Abstract
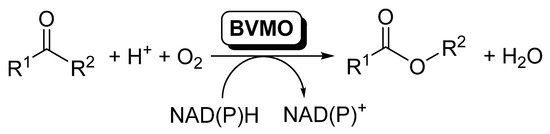
Baeyer–Villiger monooxygenases (BVMOs) are flavin-dependent oxidative enzymes capable of catalyzing the insertion of an oxygen atom between a carbonylic Csp2 and the Csp3 at the alpha position, therefore transforming linear and cyclic ketones into esters and lactones. These enzymes are dependent on nicotinamides (NAD(P)H) for the flavin reduction and subsequent reaction with molecular oxygen. BVMOs can be included in cascade reactions, coupled to other redox enzymes, such as alcohol dehydrogenases (ADHs) or ene-reductases (EREDs), so that the direct conversion of alcohols or α,β-unsaturated carbonylic compounds to the corresponding esters can be achieved. In the present review, the different synthetic methodologies that have been performed by employing multienzymatic strategies with BVMOs combining whole cells or isolated enzymes, through sequential or parallel methods, are described, with the aim of highlighting the advantages of performing multienzymatic systems, and show the recent advances for overcoming the drawbacks of using BVMOs in these techniques.
1. Introduction
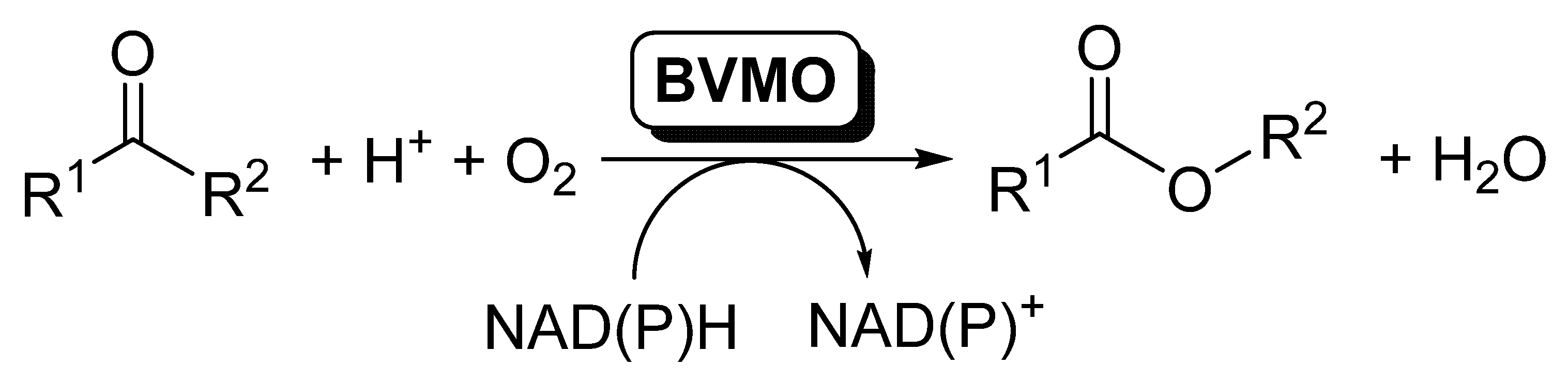
The elucidation and rational understanding of the internal organization of the different biocatalytic reactions occurring inside biological cells, in which several enzymatic reactions proceed in a concatenated manner, is one of the basis of Systems Biology [1]. The development of this discipline has fostered the design of coupled systems of biocatalytic reactions, and the number of publications dealing with this topic has increased considerably during the last years [2,3,4,5,6,7,8,9,10,11]. In this area, oxidoreductases are enzymes frequently used in cascade reactions, as usually a pair of these enzymes are simultaneously applied for the required in situ recycling of the cofactors [12,13,14,15]. Inside this type of enzymes, Baeyer–Villiger monooxygenases (BVMOs) are undoubtedly one of the most attractive members of this family; these flavin-dependent oxidative enzymes [16,17,18,19,20] are capable of catalyzing the insertion of an oxygen atom between a carbonylic Csp2 and the Csp3 at the alpha position, therefore transforming linear and cyclic ketones into esters and lactones [20,21,22,23,24,25,26,27], as schematized in Scheme 1.
Scheme 1.
BVMOs-catalyzed oxidation of ketones to furnish esters.
BVMOs require nicotinamides (NADPH) for the flavin reduction to FADH2, which reacts with molecular oxygen to form the reactive peroxyflavin responsible for the substrate oxidation. As NADPH is an expensive compound and its presence at high concentrations can inhibit the biocatalyst, it has to be recycled to obtain feasible biocatalytic procedures. In the last years, more than 100 BVMOs have been cloned and overexpressed. In many cases, the natural role of those BVMOs is not identified, while in others they seem to take part in the synthesis of secondary metabolites. Most of the BVMOs, the so-called type I BVMOs, can be included in the B subclass of flavin dependent monooxygenases [28], according to their protein sequence motifs, electron donor and type of oxygenation reaction. Thus, they are single-component enzymes possessing two α/β Rossmann-like domains for the FAD and NADPH binding, respectively, and they keep this last one bound during catalysis, while the substrate binds after the flavin-peroxide is formed [17]. BVMO catalysis started by NADPH binding and subsequent flavin reduction, after which the NADPH cofactor adopts a stable position. A stable peroxyflavin is the catalytically active specie, formed by the reaction of the reduced flavin with molecular oxygen [29]. Type II BVMOs are part of the class C of flavin dependent monooxygenases, which are two-component monooxygenases [30], but this type of BVMOs has been scarcely employed in biocatalysis due the requirement of these two components.
Most of the BVMOs are present on prokaryotes and some unicellular eukaryotic organisms such as filamentous fungi. Several bacterial BVMOs have been applied with biocatalytic purposes; even some fungal BVMOs have also been discovered and characterized in the last few years [31]. Both storage and operational stability of these biocatalysts is interesting for their biotechnological applications. For most of the BVMOs, which presents a certain stability, lyophilization in presence of different additives is a method of interest because it simplifies the enzyme transport and storage [32]. Normally, these biocatalysts perform their activity at mild reaction conditions (aqueous media at neutral pH and room temperature). In the last few years several examples of thermostable BVMOs (wild type and mutants), able to catalyze reactions at high temperatures, have been reported [33,34], with the aim of increasing the applicability of these valuable enzymes [35,36].
Baeyer–Villiger monooxygenases were first discovered in the 1960s, but they have not been widely applied with biocatalytic purposes for the preparation of high valuable compounds as esters, lactones and sulfoxides, among others. Advances in genome mining have allowed scientists to discover several BVMOs active on different types of compounds, but still nowadays, cyclohexanone monooxygenase (CHMO) from Acinetobacter calcoaceticus NCIMB 9871, discovered more than 40 years ago [37], is the BVMO with the highest applicability due to its large substrate profile and excellent selectivity. Nevertheless, CHMO presents some drawbacks as its thermal instability and its low stability to organic co-solvents [38]. Remarkably, most BVMOs display an excellent behavior when employed in multi-step cascade reactions coupled to other enzymes. In most of these reactions, lactones are obtained as final products. These cyclic esters of carboxylic acids, containing a 1-oxacycloalkan-2-one structure, are a class of secondary metabolites, thus presenting a wide range of biological activities as anti-inflammatory, antimicrobial or anticancer compounds [39]. Lactones can be also employed in cosmetics and perfume industry, in polymer chemistry, as agrochemicals or in food industry as flavoring agents. By this reason, several methodologies have been performed for their preparation. The use of biocatalysts for the synthesis of lactones presents some advantages regarding the classical methods, as only molecular oxygen is required as oxidant while working under mild (pH and temperature) reaction conditions, being achieved in general high regio- and/or enantioselectivites. The combination of two or more biocatalysts also allowed avoiding the isolation and purification of the reaction intermediates, thus increasing the atom economy of the processes. However, the use of BVMOs in these synthetic procedures can present some limitations, as low enzyme expression and stability, the NADPH-dependence, and substrate and product inhibition.
In this review, we comment on different reported cases in which enzymatic cascades, including BVMOs, have been applied for synthesizing valuable esters and lactones, aiming at stressing the applicability of this approach and promoting an increased employ of these excellent biocatalysts. For practical reasons, we have classified the reported examples into two main categories, cascades including BVMOs catalyzed by whole cells or by isolated enzymes.
2. Multi-Step Reactions Including BVMO Activity Catalyzed by Whole Cells
Generally speaking, the use of whole cells makes the cascade easier compared to those employing isolated biocatalysts, as the recycling of the required cofactors is produced inside the cell metabolic machinery, so that it is not mandatory to implement an external recycling methodology. The most attractive methodology using whole cells implies the use of à la carte engineered cells, inside which different enzymatic activities are overexpressed in order to generate the desired multi-step procedure [40]. This methodology, which has been sometimes termed as Systems Biocatalysis [41], presents several advantages [40,42,43,44,45]: (i) as already mentioned, the intra-cellular medium provides the natural enzymatic environment and the cofactors regeneration machinery; (ii) it is relatively easy and economical to have available cells by cultivation without any additional downstream process; (iii) enzymes inside cell walls and membranes are somehow protected from extreme reaction conditions, and (iv) the different enzymes involved in the cascade are co-localized inside cells, so that their local concentration is increased, therefore reducing the diffusion of intermediates.
2.1. Multi-Step Reactions Including Alcohol Dehydrogenases/BVMO Activity Catalyzed by Whole Cells
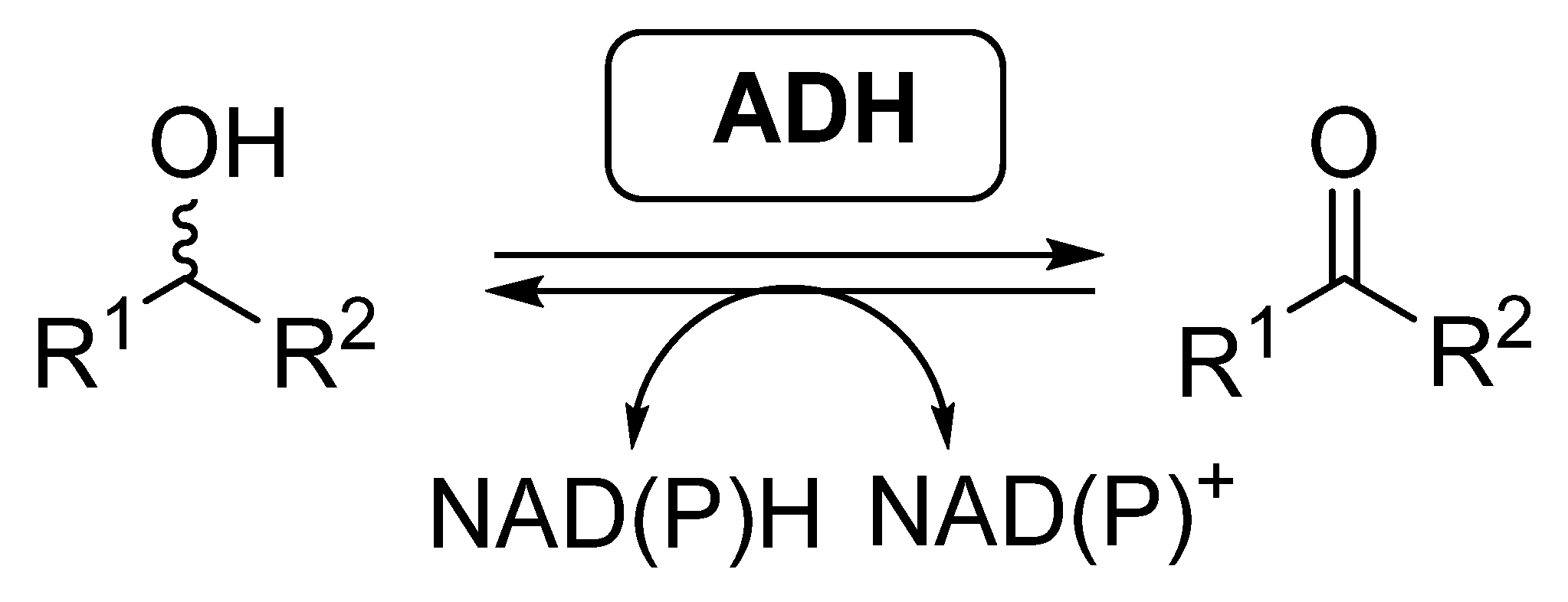
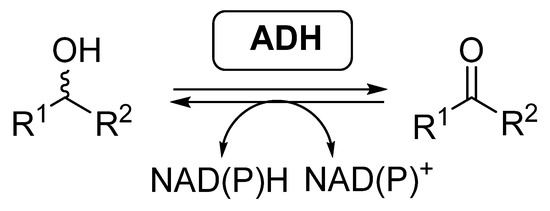
Most of the examples of biocatalytic cascades employing BVMOs have been developed together with alcohol dehydrogenases (ADHs, EC 1.1.1.x), also called ketoreductases (KREDs). These enzymes are able to catalyze the reversible reduction of carbonyl compounds into the corresponding alcohols (Scheme 2), in general with high selectivity [46,47,48,49]. ADHs require the presence of nicotinamide cofactors for performing their activity, being required to employ effective regeneration systems if these enzymes are employed as isolated biocatalysts.
Scheme 2.
Alcohol dehydrogenases catalyzed reversible reduction of carbonyl compounds and oxidation of alcohols.
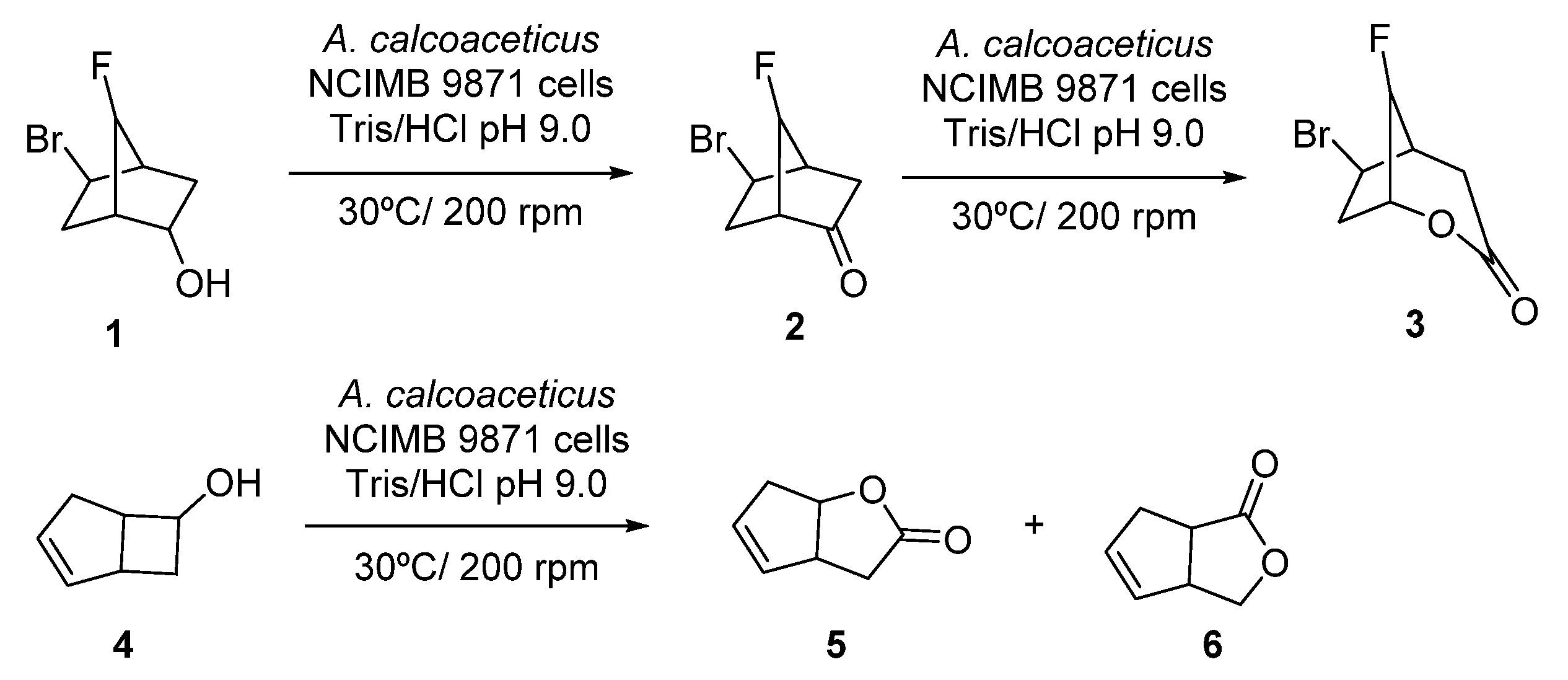
Initial studies on linear cascades combining ADHs and BVMOs were performed at the beginning of the 1990s, using whole cells systems for the direct bio-oxidation of alcohols to the corresponding lactones. Thus, the starting alcohol was oxidized to the corresponding ketones by the ADH at the expense of NAD(P)+, whereas the obtained ketone was further oxidized in presence of the BVMO to the desired lactone, employing NAD(P)H which was converted again into NAD(P)+. A pioneer example is the oxidation of alcohol 1, shown in Scheme 3, employing fractured cells of Acinetobacter calcoacetitus NCIMB to the corresponding lactone 3, described in 1991 [50]. At short reaction times, 1 was converted into ketone 2 in a great extent (80% after 6 h). Longer reaction time led to an increase in the lactone production, reaching a maximum value of 40% after 48 h, whereas concentration of 2 started to decrease after 10 h. A similar pattern was observed in the bio-oxidation of both endo- and exo-bicyclo [2.2.1]heptan-2-ols. Alcohol concentration decreased as the ketone became formed and lactone concentration started to increase during the course of the biocatalytic process. Fractured cells of A. calcoaceticus were also employed in the bio-oxidation of 6-endo-bicyclo [3.2.0]hept-2-en-6-ol 4 to obtain regioisomeric lactones 5 and 6 with complete conversion after 1 h, as shown in Scheme 3. A 15% of a by-product, which authors assumed that was exo-4, was observed in the crude mixture.
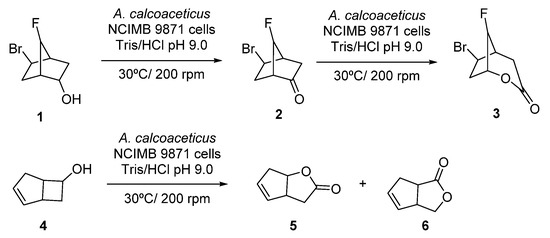
Scheme 3.
Acinetobacter calcoaceticus cells catalyzed biotransformations of alcohols into lactones.
