A Study on the Effect of Different Ball Milling Methods on the NH3-SCR Activity of Aluminum-Laden Bayan Obo Tailings
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRF Multi-Element Analysis Results
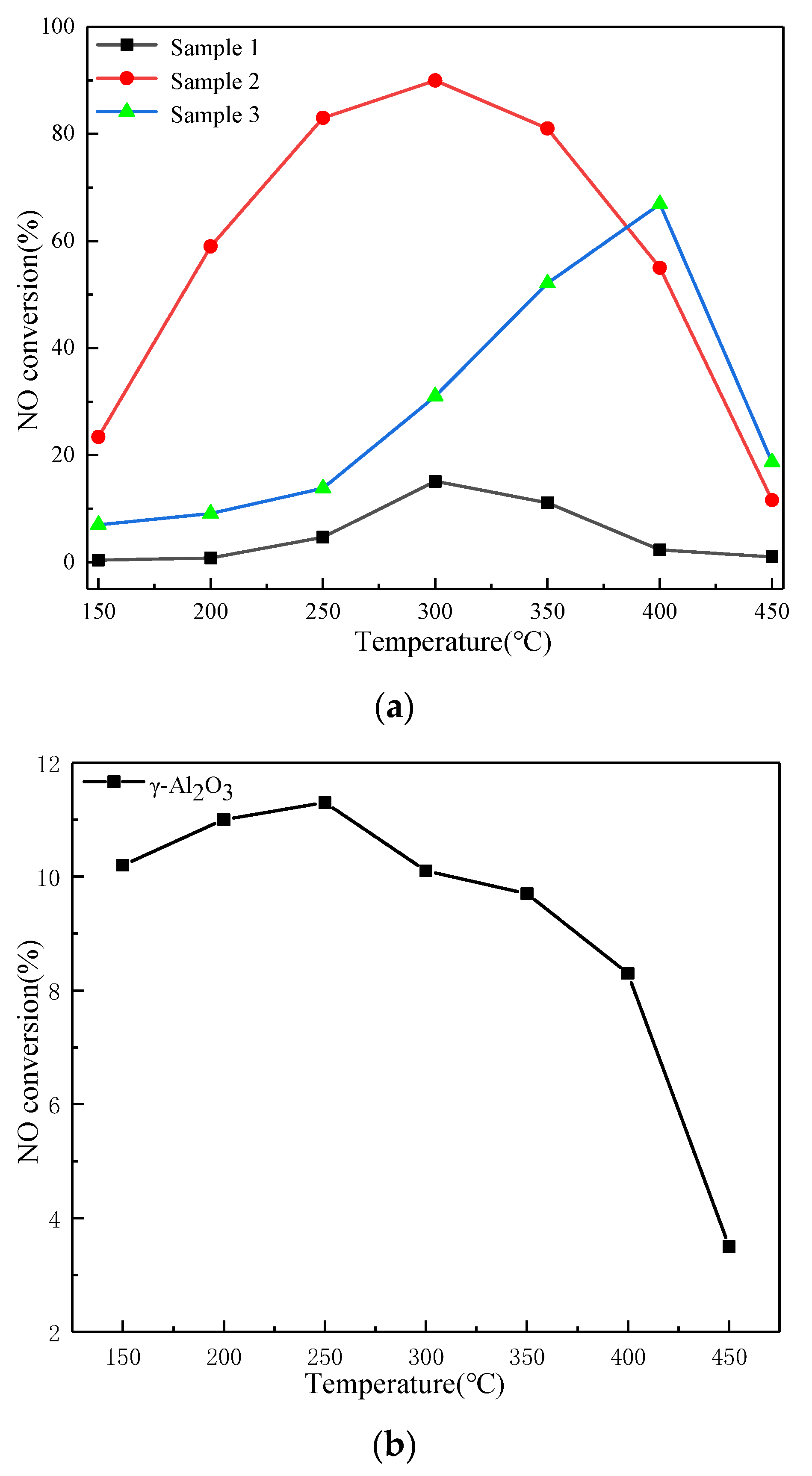
