CuO-Fe2O3 Nanoparticles Supported on SiO2 and Al2O3 for Selective Hydrogenation of 2-Methyl-3-Butyn-2-ol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization
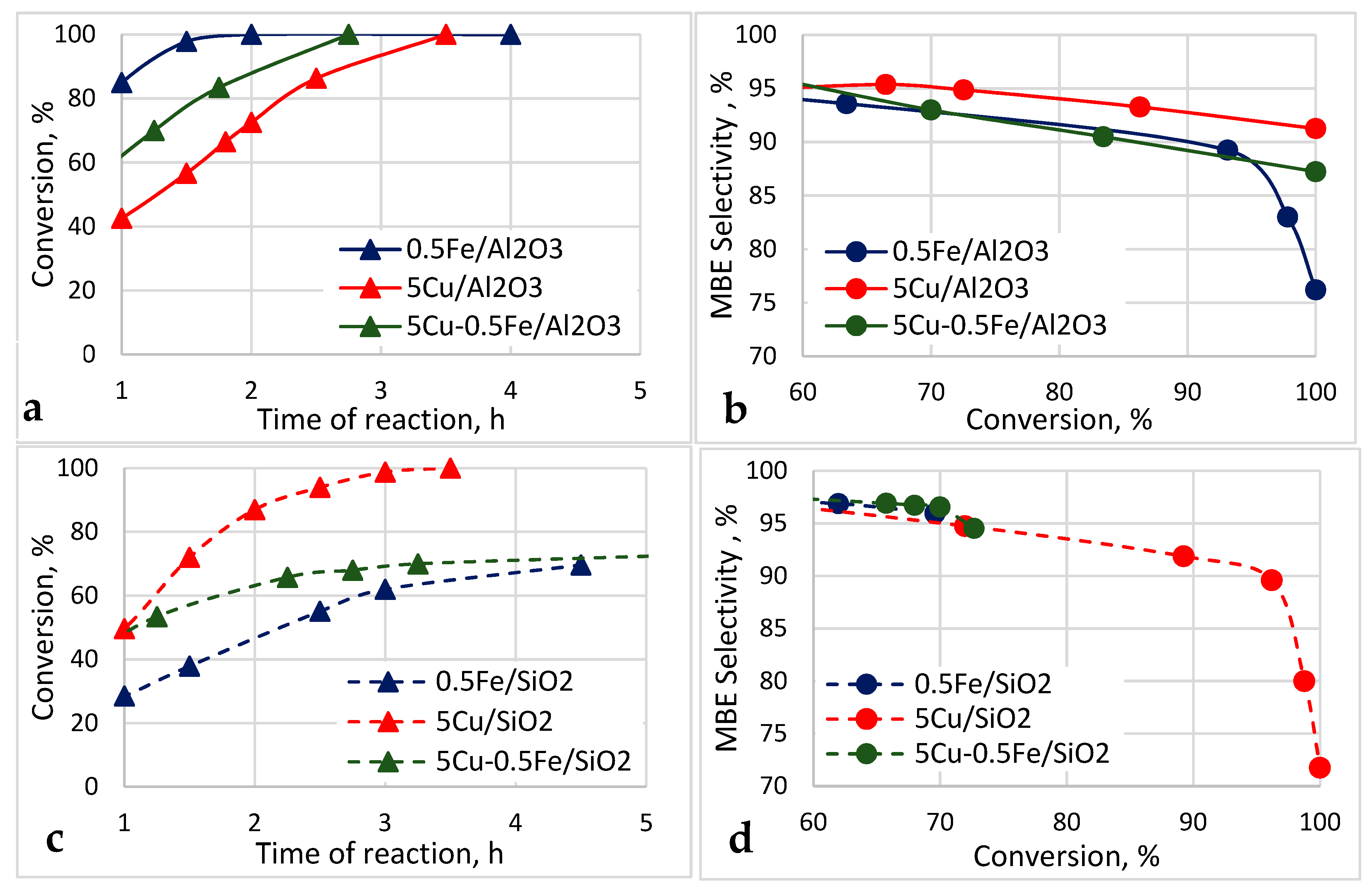
2.2. Catalytic Activity
3. Experimental
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, B.; Dingerdissen, U.; Krauter, J.; Rotgerink, H.L.; Möbus, K.; Ostgard, D.; Panster, P.; Riermeier, T.; Seebald, T.; Tacke, T.; et al. New developments in hydrogenation catalysis particularly in synthesis of fine and intermediate chemicals. Appl. Catal. A 2005, 280, 17–46. [Google Scholar] [CrossRef]
- Garcia-Mota, M.; Gomez-Diaz, J.; Novell-Leruth, G.; Vargas-Fuentes, C.; Bellarosa, L.; Bridier, B.; Perez-Ramirez, J.; Lopez, N. A density functional theory study of the ‘mythic’ Lindlar hydrogenation catalyst. Theor. Chem. Acc. 2011, 128, 663–673. [Google Scholar] [CrossRef]
- Rapeti, S.K.; Kasina, K.C.; Gundepaka, P.; Birudaraju, S.; Sailaja, B.B.V. Efficient insitu palladium nano catalysis for Z-selective semi transfer hydrogenation of internal alkynes using safer 1, 4-butanediol. Tetrahedron Lett. 2020, 61, 151395. [Google Scholar] [CrossRef]
- González-Fernández, A.; Pischetola, C.; Cárdenas-Lizana, F. Gas Phase Catalytic Hydrogenation of C4 Alkynols over Pd/Al2O3. Catalysts 2019, 9, 924. [Google Scholar] [CrossRef] [Green Version]
- Delgado, J.A.; Benkirane, O.; Claver, C.; Curulla-Ferré, D.; Godard, C. Advances in the Preparation of Highly Selective Nanocatalysts for the Semi-Hydrogenation of Alkynes Using Colloidal Approaches. Dalton Trans. 2017, 46, 12381–12403. [Google Scholar] [CrossRef] [Green Version]
- Mashkovsky, I.S.; Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Rassolov, A.V.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Stakheev, A.Y. PdZn/α-Al2O3 Catalyst for Liquid-Phase Alkyne Hydrogenation: Effect of the Solid-State Alloy Transformation into Intermetallics. Mendeleev Commun. 2018, 28, 152–154. [Google Scholar] [CrossRef]
- Vernuccio, S.; Goy, R.; Meier, A.; Rudolf von Rohr, P.; Medlock, J. Kinetics and mass transfer of the hydrogenation of 2-methyl-3-butyn-2-ol in a structured Pd/ZnO/Al2O3 reactor. Chem. Eng. J. 2017, 316, 121–130. [Google Scholar] [CrossRef]