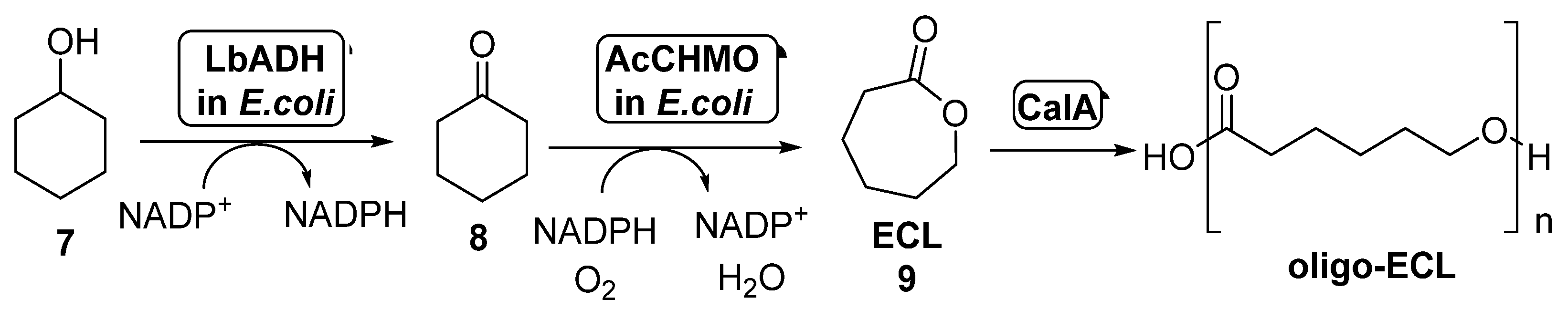
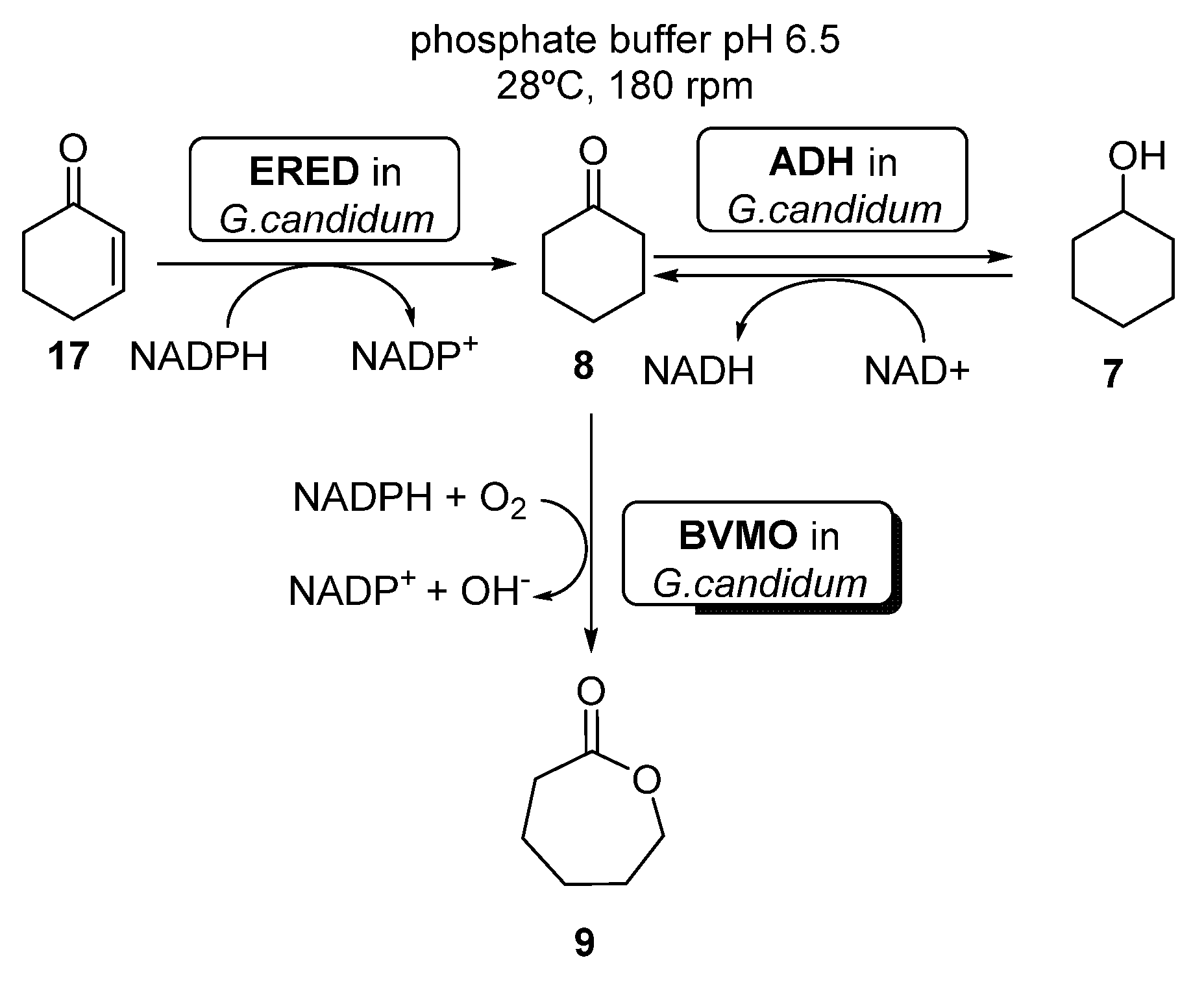
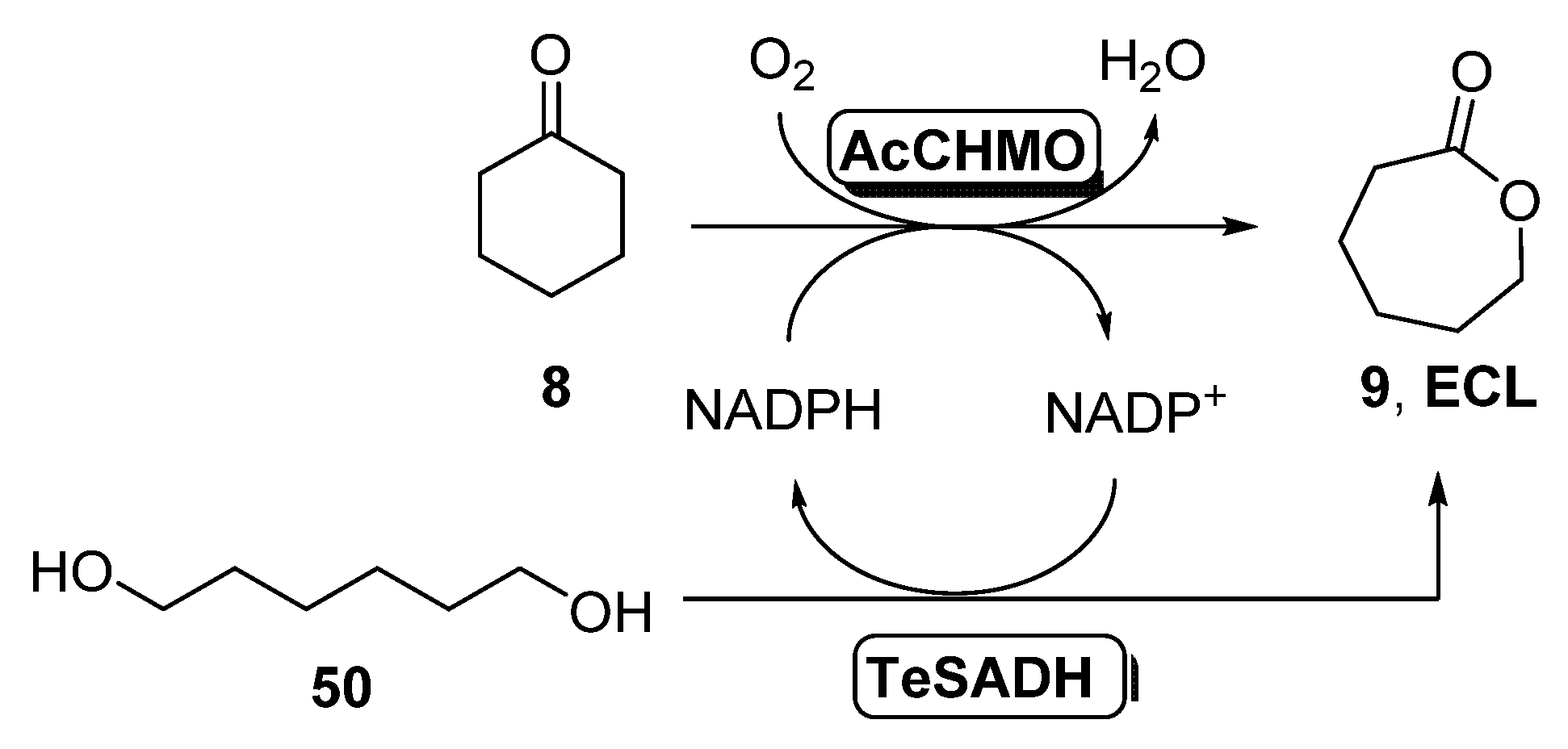
The combination of BVMOs and ADHs has been further developed for the preparation of ε-caprolactone (ECL, 9, Scheme 4). This compound is a valuable material with several applications in the field of colorants, adhesives and coating materials, as ECL can be easily polymerized yielding biodegradable thermoplastics and elastomeric polymers [51,52]. By these reasons, several approaches have been studied for its preparation under sustainable reaction conditions. BVMOs and ADHs have been employed well as whole cells or well as isolated enzymes for the synthesis of ECL starting from cyclohexanol 7, in a process in which this alcohol is oxidized by the ADH to cyclohexanone 8, which is converted into ECL in presence of the BVMO, with an in situ NAD(P)H cofactor regeneration. When employing this approach, the stability of the BVMO is critical for the process, as this biocatalyst is very sensitive to the cyclohexanol and ECL concentration in the reaction medium. For this reason, several attempts have been made in order to overcome this bottleneck.
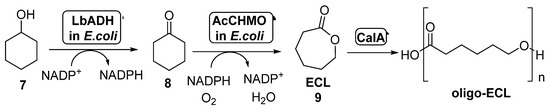
Scheme 4.
Preparation of ECL 9 and its oligomers through a biocatalytic procedure employing whole cells of Lactobacillus brevis ADH and Acinetobacter calcoaceticus CHMO.
Thus, the bienzymatic system employing whole cells of ADH-BVMO for the preparation of ECL can be coupled with the lipase-catalyzed hydrolysis of this compound in order to obtain oligo-caprolactone. This approach was performed for the first time in 2015 [53]. In order to overcome the BVMO deactivation caused by the presence of ECL in the reaction medium, this product subjected to in situ ring-opening oligomerization catalyzed by CALA (lipase from Candida antarctica, Scheme 4). The formed oligo-ECLs were easily removed from the reaction by extraction or precipitation. CALA is able to catalyze the formation of the polymers even in presence of high amounts of water, not showing any hydrolytic activity on ECL at high substrate concentrations (1.0 M). After a few hours in presence of CALA, the lactone was converted into oligomers with a maximum molecular weight of 1200 g/mol, which can be transformed into high-molecular weight polymer. Recombinant E. coli cells of Lactobacillus brevis ADH (LbADH) and the stable mutant C376L/M400I of Acinetobacter calcoaceticus cyclohexanone monooxygenase (AcCHMO), at 100 g wet cells weight/mL concentration, were combined with lipase CALA (10 mg/mL lyophilized) in a one-pot process at different concentrations of cyclohexanol. A decrease in the ECL concentration in these reactions was detected when compared to those in absence of the lipase, thus indicating the beneficiary effect of the conversion of the ECL into oligo-ECL. When both the ADH and the BVMO were employed as separate cells, results were better than expressing both biocatalysts into the same cell-system. LbADH/AcCHMO ratio was optimized 1:10 in order to achieve the highest conversion. Addition of acetone and glucose as co-substrates was also positive for a faster regeneration of the endogenous NADPH in the cell systems. The optimized cascade was performed at preparative scale and, after 48 h, a complete conversion was achieved when starting from 200 mM of 7, yielding a 75% of oligo-ECL and a 25% of ECL. Higher substrate concentrations, 300 mM and 500 mM, afforded lower conversions, 74% and 43% respectively.
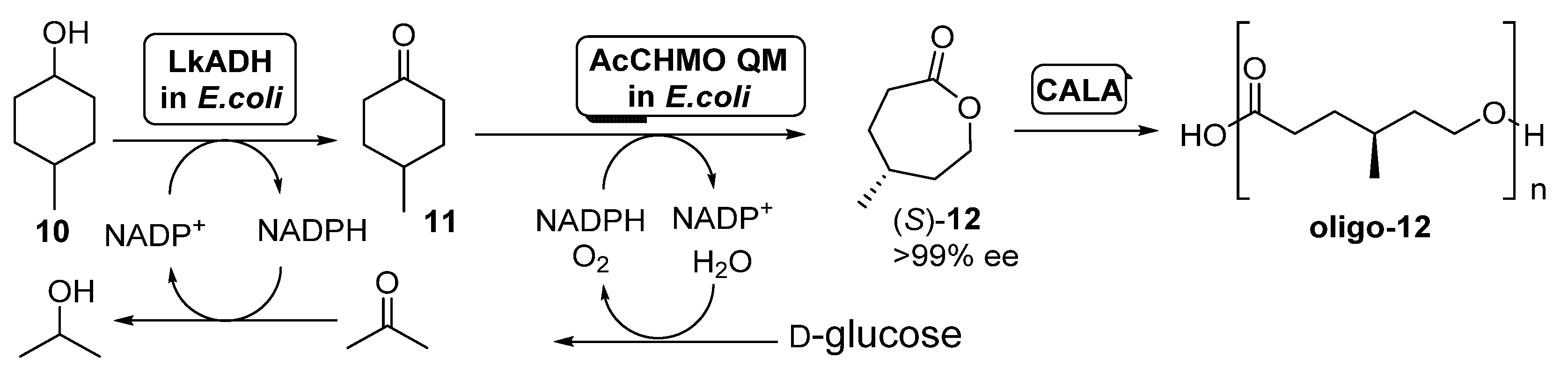
In a further development of this trienzymatic system, the same authors described the preparation of methyl-substituted ECL derivatives in a one-pot two-step process [54], as shown in Scheme 5.
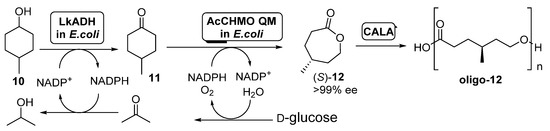
Scheme 5.
Preparation of (S)-4-methylcaprolactone (S)-12 and its oligomers through a biocatalytic procedure employing whole cells of Lactobacillus kefir ADH and Acinetobacter calcoaceticus CHMO mutant QM.
A diastereomeric mixture of 4-methylcyclohexanol (10) was employed as starting substrate; under those conditions, AcCHMO was able to convert 4-methylcyclohexanone 11 into the enantiopure lactone (S)-12 with high yield and complete selectivity. Initial experiments were performed with E. coli cells expressing Lactobacillus kefir ADH (LkADH) and wild type AcCHMO in 1:10 ratio (100 g wet cells weight/mL), but the conversions at all concentrations tested (5–20 mM) were low, achieving only a 34% of the enantiopure lactone as highest value when working at 5 mM. Poor conversion values were caused by the low conversion in the ADH catalyzed oxidation, due to the inhibition of this biocatalyst by the ketone 11 formed. In order to improve this process, a faster second oxidation process, with the aim of the fast removing of 11 from the reaction medium was envisioned. Thus, a more stable mutant of AcCHMO was employed (C376L/M400I/T415C/A463C, the so-called AcCHMO QM) and the ratio of the E. coli cells for both biocatalysts was optimized to 1:1. The supply of pure oxygen to the reaction also resulted in a higher conversion, close to 90% after 24 h at 25 °C. The addition of CALA (10 mg/mL) to the enzymatic system led to a further decrease in (S)-12 concentration, as it was being hydrolyzed into the oligo-(S)-lactone.
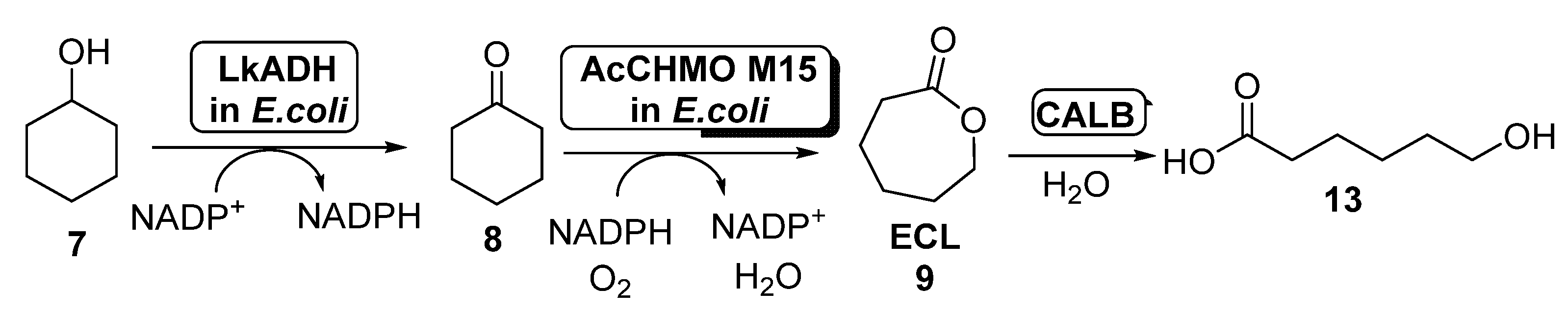
The production of ECL starting from cyclohexanol employing E. coli cells of ADH and AcCHMO has been studied by using a computational approach in 2017 [55], applying a kinetic model for the study this cascade reaction in both batch and fed-batch synthesis. To this purpose, a fed-batch synthesis was developed in order to circumvent the CHMO inhibition caused by cyclohexanol, which hampered the use of this substrate at concentrations higher than 60 mM when employed in batch. Thus, 7 was added to the reaction medium at different ratios, from 14.2 to 19.0 μmol/min; feed rates higher than 17.5 μmol/min led to the accumulation of 7, therefore reducing the rate formation of ECL due to CHMO inhibition. The amount of ECL in the reaction medium was reduced by hydrolyzing this compound to 6-hydroxyhexanoic acid (13, Scheme 6) in a process catalyzed by Candida antarctica lipase B (CALB). After 6 h, 162 mM of 13 was obtained with a feed rate of 19 μmol/min. The addition of CALB maintained a low ECL concentration in the reaction medium (lower than 10 mM).
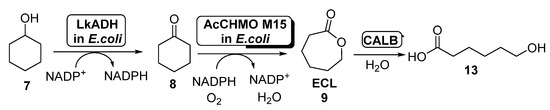
Scheme 6.
Preparation of ECL 9 and its oligomers through a biocatalytic procedure employing whole cells of Lactobacillus brevis ADH and Acinetobacter calcoaceticus CHMO M15.
The production of ECL has been optimized by performing the co-expression of the BVMO and the ADH in E. coli, employing a DuetTM vector [56], in order to obtain a higher efficiency for the enzymatic cascade. The pRSFDuet plasmid form Novagen was selected as dual expression vector, cloning the AcCHMO QM gene into the first multiple cloning site and the LkADH gene into the second multiple cloning site. With the aim of balancing the expression levels of the cascade enzymes, the ADH gene was subjected to engineering of the ribosome binding site (RBS), employing the native RBS sequence as well as two point mutations. In order to compare the results, both types of enzymes were expressed separately into E. coli cells, performing the biotransformations with an optimized ratio of CHMO: ADH cells 5:1 (100 g wet cells weight/mL) and in presence of glucose and acetone and co-substrates, as higher conversions were obtained than in absence of these compounds. This result can be explained by the difficult diffusion of the NADPH formed in the E. coli cells of the LkADH to the AcCHMO QM cells. After 16 h, it was possible to achieve a 90% conversion from 20 mM of cyclohexanol employing wild type RBS, while much lower conversions were measured with the other two RBS mutations, indicating that a decreased LkADH expression is required to obtain a similar enzyme ratio for optimal conversion. When the same process was carried out with the E. coli cells containing the double gene, a complete conversion was obtained after 16 h for all the three RBS preparations in the absence of co-substrates. In presence of glucose and acetone, complete conversion was also observed for the CHMO co-expressed with one of the RBS mutations of the ADH after 16 h, whereas after 2 h, a 54% conversion was reached. This system was the most efficient for the co-expression of both biocatalysts, suggesting that the ratios of expressed AcCHMO QM and LkADH were similar in this system. When higher substrate concentrations were employed, lower conversion values were achieved, mainly debt to the inhibitory effect of both 7 and 9 on AcCHMO.