2.2. SCR Catalyst Performance Testing
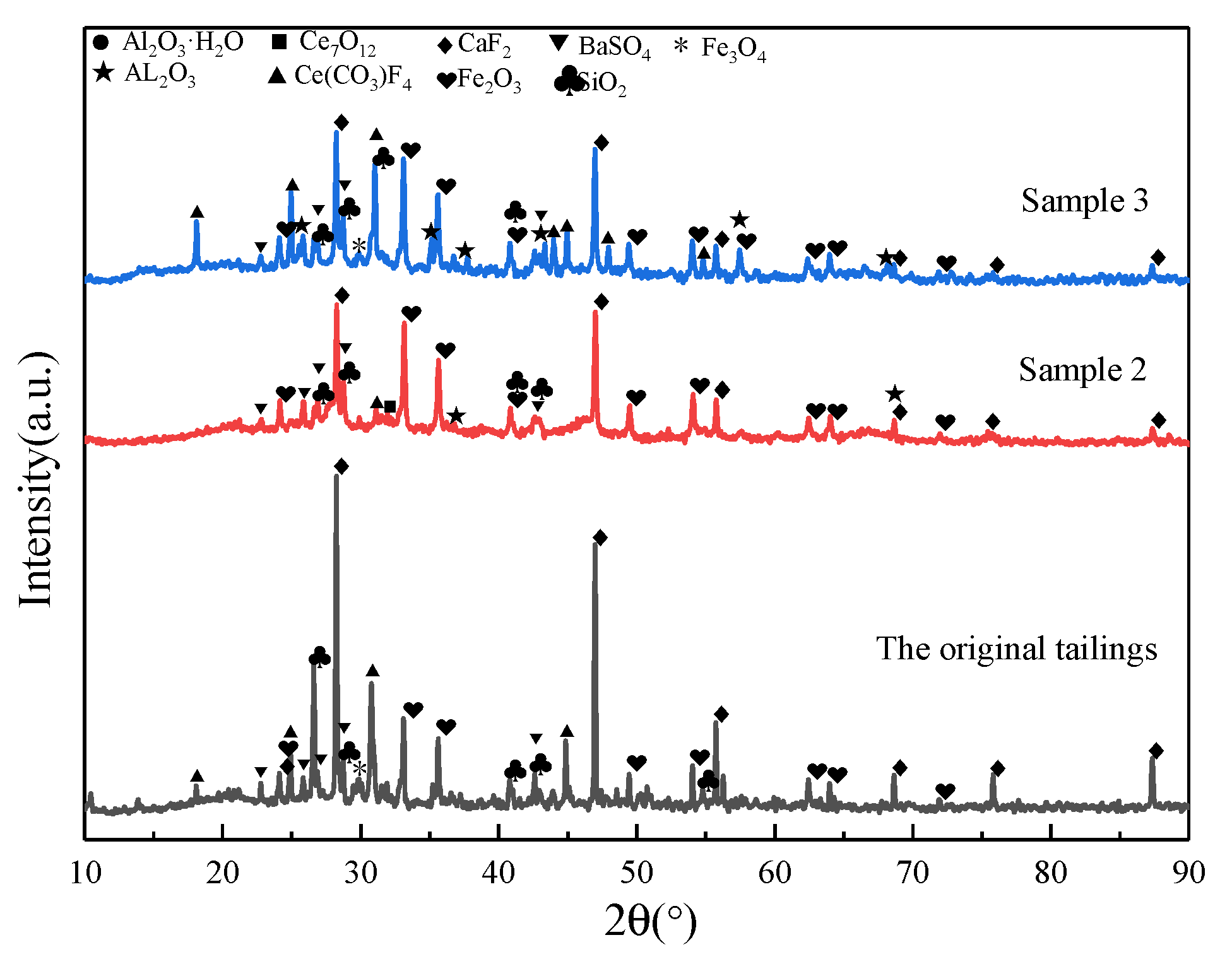
2.3. Analysis of Phase Composition
2.4. Particle Size and Morphology of Catalysts
2.5. Analysis of Specific Surface Area, Pore Volume and Pore Size of the Catalyst
