Surface-Modified Ta3N5 Photoanodes for Sunlight-Driven Overall Water Splitting by Photoelectrochemical Cells
Abstract
:1. Introduction
2. Results and Discussion
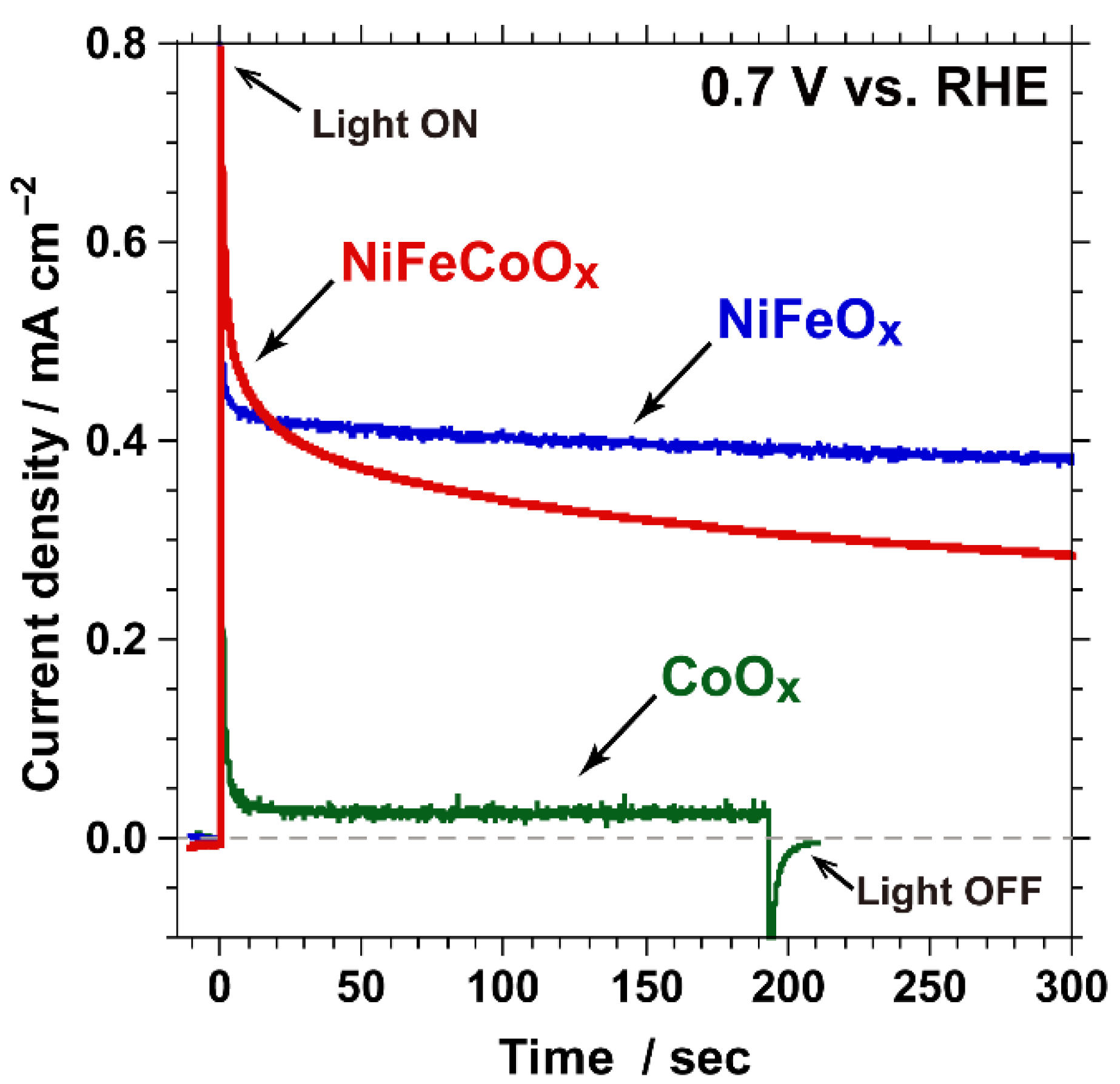
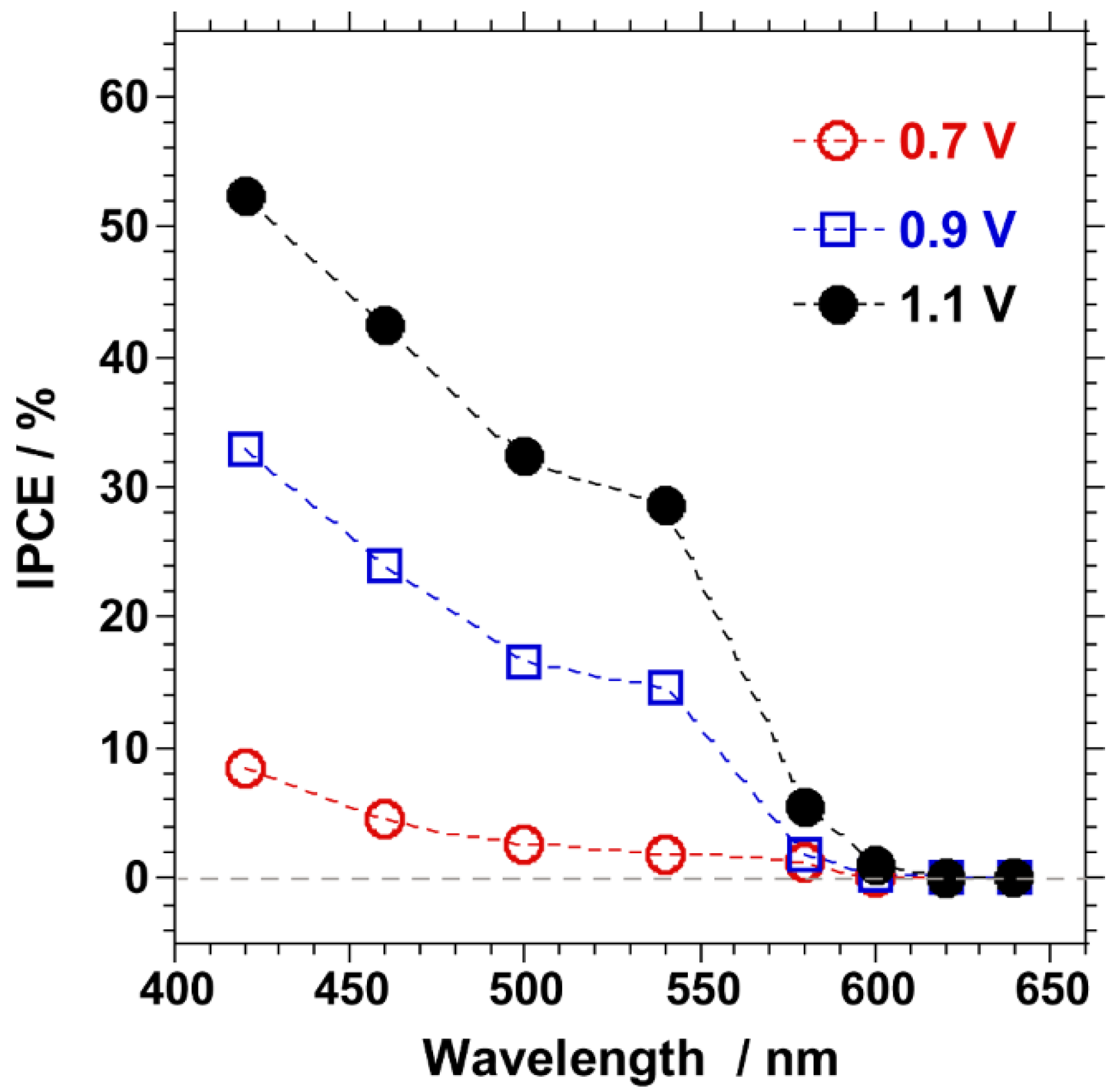
2.1. PEC Characteristics of Surface-Modified Ta3N5 Photoanodes
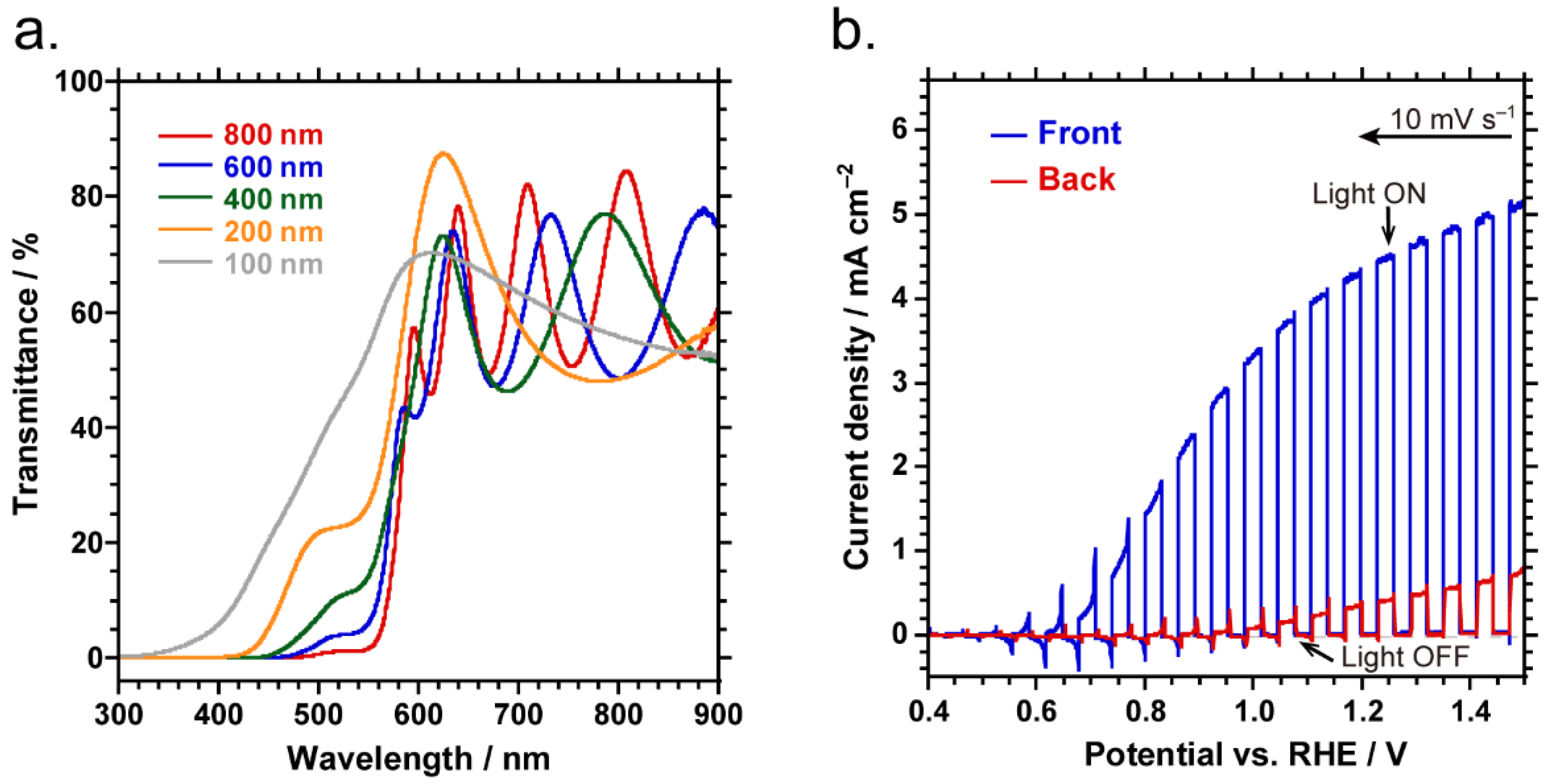
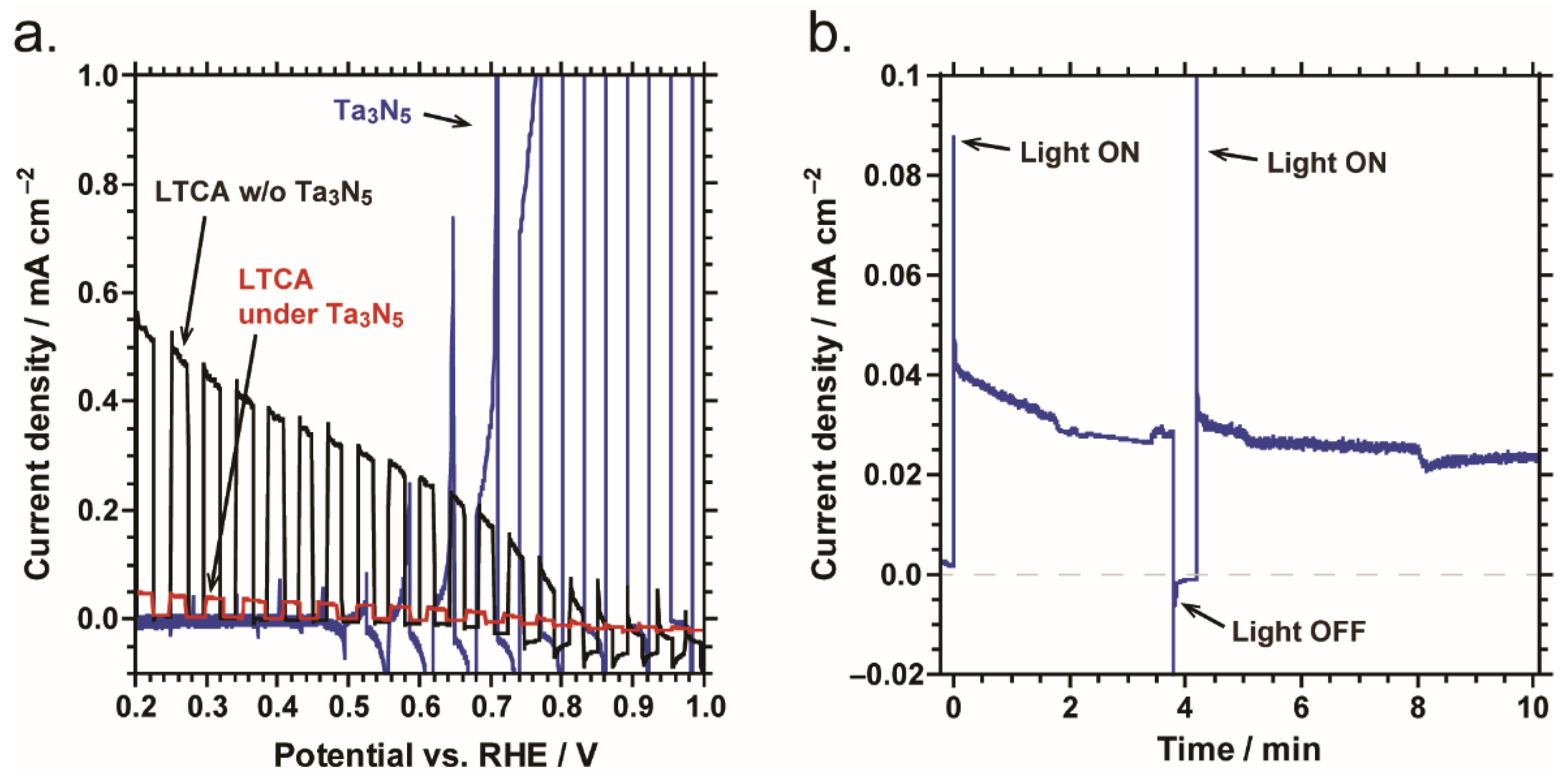
2.2. Overall Water Splitting by Parallel PEC Cell
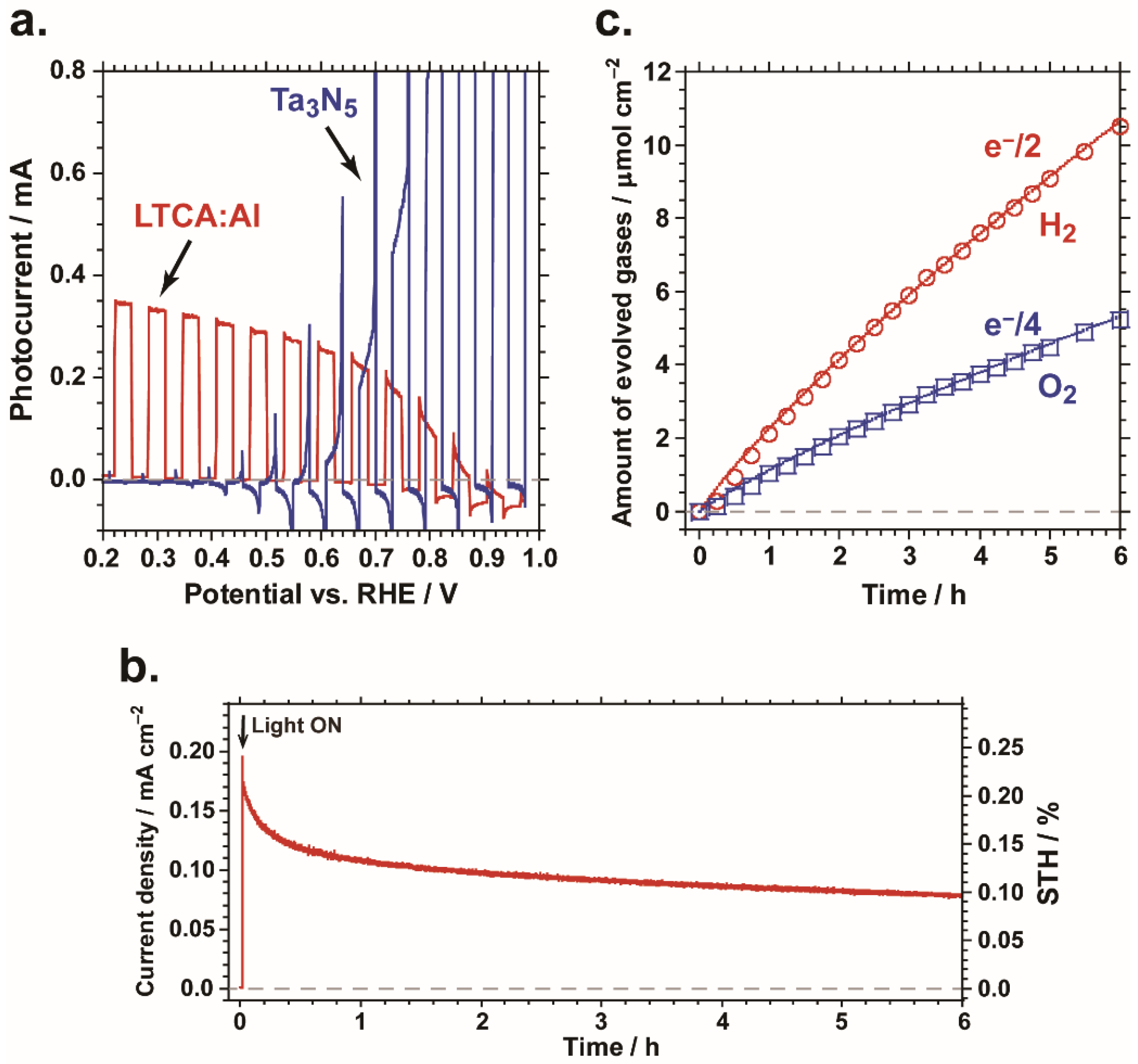
2.3. Overall Water Splitting by Tandem PEC Cell
3. Materials and Methods
3.1. Preparation of Ta3N5-Based Photoanodes
3.2. Surface Modification of Ta3N5-Based Photoanodes
3.3. Characterization
3.4. PEC Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Pinaud, B.A.; Benck, J.D.; Seitz, L.C.; Forman, A.J.; Chen, Z.; Deutsch, T.G.; James, B.D.; Baum, K.N.; Baum, G.N.; Ardo, S.; et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 2013, 6, 1983–2002. [Google Scholar] [CrossRef] [Green Version]
- McKone, J.R.; Lewis, N.S.; Gray, H.B. Will Solar-Driven Water-Splitting Devices See the Light of Day? Chem. Mater. 2014, 26, 407–414. [Google Scholar] [CrossRef]
- Jia, J.; Seitz, L.C.; Benck, J.D.; Huo, Y.; Chen, Y.; Ng, J.W.D.; Bilir, T.; Harris, J.S.; Jaramillo, T.F. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 2016, 7, 13237. [Google Scholar] [CrossRef]
- Shaner, M.R.; Atwater, H.A.; Lewis, N.S.; McFarland, E.W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 2016, 9, 2354–2371. [Google Scholar] [CrossRef] [Green Version]
- Montoya, J.H.; Seitz, J.H.M.L.C.; Chakthranont, P.; Vojvodic, A.; Jaramillo, T.F.; Nørskov, J.K. Materials for solar fuels and chemicals. Nat. Mater. 2017, 16, 70–81. [Google Scholar] [CrossRef]