Very recently, the conversion of cyclohexanol to ECL by combining LkADH with two stable mutants of CHMO has been studied in order to obtain a suitable process at 200 mM scale. Different parameters that affect the process were analyzed and optimized in order to achieve the most productive process for the preparation of the final lactone [57]. The reaction was carried out in a stirred-tank reactor with maintained temperature and pH, bubbling a mixture of oxygen with synthetic air and pumping cyclohexanol at a constant rate proportional to the whole cell cascade activity. As both 7 and 8 present a high vapor pressure, they can be stripped out of the system by the off gas. The key component of the complete system, as previously stated, is CHMO, due to its low stability and the requirement of a proper oxygen supply. This last parameter was optimized by performing a bubble aeration technique. BVMO stability was improved by assessing two CHMO mutants; thus, AcCHMO QM showed a 40% higher long-term stability, whereas AcCHMO M15, containing eight mutations, has a higher oxidative stability combined with an improved thermostability. These two variants were compared employing E. coli cells co-expressing ADH and the CHMO at pH 7.5 and 30 °C, with 200 mM cyclohexanol fed at 4.5 mM/h. When using AcCHMO QM, the formation of ECL achieved a value of 45 mM after 13 h, but at longer reaction times this concentration decreased to 39 mM, probably debt to the ECL autohydrolysis to yield 13. The same experiment was performed with AcCHMO M15, reaching a maximum concentration of ECL after 18 h (79 mM). Longer reactions times resulted in substrate accumulation in the reactor with no further formation of the desired product. AcCHMO M15 showed longer activity, but it was not possible to achieve complete conversion. The stability of both CHMO mutants under the process conditions was analyzed; when employing AcCHMO QM, the biocatalyst retained its complete activity for one hour and it decreased to 85% after 8 h. After this reaction time, there was an important drop in the biocatalyst activity. On the other hand, AcCHMO M15 maintained its activity (98% of the initial one) even after 18 h. Thus, when working with AcCHMO QM, fresh cells were added after 8 h, whereas no extra addition was required for AcCHMO M15. Further experiments showed that an increase in the cells loading in the reactor led to a decrease in the volumetric mass transfer, thus resulting in a lower efficiency. The complete optimization of the biocatalytic process allowed scientists to achieve conversions higher than 98% for both CHMO mutants, being possible to obtain a higher final product concentration (21.1 g/L), space time yield (1.1 g/L h) and isolated ECL amount (9.1 g) employing AcCHMO M15, due to its higher operational stability. These values represent a 100% increase regarding the non-optimized processes, indicating the development of a more efficient process for the preparation of ECL.
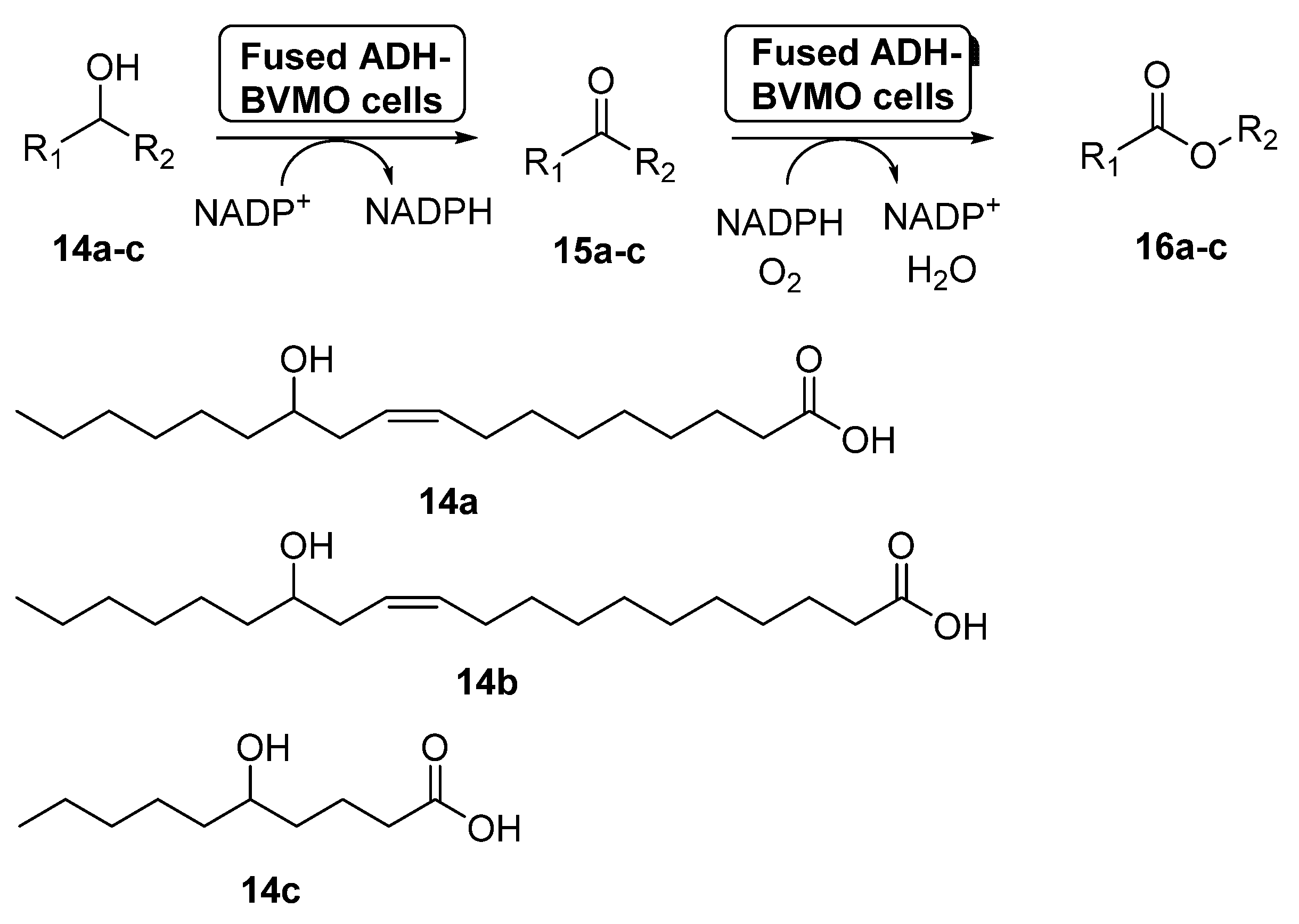
Fusion proteins have been initially employed to enhance the soluble expression of proteins or to improve the enzyme purification, but in the last years some examples have appeared in which these systems have been used with synthetic purposes [58]. In 2015, the preparation of an enzyme fusion formed by the ADH from Micrococcus luteus NCTC2665 and the BVMO from Pseudomonas putida KT2440 (3.0 g dry cells/L), able to catalyze the double oxidation of long-chain unsaturated secondary alcohols (14a–c) to the corresponding esters (16a–c) through the corresponding ketones 15a–c, was reported [59], as shown in Scheme 7. These products can be hydrolyzed into valuable ω-hydroxycarboxylic acids and n-alkanoic acids, valuable compounds. The design of the fusion enzyme showed the highest expression level when using a glycine-rich linker, formed by 12 aminoacids, between the two biocatalysts. When the bio-oxidations were carried out with the fused biocatalyst expressed in E. coli, higher conversions were achieved for all the substrates when compared with the independent ADH and BVMO expressed in E. coli. Thus, esters 16a–c can be recovered with conversions higher than 75%, with activities higher than 22 µmol/g dry cells min. This positive effect of the fusion enzyme in the cascade activity can be debt to a higher functional expression of the BVMO and/or to a better mass transport efficiency, due to the presence of both active centers at close positions.
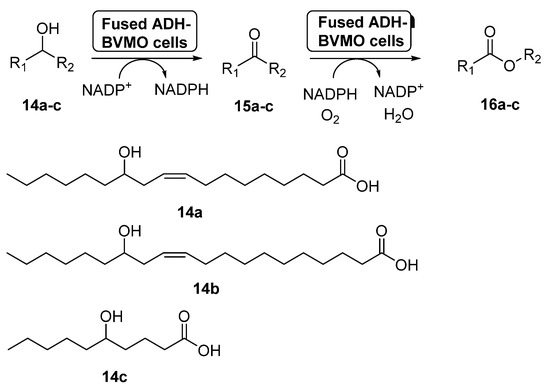
Scheme 7.
Preparation of ECL 9 and its oligomers through a biocatalytic procedure employing whole cells of Lactobacillus brevis ADH and Acinetobacter calcoaceticus CHMO M15.
2.2. Multi-Step Reactions Including ERED/BVMO Activity Catalyzed by Whole Cells
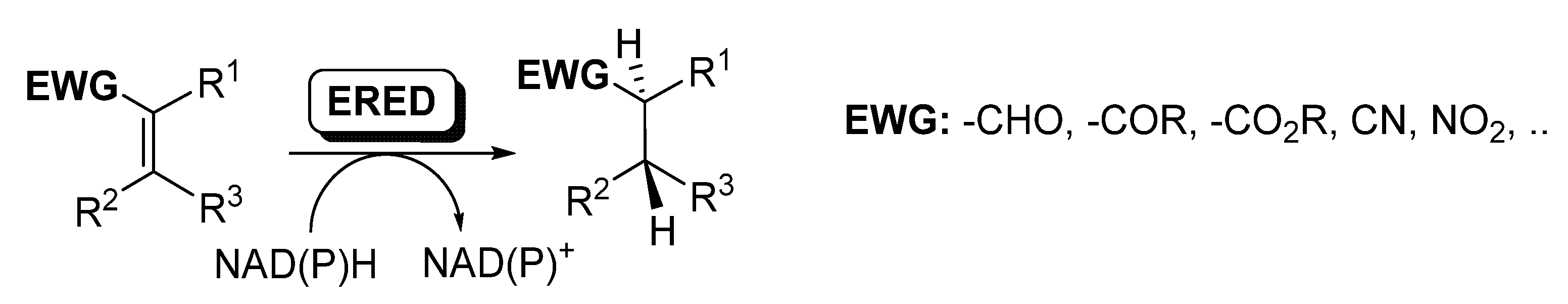
Ene-reductases (EREDs) from the old yellow enzyme (OYE) family are flavin-dependent enzymes that catalyze the chemo- and stereo-selective asymmetric reduction of electronically activated carbon–carbon double bonds [25,27,60,61,62], as depicted in Scheme 8.
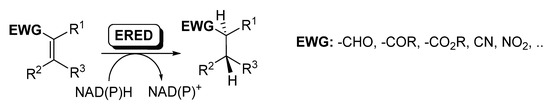
Scheme 8.
Ene-reductases for the reduction of C=C.
Several examples can be found in literature illustrating the use of whole cells-catalyzed cascades coupling BVMO and ERED activities. For instance, Silva et al. [63] reported the preparation of ECL 9 starting from either cyclohexenone 17, cyclohexanone 8 or cyclohexanol 7, using whole cells of Brazilian Geotrichum candidum CCT 1205 (Scheme 9).
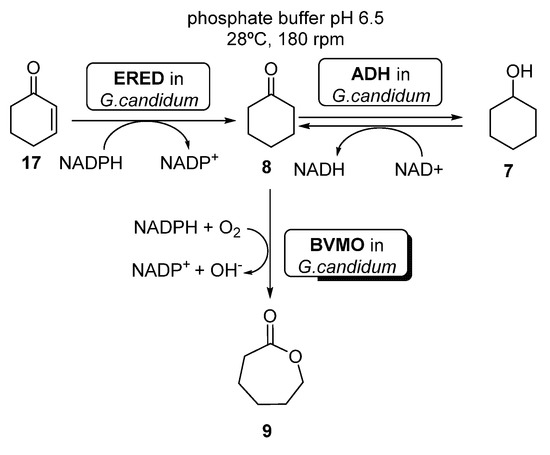
Scheme 9.
Preparation of ε-caprolactone 9, using whole cells of Geotrichum candidum CCT 1205.
When using cyclohexanone 8 as substrate, these authors reported quantitative yields of the desired ε-caprolactone 9 after only 5 h, using a higher amount (3.0 g versus 1.0 g) of whole cells (otherwise, the cascade accumulated 7 and 8). Using 7 or 8 as substrates, the reaction was even faster (4 h) than using only 10 g of cells. These results are better than those previously reported by Mihovilovic et al. [64], leading to 52% of 9 in 48 h, using BVMO expressed in E. coli. Recently, Silva et al. [65] have shown that it is possible to immobilize the whole cells from Geotrichum candidum CCT 1205 in modified silica (n SiO2–Cl, SiO2–NH2 and SiO2–SH supports), without altering their catalytic performance and also allowing the stabilization and reuse of the cells.
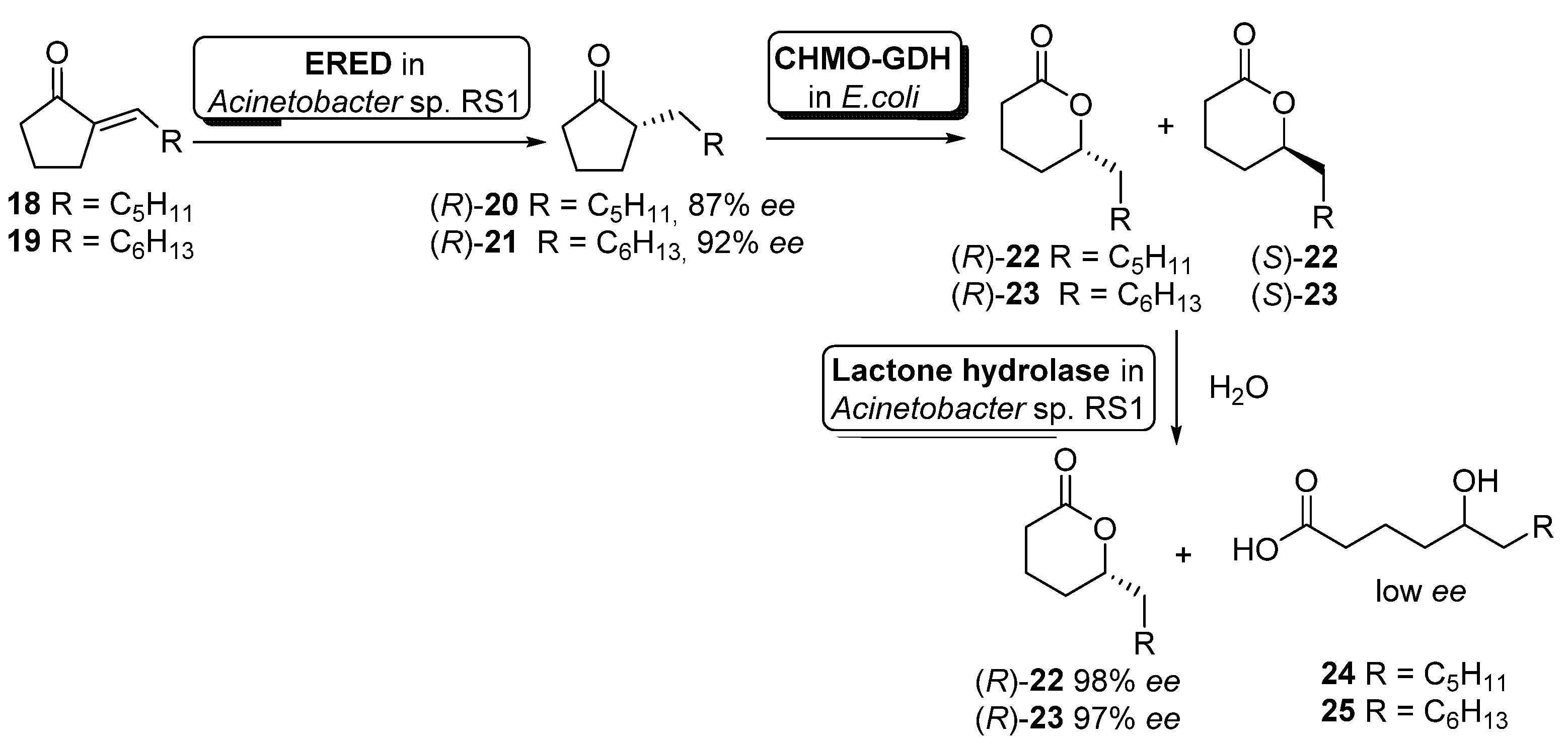
Although the previous example illustrates the use of non-engineered cells, it is becoming more usual the use of genetically modified cells. Hence, Liu and Li described an enantioselective reduction−oxidation−hydrolysis cascade catalyzed by engineered whole cells for the synthesis of (R)-2-alkyl-δ-lactones 22–23 (useful flavor and fragrance materials) starting from the corresponding 2-alkylidenecyclopentanones 18–19 (Scheme 10) [66].
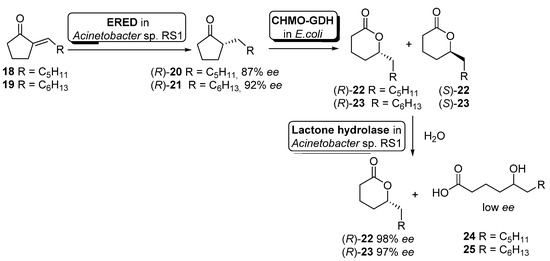
Scheme 10.
Preparation of enantiopure (R)-2-alkyl-δ-lactones by using a whole-cell-catalyzed cascade involving BVMO activity.
In this example, the cascade catalysis was started with 50 mL of cell suspension of Acinetobacter sp. RS1 in Tris buffer (cell density 12 g cdw/L) containing 40 mg of alkylidene ketones 18 or 19 and 20 mg/mL glucose. After 3 h of reaction to allow the ERED-mediated accumulation of alkyl ketones 20 and 21 (R-configuration), 150 mL of Tris buffer containing engineered cells of E. coli (CHMO−GDH) (10 g cdw/L) was added to start the Baeyer–Villiger oxidation. After simultaneous oxidation and hydrolysis for 1.5 h, extraction with ethyl acetate and purification by flash chromatography, compounds (R)-22 (56% yield, 98% ee) or (R)-23 (41% yield, 97% ee) could be obtained. The presence of a hydrolytic activity inside Acinetobacter sp. RS1 cells was responsible for the hydrolysis of the intermediate (S)-22 or (S)-23 leading to δ-hydroxyacids 24 or 25, obtained with low enantioselectivity (E = 8–11).
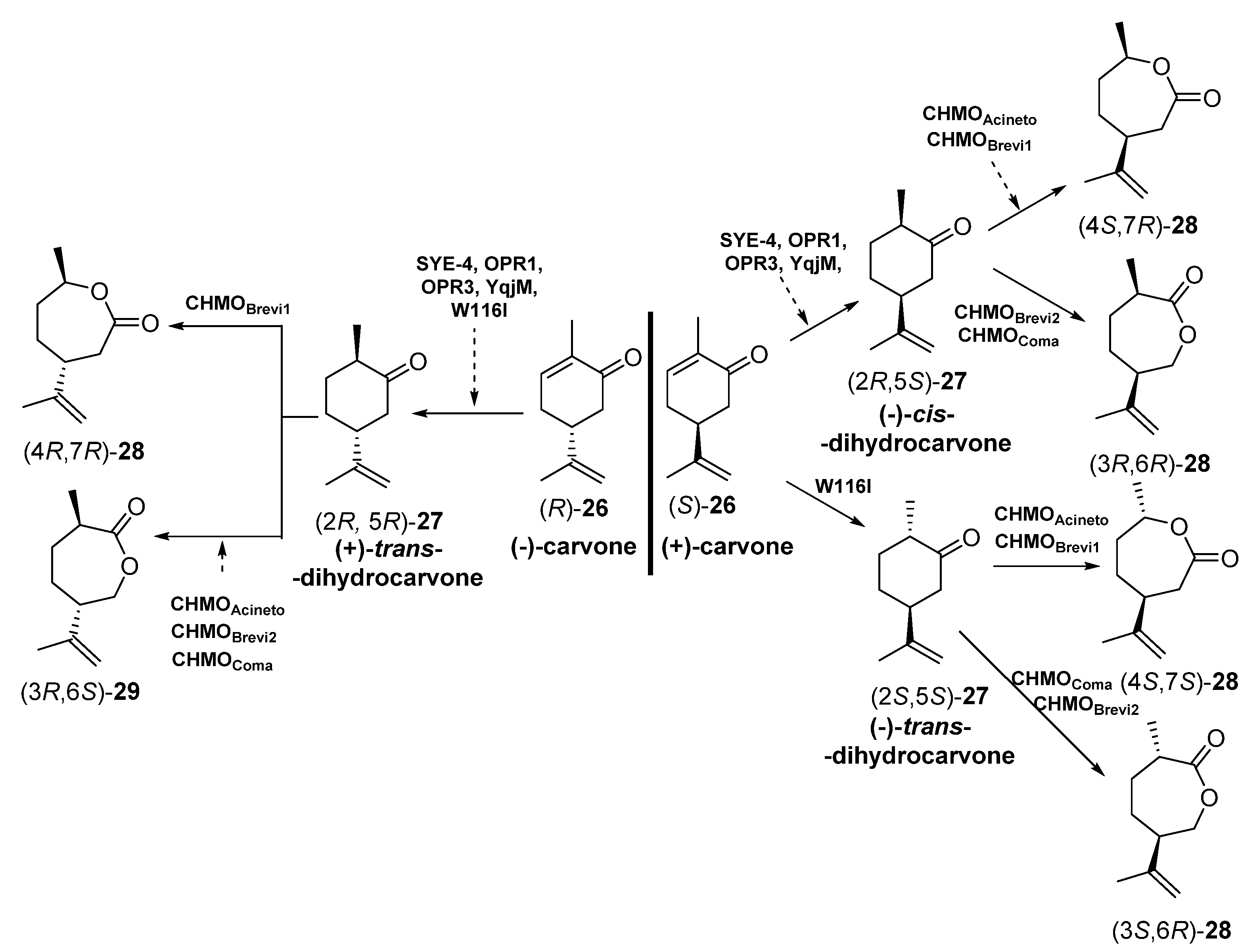
The different enantiomers of carvolactones (7-methyl-4-(prop-1-en-2-yl)oxepan-2-ones) are gaining importance as monomers for the preparation of polymeric thermoplastic elastomers (shape-memory polymers) and pressure-sensitive adhesive components [67,68,69]. These carvolactones can be obtained starting from biogenic carvones after a C=C reduction and a Baeyer–Villiger oxidation. To this purpose, Iqbal et al. [70] have reported the concurrent redox cascade to transform (-) carvone and (+)-carvone [(R) and (S)-26] into the correspondent lactones, as depicted in Scheme 11.