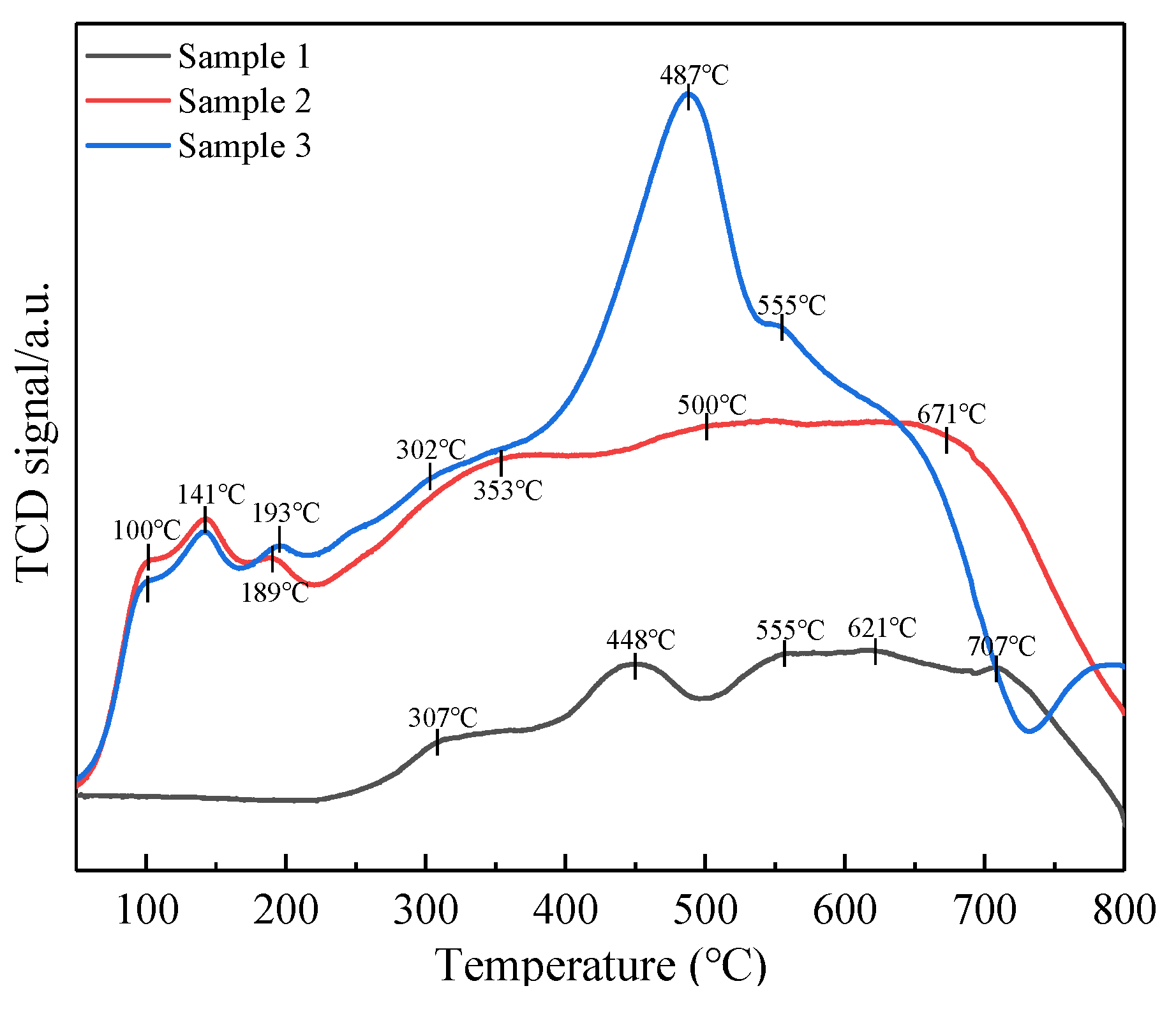
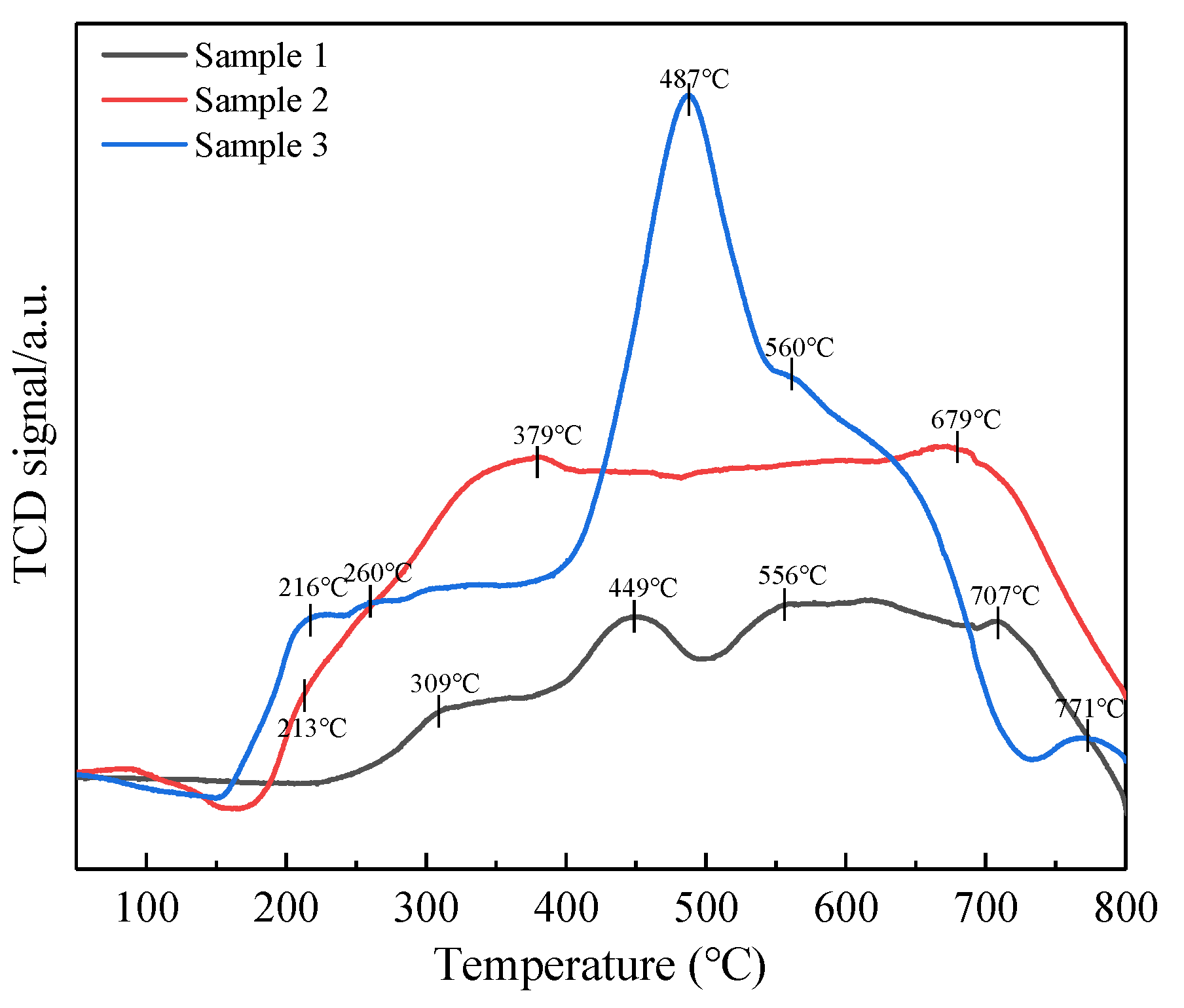
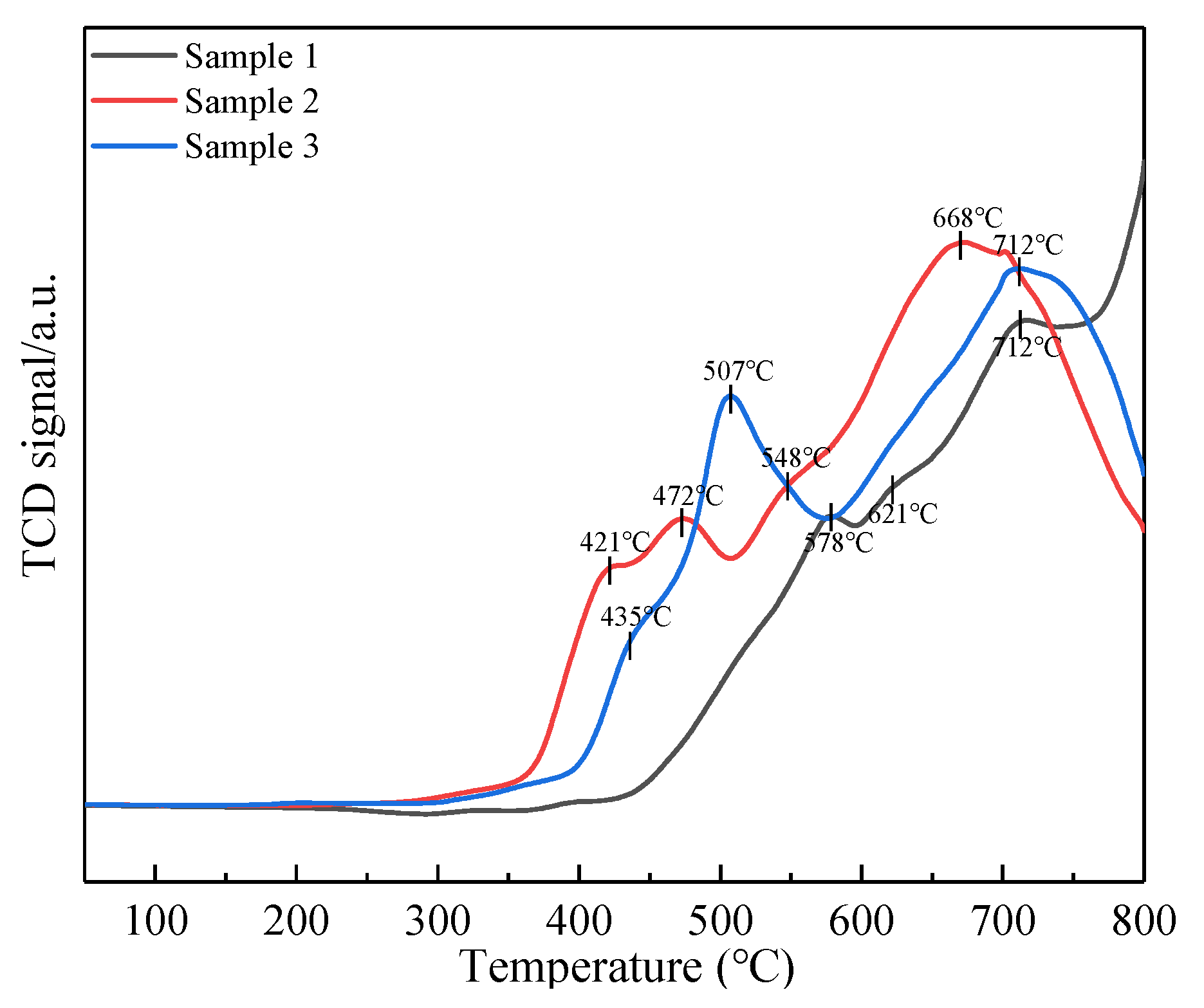
2.6. NH3-TPD, NO-TPD and H2-TPR Analyses
2.6.1. NH3-Temperature-Programed Desorption
2.6.2. NO-Temperature-Programed Desorption
2.6.3. H2-Temperature-Programed Reduction
3. Materials and Methods
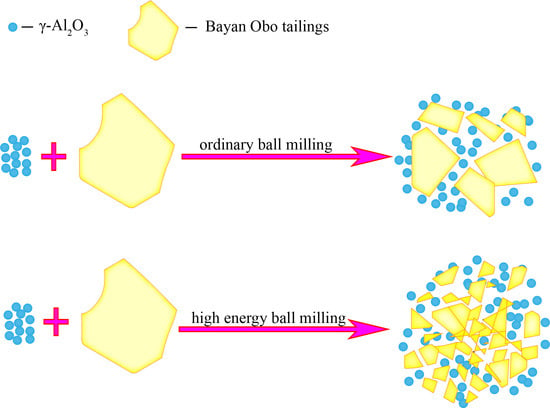
3.1. Preparation of Catalyst Materials
3.2. Methods
3.2.1. Detection of Catalytic Activity
- η—conversion rate of NO under this working condition, %;
- (NO)in—inlet concentration of NO under this working condition, ppm;
- (NO)out—outlet concentration of NO under this working condition, ppm.