- Okhlopkova, L.B.; Prosvirin, I.P.; Kerzhentsev, M.A.; Ismagilov, Z.R. Capillary microreactor with PdZn/(Ti, Ce)O2 coating for selective hydrogenation of 2-methyl-3-butyn-2-ol. Chem. Eng. Process. 2021, 159, 108240. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Kustov, L.M.; Strekalova, A.A.; Kazansky, V.B. Heterogeneous Iron-Containing Nanocatalysts—Promising Systems for Selective Hydrogenation and Hydrogenolysis. Catal. Sci. Technol. 2020, 10, 3160–3174. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Reddy, A.V.B.; Yusop, Z.; Jaafar, J.; Reddy, Y.V.M.; Aris, A.B.; Majid, Z.A.; Talib, J.; Madhavi, G. Recent progress on Fe-based nanoparticles: Synthesis, properties, characterization and environmental applications. J. Environ. Chem. Eng. 2016, 4, 3537–3553. [Google Scholar] [CrossRef]
- Redina, E.A.; Kirichenko, O.A.; Shesterkina, A.A.; Kustov, L.M. Unusual Behavior of Bimetallic Nanoparticles in Catalytic Processes of Hydrogenation and Selective Oxidation. Pure Appl. Chem. 2020, 92, 989–1006. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Kirichenko, O.A.; Kozlova, L.M.; Kapustin, G.I.; Mishin, I.V.; Strelkova, A.A.; Kustov, L.M. Liquid-Phase Hydrogenation of Phenylacetylene to Styrene on Silica-Supported Pd–Fe Nanoparticles. Mendeleev Commun. 2016, 26, 228–230. [Google Scholar] [CrossRef]
- Huang, X.; Liu, K.; Vrijburg, W.L.; Ouyang, X.; Dugulan, I.A.; Liu, Y.; Verhoeven, M.W.G.M.T.; Kosinov, N.A.; Pidko, E.A.; Hensen, E.J.M. Hydrogenation of levulinic acid to γ-valerolactone over Fe-Re/TiO2 catalysts. Appl. Catal. B Environ. 2020, 278, 119314. [Google Scholar] [CrossRef]
- Tejeda-Serrano, M.; Cabrero-Antonino, J.R.; Mainar-Ruiz, V.; Lopez-Haro, M.; Hernandez-Garrido, J.C.; Calvino, J.J.; Leyva-Perez, A.; Corma, A. Synthesis of Supported Planar Iron Oxide Nanoparticles and Their Chemo- and Stereoselectivity for Hydrogenation of Alkynes. ACS Catal. 2017, 7, 3721–3729. [Google Scholar] [CrossRef]
- Yan, K.; Chen, A. Selective hydrogenation of furfural and levulinic acid to biofuels on the ecofriendly Cu–Fe catalyst. Fuel 2014, 115, 101–108. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Shuvalova, E.V.; Kirichenko, O.A.; Strelkova, A.A.; Nissenbaum, V.D.; Kapustin, G.I.; Kustov, L.M. Using Fe–Cu nanoparticles applied to silica gel in the reaction of the selective hydrogenation of p-Dinitrobenzene to p-Phenylenediamine. Russ. J. Phys. Chem. A 2017, 91, 201–204. [Google Scholar] [CrossRef]
- He, L.; Gong, X.; Ye, L.; Duan, X.; Yuan, Y. Synergistic effects of bimetallic Cu-Fe/SiO2 nanocatalysts in selective hydrogenation of diethyl malonate to 1,3-propanediol. J. Energy Chem. 2016, 25, 1038–1044. [Google Scholar] [CrossRef]
