Integrated Utilization of Dairy Whey in Probiotic β-Galactosidase Production and Enzymatic Synthesis of Galacto-Oligosaccharides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Whey-Based Medium Optimization by RSM for β-Galactosidase Production
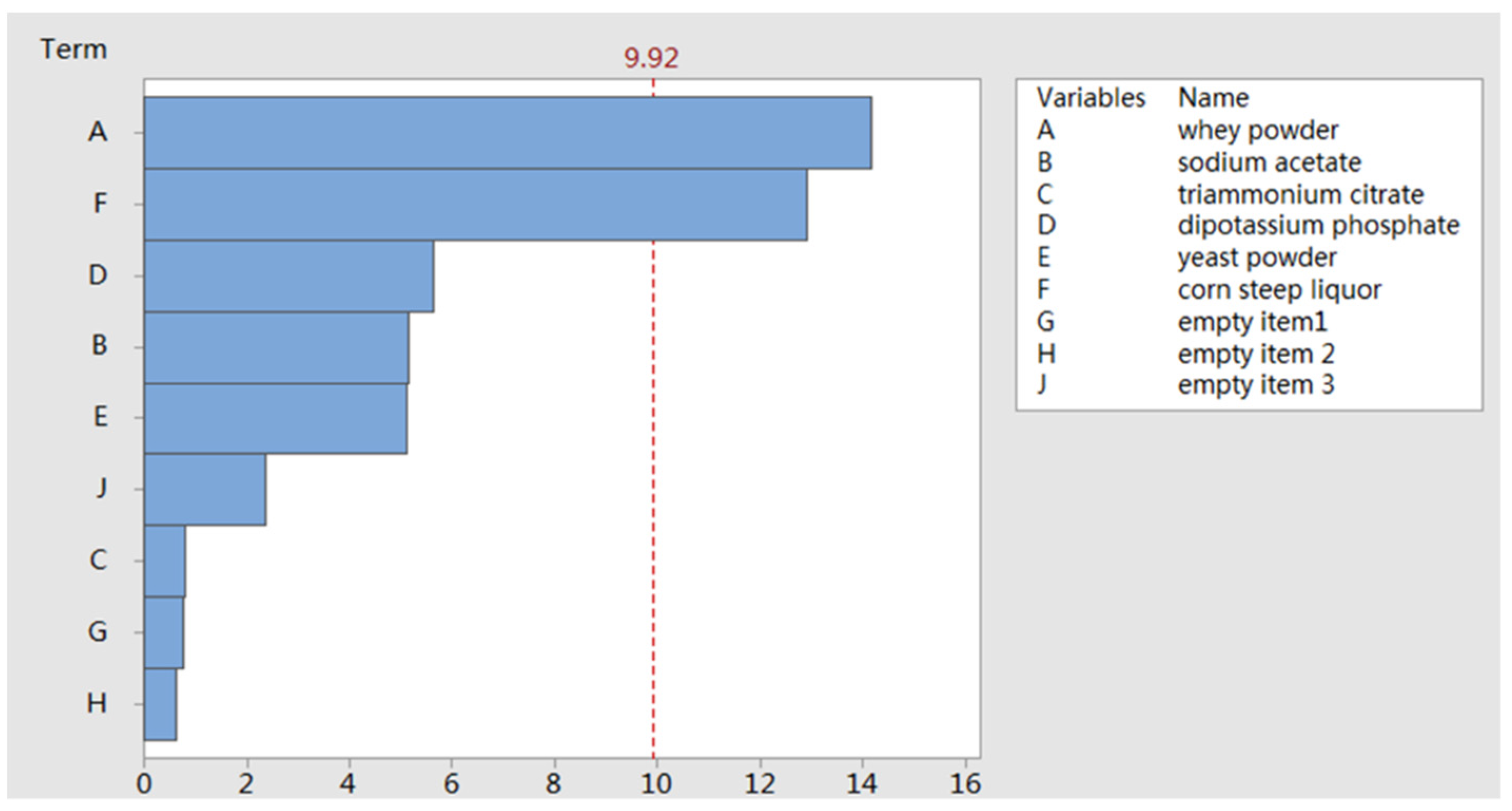
2.1.1. PB Design
2.1.2. Path of Steepest Ascent
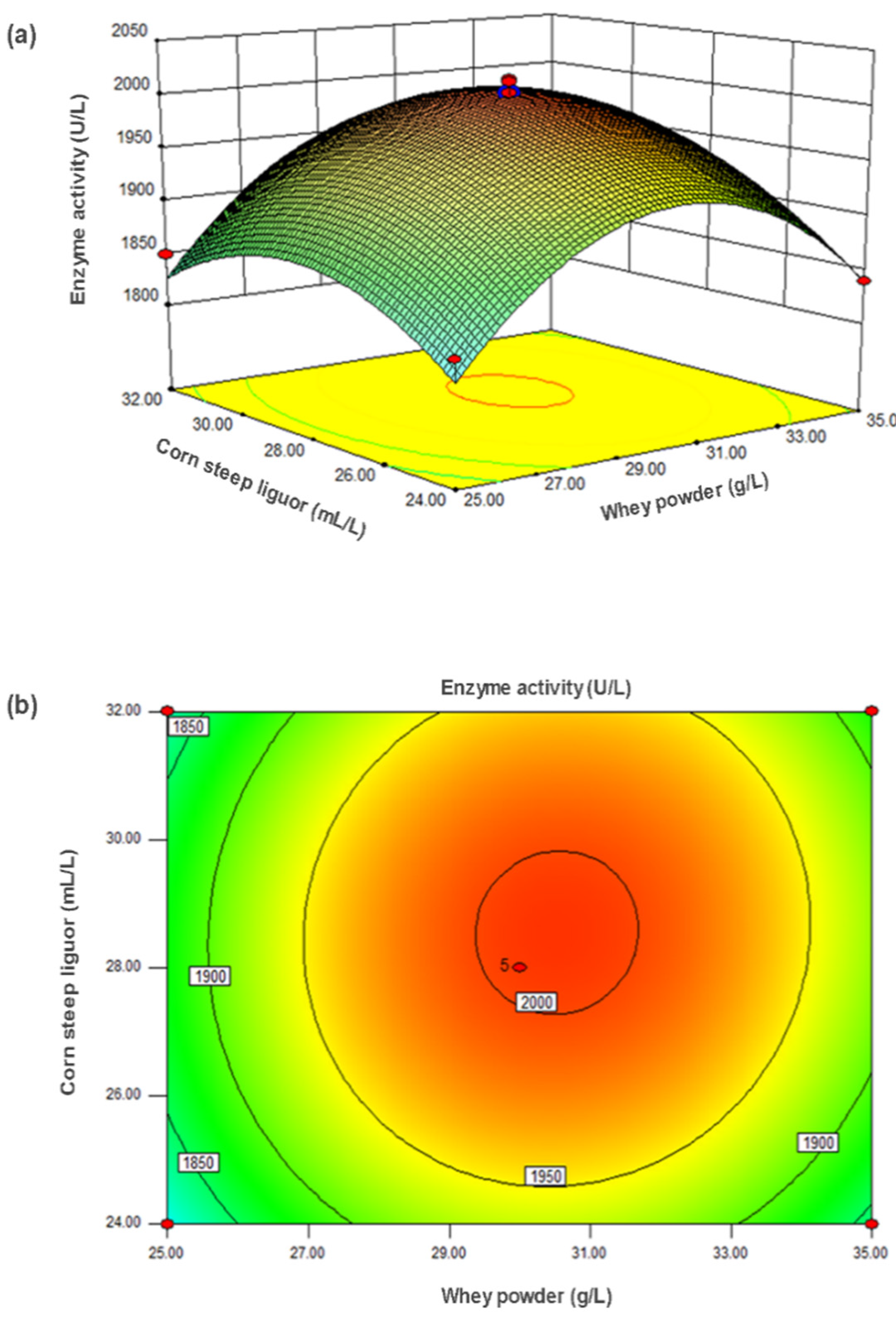
2.1.3. CCD and Response Surface Analysis
2.1.4. Model Verification
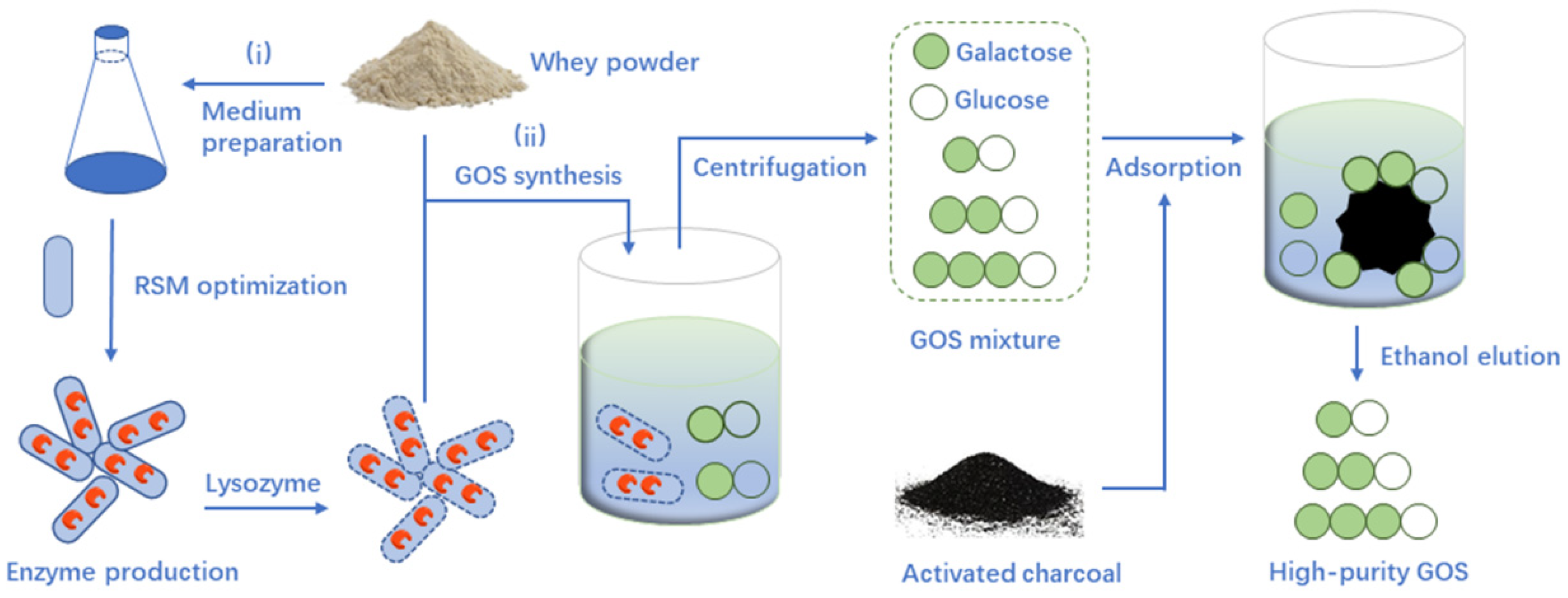
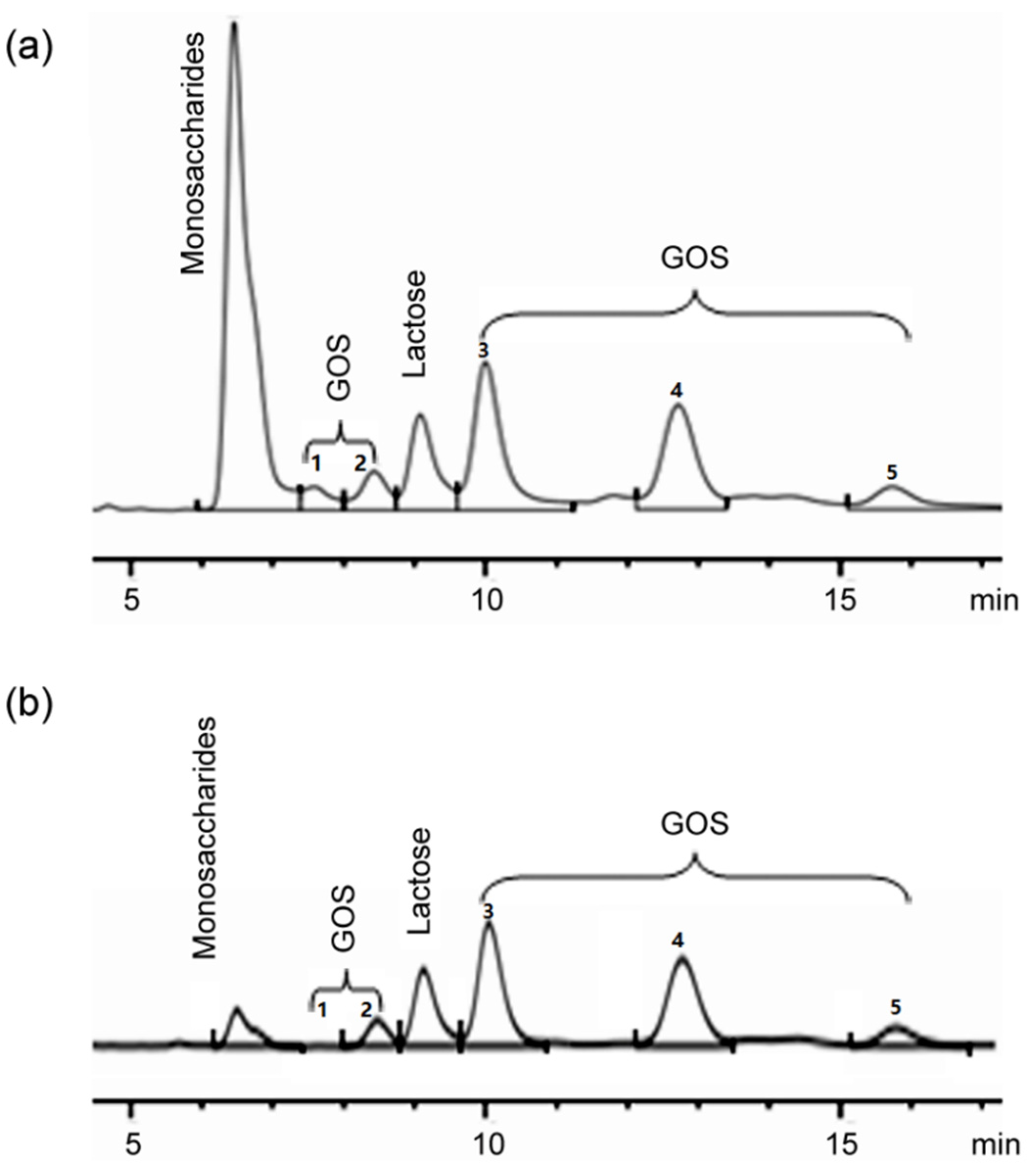
2.2. GOS Synthesis from Whey Powder by β-Galactosidase
2.3. GOS Purification by Activated Charcoal Adsorption
3. Materials and Methods
3.1. Materials
3.2. Strains and Mediums
3.3. β-Galactosidase Assays
3.4. Plackett–Burman (PB) Design
3.5. Steepest Ascent Experiment
3.6. Central Composite Design (CCD)
3.7. GOS Synthesis by Lysozyme-Treated Cells from Whey Powder
3.8. GOS Purification by Activated Charcoal Adsorption
3.9. TLC and HPLC Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lu, L.; Xiao, M. Recent Progress on galacto-oligosaccharides synthesis by microbial β-galactosidase. In Functional Carbohydrates; Chen, J., Zhu, Y., Liu, S., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 2017; Chapter 5. [Google Scholar]
- Torres, D.P.M.; Goncalves, M.P.; Teixeira, J.A.; Rodrigues, L.R. Galacto-oligosaccharides: Production, properties, applications, and significance as prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, H.R.; de Slegte, J.; Gibson, G.R.; Rastall, R.A. Galactooligosaccharides (GOS) inhibit Vibrio cholerae toxin binding to its GM1 receptor. J. Agric. Food Chem. 2009, 57, 3113–3119. [Google Scholar] [CrossRef]
