Preparation of TiO2/Ag[BMIM]Cl Composites and Their Visible Light Photocatalytic Properties for the Degradation of Rhodamine B
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.1.1. TEM Characterization
2.1.2. XRD Analysis
2.1.3. Zeta Potential Characterization
2.2. Photocatalytic Degradation Properties of TiO2/Ag[BMIM]Cl
2.3. Catalytic Effect of Ag+ and IL Dosages during the TiO2/Ag[BMIM]Cl Processing
2.4. Evaluation of Photocatalytic Degradation Effects
2.4.1. Effect of pH
2.4.2. Effect of Catalyst Dosage
2.4.3. Effect of Degradation on Initial Rh B Concentration
2.4.4. Kinetics of Photocatalytic Reaction
2.4.5. Stability and Reusability of a Photocatalyst
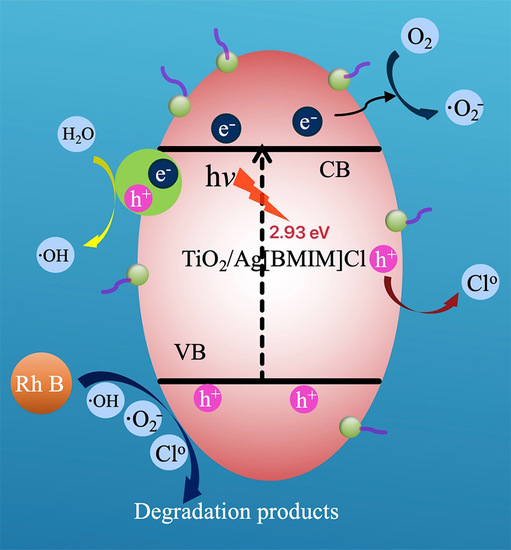
2.5. Mechanism of Photocatalytic Degradation
3. Materials and Methods
3.1. Materials
3.2. Preparation of Mesoporous TiO2
3.3. Synthesis of TiO2/Ag[BMIM]Cl
3.4. Photocatalytic Degradation of Rh B
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mikulcic, H.; Wang, X.; Duic, N.; Dewil, R. Environmental problems arising from the sustainable development of energy, water and environment system. J. Environ. Manag. 2020, 259, 109666. [Google Scholar] [CrossRef]
- Gover, H.C.; Fahmie, T.A.; McKeown, C.A. A review of environmental enrichment as treatment for problem behavior maintained by automatic reinforcement. J. Appl. Behav. Anal. 2019, 52, 299–314. [Google Scholar] [CrossRef]
- Wen, M.; Li, G.; Liu, H.; Chen, J.; An, T.; Yamashita, H. Metal–organic framework-based nanomaterials for adsorption and photocatalytic degradation of gaseous pollutants: Recent progress and challenges. Environ. Sci. Nano 2019, 6, 1006–1025. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Kheirabadi, M.; Ebrahimi, M.; Moshfegh, A.Z. Design and tailoring of one-dimensional ZnO nanomaterials for photocatalytic degradation of organic dyes: A review. Res. Chem. Intermed. 2019, 45, 2197–2254. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Wang, G. Photocatalytic Advanced Oxidation Processes for Water Treatment: Recent Advances and Perspective. Chem. Asian J. 2020, 15, 3239–3253. [Google Scholar] [CrossRef] [PubMed]
- Kusworo, T.D.; Susanto, H.; Aryanti, N.; Rokhati, N.; Widiasa, I.N.; Aziz, A.; Utomo, D.P.; Masithoh, D.; Kumoro, A.C. Preparation and Characterization of Photocatalytic PSf-TiO2 /GO Nanohybrid Membrane for the Degradation of Organic Contaminants in Natural Rubber Wastewater. J. Environ. Chem. Eng. 2021, 9, 105066. [Google Scholar] [CrossRef]
- Yang, F.L.; Song, D.; Ma, A.B.; Jiang, J.H.; Cheng, Z.J. Recent Studies of Surface Self-Nanocrystallization (SSNC) of Metallic Materials. Mater. Sci. Forum 2019, 956, 160–168. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.S.W.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2015, 7, 1569–1578. [Google Scholar] [CrossRef]
- Yang, X.; Koziel, J.A.; Laor, Y.; Zhu, W.; van Leeuwen, J.; Jenks, W.S.; Hoff, S.J.; Zimmerman, J.; Zhang, S.; Ravid, U.; et al. VOC Removal from Manure Gaseous Emissions with UV Photolysis and UV-TiO2 Photocatalysis. Catalysts 2020, 10, 607. [Google Scholar] [CrossRef]