- Prévot, M.S.; Sivula, K. Photoelectrochemical Tandem Cells for Solar Water Splitting. J. Phys. Chem. C 2013, 117, 17879–17893. [Google Scholar] [CrossRef]
- Higashi, T.; Kaneko, H.; Minegishi, T.; Kobayashi, H.; Zhong, M.; Kuang, Y.; Hisatomi, T.; Katayama, M.; Takata, T.; Nishiyama, H.; et al. Overall water splitting by photoelectrochemical cells consisting of (ZnSe)0.85(CuIn0.7Ga0.3Se2)0.15 photocathodes and BiVO4 photoanodes. Chem. Commun. 2017, 53, 11674–11677. [Google Scholar] [CrossRef]
- Higashi, T.; Shinohara, Y.; Ohnishi, A.; Liu, J.; Ueda, K.; Okamura, S.; Hisatomi, T.; Katayama, M.; Nishiyama, H.; Yamada, T.; et al. Sunlight-Driven Overall Water Splitting by the Combination of Surface-Modified La5Ti2Cu0.9Ag0.1S5O7 and BaTaO2N Photoelectrodes. ChemPhotoChem 2017, 1, 167–172. [Google Scholar] [CrossRef]
- Kaneko, H.; Minegishi, T.; Nakabayashi, M.; Shibata, N.; Kuang, Y.; Yamada, T.; Domen, K. A Novel Photocathode Material for Sunlight-Driven Overall Water Splitting: Solid Solution of ZnSe and Cu(In,Ga)Se2. Adv. Funct. Mater. 2016, 26, 4570–4577. [Google Scholar] [CrossRef]
- Hisatomi, T.; Okamura, S.; Liu, J.; Shinohara, Y.; Ueda, K.; Higashi, T.; Katayama, M.; Minegishi, T.; Domen, K. La5Ti2Cu1−xAgxS5O7 photocathodes operating at positive potentials during photoelectrochemical hydrogen evolution under irradiation of up to 710 nm. Energy Environ. Sci. 2015, 8, 3354–3362. [Google Scholar] [CrossRef] [Green Version]