- Rycroft, C.; Jones, M.; Gibson, G.; Rastall, R. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J. Appl. Microbiol. 2001, 91, 878–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mano, M.C.R.; Neri-Numa, I.A.; da Silva, J.B.; Paulino, B.N.; Pessoa, M.G.; Pastore, G.M. Oligosaccharide biotechnology: An approach of prebiotic revolution on the industry. Appl. Microbiol. Biotechnol. 2018, 102, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Guo, L.; Wang, K.; Liu, Y.; Xiao, M. β-Galactosidases: A great tool for synthesizing galactose-containing carbohydrates. Biotechnol. Adv. 2020, 39, 107465. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xu, S.; Zhao, R.; Zhang, D.; Li, Z.; Li, Y.; Xiao, M. Synthesis of galactooligosaccharides by CBD fusion β-galactosidase immobilized on cellulose. Bioresour. Technol. 2012, 116, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Carević, M.; Vukašinović-Sekulić, M.; Ćorović, M.; Rogniaux, H.; Ropartz, D.; Veličković, D.; Bezbradica, D. Evaluation of β-galactosidase from Lactobacillus acidophilus as biocatalyst for galacto-oligosaccharides synthesis: Product structural characterization and enzyme immobilization. J. Biosci. Bioeng. 2018, 126, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Raychaudhuri, A.; Ghosh, S.K. Supply chain of bioethanol production from whey: A review. Procedia Environ. Sci. 2016, 35, 833–846. [Google Scholar] [CrossRef]
- Pescuma, M.; de Valdez, G.F.; Mozzi, F. Whey-derived valuable products obtained by microbial fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 6183–6196. [Google Scholar] [CrossRef]
