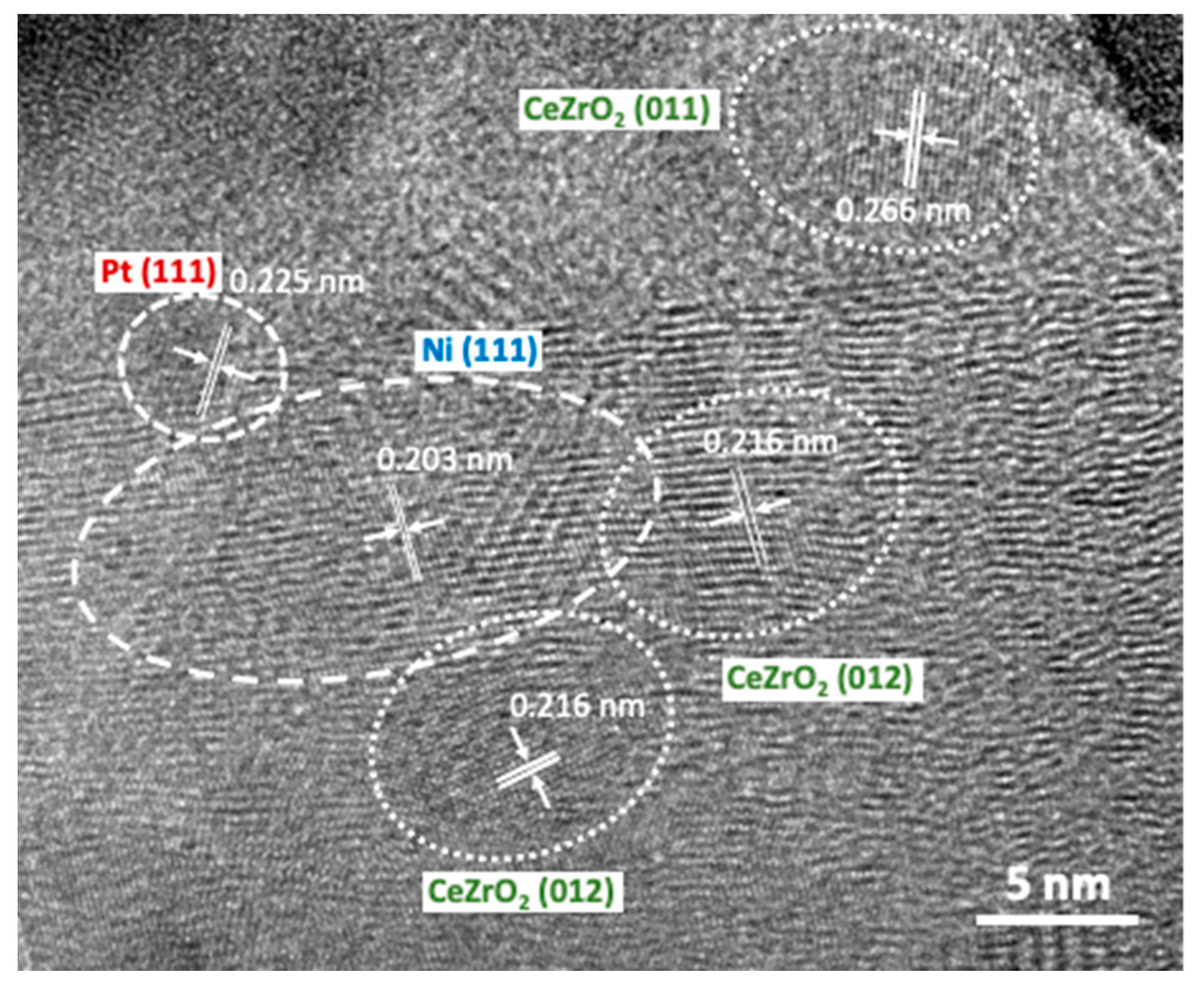
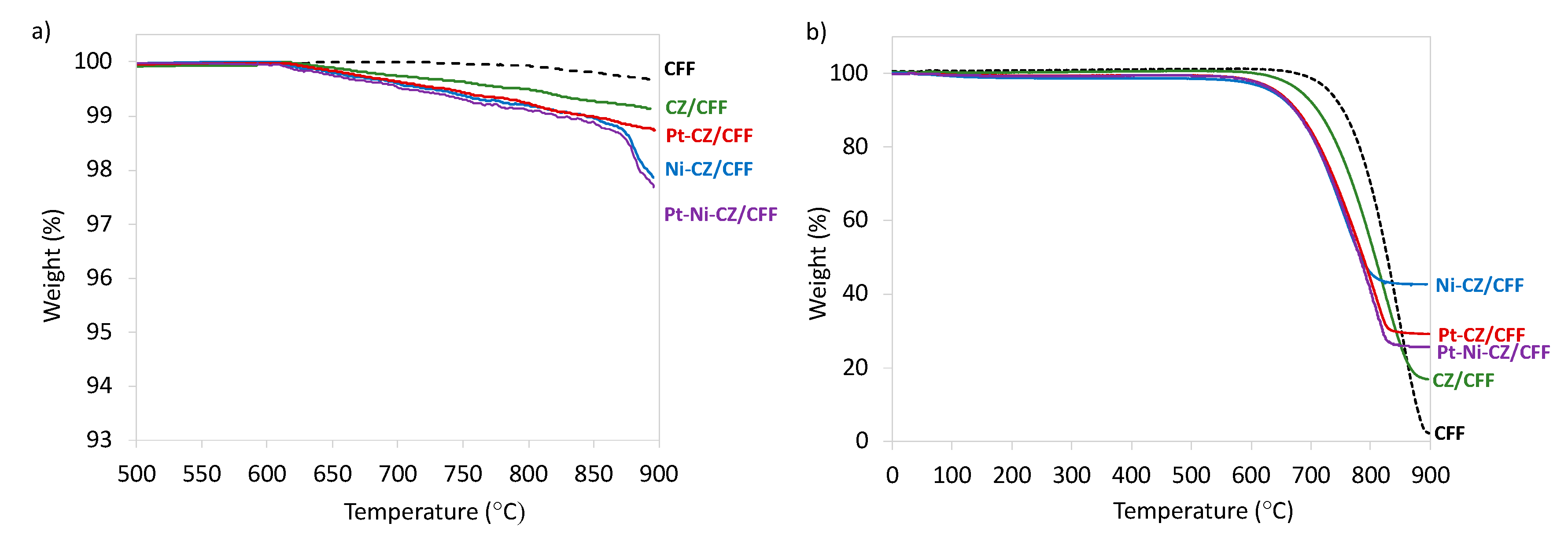
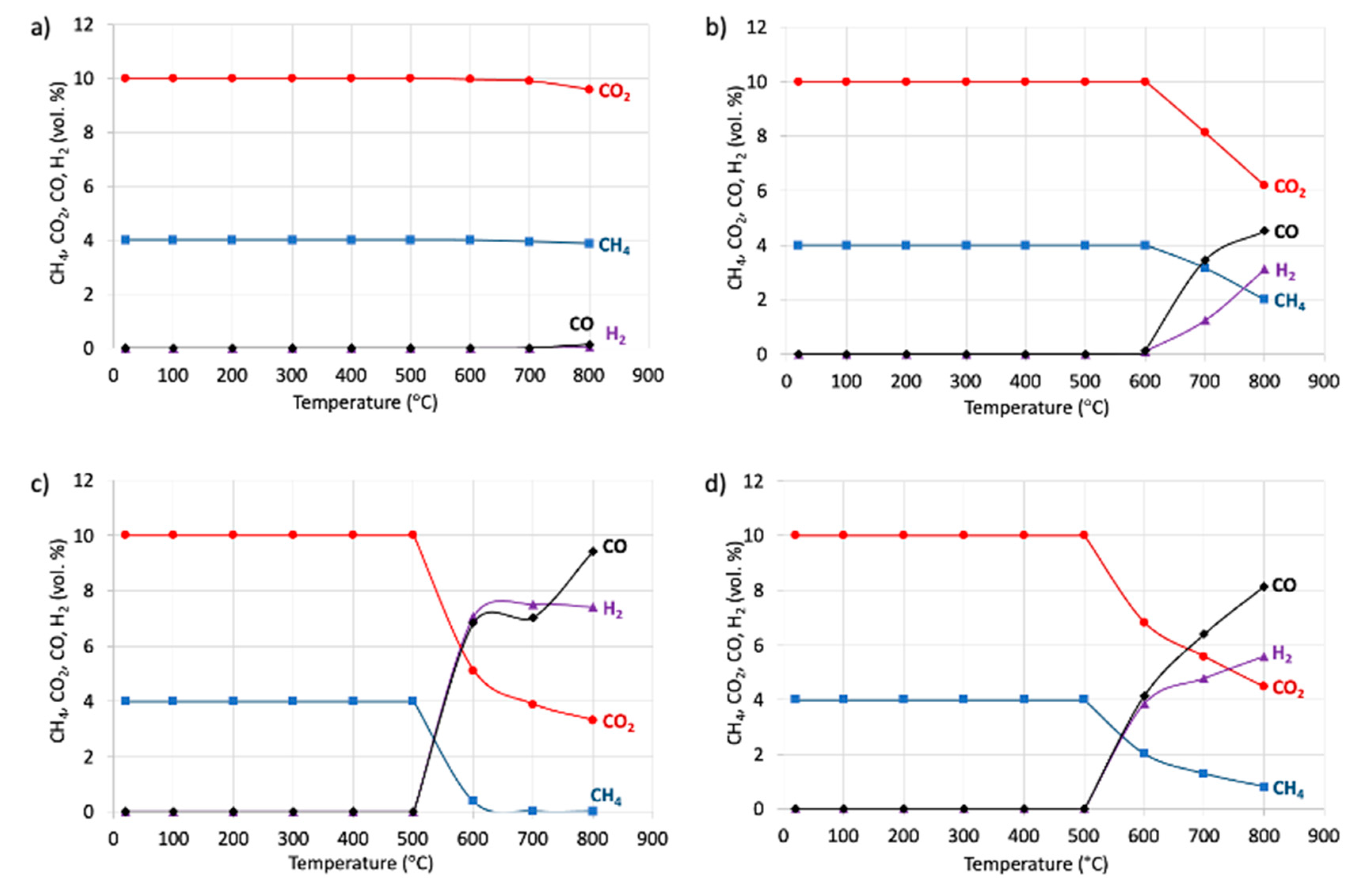

| Sample | D111 (nm) | D200 (nm) | D220 (nm) |
|---|---|---|---|
| CZ/CFF | 3.50 | 2.43 | 3.18 |
| Ni-CZ/CFF | 3.42 | 2.48 | 3.20 |
| Pt-CZ/CFF | 3.40 | 2.46 | 3.22 |
| Pt-Ni-CZ/CFF | 3.37 | 2.40 | 3.40 |
| Sample | SSA (m2/g) | Vt (cc/g) | d (nm) |
|---|---|---|---|
| CF (pristine) | 26 | 0.10 | 3.0 |
| CFF | 100 | 0.17 | 3.7 |
| CZ/CFF | 69 | 0.23 | 3.9 |
| Ni-CZ/CFF | 51 | 0.25 | 1.9 |
| Pt-CZ/CFF | 52 | 0.12 | 3.7 |
| Pt-Ni-CZ/CFF | 44 | 0.15 | 2.5 |
| Sample | D (nm) CeZrO2 (220) | D (nm) Ni (111) |
|---|---|---|
| CZ/CFF(s) | 4.99 | - |