- Song, A.; Bogdanoff, P.; Esau, A.; Ahmet, I.Y.; Levine, I.; Dittrich, T.; Unold, T.; van de Krol, R.; Berglund, S.P. Assessment of a W:BiVO4–CuBi2O4Tandem Photoelectrochemical Cell for Overall Solar Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 13959–13970. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Sato, N.; Orita, M.; Kuang, Y.; Kaneko, H.; Minegishi, T.; Yamada, T.; Domen, K. Development of highly efficient CuIn0.5Ga0.5Se2-based photocathode and application to overall solar driven water splitting. Energy Environ. Sci. 2018, 11, 3003–3009. [Google Scholar] [CrossRef]
- Huang, D.; Wang, K.; Li, L.; Feng, K.; An, N.; Ikeda, S.; Kuang, Y.; Ng, Y.; Jiang, F. 3.17% efficient Cu2ZnSnS4–BiVO4 integrated tandem cell for standalone overall solar water splitting. Energy Environ. Sci. 2021, 14, 1480–1489. [Google Scholar] [CrossRef]
- Jiang, F.; Harada, T.; Kuang, Y.; Minegishi, T.; Domen, K.; Ikeda, S. Pt/In2S3/CdS/Cu2ZnSnS4 Thin Film as an Efficient and Stable Photocathode for Water Reduction under Sunlight Radiation. J. Am. Chem. Soc. 2015, 137, 13691–13697. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kaneko, H.; Minegishi, T.; Kubota, J.; Domen, K.; Lee, J.S. Overall Photoelectrochemical Water Splitting using Tandem Cell under Simulated Sunlight. ChemSusChem 2016, 9, 61–66. [Google Scholar] [CrossRef]
- Chun, W.-J.; Ishikawa, A.; Fujisawa, H.; Takata, T.; Kondo, J.N.; Hara, M.; Kawai, M.; Matsumoto, A.Y.; Domen, K. Conduction and Valence Band Positions of Ta2O5, TaON, and Ta3N5 by UPS and Electrochemical Methods. J. Phys. Chem. B 2003, 107, 1798–1803. [Google Scholar] [CrossRef]
- Higashi, M.; Domen, K.; Abe, R. Fabrication of efficient TaON and Ta3N5 photoanodes for water splitting under visible light irradiation. Energy Environ. Sci. 2011, 4, 4138–4147. [Google Scholar] [CrossRef]