- Nitta, Y.; Matsugi, S.; Imanaka, T. Partial hydrogenation of phenylacetylene on copper-promoted iron catalyst. Catal. Lett. 1990, 5, 67–72. [Google Scholar] [CrossRef]
- Kirichenko, O.; Kapustin, G.; Nissenbaum, V.; Strelkova, A.; Shuvalova, E.; Shesterkina, A.; Kustov, L. Thermal decomposition and reducibility of silica-supported precursors of Cu, Fe and Cu–Fe nanoparticles. J. Therm. Anal. Calorim. 2018, 134, 233–251. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Shuvalova, E.V.; Redina, E.A.; Kirichenko, O.A.; Tkachenko, O.P.; Mishin, I.V.; Kustov, L.M. Silica-supported iron oxide nanoparticles: Unexpected catalytic activity in hydrogenation of phenylacetylene. Mendeleev Commun. 2017, 27, 512–514. [Google Scholar] [CrossRef]
- Šmit, G.; Strukan, N.; Crajé, M.W.J.; Lázár, K. A comparative study of CO adsorption and oxidation on Au/Fe2O3 catalysts by FT-IR and in situ DRIFTS spectroscopies. J. Mol. Catal. A Chem. 2016, 252, 163–170. [Google Scholar] [CrossRef]
- Lu, R.; Mao, D.; Yu, J.; Guo, Q. Enhanced activity of CuFe/SiO2 catalyst for CO hydrogenation to higher alcohols by pretreating the support with ammonia. J. Ind. Eng. Chem. 2015, 25, 338–343. [Google Scholar] [CrossRef]
- Xu, R.; Ma, Z.; Yang, C.; Wei, W.; Li, W.; Sun, Y. The effect of iron on the adsorption properties of CuMnZrO2 catalysts studied by temperature-programmed desorption and FTIR spectroscopy. J. Mol. Catal. A Chem. 2004, 218, 133–140. [Google Scholar] [CrossRef]
- Fisher, I.A.; Bell, A.T. In Situ Infrared Study of Methanol Synthesis from H2/CO over Cu/SiO2 and Cu/ZrO2/SiO2. J. Catal. 1988, 178, 153–173. [Google Scholar] [CrossRef]
- Sun, C.; Mao, D.; Han, L.; Yu, J. Effect of impregnation sequence on performance of SiO2 supported Cu-Fe catalysts for higher alcohols synthesis from syngas. Catal. Commun. 2016, 84, 175–178. [Google Scholar] [CrossRef]
- Larrubia Vargas, M.A.; Busca, G.; Costantino, U.; Marmottini, F.; Montanari, T.; Patrono, P.; Ramis, G. An IR study of methanol steam reforming over ex-hydrotalcite Cu–Zn–Al catalysts. J. Mol. Catal. A Chem. 2007, 266, 188–197. [Google Scholar] [CrossRef]
- Lingaiah, N.; Babu, N.S.; Gopinath, R.; Reddy, P.S.S.; Prasad, P.S.S. Hydrodechlorination of chlorobenzene over supported metal catalysts. Catal. Surv. Asia 2006, 10, 29–39. [Google Scholar] [CrossRef]
- dos Santos Monteiro, D.; da Guarda Souza, M.O. Thermal decomposition of precursors and iron oxide properties. J. Therm. Anal. Calorim. 2016, 123, 955–963. [Google Scholar] [CrossRef]