3.2.2. Characterization Method
3.2.3. Experimental Equipment and Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, X.-L.; Bai, L.; Wang, Q.-C.; Liu, J.; Chi, M.-Y.; Wang, Z.-C. Recovery of Rare Earths, Niobium, and Thorium from the Tailings of Giant Bayan Obo Ore in China. Metall. Mater. Trans. B 2012, 43, 485–493. [Google Scholar] [CrossRef]
- Song, I.; Youn, S.; Kim, H. Characteristics of Manganese Supported on Hydrous Titanium Oxide Catalysts for the Selective Catalytic Reduction of NOx with Ammonia. Top. Catal. 2016, 59, 1008–1012. [Google Scholar] [CrossRef]
- Mohammad, A.A.; Tabbi, W.; Khaled, E. Transition metal carbides and nitrides as oxygen reduction reaction catalyst or catalyst support in proton exchange membrane fuel cells (PEMFCs). Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Nikolopowlos, A.A.; Stergioula, E.S.; Efthimladis, E.A. Selective catalytic reduction of NO by propane excess oxygen on Pt- and Rh-supported alumina catalysts. J. Catal Today 1999, 54, 439–450. [Google Scholar] [CrossRef]
- László, H.; Kristóf, S.M.; István, E.S. Poisoning and Reuse of Supported Precious Metal Catalysts in the Hydrogenation of N -Heterocycles Part I: Ruthenium-Catalysed Hydrogenation of 1-Methylpyrrole. Catal. Lett. 2018, 148, 1939–1950. [Google Scholar]
- Wang, L. Study on Preparation of Rare Earth-based Doped Oxide Composites Catalytic Materials of from Bayan Obo Rare Earth Tailings; Inner Mongolia University: Hohhot, China, 2017; pp. 30–32. [Google Scholar]
- Duan, L.P.; Wang, W.C.; Shen, M.S. Study on The Process Mineralogy of Nb-bearing Minerals of Fe-separation Tailings in Bayan Obo Ore. J. Disper. Sci. Technol. 2015, 34, 9–12. [Google Scholar]
- Zhang, Y.; Lin, H.; Dong, Y.B.; Xu, X.F.; Wang, X.; Gao, Y.J. Comprehensive Recovery of Iron, Niobium, Rare Earth and Fluorite in Bayan Obo Tailings. Rare Met. 2017, 41, 799–809. [Google Scholar]
- Wang, S.H.; Yang, Z.F.; Wang, Z.J. Study on Niobium and Rare Earth Occurrence State in Bayan Obo Tailings. Nonferr. Met. Eng. 2019, 9, 60–66. [Google Scholar]
- Irene, L.H.; Jesús, M.; Palomares, A.E. The Influence of the Support on the Activity of Mn–Fe Catalysts Used for the Selective Catalytic Reduction of NOx with Ammonia. J. Catal. 2020, 10, 63–68. [Google Scholar]
- Wang, Z.; Huang, Y.; Luo, H.; Gong, Z.; Zhang, K.; Li, N.; Wu, W. Denitrification performance of rare earth tailings-based catalysts. Green Process. Synth. 2019, 8, 865–872. [Google Scholar] [CrossRef]
- Liu, C.; Yang, S.; Ma, L.; Peng, Y.; Hamidreza, A.; Chang, H.; Li, J. Comparison on the Performance of α-Fe2O3 and γ-Fe2O3 for Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. Catal. Lett. 2013, 143, 697–704. [Google Scholar] [CrossRef]
- Ye, D.; Ren, X.; Qu, R.; Liu, S.; Zheng, C.; Gao, X. Designing SO2-resistant cerium-based catalyst by modifying with Fe2O3 for the selective catalytic reduction of NO with NH3. Mol. Catal. 2019, 462, 10–18. [Google Scholar] [CrossRef]