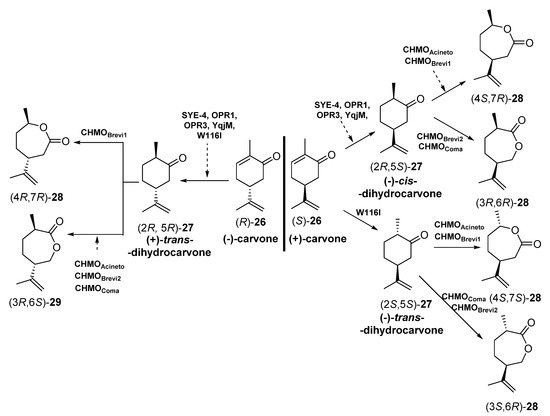
Scheme 11.
Preparation of carvolactone stereoisomers 28 and 29 by a concurrent redox cascade.
Crude cell extracts (5.0 mg in 200 µL) from E. coli BL21 (DE3) containing the corresponding EREDs or BVMOs were used. Initially, each biotransformation step was carried out independently, starting with bioreductions of enantiomerically pure carvones (R) or (S)-26 and subsequent bio-oxidation with different BVMOs to furnish different stereoisomers of carvolactones 28 and 29. Finally, the reduction-oxidation steps were performed in a concurrent one-pot cascade. Both for the individual steps and the cascade, NADP+, glucose -6-phosphate and glucose -6-phosphate dehydrogenase were used for cofactor recycling. The EREDs that expressed E. coli BL21 (DE3) were SYE-4 (from Shewanella oneidensis [71]), OPR1 and OPR3 (12-oxophytodienoate reductase from Lycopersicon esculentum, tomato [72]), YqjM (from Bacillus subtilis [73]) and the variant W116I (replacement of tryptophan-116 by isoleucine in the OYE from Saccharomyces pastorianus [74]). On the other hand, the BVMOs were either cyclohexanone monooxygenases (CHMOs: AcCHMO [75], CHMOBrevi1 from Brevibacterium sp. [76] or cyclopentanone monooxygenases (CPMOs-type enzymes: CHMOBrevi2 from Brevibacterium sp. [76] and CPMOComa from Comamonas sp. [77]). Regarding the reaction stereochemistry of the bioreduction, as depicted in Scheme 5, when starting from (-)-carvone (R)-26 all the EREDs catalyzed the trans C=C bioreduction to afford (+)-trans-dihydrocarvone (2R,5R)-27. Opposite, if (+)-carvone (S)-26 was the initial substrate, most of the EREDs catalyzed the cis C=C bioreduction to afford (+)-cis-dihydrocarvone (2R,5S)-27, while the trans bioreduction, leading to (-)-trans-dihydrocarvone (2S,5S)-27, was observed only when employing W116I. Analyzing the regioselectivity of the BVMOs, depending on the absolute configuration of 27, some of the enzymes were leading to the “normal” lactones 28 (classical migration of the Criegge intermediate leading to the oxygen insertion in the most substituted alpha carbon [78]) or the “abnormal” ones 29, in good yields and enantiopurity.
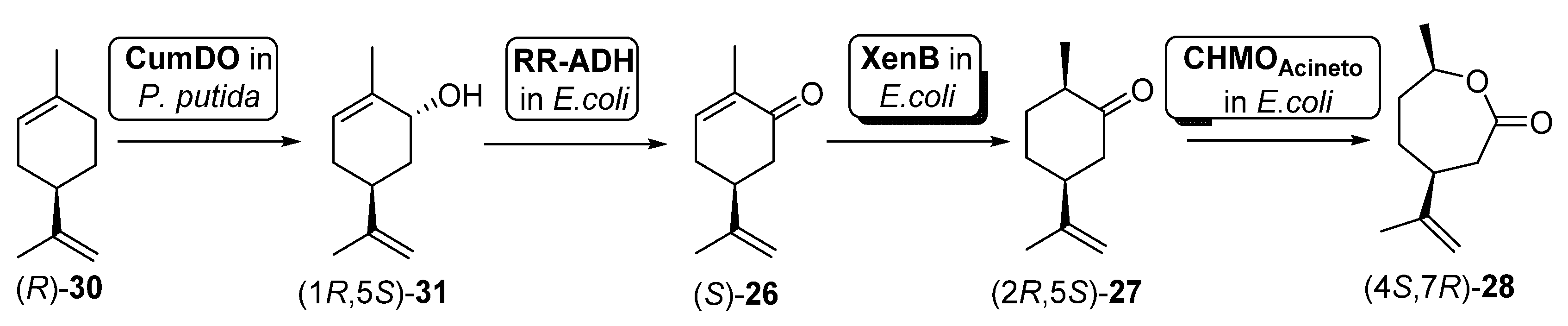
In another example, Oberleitner et al. [79] reported the preparation of carvolactone (4S,7R)-28 starting from (R)-limonene ((R)-30) directly extracted from orange peel by means of a biocatalytic cascade shown in Scheme 12.
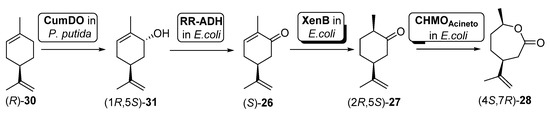
Scheme 12.
Preparation of carvolactone (4S,7R)-28, using a whole cells-catalyzed cascade involving a BVMO.
To this purpose, two different types of cells were used: (i) an engineered Pseudomona putida S12 expressing cumene dioxygenase (CumDO) and (ii) E. coli BL21 (DE3) cells possessing different oxidative activities. These last ones were previously reported as being capable of catalyzing the conversion from carveol (1R,5S)-31 into (4S,7R)-28 [80], as they contain the expression of an alcohol dehydrogenase (RR-ADH from Rhodococcus ruber), an ERED (XenB from Pseudomonas sp.) and a BVMO (AcCHMO). Through the mixed culture approach, combining the two bacterial strains (different concentrations tested) in one pot, 47% of (4S,7R)-28 was produced after 20 h. They used a sequential approach, where hydroxylation of (R)-30 by CumDO was performed first, and E. coli BL21(DE3) resting cells were added to the reaction vessel after 10 h, observing full conversion to (4S,7R)-28 in 20 h. Then, these authors explored the direct use of waste product orange peel (biomass loading of about 3% (w/v), obtaining 3.2 mg of (4S,7R)-28 per g orange peel. Anyhow, by lowering the orange peel amount to 1.5% (w/v) and using the mixed-culture sequential combination, it was possible to furnish 6.3 mg of carvolactone per g orange peel (29% of (4S,7R)-28 from (R)-30 over four biocatalytic steps, 73% per step), only relying on orange peel as the substrate reservoir in aqueous buffer without any additives.
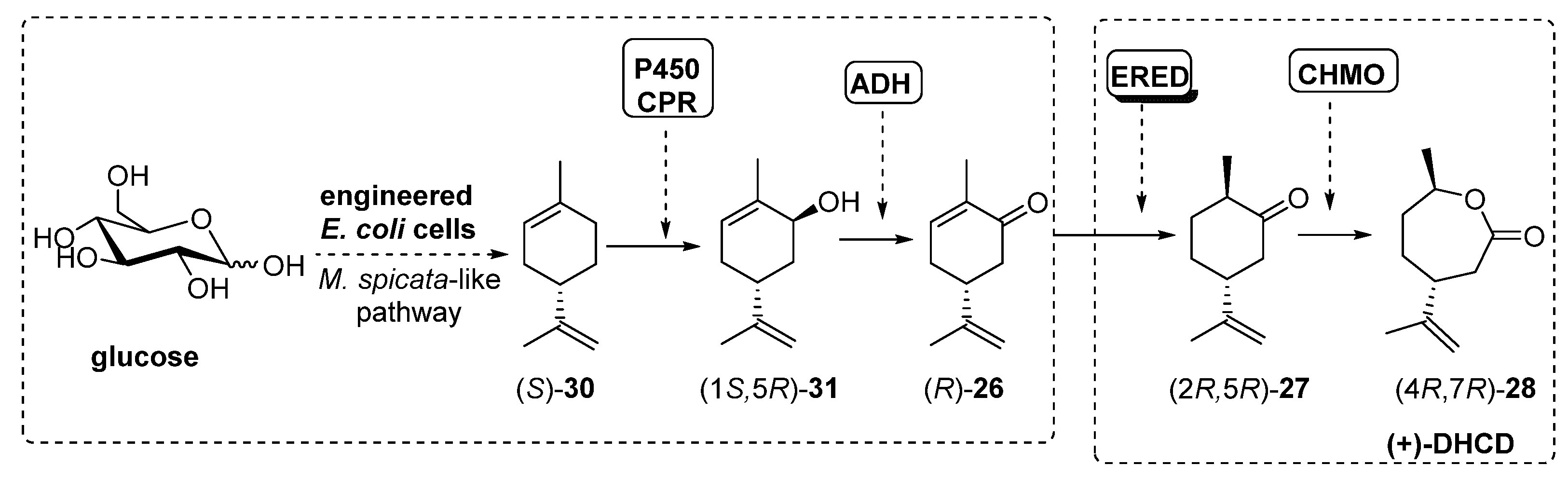
Recently, Avalos et al. [81] have described a similar process, using engineered E. coli cells, already designed to follow the Mentha spicata route leading to (-)-carvone (R)-26, in which the addition of a ERED and a BVMO (10 µM) activity finally furnished (4R,7R)-28, also known as (+)-dihydrocarvide (+)-DHCD, as shown in Scheme 13.
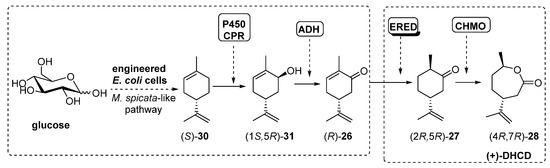
Scheme 13.
Preparation of (+)-DHCD, using engineered whole-cells-catalyzed cascade.
The initial step into the M. spicata biosynthesis of (-)-carvone (R)-26 is the hydroxylation of (S)-limonene (S)-30 to furnish carveol (1S,5R)-31 catalyzed by limonene-6-hydroxylase (L6H, a P450 enzyme) coupled to SmCPR, a cytochrome P450 reductase from Salvia miltiorrhiza. Based on this system, authors constructed the rest of the enzymatic system by adding genes from ADH (RR-ADH was the best option), ERED (PETNR, pentaerythriol tetranitrate reductase from Enterobacter cloacae PB2 [82,83]) and BVMO (CHMO3M, a triple mutant from Rhodococcus sp. Phi1 CHMO [84]). Under optimal conditions, the complete biosynthesis of (+)-DHCD from glucose in E. coli was reported at 6.6 mg/L.
3. Multi-Step Reactions Including BVMO Activity Catalyzed by Isolated Enzymes
3.1. Linear ADHs/BVMOs Cascades
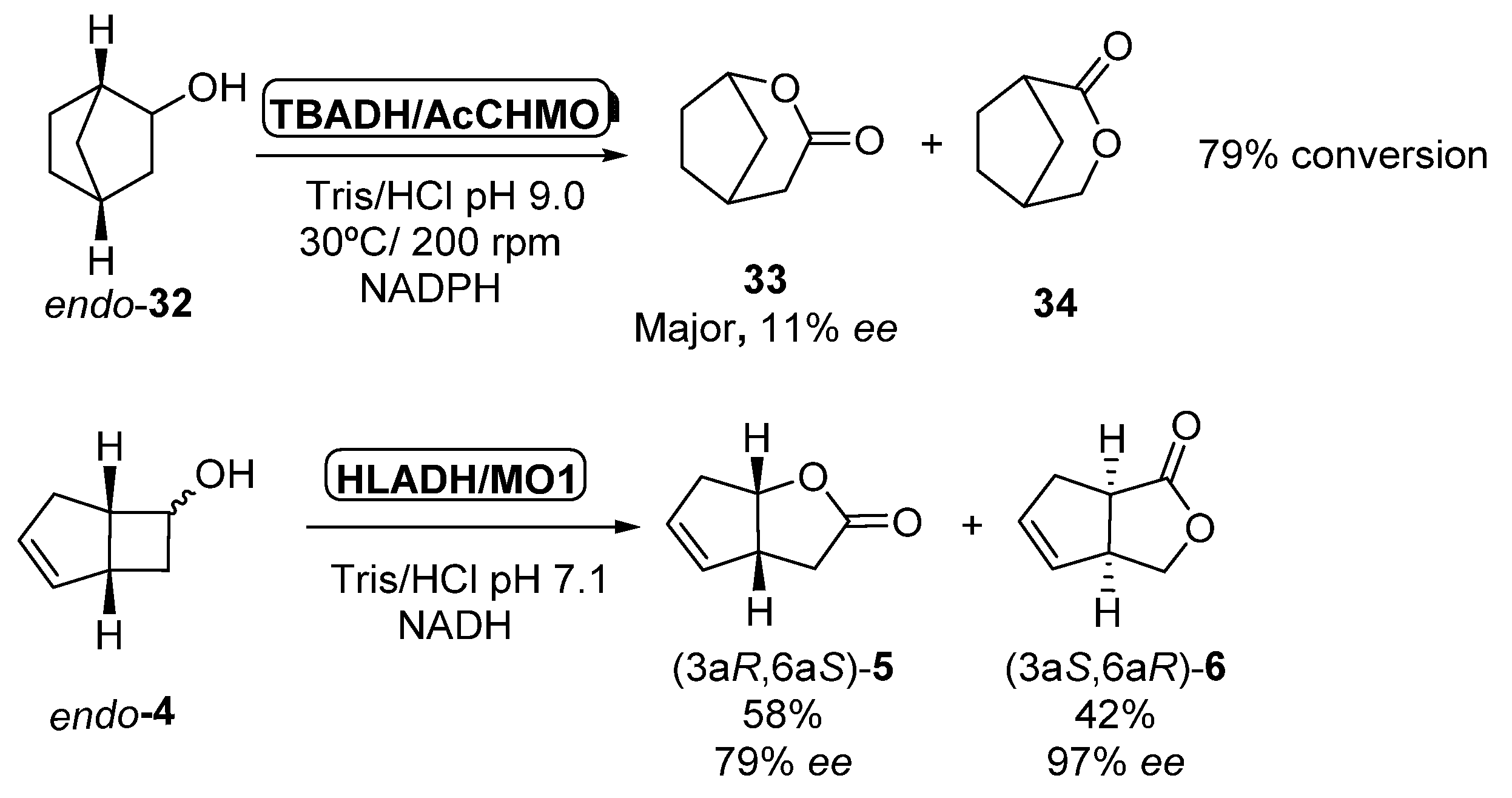
The initial experiments in which multienzymatic processes involving ADHs and BVMOs were described [50] were also carried out by using isolated enzymes, combining purified Thermoanaerobium brockii ADH (TBADH) with purified CHMO form Acinetobacter sp. NCIMB 9871 (40 units) in the presence of a catalytic amount of NADPH, which was recycled by both enzymes. As depicted in Scheme 14, oxidation of endo-32 afforded a regioisomeric mixture of lactones 33 and 34 with 79% conversion (67% yield) after 5.5 h. Lactone 33 was the major product in the mixture, being recovered with low optical purity (>95% purity, 11% ee). Same conditions were applied to the biotransformation of endo-bicyclo[3.2.0]hept-2-en-6-ol 4 leading to a 41% of a mixture of lactones 5:6 in 2:1 ratio after 8.5 h; (1S,5R)-5 was recovered with 86%% ee. The same approach was performed three years later in the oxidation of racemic norbornenol 32 to the corresponding lactone 33 [85] as solely product. Starting alcohol was non-selectively oxidized to norbornanone by TBADH, which was oxidized to 33 in presence of Acinetobacter calcoaceticus NCIMB 9871 with a 67% global yield and 11% ee. Only 3.5 mol% of NADPH cofactor was required to ensure the complete conversion of the starting alcohol. The same authors described the formation of two regioisomeric lactones (5 and 6) in the bio-oxidation of alcohol endo-4 in presence of horse liver alcohol dehydrogenase (HLADH) and Pseudomonas putida monooxygenase MO1. HLADH catalyzed the non-selective oxidation of the alcohol to the ketone, which was selectively oxidized to a mixture of (3aR,6aS)-5 (58% yield, 79% ee) and (3aS,6aR)-6 (42% yield, 97% ee) by MO1 using in this system NADH as coenzyme.
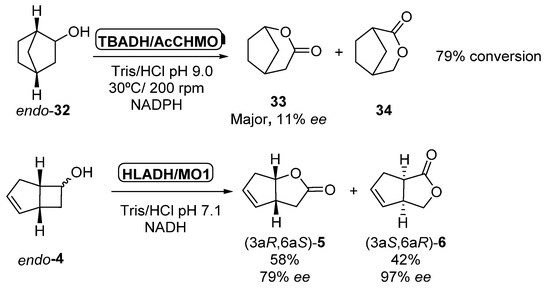
Scheme 14.
Biocatalyzed cascades employing isolated ADHs and BVMOs for the synthesis of bicyclic lactones.
The preparation of ECL combining isolated ADHs and BVMOs have also been widely studied. One of the first examples used a chimeric polyol dehydrogenase (PDH), formed by introducing a substrate recognition loop from the Rhodobacter sphaeroides PDH into the thermostable PDH from Deinococcus goethermalis [86]. When the resulting PDH was tested together with AcCHMO, conversions around 80% were obtained when working at cyclohexanol concentrations of 5–10 mM. As previously shown, higher substrate concentrations led to lower BVMO activities, resulting this enzyme critical for the development of a valuable procedure. When the process was carried out at a preparative scale, a 55% yield of ECL with complete purity was obtained after 2 h, employing 40 units of AcCHMO and 3.6 units of PDH. Authors sustained that this low yield was caused by the volatility of cyclohexanol. In order to increase the BVMO stability, both CHMO and PDH were co-immobilized onto RelizymeTM HA403 using glutaraldehyde as linker. A CHMO: PDH ratio 10:1 led to the highest activity of the biocatalytic system, but still CHMO showed a low stability in this preparation. Only a 34% conversion was achieved after 5.5 h. The immobilized biocatalyst was reused for several cycles, being measured a residual activity of 10% after the first cycle. This loss was debt to the instability of the CHMO, as the addition of fresh BVMO after each cycle reduced the loss in activity observed.