- Pino, N.; Sitthisa, S.; Tan, Q.; Souza, T.; López, D.; Resasco, D.E. Structure, activity, and selectivity of bimetallic Pd-Fe/SiO2 and Pd-Fe/γ-Al2O3 catalysts for the conversion of furfural. J. Catal. 2016, 350, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Kirichenko, O.; Kapustin, G.; Nissenbaum, V.; Mishin, I.; Kustov, L. Evaluation of Stability of Silica-Supported Fe–Pd and Fe–Pt Nanoparticles in Aerobic Conditions Using Thermal Analysis. J. Therm. Anal. Calorim. 2014, 118, 749–758. [Google Scholar] [CrossRef]





| Catalyst | Peak Temperature (◦C) | H2 Uptake (mmol/g.cat) | H2/Cu | H2/Fe |
|---|---|---|---|---|
| 5Cu/SiO2 | 255 | 0.89 | 1.13 | – |
| 5Cu/Al2O3 | 230 | 1.52 | 1.97 | – |
| 5Fe/Al2O3 | 416 628 | 0.23 0.51 | – | 0.83 |
| 5Cu-5Fe/SiO2 | 223 260 470 | 0.54 0.49 0.71 | 0.69 | 1.34 |
| 5Cu-5Fe/Al2O3 | 218 486 | 1.24 0.33 | 1.01 | 0.88 |
| Catalyst | SBET/m2g–1 | Dpore, nm | t-plot Vmicro/cm3g–1 | Vtotal/cm3g–1 (at P/P0 = 0.99) |
|---|---|---|---|---|
| SiO2 | 108 | 26 | 0.006 | 0.639 |
| 5Cu/SiO2 | 134 | 31 | 0.002 | 0.636 |
| 5Cu-5Fe/SiO2 | 124 | 24 | 0.005 | 0.560 |
| 5Cu-5Fe/SiO2-DPU | 169 | 22 | 0.006 | 0.676 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shesterkina, A.A.; Strekalova, A.A.; Shuvalova, E.V.; Kapustin, G.I.; Tkachenko, O.P.; Kustov, L.M. CuO-Fe2O3 Nanoparticles Supported on SiO2 and Al2O3 for Selective Hydrogenation of 2-Methyl-3-Butyn-2-ol. Catalysts 2021, 11, 625. https://doi.org/10.3390/catal11050625
Shesterkina AA, Strekalova AA, Shuvalova EV, Kapustin GI, Tkachenko OP, Kustov LM. CuO-Fe2O3 Nanoparticles Supported on SiO2 and Al2O3 for Selective Hydrogenation of 2-Methyl-3-Butyn-2-ol. Catalysts. 2021; 11(5):625. https://doi.org/10.3390/catal11050625
Chicago/Turabian StyleShesterkina, Anastasiya A., Anna A. Strekalova, Elena V. Shuvalova, Gennady I. Kapustin, Olga P. Tkachenko, and Leonid M. Kustov. 2021. "CuO-Fe2O3 Nanoparticles Supported on SiO2 and Al2O3 for Selective Hydrogenation of 2-Methyl-3-Butyn-2-ol" Catalysts 11, no. 5: 625. https://doi.org/10.3390/catal11050625
APA StyleShesterkina, A. A., Strekalova, A. A., Shuvalova, E. V., Kapustin, G. I., Tkachenko, O. P., & Kustov, L. M. (2021). CuO-Fe2O3 Nanoparticles Supported on SiO2 and Al2O3 for Selective Hydrogenation of 2-Methyl-3-Butyn-2-ol. Catalysts, 11(5), 625. https://doi.org/10.3390/catal11050625