- Cheng, L.S.; Yang, R.T.; Chenb, N. Iron Oxide and Chromia Supported on Titania-Pillared Clay for Selective Catalytic Reduction of Nitric Oxide with Ammonia. J. Catal. 1996, 164, 70–81. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Zhang, C. Selective catalytic reduction of NO with NH3 over iron titanate catalyst: Catalytic performance and charac-terization. J. Appl. Catal. B Environ. 2010, 96, 408–420. [Google Scholar] [CrossRef]
- Gongshin, Q.; Ralph, Y.T. A Superior Catalyst for Low-temperature NO Reduction with NH3. Chem. Commun. 2003, 848–849. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Feng, Y.; Si, Z.; Weng, D.; Shi, L. Low-temperature SCR activity and SO2 deactivation mechanism of Ce-modified V2O5–WO3/TiO2 catalyst. Prog. Nat. Sci. 2015, 25, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Shan, W.P.; Xiong, S.C.; Liao, Y.; Yang, S.J.; Liu, F.D. Effect of CeO2 for a High-efficiency CeO2/WO3–TiO2 Catalyst on N2O Formation in NH3-SCR. Catal. Sci. Technol. 2016, 6, 3149–3155. [Google Scholar]
- Chen, Y.; Williams, J.; Campbell, S.; Wang, G. Increased dissolution of ilmenite induced by high-energy ball milling. Mater. Sci. Eng. 1999, 271, 485–490. [Google Scholar] [CrossRef]
- Yin, Z.; Peng, Y.; Zhu, Z.; Ma, C.; Yu, Z.; Wu, G. Effect of mill speed and slurry filling on the charge dynamics by an instrumented ball. Adv. Powder Technol. 2019, 30, 1611–1616. [Google Scholar] [CrossRef]
- Chen, Y. Low-temperature oxidation of ilmenite (FeTiO3) induced by high energy ball milling at room temperature. J. Alloys Compd. 1997, 257, 156–160. [Google Scholar] [CrossRef]
- Ba, X.L. Synthesis, Microstructure and Magnetic Properties of Fe-SiO2 Nanocomposite Materials; Lanzhou University: Lanzhou, China, 2008; pp. 12–24. [Google Scholar]
- Ju, Y.; Li, Z.Q. The Mechanisms of Mechanical Alloying and the Application in the Field of Magnetic Materials. Adv. Funct. Mater. 2002, 33, 12–14. [Google Scholar]
- Huang, X.; Zhang, G.; Lu, G.; Tang, Z. Recent Progress on Establishing Structure–Activity Relationship of Catalysts for Selective Catalytic Reduction (SCR) of NOx with NH3. Catal. Surv. Asia 2017, 22, 1–19. [Google Scholar] [CrossRef]
- Qian, Y.J.; Gui, K.T.; Liang, H. Performance of Low-temperature Selective Catalytic Reduction of NO Over Supported Fe-Ce Catalysts. J. Eng. Thermophy. 2015, 36, 101–105. [Google Scholar]
- Wang, S.-X.; Guo, R.-T.; Pan, W.-G.; Li, M.-Y.; Sun, P.; Liu, S.-M.; Liu, S.-W.; Sun, X.; Liu, J. The deactivation mechanism of Pb on the Ce/TiO2 catalyst for the selective catalytic reduction of NOx with NH3: TPD and DRIFT studies. J. Phys. Chem. Chem. 2017, 19, 5333–5342. [Google Scholar] [CrossRef]
- Zhou, R. Low Temperature Selective Catalytic Reduction(SCR) of NOx on Cerium-based Catalysts. Master’s Thesis, Shanghai Electric Power University, Shanghai, China, 1 March 2013. [Google Scholar]
- Han, J.; Meeprasert, J.; Maitarad, P.; Nammuangruk, S.; Shi, L.; Zhang, D.; Namuangruk, S. Investigation of the Facet-Dependent Catalytic Performance of Fe2O3/CeO2 for the Selective Catalytic Reduction of NO with NH3. J. Phys. Chem. C 2016, 120, 1523–1533. [Google Scholar] [CrossRef]