- Vidra, A.; Németh, A. Whey utilization in a two-stage fermentation process. Waste Treat. Recovery 2017, 2, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of cheese whey using microbial fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Abbaspourrad, A. Production of galacto-oligosaccharides from whey permeate using β-galactosidase immobilized on functionalized glass beads. Food Chem. 2018, 251, 115–124. [Google Scholar] [CrossRef]
- Seo, Y.H.; Sung, M.; Han, J.I. Lactulose production from cheese whey using recyclable catalyst ammonium carbonate. Food Chem. 2016, 197, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Roy, A.P.; Bhattacharjee, S.; Chakraborty, S.; Bhattacharjee, C. Lactose hydrolysis by β-galactosidase enzyme: Optimization using response surface methodology. Ecotoxicol. Environ. Saf. 2015, 121, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Priyadharshini, S.D.; Bakthavatsalam, A.K. Optimization of phenol degradation by the microalga Chlorella pyrenoidosa using Plackett-Burman Design and Response Surface Methodology. Bioresour. Technol. 2016, 207, 150–156. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Jiang, Z.; Yan, Q.; Yang, S. Biochemical characterization of a novel β-galactosidase from Paenibacillus barengoltzii suitable for lactose hydrolysis and galactooligosaccharides synthesis. Int. J. Biol. Macromol. 2017, 104, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Shin, H.J.; Bucke, C. Purification and biochemical properties of a galactooligosaccharide producing beta-galactosidase from Bullera singularis. Biotechnol. Lett. 2003, 25, 2107–2111. [Google Scholar] [CrossRef]