- Pinaud, B.A.; Vesborg, P.C.K.; Jaramillo, T.F. Effect of Film Morphology and Thickness on Charge Transport in Ta3N5/Ta Photoanodes for Solar Water Splitting. J. Phys. Chem. C 2012, 116, 15918–15924. [Google Scholar] [CrossRef]
- Liu, G.; Ye, S.; Yan, P.; Xiong, F.; Fu, P.; Wang, Z.; Chen, Z.; Shi, J.; Li, C. Enabling an integrated tantalum nitride photoanode to approach the theoretical photocurrent limit for solar water splitting. Energy Environ. Sci. 2016, 9, 1327–1334. [Google Scholar] [CrossRef]
- Takanabe, K. Photocatalytic Water Splitting: Quantitative Approaches toward Photocatalyst by Design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Jing, T.; Dai, Y.; Ma, X.; Wei, W.; Huang, B. Effects of intrinsic defects and extrinsic doping on the electronic and photocatalytic properties of Ta3N5. RSC Adv. 2015, 5, 59390–59397. [Google Scholar] [CrossRef]
- Fu, G.; Yan, S.; Yu, T.; Zou, Z. Oxygen related recombination defects in Ta3N5 water splitting photoanode. Appl. Phys. Lett. 2015, 107, 171902. [Google Scholar] [CrossRef]
- Harb, M.; Sautet, P.; Nurlaela, E.; Raybaud, P.; Cavallo, L.; Domen, K.; Basset, J.-M.; Takanabe, K. Tuning the properties of visible-light-responsive tantalum (oxy)nitride photocatalysts by non-stoichiometric compositions: A first-principles viewpoint. Phys. Chem. Chem. Phys. 2014, 16, 20548–20560. [Google Scholar] [CrossRef] [PubMed]
- Nurlaela, E.; Ould-Chikh, S.; Harb, M.; Del Gobbo, S.; Aouine, M.; Puzenat, E.; Sautet, P.; Domen, K.; Basset, J.-M.; Takanabe, K. Critical Role of the Semiconductor–Electrolyte Interface in Photocatalytic Performance for Water-Splitting Reactions Using Ta3N5 Particles. Chem. Mater. 2014, 26, 4812–4825. [Google Scholar] [CrossRef]