In 2013, Gröger et al. also described a linear cascade for the synthesis of ECL starting from cyclohexanol [87]. For the oxidation of cyclohexanol to cyclohexanone, a crude extract of LkADH was employed, whereas a commercial preparation of AcCHMO (Enzymicals AG; 0.153 U/mg) catalyzed the ketone oxidation. When starting from 20 mM cyclohexanol, it was possible to obtain ECL with an excellent conversion (97%) after 24 h. Increasing substrate concentration up to 60 mM also led to excellent conversion values, whereas higher values led to high drop for product obtained. Further studies on these results showed a significant inhibition on the BVMO at high ECL concentrations, as well as a negative impact on this enzyme of both cyclohexanol and cyclohexanone.
In 2017, the two redox enzymes involved in the biotransformation of cyclohexanol to ECL were fused into a self-sufficient bifunctional catalyst [88]. The BVMO that was used was the cyclohexanone monooxygenase form Thermocrispum municipale (TmCHMO) [89], whereas three NADPH-dependent alcohol dehydrogenases were tested: TBADH, ADH from Pyrococccus furiosus and the ADHMi from Mesotoga infera. Fusion catalysts (5–20 μM) were prepared in both orientations, with the TmCHMO either at the C- or at the N-terminus of the ADH. From the six fusion catalysts prepared, Tb-Tm, which contains TBADH at the N-terminus and TmCHMO at the C-terminus, showed the highest thermostability and was tested with synthetic purposes. When starting from 10 mM of cyclohexanol, a complete conversion was observed after 24 h at 37 °C. The use of cyclohexanol concentrations higher than 100 mM did not afford any conversion, due to the inhibition of TmCHMO by this substrate. In order to overcome this drawback, the starting alcohol was pumped into the reaction medium, achieving a 64% conversion after 24 h. Subsequently, with the aim of analyzing the effect of ECL inhibition, CALA (Candida antarctica lipase A, 10 mg/mL) was added in the reaction medium to hydrolyze the lactone product into oligo-caprolactone (see Scheme 4). In these conditions, conversion was increased up to 81%. During this reaction, a pH decrease was observed due to the hydrolytic process, and by this reason, authors employed a phosphate buffer pH 8.0 of high concentration. After all of these modifications, conversion higher than 99% was obtained when starting from 200 mM of cyclohexanol, in a process with a turnover number (TON) of 13,333. Further experiments showed that TmCHMO was the slowest enzyme of the fusion protein, being the oxidation of the cyclohexanone to ECL the rate-limiting step. The fusion protein seemed to have a better performance than both individual biocatalysts. This result can be attributed to several factors, as the lower stability of the non-fused TmCHMO, higher activity of the BVMO or the localization of both enzymes at closer positions in the fused biocatalyst.
In order to optimize the preparation of ECL through the cascade involving ADHs and BVMOs, a solvent engineering study has been developed by carrying out the oxidation of cyclohexanol to ECL combining isolated Lactobacillus kefir ADH (LkADH) and AcCHMO (3.82 units per 5 mL of reaction) [90]. Different hydrophobic organic solvents, including ethyl acetate, methyl tert-butyl ether, toluene, methylcyclohexane, n-heptane and isooctane were tested in this process at 10% v/v. With most of the solvents, lower conversions were obtained, but in presence of isooctane, complete conversion was achieved after 23 h, the same result to the one obtained in buffer alone. The time course of the reaction when employing 40 mM of starting material revealed that the process was faster in presence of 10% v/v of the organic co-solvent, as higher conversions were measured after 1, 2, 3 and 4 h, a result that is maintained at higher substrate concentration (80 mM). The physical state of the BVMO was also tested in the reactions with 10% v/v of isooctane. When employing cell free extracts of AcCHMO, a slightly lower reaction course was obtained when compared with the purified enzyme, whereas the use of the whole cells of the BVMO only led to a 7% conversion after 23 h.
The preparation of lactones through a multicatalytic process in which a BVMO is involved has been developed starting from cycloalkanes [91]. In a three-step procedure, shown in Scheme 15, the alkane was oxidized to the cyclic alcohol in a reaction catalyzed by a cytochrome P450 monooxygenase (CYP450, 6.3 μM). These biocatalysts are able to perform a wide set of oxyfunctionalizations, in general with high regio- and enantioselectivity, which makes them useful enzymes in organic synthesis [92,93]. In the third step, the alcohol led to the corresponding lactone by described bienzymatic system ADH-BVMO. As this process is not redox balanced, the biocatalysts were employed as cell-free extracts, which contains endogenous NAD(P)H required for cofactor regeneration, together with glycerol and glucose and co-substrates.
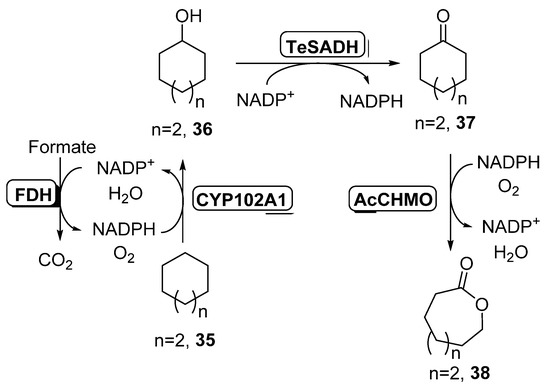
Scheme 15.
Enzymatic preparation of lactones in a three-step biocatalytic cascade combining a CYP450, an ADH and a BVMO.
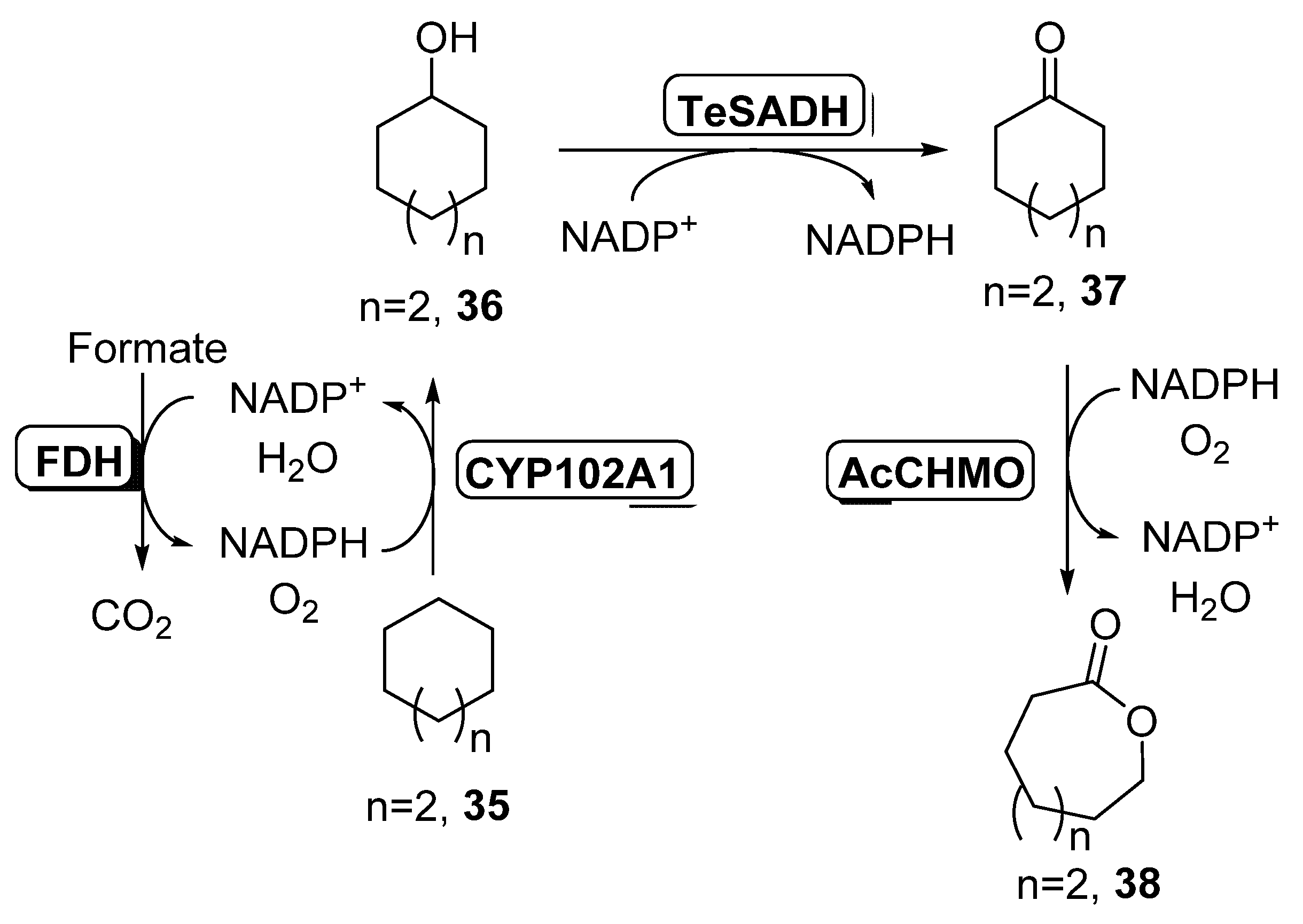
Initial experiments were carried out with cycloheptane 35, at a high concentration (165 mM), in order to have a second phase at the reaction medium. The best conditions were found employing the CYP102A1 mutant of cytochrome P450 BM3, a NADH-dependent CYP450 able to convert 35 into cycloheptanol 36, together with Thermoanaerobacter ethanolicus ADH (TeSADH) which converts 36 into ketone 37 and AcCHMO, NADPH-dependent enzymes, recovering 0.41 g/L of final lactone 38. The NADH required for CYP102A1 activity was recycled by the E. coli extract, whereas the ADH and the BVMO, using 0.75 g wet cells weight, were the responsible for the NADPH regeneration. In order to prepare a redox balance cascade, formate dehydrogenase (FDH), a NADH dependent enzyme, was added to the reaction system, allowing an increase in the production of oxocan-2-one 38 (1.4 g/L). Addition of external NADH and NADPH also led to a higher formation of 38. Finally, the use of higher biocatalyst concentration by employing highly concentrated cells suspensions in the formation of the cell free extracts allowed a high productivity at reaction times of 12 h (2.9 g/L), whereas longer times led to a deactivation of both CYP450 and CHMO. The optimized cascade was also applied to the formation of ECL (n = 1) and caprylolactone (oxonan-2-one, n = 3), with productivities lower for these compounds (around 0.6 g/L).
A multienzymatic system for the conversion of cyclohexanol into aminohexanoic acid (42, Scheme 16), the monomer of nylon-6, was developed by combining one mutant of AcCHMO with different biocatalysts [94].
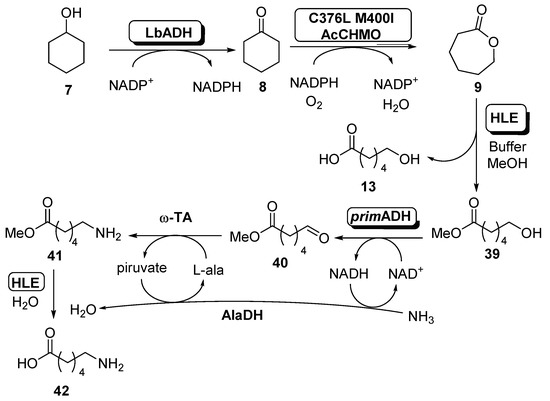
Scheme 16.
Multienzymatic system for the preparation of 6-aminohexanoic acid (42).
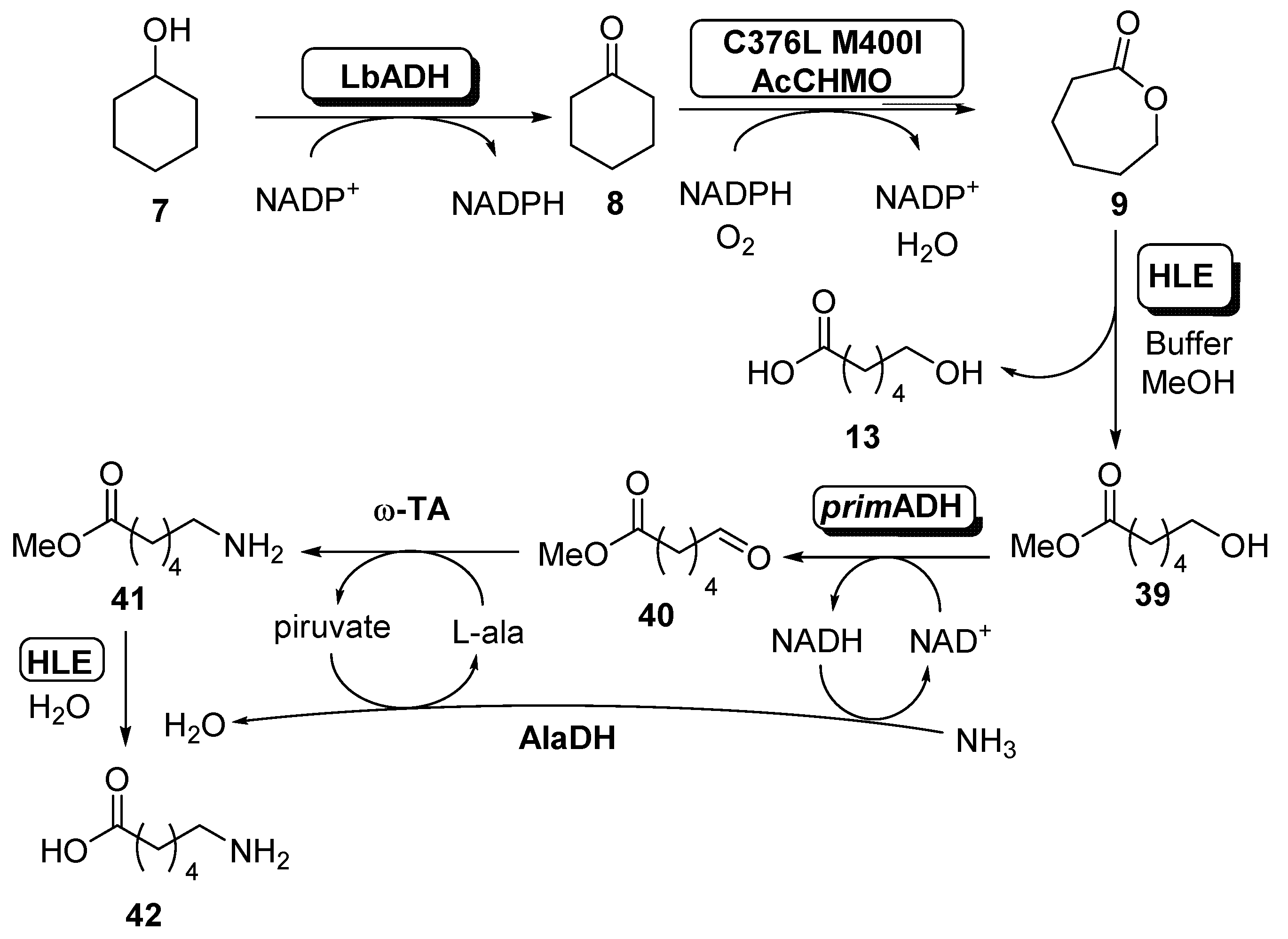
The complete system was based in two different modules. In the first one, which was well described in the present paper (see Scheme 4, Scheme 6 and Scheme 8), ADH from Lactobacillus brevis (LbADH, 0.2 units) was combined with the C376L M400I mutant of AcCHMO (0.2 units) with recycling of NADPH in order to generate ECL with excellent conversion (98%), even at high cyclohexanol concentration (96% conversion at 200 mM). When this process was carried out at preparative scale, a 75% yield was achieved. The second module comprises the transformation of ECL into 6-aminohexanoic acid and initially was studied as a combination of a lactonase (from Rhodococcus sp.), to open the ECL into compound 13, an ADH from E. coli for the oxidation of 13, an alanine dehydrogenase (AlaDH) from Bacillus subtilis and different transaminases for the amination reaction to obtain 42. When this system was applied, the formation of compound 13 occurred with excellent conversion, but a very low amount of final amine was recovered. This result was caused by the carboxylic acid moiety of 6-hydroxyhexanoic acid, which inhibited the ADH-catalyzed oxidation. In order to overcome this drawback, a novel synthetic route was analyzed, thus converting ECL into a methyl ester 39 employing methanol as nucleophile for the opening of the lactone. In this way, the carboxylic acid 13 was not presented in the reaction medium, allowing the reaction to occur with higher conversions. Different esterases were tested for this purpose, obtaining the best results with the horse liver esterase (HLE). When the treatment of ECL 9 was carried out in buffer containing 10% v/v of methanol as co-solvent in presence of this biocatalyst, it was observed the formation of double amount of the methyl ester 39 until all the ECL was consumed, before starting with a slow hydrolysis of the methyl ester to the carboxylic acid. Once obtained, this compound was oxidized by an ADH (prim-ADH from Bacillus stearothermophilus) to aldehyde 40, which was subsequently aminated by a ω-transaminase (ω-TA) in presence of an alanine dehydrogenase (AlaDH) into compound 41. This reaction requires L-alanine as amine donor, which generates pyruvate, that is recycled by AlaDH, consuming ammonia and the NADH generated in the ADH oxidation, thus regenerating this cofactor. The final step is the esterase-catalyzed hydrolysis of the aminoester 41 to furnish 6-aminehexanoic acid 42. When the complete system was tested, it was observed that the presence of methanol in the medium was a problem for the AcCHMO stability. Thus, this solvent concentration was lowered down to 2% v/v, being possible to obtain after 24 h a 24% of 6-aminohexanoic acid, with a 35% of starting cyclohexanol, 30% 6-hydroxyhexanoic acid and a 10% of cyclohexanone.
3.2. Parallel ADHs/BVMOs Cascades
Apart from linear cascades, in which one reaction is conducted before the start of the subsequent reaction, parallel or tandem cascades, with both reactions occurring simultaneously, have been developed combining ADHs and BVMOs.
One of the first examples of parallel cascades with BVMOs was shown in 2010. These isolated catalysts and ADHs have been employed in a parallel fashion for the simultaneous preparation of optically active compounds [95]. This methodology, called Parallel Interconnected Kinetic Asymmetric Transformations (PIKAT), allowed minimizing the quantity of reagents, thus maximizing the redox economy of the system. The PIKAT was performed by coupling two different asymmetric transformations: (a) via two kinetic resolutions (Scheme 17) or (b) via a kinetic resolution and a desymmetrization reaction (Scheme 18).
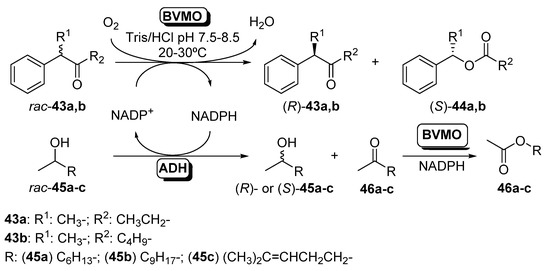
Scheme 17.
Parallel interconnected kinetic asymmetric transformation for the concurrent synthesis of optically active alcohols, ketones and esters combining ADHs and BVMOs in a double kinetic resolution.
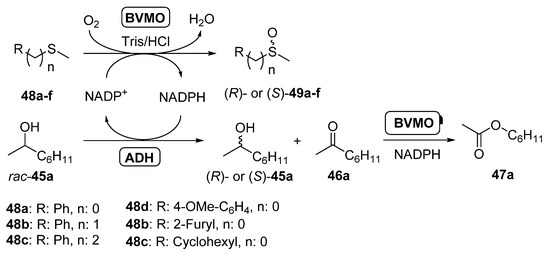
Scheme 18.
Combination of ADHs and BVMOs for the kinetic resolution of racemic 2-octanol and the enantioselective sulfoxidation of prochiral sulfides in a concurrent fashion.