- Anatriello, E.; Cunha, M.; Nogueira, J.; Carvalho, J.L.; Sá, A.K.; Miranda, M.; Castro-Faria-Neto, H.; Keller, A.C.; Aimbire, F. Oral feeding of Lactobacillus bulgaricus N45.10 inhibits the lung inflammation and airway remodeling in murine allergic asthma: Relevance to the Th1/Th2 cytokines and STAT6/T-bet. Cell. Immunol. 2019, 341, 103928. [Google Scholar] [CrossRef]
- Ghosh, T.; Beniwal, A.; Semwal, A.; Navani, N.K. Mechanistic insights into probiotic properties of lactic acid bacteria associated with ethnic fermented dairy products. Front. Microbiol. 2019, 10, 502. [Google Scholar] [CrossRef] [Green Version]
- Wollowski, I.; Rechkemmer, G.; Pool-Zobel, B.L. Protective role of probiotics and prebiotics in colon cancer. Am. J. Clin. Nutr. 2001, 73, 451S–455S. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.; Tomar, S.K.; Ali, B.; Singh, R.R.; Singh, A.K. Production of β-galactosidase from Streptococcus thermophilus for galactooligosaccharides synthesis. J. Food Sci. Technol. 2015, 52, 4206–4215. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, O.; Ruiz-Matute, A.I.; Olano, A.; Moreno, F.J.; Sanz, M.L. Comparison of fractionation techniques to obtain prebiotic galactooligosaccharides. Int. Dairy J. 2009, 19, 531–536. [Google Scholar] [CrossRef]
- Nobre, C.; Teixeira, J.A.; Rodrigues, L.R. New trends and technological challenges in the industrial production and purification of fructo-oligosaccharides. Crit. Rev. Food Sci. Nutr. 2015, 55, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, A.; Qin, J.; Li, L.; Ai, X.; Jiang, T.; Tang, H.; Xu, P. Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM. Appl. Microbiol. Biotechnol. 2009, 82, 49–57. [Google Scholar] [CrossRef] [PubMed]