- Murthy, D.H.K.; Matsuzaki, H.; Wang, Z.; Suzuki, Y.; Hisatomi, T.; Seki, K.; Inoue, Y.; Domen, K.; Furube, A. Origin of the overall water splitting activity of Ta3N5 revealed by ultrafast transient absorption spectroscopy. Chem. Sci. 2019, 10, 5353–5362. [Google Scholar] [CrossRef] [Green Version]
- Ziani, A.; Nurlaela, E.; Dhawale, D.S.; Silva, D.A.; Alarousu, E.; Mohammed, O.F.; Takanabe, K. Carrier dynamics of a visible-light-responsive Ta3N5 photoanode for water oxidation. Phys. Chem. Chem. Phys. 2014, 17, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Luo, W.; Cao, D.; Zhao, X.; Li, Z.; Yu, T.; Zou, Z. A Co-catalyst-Loaded Ta3N5 Photoanode with a High Solar Photocurrent for Water Splitting upon Facile Removal of the Surface Layer. Angew. Chem. Int. Ed. 2013, 52, 11016–11020. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Chen, R.; Zhao, Y.; Li, Z.; Zong, X.; Li, C. Reducing the surface defects of Ta3N5 photoanode towards enhanced photoelectrochemical water oxidation. J. Mater. Chem. A 2020, 8, 23274–23283. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Nguyen, N.T.; Hwang, I.; Schmuki, P. Strongly Enhanced Water Splitting Performance of Ta3N5 Nanotube Photoanodes with Subnitrides. Adv. Mater. 2016, 28, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Feng, J.; Luo, W.; Wang, Z.; Zhang, J.; Li, Z.; Yu, T.; Zou, Z. Co3O4 Nanoparticles as Robust Water Oxidation Catalysts Towards Remarkably Enhanced Photostability of a Ta3N5 Photoanode. Adv. Funct. Mater. 2012, 22, 3066–3074. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Z.; Yang, C.; Cheng, H.; Jiao, S.; Zhu, H. Cobalt-bilayer catalyst decorated Ta3N5 nanorod arrays as integrated electrodes for photoelectrochemical water oxidation. Energy Environ. Sci. 2013, 6, 3322–3330. [Google Scholar] [CrossRef]