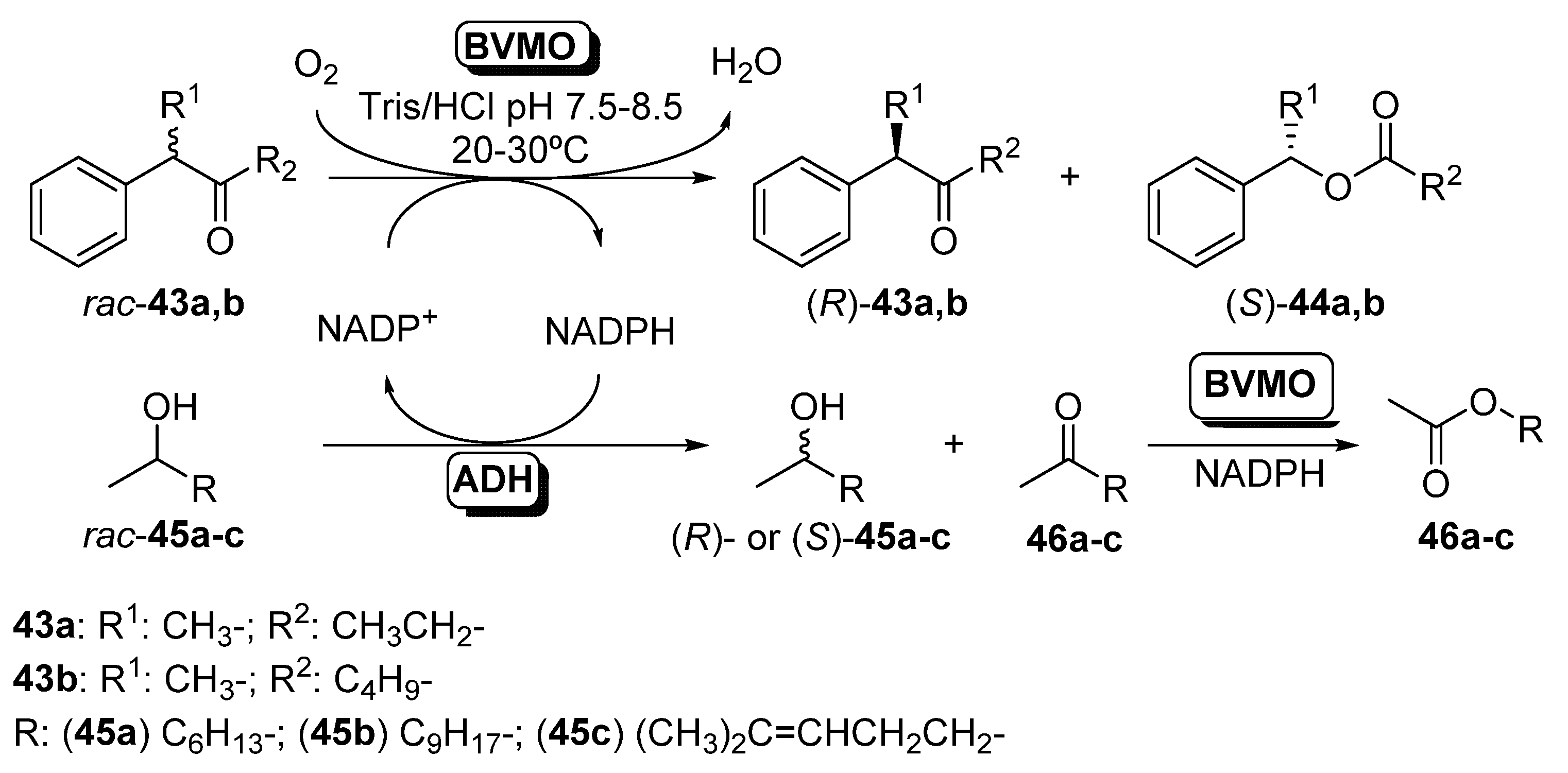
By the proper selection of both catalysts (an ADH and a BVMO, 2 U) and using a catalytic amount of NADPH, which acts as the connector between the two transformations, all possible enantiomers can be obtained in a one-pot process. Phenylacetone monooxygenase (PAMO) from Thermobifida fusca [96] and 4-hydroxyacetophenone monooxygenase (HAPMO) from Pseudomonas fluorescens ACB [97] were selected as BVMOs, due to their excellent behavior in the kinetic resolution of racemic ketones [98], whereas two NADPH-dependent ADHs with opposite selectivity were employed: LbADH and ADH from Thermoanaerobacter sp. (ADH-T). Initial experiments were carried out in the interconnected kinetic resolution of racemic 2-phenylpentan-3-one (43a) and 2-octanol (45a) with BVMOs and ADHs in presence of a catalytic amount of NADPH (0.2 mM). When using HAPMO and ADH-T at 20 °C and pH 7.5, it was possible to obtain (R)-43a and (S)-44a in a process with excellent enantioselectivity (E > 200). ADH-T was able to oxidize (S)-45a to ketone 46a, remaining (R)-45a with high enantiomeric excess. When LbADH was used instead of ADH-T, (S)-45a was recovered in an excellent double kinetic resolution when the PIKAT was carried out at pH 8.5. PAMO was also tested in this process. When combined with ADH-T, (R)-43a, (S)-44a and (R)-45a were obtained with conversions around 50% and excellent enantioselectivities. The pH and the temperature effect were studied in the coupled system PAMO/LbADH, being observed that the system can work even at 60 °C, but the best results were achieved at pH 8.5 and 30 °C. A small amount of hexyl acetate (47a) was recovered in the reaction medium. This compound was obtained by the PAMO-catalyzed oxidation of 2-octanone 46a in a secondary reaction. The parallel resolution was extended to other secondary alcohols such as undecanol (45b) or sulcatol (45c) in combination with the BVMO-catalyzed resolution of rac-43a. For all the BVMOs and ADHs, excellent results were obtained in the resolution of racemic 45b and 43a, being observed that PAMO led to better conversions and enantioselectivities than with HAPMO. (R)-sulcatol was obtained with low optical purities in the ADH-T-biocatalyzed reactions, as this enzyme was not able to perform a selective bio-oxidation of the racemic alcohol. When the reactions were carried out with LbADH, excellent resolutions were achieved. 4-Phenylhexan-3-one (rac-43b) was resolved in presence of 45a, with excellent selectivity and high optical purities for all the biocatalysts combinations, thus recovering (R)-43b, (S)-44b and (R)- or (S)-45a depending on the enzymes employed. In a further development of the PIKAT system, the kinetic resolution of racemic alcohols catalyzed by isolated ADHs was coupled with the desymmetrization of different prochiral sulfides employing BVMOs [99], as shown in Scheme 17.
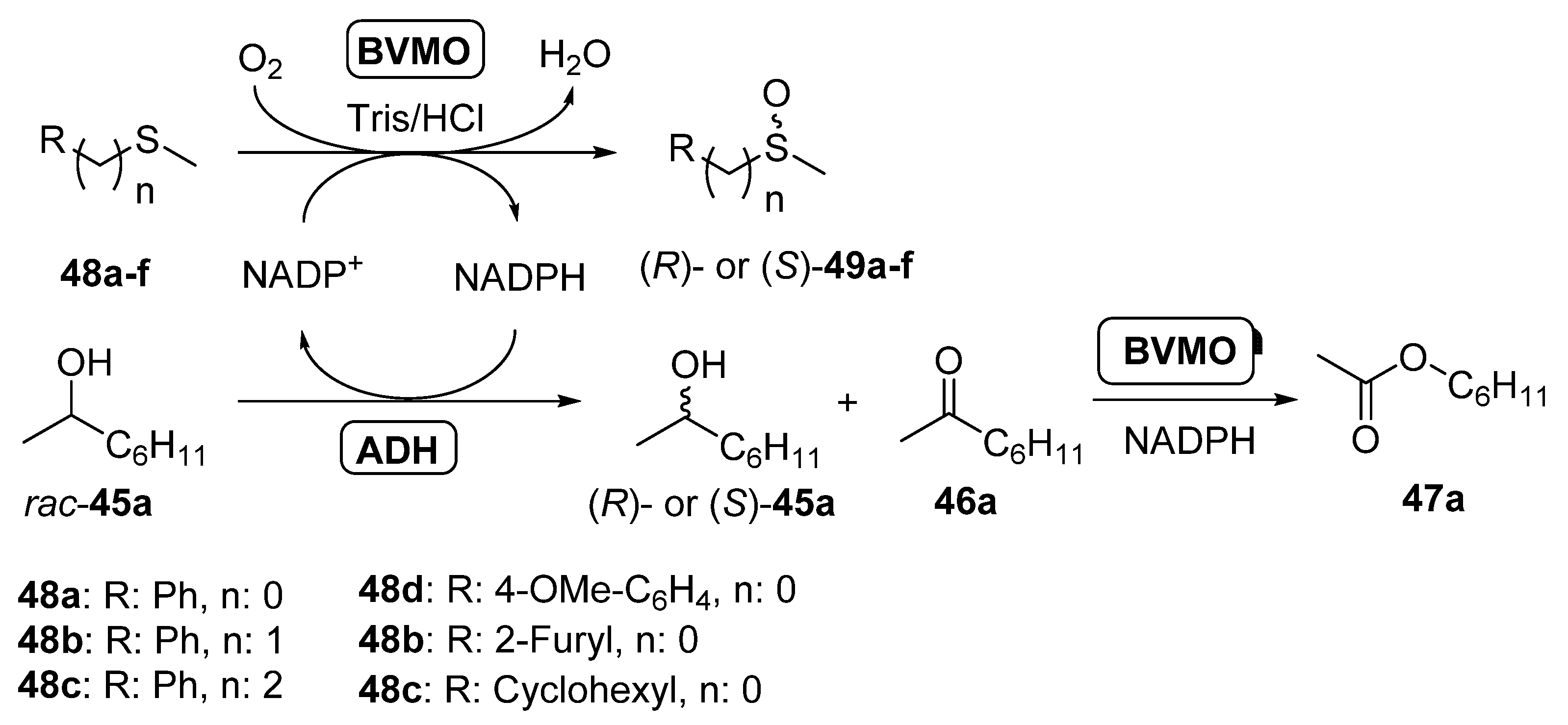
Thus, racemic 2-octanol was selectively oxidized by both LBADH and ADH-T in a process coupled with the enantioselective oxidation of sulfides 48a–f catalyzed by PAMO, its M446G mutant [100] or HAPMO. In order to ensure the best performance for these biocatalysts, PAMO and its mutant were used at 30 °C whereas HAPMO was employed at 20 °C. Aromatic sulfoxides (49a–d) were obtained with moderate to good conversions and high optical purity, whereas the oxidation of rac-45a led to the (R)- or (S)-alcohol with high enantiomeric excess and conversions around 50%. Some amount of ester 44a is formed, due to the BVMO oxidation of ketone 43a. The system was also applied to the sulfoxidation of a heteroaryl alkyl sulfide (45e), being possible to obtain both enantiomers of the sulfoxide with high selectivity, whereas (S)-cyclohexyl methyl sulfoxide (49f) was obtained with excellent optical purity and high conversion in the system catalyzed by HAPMO and both of the ADHs. The concurrent synthesis of (S)-methyl phenyl sulfoxide (49a; R = Ph, n = 0) catalyzed by HAPMO was coupled with the kinetic resolution of several secondary alcohols in presence of LBADH, achieving excellent results in the oxidation of 2-octanol, 2-undecanol and sulcatol to yield the (S)-enantiomers. When the hydroxyl moiety was in position 3 of the starting alcohol (3-octanol) or diols were employed, lower optical purities were measured. Finally, the NADPH cofactor in the reaction medium was optimized, in order to ensure an effective large-scale application. Studies revealed that when the cofactor concentration was 5 µM the system performance was optimal in the concurrent sulfoxidation of thioanisole (48a) and the kinetic resolution of 2-octanol combining HAPMO and LBADH. Lower concentrations (1 µM) can be used in the double kinetic resolution of rac-43b and rac-45a catalyzed by PAMO and LBADH, respectively, as this ketone is a very good substrate for the BVMO.
The production of ε-caprolactone combining isolated BVMOs and ADHs has also been described through a convergent cascade [101], depicted in Scheme 19. In this approach, two different substrates were employed, which leads to the final product without the formation of any intermediate.
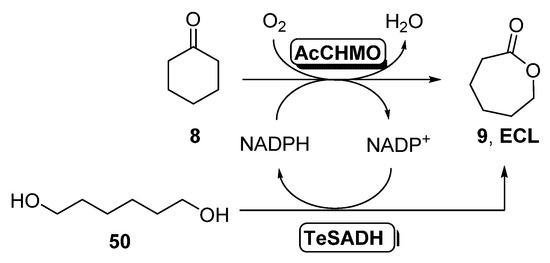
Scheme 19.
Convergent cascade for the synthesis of ECL starting from cyclohexanone and 1,6-hexanodiol employing BVMOs (AcCHMO) and ADHs (TeSADH).
Thus, two equivalents of cyclohexanone 8 were oxidized by a BVMO (0.1–1.0 mg/mL) to caprolactone 9, whereas the ADH-catalyzed oxidation of one equivalent of 1,6-hexanediol 50 afforded the same lactone, with simultaneous regeneration of the NAD(P)H cofactor, as shown in Scheme 18. Biocatalysts were employed as crude extracts free of cells. The initial study for finding the best ADH in this oxidation pointed towards the Thermus sp. ATN1 ADH (TADH) as a promising candidate for this reaction. Unfortunately, this biocatalyst is NADH dependent and no suitable BVMOs with preference for this cofactor were found for the cascade proposed. Then, different bienzymatic systems were tested, achieving the highest concentration of final lactone when combining AcCHMO with TeSADH (0.01–0.1 mg/mL), a NADPH-dependent biocatalyst. Thus, 10 mM of ECL were formed when starting from 20 mM of cyclohexanone and 10 mM of 1,6-hexanediol. Reactions in absence of one or two of the catalytic members (cofactor, CHMO and ADH) were carried out. ECL was obtained in 6 mM concentration after 72 h in absence of TeSADH, indicating that some endogenous ADH presented in the crude CHMO preparation can present some activity on 50. A very low concentration of ECL was recovered (2 mM) in absence of NADPH, maybe owing to the cofactor present in the extracts. No lactone formation was observed in absence of CHMO. In addition, some ECL hydrolysis was observed in the reaction conditions. The CHMO-TeSADH system was optimized by increasing the concentration of substrates and biocatalysts. Addition of extra CHMO after 48 h allowed obtaining complete conversion after 72 h, whereas doubling the starting amount of enzymes led to the same conversion after 48 h, but with a lower turnover frequency (2.0 vs. 4.8 h−1). Increasing cyclohexanone concentration to 100 mM led to the same TOF (1.8 h−1) with only 32% conversion after 72 h. The study of the reaction showed that cyclohexanol was formed during the process as a by-product, achieving a maximum value after 24 h, and then decreasing its concentration until it disappearance at 72 h. The convergent cascade was run at 50 mL scale, starting with 98 mg of ketone (20 mM) and 59 mg of diol (10 mM). After 18 h, complete cyclohexanone conversion was observed, with 19.7 mM of ECL. This result can be debt to a better oxygenation in the multimilligram scale. After 24 h, lactone concentration was 19 mM and 3.7 mM of diol were measured. Reaction work up afforded 150 mg of a mixture containing ECL, poly-ECL and diol at 1:1.5:0.6 ratio.
3.3. Sequential EREDs/BVMOs Cascades
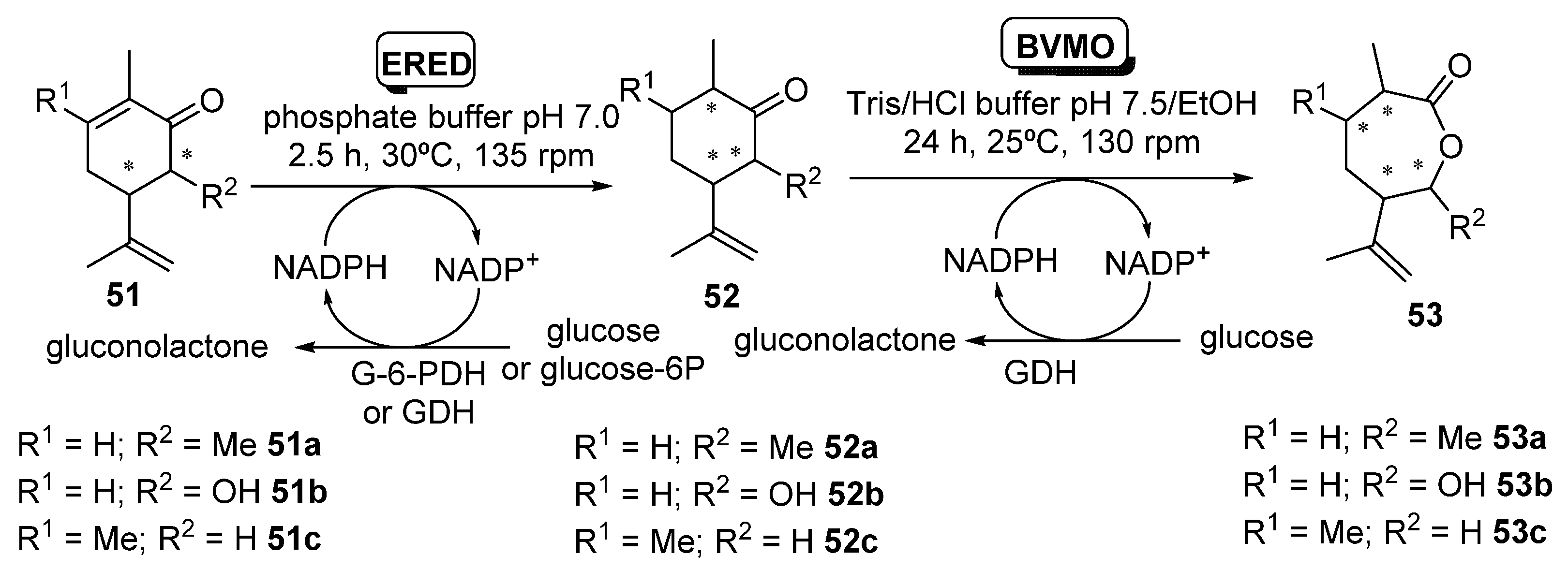
Issa et al. [102] have recently studied the enzymatic bioreduction of synthetic analogies of (-)-carvone (R)-13 (Scheme 5) decorated with different substituents at position C3 and/or C6, and their subsequent transformation into the corresponding carvolactones by means of BVMOs, as shown in Scheme 20.
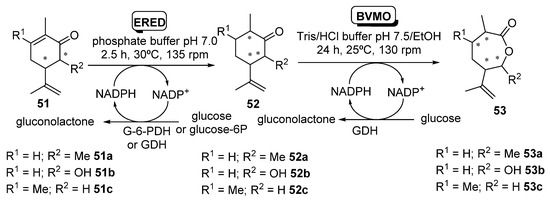
Scheme 20.
Enzymatic sequential conversion of synthetic analogues of carvones 51 into dihydrocarvones 52 and carvolactones 53.