| Variables | Whey Powder (g/L) | CH3COONa (g/L) | Triammonium Citrate (g/L) | K2HPO4 (g/L) | Yeast Powder (g/L) | Corn Steep Liquor (mL/L) | Enzyme Activity (U/L) | |
|---|---|---|---|---|---|---|---|---|
| Symbol | A | B | C | D | E | F | ||
| Coded levels | −1 | 16 | 2 | 1.6 | 1.6 | 3.2 | 16 | |
| +1 | 24 | 3 | 2.4 | 2.4 | 4.8 | 24 | ||
| Run | 1 | −1 | +1 | +1 | −1 | +1 | −1 | 1002 |
| 2 | −1 | +1 | −1 | −1 | +1 | +1 | 1229 | |
| 3 | +1 | −1 | −1 | −1 | +1 | +1 | 1456 | |
| 4 | +1 | +1 | +1 | +1 | +1 | −1 | 1420 | |
| 5 | −1 | −1 | +1 | +1 | +1 | +1 | 1205 | |
| 6 | −1 | −1 | +1 | +1 | −1 | +1 | 1313 | |
| 7 | +1 | +1 | −1 | +1 | −1 | +1 | 1861 | |
| 8 | +1 | +1 | +1 | −1 | −1 | +1 | 1682 | |
| 9 | +1 | −1 | −1 | +1 | +1 | −1 | 1324 | |
| 10 | −1 | −1 | −1 | −1 | −1 | −1 | 1038 | |
| 11 | −1 | +1 | −1 | +1 | −1 | −1 | 1157 | |
| 12 | +1 | −1 | +1 | −1 | −1 | −1 | 1253 |
| Step | Whey Powder (g/L) | Corn Steep Liquor (mL/L) | Enzyme Activity (U/L) |
|---|---|---|---|
| 1 | 20 | 20 | 1718 |
| 2 | 25 | 24 | 1849 |
| 3 | 30 | 28 | 1993 |
| 4 | 35 | 32 | 1885 |
| 5 | 40 | 36 | 1778 |
| Run | Actual Level of Variables | Coded Levels | Enzyme Activity (U/L) | ||
|---|---|---|---|---|---|
| Whey Powder (g/L) | Corn Steep Liquor (mL/L) | X1 | X2 | ||
| 1 | 25 | 24 | −1 | −1 | 1828 |
| 2 | 35 | 24 | 1 | −1 | 1840 |
| 3 | 25 | 32 | −1 | 1 | 1849 |
| 4 | 35 | 32 | 1 | 1 | 1885 |
| 5 | 22.93 | 28 | −α | 0 | 1731 |