- Suzuki, S.; Yanai, M.; Yamada, T.; Wagata, H.; Sasaki, Y.; Oishi, S.; Domen, K.; Teshima, K. Ta3N5 Photoanodes Fabricated by Providing NaCl–Na2CO3 Evaporants to Tantalum Substrate Surface under NH3 Atmosphere. ACS Appl. Energy Mater. 2018, 1, 6129–6135. [Google Scholar] [CrossRef]
- Zhong, M.; Hisatomi, T.; Sasaki, Y.; Suzuki, S.; Teshima, K.; Nakabayashi, M.; Shibata, N.; Nishiyama, H.; Katayama, M.; Yamada, T.; et al. Highly Active GaN-Stabilized Ta3N5 Thin-Film Photoanode for Solar Water Oxidation. Angew. Chem. Int. Ed. 2017, 56, 4739–4743. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, Y.; Ding, C.; Fan, D.; Liu, G.; Zhao, Y.; Li, C. Insight into the charge transfer in particulate Ta3N5 photoanode with high photoelectrochemical performance. Chem. Sci. 2016, 7, 4391–4399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Fu, P.; Zhou, L.; Yan, P.; Ding, C.; Shi, J.; Li, C. Efficient Hole Extraction from a Hole-Storage-Layer-Stabilized Tantalum Nitride Photoanode for Solar Water Splitting. Chem. A Eur. J. 2015, 21, 9624–9628. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.A.; Majumder, S.; Perumandla, N.; Zahran, Z.N.; Naruta, Y. Enhanced Performance of Pristine Ta3N5 Photoanodes for Solar Water Splitting by Modification with Fe–Ni–Co Mixed-Metal Oxide Cocatalysts. J. Phys. Chem. C 2017, 121, 20093–20100. [Google Scholar] [CrossRef]
- Haleem, A.A.; Perumandla, N.; Naruta, Y. Preparation of Nanostructured Ta3N5 Electrodes by Alkaline Hydrothermal Treatment Followed by NH3 Annealing and Their Improved Water Oxidation Performance. ACS Omega 2019, 4, 7815–7821. [Google Scholar] [CrossRef]