- Neri, G.; Visco, A.; Galvagno, S.; Donato, A.; Panzalorto, M. Au/iron oxide catalysts: Temperature programmed reduction and X-ray diffraction characterization. Thermochim. Acta 1999, 329, 39–46. [Google Scholar] [CrossRef]
- Yang, H.; Rong, Y.; Tang, R.; Xue, X.-X.; Li, Y. Recovery of iron from Baotou rare earth tailings by magnetizing roast. Rare Met. 2013, 32, 616–621. [Google Scholar] [CrossRef]




| Components | Content |
|---|---|
| Fe2O3 | 28.62 |
| CaO | 21.97 |
| F | 14.03 |
| SiO2 | 11.48 |
| SO3 | 4.88 |
| BaO | 3.78 |
| MgO | 3.67 |
| P2O5 | 2.80 |
| MnO | 1.76 |
| La2O3 | 1.58 |
| Nd2O3 | 1.32 |
| Al2O3 | 1.12 |
| TiO2 | 1.04 |
| Na2O | 0.75 |
| K2O | 0.50 |
| NbO | 0.17 |
| Pr6O11 | 0.14 |
| ZnO | 0.11 |
| SrO | 0.10 |
| PbO | 0.08 |
| Y2O3 | 0.03 |
| ThO2 | 0.03 |
| CuO | 0.007 |
| CO2 | 0.01 |
| Sample Name | Specific Surface Area (m2/g) | Pore Volume (ml/g) | Pore Size (nm) |
|---|---|---|---|
| Sample 1 | 0.92 | 0.0018 | 11.73 |
| Sample 2 | 200.93 | 0.23 | 13.62 |
| Sample 3 | 195.26 | 0.18 | 5.69 |
| γ-Al2O3 | 367.84 | 0.42 | 6.68 |
| Equipment Name | Model | Factory |
|---|---|---|
| Electronic balance | TE2101-L | Germany Sartourius (Guxhagen, Germany) |
| BSA124S | Germany Sartourius | |
| Ordinary ball mill | BM04 | Nanjing Kangye Optical Technology Co., Ltd. (Nanjing, China) |
| High energy ball mill | PM0.4L | Changsha Deke Instrument Equipment Co., Ltd. (Changsha, China) |
| Microwave roaster | CY-R01000C-S | Hunan Changyi Microwave Technology Co., Ltd. (Changsha, China) |
| 20 mesh standard sieve | Shaoxing Shangyu Zhangxing Yarn Screen Factory (Shaoxing, China) | |
| X-ray diffractometer | D/MAX2500 | PANalytical (PANalytical, Almelo, Netherlands) |
| X-ray fluorescence | LABCENTER XRF-1800 | Shimadzu (Kyoto, Japan) |
| scanning electron microscope | X-3400 | Hitachi (Tokyo, Japan) |
| Energy spectrometer | INCA 3294 | Oxford Instruments (Oxford, United Kingdom) |
| Chemisorption Apparatus | PCA-1200 | Biod Electronics (Beijing, China) |
| Vertical tube furnace | VTL1600 | Nanjing Boyuntong Instrument Technology Co., Ltd. (Nanjing, China) |
| Blast drying box | FCD-3000 | Huitai Instruments (Dongwan, China) |
| Specific surface area and pore size analyzer | 3H-2000PS1 | Best Instrument Technology Co., Ltd. (Changsha, China) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Lin, J.; Chen, Z.; Hou, L.; Wu, W. A Study on the Effect of Different Ball Milling Methods on the NH3-SCR Activity of Aluminum-Laden Bayan Obo Tailings. Catalysts 2021, 11, 568. https://doi.org/10.3390/catal11050568
Bai X, Lin J, Chen Z, Hou L, Wu W. A Study on the Effect of Different Ball Milling Methods on the NH3-SCR Activity of Aluminum-Laden Bayan Obo Tailings. Catalysts. 2021; 11(5):568. https://doi.org/10.3390/catal11050568
Chicago/Turabian StyleBai, Xinrui, Jiawei Lin, Zedong Chen, Limin Hou, and Wenfei Wu. 2021. "A Study on the Effect of Different Ball Milling Methods on the NH3-SCR Activity of Aluminum-Laden Bayan Obo Tailings" Catalysts 11, no. 5: 568. https://doi.org/10.3390/catal11050568