| Ni-CZ/CFF(s) | 4.83 | 9.87 |
| Pt-CZ/CFF(s) | 4.99 | - |
| Pt-Ni-CZ/CFF(s) | 5.34 | 7.35 |
| Sample | CeZrO2 (wt. %) | Ni (wt. %) | Pt (wt. %) |
|---|---|---|---|
| CF (pristine) | - | - | - |
| CFF | - | - | - |
| CZ/CFF | 20 | - | - |
| Ni-CZ/CFF | 20 | 1.6 | - |
| Pt-CZ/CFF | 20 | - | 0.16 |
| Pt-Ni-CZ/CFF | 20 | 1.6 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagódka, P.; Matus, K.; Sobota, M.; Łamacz, A. Dry Reforming of Methane over Carbon Fibre-Supported CeZrO2, Ni-CeZrO2, Pt-CeZrO2 and Pt-Ni-CeZrO2 Catalysts. Catalysts 2021, 11, 563. https://doi.org/10.3390/catal11050563
Jagódka P, Matus K, Sobota M, Łamacz A. Dry Reforming of Methane over Carbon Fibre-Supported CeZrO2, Ni-CeZrO2, Pt-CeZrO2 and Pt-Ni-CeZrO2 Catalysts. Catalysts. 2021; 11(5):563. https://doi.org/10.3390/catal11050563
Chicago/Turabian StyleJagódka, Paulina, Krzysztof Matus, Michał Sobota, and Agata Łamacz. 2021. "Dry Reforming of Methane over Carbon Fibre-Supported CeZrO2, Ni-CeZrO2, Pt-CeZrO2 and Pt-Ni-CeZrO2 Catalysts" Catalysts 11, no. 5: 563. https://doi.org/10.3390/catal11050563
APA StyleJagódka, P., Matus, K., Sobota, M., & Łamacz, A. (2021). Dry Reforming of Methane over Carbon Fibre-Supported CeZrO2, Ni-CeZrO2, Pt-CeZrO2 and Pt-Ni-CeZrO2 Catalysts. Catalysts, 11(5), 563. https://doi.org/10.3390/catal11050563