- Seo, J.; Takata, T.; Nakabayashi, M.; Hisatomi, T.; Shibata, N.; Minegishi, T.; Domen, K. Mg–Zr Cosubstituted Ta3N5 Photoanode for Lower-Onset-Potential Solar-Driven Photoelectrochemical Water Splitting. J. Am. Chem. Soc. 2015, 137, 12780–12783. [Google Scholar] [CrossRef] [PubMed]
- Akagi, D.; Kageshima, Y.; Hashizume, Y.; Aoi, S.; Sasaki, Y.; Kaneko, H.; Higashi, T.; Hisatomi, T.; Katayama, M.; Minegishi, T.; et al. A Semitransparent Nitride Photoanode Responsive up to λ = 600 nm Based on a Carbon Nanotube Thin Film Electrode. ChemPhotoChem 2019, 3, 521–524. [Google Scholar] [CrossRef]
- Kim, Y.W.; Cha, S.; Kwak, I.; Kwon, I.S.; Park, K.; Jung, C.S.; Cha, E.H.; Park, J. Surface-Modified Ta3N5 Nanocrystals with Boron for Enhanced Visible-Light-Driven Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 36715–36722. [Google Scholar] [CrossRef] [PubMed]
- Nurlaela, E.; Shinagawa, T.; Qureshi, M.; Dhawale, D.S.; Takanabe, K. Temperature Dependence of Electrocatalytic and Photocatalytic Oxygen Evolution Reaction Rates Using NiFe Oxide. ACS Catal. 2016, 6, 1713–1722. [Google Scholar] [CrossRef] [Green Version]
- Higashi, T.; Nishiyama, H.; Otsuka, Y.; Kawase, Y.; Sasaki, Y.; Nakabayashi, M.; Katayama, M.; Minegishi, T.; Shibata, N.; Takanabe, K.; et al. Efficient Water Oxidation Using Ta3N5 Thin Film Photoelectrodes Prepared on Insulating Transparent Substrates. ChemSusChem 2020, 13, 1974–1978. [Google Scholar] [CrossRef]
- Higashi, T.; Nishiyama, H.; Suzuki, Y.; Sasaki, Y.; Hisatomi, T.; Katayama, M.; Minegishi, T.; Seki, K.; Yamada, T.; Domen, K. Transparent Ta3N5 Photoanodes for Efficient Oxygen Evolution toward the Development of Tandem Cells. Angew. Chem. Int. Ed. 2019, 58, 2300–2304. [Google