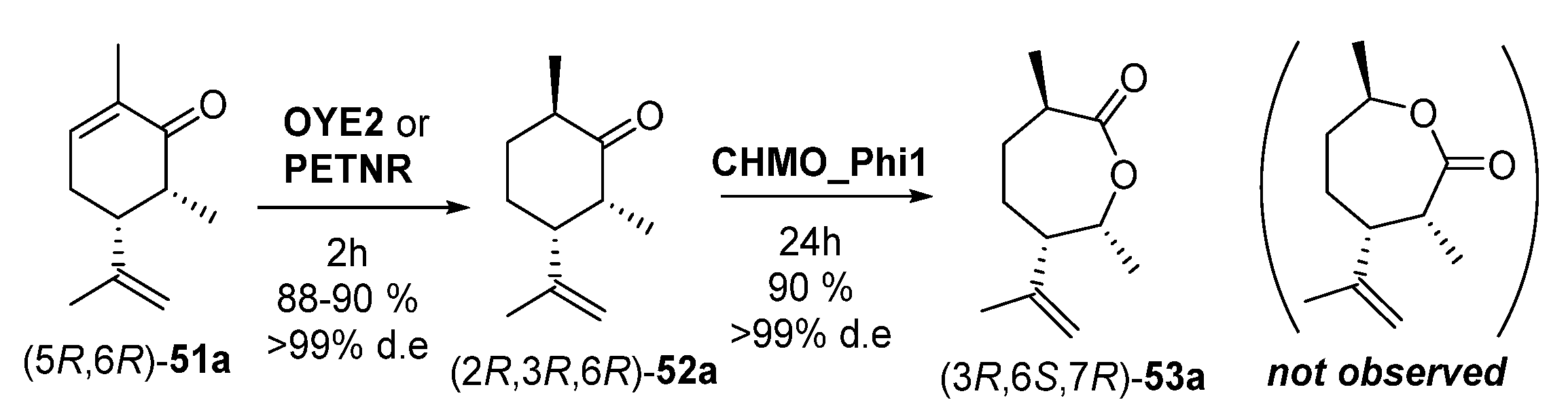
EREDs tested (2–10 µm), were OYE2 and OYE3 [103] from S. cerevisiae and PETNR [82,83], while CHMO_Ph1 [84] was used for the Baeyer–Villiger oxidation. Data provided by these authors [102] showed that the configuration of the extra methyl (R1) at C6 did not affect the binding and/or orientation of the substrate in the bioreduction compared to the parent compound (R)-13. Thus, (6S) stereochemistry of 51a was always leading to (2S) stereoisomers of 52a, and (6R) isomers of 51a were furnishing (2R) stereoisomers of 51a. Notably, the stereo-configuration of the methyl at C6 does significantly impact the rate of conversion; in fact, (6S)-51a was converted more slowly and to a lower yield (15% with OYE2 in 24 h, 17% with PETNR in 24 h) compared to (6R)-51a (90% with OYE2 in 2 h, 88% with PETNR at the same time). This reduction in conversion upon transforming the pure diastereomers (5R,6S)-51a and (5R,6R)-51a was also observed using a mixture of them, where a more rapid depletion of (5R,6R)-51a and a quick formation of (2R,3R,6R)-52a was reported. Remarkably, a computational model to understand this stereochemical pattern was proposed [102].
In a second step, the effect of heteroatom substitution at C6 was explored, using 6-OH substitution instead of 6-Me (substrates 51b). In this case, (5S,6S)-51b and (5S,6R)-51b were respectively converted to the corresponding 6-hydroxydihydrocarvones (2S,3S,6R)-52b and (2R,3S,6R)-52b, by OYE2 with moderate yields (40% and 30% after 24 h) but with very high diastereomeric excesses (≥99% de) in all cases. On the other hand, PETNR performed better, affording 66 and 80% yields of (2S,3S,6R)-52b and (2R,3S,6R)-52b, respectively, after 2 h, also with ≥99% de. As with the 6-methyl derivatives, these were poorer substrates for OYE3 under the same conditions. The corresponding 6-hydroxycarvone diastereoisomers derived from (+)-carvone (S)-13, (5R,6S)-51b and (5R,6R)-51b, were also poor substrates for OYE2, affording <10% yields at 2–24 h. A similar behavior was reported with PETNR on the (5R,6S)-51b (5–10% maximum yield), although this enzyme allowed yields (85–95%) of (2R,3R,6R)-52b when starting from (5R,6R)-51b, but with much reduced de (<30%). Finally, synthetic C3-Me-(-)-carvone analogue 51c is a regioisomer substrate of 51a, but introducing a methyl at the site of enzymatic conjugate reductive attack. Bioreductions catalyzed by OYE2 and PETNR proceeded with low yields (≤ 10%), although with high diastereoselectivity (de ≥ 99) for compound (2S,3R,6R)-52c.
After this exhaustive stereochemical study of the EREDs performance, Issa et al. [102] assessed the BV oxidation with the different diastereomers of 52a and 52b. The (2R,3R,6R)-52a isomer was completely converted to lactone (3R,6S,7R)-53a (oxygen insertion between C1–C2), leading to with apparently complete regioselectivity (≥99%), with no traces of the lactone coming from the oxygen insertion between C1-C6. Remarkably, there was no reaction at all when starting with (2S,3R,6R)-52a; this indicates a remarkable diastereoisomer-selectivity, as an inversion of 6-methyl absolute configuration avoids the enzymatic transformation. Thus, a cascade reaction was carried out at 50 mg scale, as shown in Scheme 21.
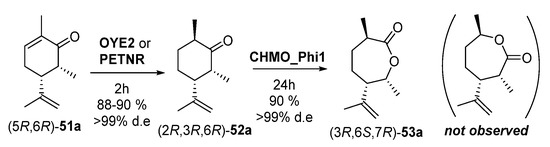
Scheme 21.
Enzymatic sequential conversion of (5R,6R)-51a into dihydrocarvone (2R,3R,6R)-52a and carvolactone (3R,6S,7R)-53a.
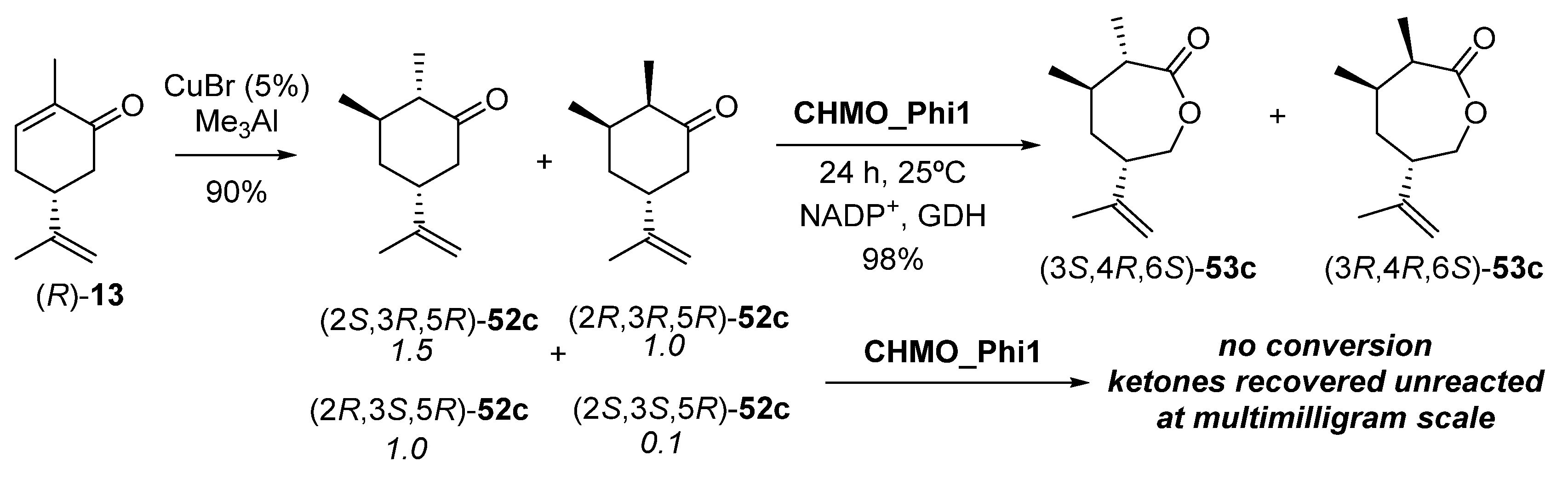
Regarding 3-methyl modified dihydrocarvones 52c, they were chemically synthesized staring from (R)-13, as shown in Scheme 22. This chemical reduction with CuBr and trimethylaluminium was carried out because of the poor ERED outcome using OYE2 and PETNR on 51c, so that a mixture of four isomers with a diastereomeric ratio of 1.5:1:1:0.1 was obtained.
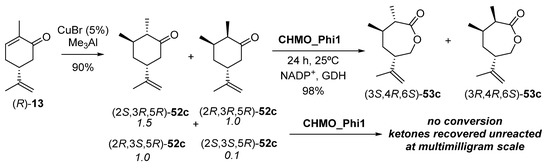
Scheme 22.
Chemical synthesis of dihydrocarvones 49c and subsequent enzymatic Baeyer–Villiger oxidation to carvolactones 50c.
The two 3R diastereomers ((2S,3R,5R)-52c and (2R,3R,5R)-52c, about 70–75% of total) were separated from the two (3S)-isomers, so that it was possible to evaluate valuation of all four isomers, and of the separate pairs of C3 diastereomers with CHMO_Phi1. Bioreductions were run at 25 °C for 24 h, with the system NADP+/GDH employed as the hydride donor, leading to a complete conversion of (2S,3R,5R)-52c and (2R,3R,5R)-52c to their corresponding enantiopure lactones (3S,4R,6S)-53c and (3R,4R,6S)-53c in 98% yield (Scheme 21). This process was scaled up to 50 mg. However, no lactone formation was observed from the bio-oxidation of (2R,3S,5R)-52c and (2S,3S,5R)-52c diastereomers.
4. Conclusions
In the last few years, the development of multienzymatic processes has been largely spread, being possible to design novel biotransformations avoiding the isolation of reaction intermediates and reducing the consumption of solvents and energy, thus maximizing the atom economy of the reactions. For this reason, several processes in which two or more biocatalysts are employed in a sequential or concurrent fashion have been designed. Among these biocatalysts, Baeyer–Villiger monooxygenases have been demonstrated to be useful biocatalysts for the synthesis of valuable compounds employing mild and environmentally friendly conditions. Thus, these enzymes have been employed in multienzymatic reactions in combination with other oxidoreductases, mainly coupled to enoate reductases or alcohol dehydrogenases, being described in some examples in which cytochrome P450 are also involved. EREDs have been generally employed for the hydrogenation of α,β-unsaturated cycloketones to the corresponding cyclic ketones, which are then oxidized to valuable lactones.
When combined with alcohol dehydrogenases, most of the synthetic procedures described balance redox systems in which the nicotinamide co-factor required by both biocatalysts is recycled through an initial ADH-catalyzed oxidation of an alcohol to a ketone, with a subsequent BVMO-catalyzed formation of ester or lactone. Most of these examples involved the preparation of ε-caprolactone or some of its analogues from cyclohexanols in a bienzymatic transformation combining the ADH-catalyzed oxidation of the alcohol with the BVMO-catalyzed oxidation of the ketone with in situ cofactor regeneration. This model system has been studied by employing both whole-cell and cell-free systems, but, in all the cases, a critical parameter is the low operational stability of the Baeyer–Villiger monooxygenase, very sensitive to the substrate and product concentration or to the reaction conditions. Different approaches have been made to overcome this drawback, including the application of more stable biocatalysts able to tolerate higher concentrations of substrates and products, the use of organic co-solvents or the inclusion of a further biocatalytic step with the aim of diminishing the ECL concentration in the reaction medium, as the lipase-catalyzed conversion of ECL to oligo-ECL or 6-hydroxyhexanoic acid. After all of these improvements, the results obtained indicate the requirement of further developments are still required to ensure the productive biocatalytic synthesis of ECL starting from cyclohexanol.
Author Contributions
Conceptualization, G.d.G. and A.R.A.; writing—original draft preparation, G.d.G. and A.R.A.; writing—review and editing, G.d.G. and A.R.A.; visualization, G.d.G. and A.R.A.; supervision, G.d.G. and A.R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by Spanish Ministry of Science and Innovation (PID2019-105337RB-C22) and Banco de Santander-Complutense Research Projects (PR87/19-22676).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kitano, H. Systems biology: A brief overview. Science 2002, 295, 1662–1664. [Google Scholar] [CrossRef]
- García-Junceda, E. Multi-Step Enzyme Catalysis: Biotransformations and Chemoenzymatic Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]
- García-Junceda, E.; Lavandera, I.; Rother, D.; Schrittwieser, J.H. (Chemo)enzymatic cascades-Nature’s synthetic strategy transferred to the laboratory. J. Mol. Catal. B Enzym. 2015, 114, 1–6. [Google Scholar] [CrossRef]
- Oroz-Guinea, I.; Fernández-Lucas, J.; Hormigo, D.; García-Junceda, E. Designed Enzymatic Cascades. In Science of Synthesis: Biocatalysis in Organic Synthesis 3; Faber, K., Fessner, W.D., Turner, N.J., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2015; Volume 3. [Google Scholar]
- Schrittwieser, J.H.; Velikogne, S.; Hall, M.; Kroutil, W. Artificial Biocatalytic Linear Cascades for Preparation of Organic Molecules. Chem. Rev. 2018, 118, 270–348. [Google Scholar] [CrossRef]
- Schmidt, S.; Castiglione, K.; Kourist, R. Overcoming the Incompatibility Challenge in Chemoenzymatic and Multi-Catalytic Cascade Reactions. Chem. Eur. J. 2018, 24, 1755–1768. [Google Scholar] [CrossRef]
- Schrittwieser, J.H.; Velikogne, S.; Kroutil, W. Artificial Biocatalytic Cascades to Alcohols and Amines. In Modern Biocatalysis: Advances Towards Synthetic Biological Systems; Williams, G., Hall, M., Eds.; Royal Soc Chemistry: Cambridge, UK, 2018; Volume 32, pp. 387–438. [Google Scholar]
- Walsh, C.T.; Moore, B.S. Enzymatic Cascade Reactions in Biosynthesis. Angew. Chem. Int. Ed. 2019, 58, 6846–6879. [Google Scholar] [CrossRef]
- Kara, S.; Rudroff, F. (Eds.) Enzyme Cascade Design and Modelling; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- McIntosh, J.A.; Owens, A.E. Enzyme engineering for biosynthetic cascades. Curr. Opin. Green Sustain. Chem. 2021, 29, 100448. [Google Scholar] [CrossRef]
- Nazor, J.; Liu, J.; Huisman, G. Enzyme evolution for industrial biocatalytic cascades. Curr. Opin. Biotechnol. 2021, 69, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Uppada, V.; Bhaduri, S.; Noronha, S.B. Cofactor regeneration—An important aspect of biocatalysis. Curr. Sci. 2014, 106, 946–957. [Google Scholar]
- Berenguer-Murcia, A.; Fernandez-Lafuente, R. New Trends in the Recycling of NAD(P)H for the Design of Sustainable Asymmetric Reductions Catalyzed by Dehydrogenases. Curr. Org. Chem. 2010, 14, 1000–1021. [Google Scholar] [CrossRef]
- Truppo, M.D. 7.4 Cofactor Recycling for Enzyme Catalyzed Processes. In Comprehensive Chirality; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; Volume 7, pp. 46–70. [Google Scholar]
- Mordhorst, S.; Andexer, J.N. Round, round we go-strategies for enzymatic cofactor regeneration. Nat. Prod. Rep. 2020, 37, 1316–1333. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.A.G.; Li, H.; Johnson, M.; Sobrado, P. New frontiers in flavin-dependent monooxygenases. Arch. Biochem. Biophys. 2021, 699, 14. [Google Scholar] [CrossRef] [PubMed]
- Toplak, M.; Matthews, A.; Teufel, R. The devil is in the details: The chemical basis and mechanistic versatility of flavoprotein monooxygenases. Arch. Biochem. Biophys. 2021, 698, 14. [Google Scholar] [CrossRef]
- Dockrey, S.A.B.; Narayan, A.R.H. Flavin-dependent biocatalysts in synthesis. Tetrahedron 2019, 75, 1115–1121. [Google Scholar] [CrossRef]
- Hall, M. Flavoenzymes for biocatalysis. In Enzymes; Chaiyen, P., Tamanoi, F., Tamanoi, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 47, pp. 37–62. [Google Scholar]
- Schmidt, S.; Bornscheuer, U.T. Baeyer-Villiger monooxygenases: From protein engineering to biocatalytic applications. In Enzymes; Chaiyen, P., Tamanoi, F., Tamanoi, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 47, pp. 231–281. [Google Scholar]
- de Gonzalo, G.; Mihovilovic, M.D.; Fraaije, M.W. Recent Developments in the Application of Baeyer-Villiger Monooxygenases as Biocatalysts. ChemBioChem 2010, 11, 2208–2231. [Google Scholar] [CrossRef]
- Leisch, H.; Morley, K.; Lau, P.C.K. Baeyer-Villiger Monooxygenases: More Than Just Green Chemistry. Chem. Rev. 2011, 111, 4165–4222. [Google Scholar] [CrossRef] [PubMed]
- Balke, K.; Kadow, M.; Mallin, H.; Sass, S.; Bornscheuer, U.T. Discovery, application and protein engineering of Baeyer-Villiger monooxygenases for organic synthesis. Org. Biomol. Chem. 2012, 10, 6249–6265. [Google Scholar] [CrossRef]
- Bucko, M.; Gemeiner, P.; Schenkmayerova, A.; Krajcovic, T.; Rudroff, F.; Mihovilovic, M.D. Baeyer-Villiger oxidations: Biotechnological approach. Appl. Microbiol. Biotechnol. 2016, 100, 6585–6599. [Google Scholar] [CrossRef] [PubMed]
- Knaus, T.; Toogood, H.S.; Scrutton, N.S. Ene-reductases and their Applications. In Green Biocatalysis; Patel, R.N., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 473–488. [Google Scholar] [CrossRef]
- Furst, M.; Gran-Scheuch, A.; Aalbers, F.S.; Fraaije, M.W. Baeyer-Villiger Monooxygenases: Tunable Oxidative Biocatalysts. ACS Catal. 2019, 9, 11207–11241. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, H.; Liu, J.; Li, S.; Guo, J.; Li, H.; Jia, X.; Huo, H.; Zheng, Z.; You, S.; et al. Old yellow enzymes: Structures and structure-guided engineering for stereocomplementary bioreduction. Appl. Microbiol. Biotechnol. 2020, 104, 8155–8170. [Google Scholar] [CrossRef]
- Huijbers, M.M.E.; Montersino, S.; Westphal, A.H.; Tischler, D.; van Berkel, W.J.H. Flavin dependent monooxygenases. Arch. Biochem. Biophys. 2014, 544, 2–17. [Google Scholar] [CrossRef]
- Cummings Ryerson, C.; Ballou, D.P.; Walsh, C. Mechanistic Studies on Cyclohexanone Oxygenase. Biochemistry 1982, 21, 2644–2655. [Google Scholar] [CrossRef]
- van Berkel, W.J.H.; Kamerbeek, N.M.; Fraaije, M.W. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J. Biotechnol. 2006, 124, 670–689. [Google Scholar] [CrossRef]
- Mthethwa, K.S.; Kassier, K.; Engel, J.; Kara, S.; Smit, M.S.; Opperman, D.J. Fungal BVMOs as alternatives to cyclohexanone monooxygenase. Enzym. Microb. Technol. 2017, 106, 11–17. [Google Scholar] [CrossRef] [PubMed]
- van Beek, H.L.; Beyer, N.; Janssen, D.B.; Fraaije, M.W. Lyophilization conditions for the storage of monooxygenases. J. Biotechnol. 2015, 203, 41–44. [Google Scholar] [CrossRef]
- Balke, K.; Beier, A.; Bornscheuer, U.T. Hot spots for the protein engineering of Baeyer-Villiger monooxygenases. Biotechnol. Adv. 2018, 36, 247–263. [Google Scholar] [CrossRef]
- Fraaije, M.W.; Wu, J.; Heuts, D.P.H.M.; Van Hellemond, E.W.; Spelberg, J.H.L.; Janssen, D.B. Discovery of a thermostable Baeyer-Villiger monooxygenase by genome mining. Appl. Microbiol. Biotechnol. 2005, 66, 393–400. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Ottolina, G.; Zambianchi, F.; Fraaije, M.W.; Carrea, G. Biocatalytic properties of Baeyer-Villiger monooxygenases in aqueous-organic media. J. Mol. Catal. B Enzym. 2006, 39, 91–97. [Google Scholar] [CrossRef]
- Rodríguez, C.; de Gonzalo, G.; Fraaije, M.W.; Gotor, V. Ionic liquids for enhancing the enantioselectivity of isolated BVMO-catalysed oxidations. Green Chem. 2010, 12, 2255–2260. [Google Scholar] [CrossRef]
- Stewart, J.D. Cyclohexanone monooxygenase: A useful reagent for asymmetric baeyer-villiger reactions. Curr. Org. Chem. 1998, 2, 195–216. [Google Scholar]
- Secundo, F.; Fialà, S.; Fraaije, M.W.; De Gonzalo, G.; Meli, M.; Zambianchi, F.; Ottolina, G. Effects of water miscible organic solvents on the activity and conformation of the Baeyer-Villiger monooxygenases from Thermobifida fusca and Acinetobacter calcoaceticus: A comparative study. Biotechnol. Bioeng. 2011, 108, 491–499. [Google Scholar] [CrossRef]
- Sartori, S.K.; Diaz, M.A.N.; Diaz-Muñoz, G. Lactones: Classification, synthesis, biological activities, and industrial applications. Tetrahedron 2021, 84, 132001. [Google Scholar] [CrossRef]
- Wu, S.K.; Li, Z. Whole-Cell Cascade Biotransformations for One-Pot Multistep Organic Synthesis. ChemCatChem 2018, 10, 2164–2178. [Google Scholar] [CrossRef]