| 6 | 37.07 | 28 | α | 0 | 1821 |
| 7 | 30 | 22.34 | 0 | −α | 1859 |
| 8 | 30 | 33.66 | 0 | α | 1897 |
| 9 | 30 | 28 | 0 | 0 | 1993 |
| 10 | 30 | 28 | 0 | 0 | 2007 |
| 11 | 30 | 28 | 0 | 0 | 2020 |
| 12 | 30 | 28 | 0 | 0 | 1981 |
| 13 | 30 | 28 | 0 | 0 | 2017 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 99,800.07 | 5 | 19,960.01 | 46.74 | <0.0001 | significant |
| X1 | 3840.09 | 1 | 3840.09 | 8.99 | 0.0200 | - |
| X2 | 1792.11 | 1 | 1792.11 | 4.20 | 0.0797 | - |
| X1X2 | 144.00 | 1 | 144.00 | 0.34 | 0.5797 | - |
| X12 | 81,044.50 | 1 | 81,044.50 | 189.76 | <0.0001 | - |
| X22 | 22,542.03 | 1 | 22,542.03 | 52.78 | 0.0002 | - |
| Residual | 2989.62 | 7 | 427.09 | - | - | - |
| Lack of Fit | 1906.42 | 3 | 635.47 | 2.35 | 0.2140 | not significant |
| Pure Error | 1083.20 | 4 | 270.80 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, F.; Zhao, R.; Yang, J.; Xiao, M.; Lu, L. Integrated Utilization of Dairy Whey in Probiotic β-Galactosidase Production and Enzymatic Synthesis of Galacto-Oligosaccharides. Catalysts 2021, 11, 658. https://doi.org/10.3390/catal11060658
Duan F, Zhao R, Yang J, Xiao M, Lu L. Integrated Utilization of Dairy Whey in Probiotic β-Galactosidase Production and Enzymatic Synthesis of Galacto-Oligosaccharides. Catalysts. 2021; 11(6):658. https://doi.org/10.3390/catal11060658
Chicago/Turabian StyleDuan, Feiyu, Renfei Zhao, Jingyi Yang, Min Xiao, and Lili Lu. 2021. "Integrated Utilization of Dairy Whey in Probiotic β-Galactosidase Production and Enzymatic Synthesis of Galacto-Oligosaccharides" Catalysts 11, no. 6: 658. https://doi.org/10.3390/catal11060658