Scholar] [CrossRef]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Zhang, Z.; Sedach, P.A.; Siu, M.K.J.; Trudel, S.; Berlinguette, C.P. Photochemical Route for Accessing Amorphous Metal Oxide Materials for Water Oxidation Catalysis. Science 2013, 340, 60–63. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Kawase, Y.; Higashi, T.; Katayama, M.; Domen, K.; Takanabe, K. Maximizing Oxygen Evolution Performance on a Transparent NiFeOx/Ta3N5 Photoelectrode Fabricated on Insulating Quartz Substrates. ACS Appl. Mater. Interfaces 2021, 13, 16317–16325. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, T.; Nishimura, N.; Kubota, J.; Domen, K. Photoelectrochemical properties of LaTiO2N electrodes prepared by particle transfer for sunlight-driven water splitting. Chem. Sci. 2012, 4, 1120–1124. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashi, T.; Sasaki, Y.; Kawase, Y.; Nishiyama, H.; Katayama, M.; Takanabe, K.; Domen, K. Surface-Modified Ta3N5 Photoanodes for Sunlight-Driven Overall Water Splitting by Photoelectrochemical Cells. Catalysts 2021, 11, 584. https://doi.org/10.3390/catal11050584
Higashi T, Sasaki Y, Kawase Y, Nishiyama H, Katayama M, Takanabe K, Domen K. Surface-Modified Ta3N5 Photoanodes for Sunlight-Driven Overall Water Splitting by Photoelectrochemical Cells. Catalysts. 2021; 11(5):584. https://doi.org/10.3390/catal11050584
Chicago/Turabian StyleHigashi, Tomohiro, Yutaka Sasaki, Yudai Kawase, Hiroshi Nishiyama, Masao Katayama, Kazuhiro Takanabe, and Kazunari Domen. 2021. "Surface-Modified Ta3N5 Photoanodes for Sunlight-Driven Overall Water Splitting by Photoelectrochemical Cells" Catalysts 11, no. 5: 584. https://doi.org/10.3390/catal11050584