- Fessner, W.D. Systems Biocatalysis: Development and engineering of cell-free “artificial metabolism” for preparative multi-enzymatic synthesis. New Biotech. 2015, 32, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, J.D.; Quezada, M.A.; Hoyos, P.; Simeo, Y.; Hernaiz, M.J.; Alcantara, A.R.; Sinisterra, J.V. Microbial cells as catalysts for stereoselective red-ox reactions. Biotechnol. Adv. 2009, 27, 686–714. [Google Scholar] [CrossRef]
- Kadisch, M.; Willrodt, C.; Hillen, M.; Buhler, B.; Schmid, A. Maximizing the stability of metabolic engineering-derived whole-cell biocatalysts. Biotechnol. J. 2017, 12, 29. [Google Scholar] [CrossRef]
- Polakovic, M.; Svitel, J.; Bucko, M.; Filip, J.; Nedela, V.; Ansorge-Schumacher, M.B.; Gemeiner, P. Progress in biocatalysis with immobilized viable whole cells: Systems development, reaction engineering and applications. Biotechnol. Lett. 2017, 39, 667–683. [Google Scholar] [CrossRef]
- Garzon-Posse, F.; Becerra-Figueroa, L.; Hernandez-Arias, J.; Gamba-Sanchez, D. Whole Cells as Biocatalysts in Organic Transformations. Molecules 2018, 23, 37. [Google Scholar] [CrossRef]
- Musa, M.M.; Phillips, R.S. Recent advances in alcohol dehydrogenase-catalyzed asymmetric production of hydrophobic alcohols. Catal. Sci. Technolog. 2011, 1, 1311–1323. [Google Scholar] [CrossRef]
- Kratzer, R.; Woodley, J.M.; Nidetzky, B. Rules for biocatalyst and reaction engineering to implement effective, NAD(P)H-dependent, whole cell bioreductions. Biotechnol. Adv. 2015, 33, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.G.; Yin, H.H.; Yu, D.F.; Chen, X.; Tang, X.L.; Zhang, X.J.; Xue, Y.P.; Wang, Y.J.; Liu, Z.Q. Recent advances in biotechnological applications of alcohol dehydrogenases. Appl. Microbiol. Biotechnol. 2017, 101, 987–1001. [Google Scholar] [CrossRef]
- Gonzalo, G.; Lavandera, I. Recent advances in selective biocatalytic (Hydrogen Transfer) reductions. In Homogeneous Hydrogenation with Non-Precious Catalysts; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 227–259. [Google Scholar] [CrossRef]
- Willetts, A.J.; Knowles, C.J.; Levitt, M.S.; Roberts, S.M.; Sandey, H.; Shipston, N.F. Biotransformation of endo-bicyclo[2.2.1]heptan-2-ols and endo-bicyclo[3.2.0]-hept-2-en-6-ol into the corresponding lactones. J. Chem. Soc. Perkin Trans. 1 1991, 1608–1610. [Google Scholar] [CrossRef]
- Lipik, V.T.; Kong, J.F.; Chattopadhyay, S.; Widjaja, L.K.; Liow, S.S.; Venkatraman, S.S.; Abadie, M.J.M. Thermoplastic biodegradable elastomers based on ε-caprolactone and L-lactide block co-polymers: A new synthetic approach. Acta Biomater. 2010, 6, 4261–4270. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Aihara, K.; Yamanishi, H.; Fukuoka, H.; Tanaka, R.; Cai, Z.; Shiono, T. Synthesis of biodegradable thermoplastic elastomers from ε-caprolactone and lactide. J. Polym. Sci. Part A 2015, 53, 489–495. [Google Scholar] [CrossRef]
- Schmidt, S.; Scherkus, C.; Muschiol, J.; Menyes, U.; Winkler, T.; Hummel, W.; Groger, H.; Liese, A.; Herz, H.G.; Bornscheuer, U.T. An Enzyme Cascade Synthesis of epsilon-Caprolactone and its Oligomers. Angew. Chem. Int. Ed. 2015, 54, 2784–2787. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Buchsenschutz, H.C.; Scherkus, C.; Liese, A.; Groger, H.; Bornscheuer, U.T. Biocatalytic Access to Chiral Polyesters by an Artificial Enzyme Cascade Synthesis. ChemCatChem 2015, 7, 3951–3955. [Google Scholar] [CrossRef]
- Scherkus, C.; Schmidt, S.; Bornscheuer, U.T.; Groeger, H.; Kara, S.; Liese, A. Kinetic insights into e-caprolactone synthesis: Improvement of an enzymatic cascade reaction. Biotechnol. Bioeng. 2017, 114, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Kohl, A.; Srinivasamurthy, V.; Boettcher, D.; Kabisch, J.; Bornscheuer, U.T. Co-expression of an alcohol dehydrogenase and a cyclohexanone monooxygenase for cascade reactions facilitates the regeneration of the NADPH cofactor. Enz. Microb. Technol. 2018, 108, 53–58. [Google Scholar] [CrossRef]
- Srinivasamurthy, V.S.T.; Bottcher, D.; Engel, J.; Kara, S.; Bornscheuer, U.T. A whole-cell process for the production of epsilon-caprolactone in aqueous media. Process Biochem. 2020, 88, 22–30. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.Y.; Baek, A.H.; Bornscheuer, U.T.; Park, J.B. Enzyme fusion for whole-cell biotransformation of long-chain sec-alcohols into esters. Appl. Microbiol. Biotechnol. 2015, 99, 6267–6275. [Google Scholar] [CrossRef]
- Toogood, H.S.; Scrutton, N.S. New developments in ‘ene’-reductase catalysed biological hydrogenations. Curr. Opin. Chem. Biol. 2014, 19, 107–115. [Google Scholar] [CrossRef]
- Toogood, H.S.; Scrutton, N.S. Discovery, Characterization, Engineering, and Applications of Ene-Reductases for Industrial Biocatalysis. ACS Catal. 2018, 8, 3532–3549. [Google Scholar] [CrossRef]
- Nett, N.; Duewel, S.; Schmermund, L.; Benary, G.E.; Ranaghan, K.; Mulholland, A.; Opperman, D.J.; Hoebenreich, S. A robust and stereocomplementary panel of ene-reductase variants for gram-scale asymmetric hydrogenation. Mol. Cat. 2021, 502, 111404. [Google Scholar] [CrossRef]
- Silva, A.L.P.; Batista, P.K.; Filho, A.D.; do Nascimento Junior, C.S.; Reboucas, J.S.; Vale, J.A. Rapid conversion of cyclohexenone, cyclohexanone and cyclohexanol to epsilon-caprolactone by whole cells of Geotrichum candidum CCT 1205. Biocatal. Biotransfor. 2017, 35, 185–190. [Google Scholar] [CrossRef]
- Mihovilovic, M.D.; Snajdrova, R.; Grotzl, B. Microbial Baeyer-Villiger oxidation of 4,4-disubstituted cyclohexan—And cyclohexenones by recombinant whole-cells expressing monooxygenases of bacterial origin. J. Mol. Catal. B Enzym. 2006, 39, 135–140. [Google Scholar] [CrossRef]
- Silva, A.L.P.; Caridade, T.N.D.; Magalhaes, R.R.; de Sousa, K.T.; de Sousa, C.C.; Vale, J.A. Biocatalytic production of e-caprolactone using Geotrichum candidum cells immobilized on functionalized silica. Appl. Microbiol. Biotechnol. 2020, 104, 8887–8895. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z. Cascade Biotransformations via Enantioselective Reduction, Oxidation, and Hydrolysis: Preparation of (R)-delta-Lactones from 2-Alkylidenecyclopentanones. ACS Catal. 2013, 3, 908–911. [Google Scholar] [CrossRef]
- Shin, J.; Martello, M.T.; Shrestha, M.; Wissinger, J.E.; Tolman, W.B.; Hillmyer, M.A. Pressure-sensitive adhesives from renewable triblock copolymers. Macromolecules 2011, 44, 87–94. [Google Scholar] [CrossRef]
- Wanamaker, C.L.; O’Leary, L.E.; Lynd, N.A.; Hillmeyer, M.A.; Tolman, W.B. Renewable-resource thermoplastic elastomers based on polylactide and polymenthide. Biomacromolecules 2007, 8, 3634–3640. [Google Scholar] [CrossRef]
- Gurusamy-Thangavelu, S.A.; Emond, S.J.; Kulshrestha, A.; Hillmyer, M.A.; MacOsko, C.W.; Tolman, W.B.; Hoye, T.R. Polyurethanes based on renewable polyols from bioderived lactones. Polym. Chem. 2012, 3, 2941–2948. [Google Scholar] [CrossRef]
- Iqbal, N.; Stewart, J.D.; Macheroux, P.; Rudroff, F.; Mihovilovic, M.D. Novel concurrent redox cascades of (R)- and (S)-carvones enables access to carvo-lactones with distinct regio- and enantioselectivity. Tetrahedron 2018, 74, 7389–7394. [Google Scholar] [CrossRef]
- Brigé, A.; Van Den Hemel, D.; Carpentier, W.; De Smet, L.; Van Beeumen, J.J. Comparative characterization and expression analysis of the four Old Yellow Enzyme homologues from Shewanella oneidensis indicate differences in physiological function. Biochem. J. 2006, 394, 335–344. [Google Scholar] [CrossRef]
- Hall, M.; Stueckler, C.; Kroutil, W.; Macheroux, P.; Faber, K. Asymmetric bioreduction of activated alkenes using cloned 12-oxophytodienoate reductase isoenzymes OPR-1 and OPR-3 from Lycopersicon esculentum (tomato): A striking change of stereoselectivity. Angew. Chem. Int. Ed. 2007, 46, 3934–3937. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Stueckler, C.; Ehammer, H.; Pointner, E.; Oberdorfer, G.; Gruber, K.; Hauer, B.; Stuermer, R.; Kroutil, W.; Macheroux, P.; et al. Asymmetric bioreduction of C=C bonds using enoate reductases OPR1, OPR3 and YqjM: Enzyme-based stereocontrol. Adv. Synth. Catal. 2008, 350, 411–418. [Google Scholar] [CrossRef]
- Padhi, S.K.; Bougioukou, D.J.; Stewart, J.D. Site-saturation mutagenesis of tryptophan 116 of Saccharomyces pastorianus old yellow enzyme uncovers stereocomplementary variants. J. Am. Chem. Soc. 2009, 131, 3271–3280. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, N.A.; Norris, D.B.; Trudgill, P.W. The Purification and Properties of Cyclohexanone Oxygenase from Nocardia globerula CL1 and Acinetobacter NCIB 9871. Eur. J. Biochem. 1976, 63, 175–192. [Google Scholar] [CrossRef]
- Brzostowicz, P.C.; Gibson, K.L.; Thomas, S.M.; Blasko, M.S.; Rouvière, P.E. Simultaneous identification of two cyclohexanone oxidation genes from an environmental Brevibacterium isolate using MRNA differential display. J. Bacteriol. 2000, 182, 4241–4248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Griffin, M.; Trudgill, P.W. Purification and Properties of Cyclopentanone Oxygenase of Pseudomonas NCIB 9872. Eur. J. Biochem. 1976, 63, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Krow, G.R. The Baeyer–Villiger Oxidation of Ketones and Aldehydes. In Organic Reactions; Paquette, L.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1993; Volume 43, pp. 251–353. [Google Scholar]
- Oberleitner, N.; Ressmann, A.K.; Bica, K.; Gartner, P.; Fraaije, M.W.; Bornscheuer, U.T.; Rudroff, F.; Mihovilovic, M.D. From waste to value—Direct utilization of limonene from orange peel in a biocatalytic cascade reaction towards chiral carvolactone. Green Chem. 2017, 19, 367–371. [Google Scholar] [CrossRef]
- Oberleitner, N.; Peters, C.; Muschiol, J.; Kadow, M.; Sass, S.; Bayer, T.; Schaaf, P.; Iqbal, N.; Rudroff, F.; Mihovilovic, M.D.; et al. An Enzymatic Toolbox for Cascade Reactions: A Showcase for an In Vivo Redox Sequence in Asymmetric Synthesis. ChemCatChem 2013, 5, 3524–3528. [Google Scholar] [CrossRef]
- Avalos, G.A.A.; Toogood, H.S.; Tait, S.; Messiha, H.L.; Scrutton, N.S. From Bugs to Bioplastics: Total (+)-Dihydrocarvide Biosynthesis by Engineered Escherichia coli. ChemBioChem 2019, 20, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Fryszkowska, A.; Toogood, H.; Sakuma, M.; Gardiner, J.M.; Stephens, G.M.; Scrutton, N.S. Asymmetrie reduction of activated alkenes by pentaerythritol tetranitrate reductase: Specificity and control of stereochemical outcome by reaction optimisation. Adv. Synth. Catal. 2009, 351, 2976–2990. [Google Scholar] [CrossRef] [PubMed]
- French, C.E.; Nicklin, S.; Bruce, N.C. Sequence and properties of pentaerythritol tetranitrate reductase from Enterobacter cloacae PB2. J. Bacteriol. 1996, 178, 6623–6627. [Google Scholar] [CrossRef] [PubMed]
- Messiha, H.L.; Ahmed, S.T.; Karuppiah, V.; Suardíaz, R.; Ascue Avalos, G.A.; Fey, N.; Yeates, S.; Toogood, H.S.; Mulholland, A.J.; Scrutton, N.S. Biocatalytic Routes to Lactone Monomers for Polymer Production. Biochemistry 2018, 57, 1997–2008. [Google Scholar] [CrossRef]
- Gagnon, R.; Grogan, G.; Levitt, M.S.; Roberts, S.M.; Wan, P.W.H.; Willetts, A.J. Biological Baeyer-Villiger oxidation of some monocyclic and bicyclic ketones using monooxygenases from Acinetobacter calcoaceticus NCIMB 9871 and Pseudomonas putida NCIMB 10007. J. Chem. Soc. Perkin Trans 1 1994, 2537–2543. [Google Scholar] [CrossRef]
- Mallin, H.; Wulf, H.; Bornscheuer, U.T. A self-sufficient Baeyer-Villiger biocatalysis system for the synthesis of ε-caprolactone from cyclohexanol. Enzym. Microb. Technol. 2013, 53, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Staudt, S.; Bornscheuer, U.T.; Menyes, U.; Hummel, W.; Gröger, H. Direct biocatalytic one-pot-transformation of cyclohexanol with molecular oxygen into ε-caprolactone. Enzym. Microb. Technol. 2013, 53, 288–292. [Google Scholar] [CrossRef]
- Aalbers, F.S.; Fraaije, M.W. Coupled reactions by coupled enzymes: Alcohol to lactone cascade with alcohol dehydrogenase–cyclohexanone monooxygenase fusions. Appl. Microbiol. Biotechnol. 2017, 101, 7557–7565. [Google Scholar] [CrossRef] [PubMed]
- de Gonzalo, G.; Franconetti, A. Enantioselective sulfoxidations employing the thermostable cyclohexanone monooxygenase from Thermocrispum municipale. Enzym. Microb. Technol. 2018, 113, 24–28. [Google Scholar] [CrossRef]
- Reimer, A.; Wedde, S.; Staudt, S.; Schmidt, S.; Höffer, D.; Hummel, W.; Kragl, U.; Bornscheuer, U.T.; Gröger, H. Process Development through Solvent Engineering in the Biocatalytic Synthesis of the Heterocyclic Bulk Chemical ε-Caprolactone. J. Heterocycl. Chem. 2017, 54, 391–396. [Google Scholar] [CrossRef]
- Pennec, A.; Hollmann, F.; Smit, M.S.; Opperman, D.J. One-pot conversion of cycloalkanes to lactones. ChemCatChem 2015, 7, 236–239. [Google Scholar] [CrossRef]
- Fasan, R. Tuning P450 Enzymes as Oxidation Catalysts. ACS Catal. 2012, 2, 647–666. [Google Scholar] [CrossRef]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 Monooxygenases in Biotechnology and Synthetic Biology. Trends Biotechnol. 2019, 37, 882–897. [Google Scholar] [CrossRef]
- Sattler, J.H.; Fuchs, M.; Mutti, F.G.; Grischek, B.; Engel, P.; Pfeffer, J.; Woodley, J.M.; Kroutil, W. Introducing an In Situ Capping Strategy in Systems Biocatalysis To Access 6-Aminohexanoic acid. Angew. Chem. Int. Ed. 2014, 53, 14153–14157. [Google Scholar] [CrossRef] [PubMed]
- Rioz-Martinez, A.; Bisogno, F.R.; Rodriguez, C.; de Gonzalo, G.; Lavandera, I.; Pazmino, D.E.T.; Fraaije, M.W.; Gotor, V. Biocatalysed concurrent production of enantioenriched compounds through parallel interconnected kinetic asymmetric transformations. Org. Biomol. Chem. 2010, 8, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo, G.; Torres Pazmiño, D.E.; Ottolina, G.; Fraaije, M.W.; Carrea, G. Oxidations catalyzed by phenylacetone monooxygenase from Thermobifida fusca. Tetrahedron Asymmetry 2005, 16, 3077–3083. [Google Scholar] [CrossRef]
- De Gonzalo, G.; Torres Pazmiño, D.E.; Ottolina, G.; Fraaije, M.W.; Carrea, G. 4-Hydroxyacetophenone monooxygenase from Pseudomonas fluorescens ACB as an oxidative biocatalyst in the synthesis of optically active sulfoxides. Tetrahedron Asymmetry 2006, 17, 130–135. [Google Scholar] [CrossRef]
- Rodríguez, C.; Gonzalo, G.D.; Torres Pazmiño, D.E.; Fraaije, M.W.; Gotor, V. Baeyer-Villiger monooxygenase-catalyzed kinetic resolution of racemic α-alkyl benzyl ketones: Enzymatic synthesis of α-alkyl benzylketones and α-alkyl benzylesters. Tetrahedron Asymmetry 2009, 20, 1168–1173. [Google Scholar] [CrossRef][Green Version]
- Bisogno, F.R.; Rioz-Martinez, A.; Rodriguez, C.; Lavandera, I.; de Gonzalo, G.; Pazmino, D.E.T.; Fraaije, M.W.; Gotor, V. Oxidoreductases Working Together: Concurrent Obtaining of Valuable Derivatives by Employing the PIKAT Method. ChemCatChem 2010, 2, 946–949. [Google Scholar] [CrossRef]
- Torres Pazmiño, D.E.; Snajdrova, R.; Rial, D.V.; Mihovilovic, M.D.; Fraaije, M.W. Altering the substrate specificity and enantioselectivity of phenylacetone monooxygenase by structure-inspired enzyme redesign. Adv. Synth. Catal. 2007, 349, 1361–1368. [Google Scholar] [CrossRef]
- Bornadel, A.; Hatti-Kaul, R.; Hollmann, F.; Kara, S. A Bi-enzymatic Convergent Cascade for epsilon-Caprolactone Synthesis Employing 1,6-Hexanediol as a “Double-Smart Cosubstrate”. ChemCatChem 2015, 7, 2442–2445. [Google Scholar] [CrossRef]
- Issa, I.S.; Toogood, H.S.; Johannissen, L.O.; Raftery, J.; Scrutton, N.S.; Gardiner, J.M. C3 and C6 Modification-Specific OYE Biotransformations of Synthetic Carvones and Sequential BVMO Chemoenzymatic Synthesis of Chiral Caprolactones. Chem. Eur. J. 2019, 25, 2983–2988. [Google Scholar] [CrossRef] [PubMed]
- Niino, Y.S.; Chakraborty, S.; Brown, B.J.; Massey, V. A new old yellow enzyme of Saccharomyces cerevisiae. J. Biol. Chem. 1995, 270, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).