- He, X.; Wu, M.; Ao, Z.; Lai, B.; Zhou, Y.; An, T.; Wang, S. Metal-organic frameworks derived C/TiO2 for visible light photocatalysis: Simple synthesis and contribution of carbon species. J. Hazard. Mater. 2021, 403, 124048. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Liu, Z.; Chen, J.; Wu, Y.; Guo, M.; Na, P. In-situ deposition of Ag3PO4 on TiO2 nanosheets dominated by (001) facets for enhanced photocatalytic activities and recyclability. Ceram. Int. 2017, 43, 11588–11595. [Google Scholar] [CrossRef]
- Ma, H.; Zheng, W.; Yan, X.; Li, S.; Zhang, K.; Liu, G.; Jiang, L. Polydopamine-induced fabrication of Ag-TiO2 hollow nanospheres and their application in visible-light photocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124283. [Google Scholar] [CrossRef]
- Mittal, A.; Mari, B.; Sharma, S.; Kumari, V.; Maken, S.; Kumari, K.; Kumar, N. Non-metal modified TiO2: A step towards visible light photocatalysis. J. Mater. Sci. Mater. Electron. 2019, 30, 3186–3207. [Google Scholar] [CrossRef]
- Na, S.; Seo, S.; Lee, H. Recent Developments of Advanced Ti3+-Self-Doped TiO2 for Efficient Visible-Light-Driven Photocatalysis. Catalysts 2020, 10, 679. [Google Scholar] [CrossRef]
- Ingrosso, C.; Corcione, C.E.; Striani, R.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Frigione, M. UV-curable nanocomposite based on methacrylic-siloxane resin and surface-modified TiO2 nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 15494. [Google Scholar] [CrossRef]
- Corcione, C.E.; Ingrosso, C.; Petronella, F.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Frigione, M.; Curri, M.L. A designed UV–vis light curable coating nanocomposite based on colloidal TiO2 NRs in a hybrid resin for stone protection. Prog. Org. Coat. 2018, 122, 290–301. [Google Scholar] [CrossRef]
- Ramanathan, R.; Bansal, V. Ionic liquid mediated synthesis of nitrogen, carbon and fluorine-codoped rutile TiO2 nanorods for improved UV and visible light photocatalysis. RSC Adv. 2015, 5, 1424–1429. [Google Scholar] [CrossRef]
- Kumar, D.A.; Palanichamy, V.; Roopan, S.M. One step production of AgCl nanoparticles and its antioxidant and photo catalytic activity. Mater. Lett. 2015, 144, 62–64. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Chen, B.; Zhang, Y.; Lin, L.; Han, X.; Zou, P.; Wang, G.; Zeng, J.; Zhao, M. Fabrication of a three-dimensional visible-light-driven Ag–AgBr/TiO2/graphene aerogel composite for enhanced photocatalytic destruction of organic dyes and bacteria. New J. Chem. 2019, 43, 5088–5098. [Google Scholar] [CrossRef]
- Padervand, M. Facile Synthesis of the Novel Ag[1-butyl 3-methyl imidazolium]Br Nanospheres for Efficient Photodisinfection of Wastewaters. Chem. Eng. Commun. 2016, 203, 1532–1537. [Google Scholar] [CrossRef][Green Version]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, T.N.; Sureshkumar, K.; Dupont, J.; Ramakrishnappa, T.; Nagaraju, G. Ionic liquid-assisted hydrothermal synthesis of TiO2 nanoparticles and its applications towards the photocatalytic activity and electrochemical sensor. J. Exp. Nanosci. 2015, 10, 1358–1373. [Google Scholar] [CrossRef]
- Shahhiran, A.F.; Ramli, R.M.; Zaid, H.F.M. Development of photocatalytic degradation and kinetic study for imidazolium based ionic liquids in Fe-Cu/TiO2-AC system. IOP Conf. Ser. Earth Environ. Sci. 2020, 442, 012001. [Google Scholar] [CrossRef]
- Liu, H.; Liang, Y.; Hu, H.; Wang, M. Hydrothermal synthesis of mesostructured nanocrystalline TiO2 in an ionic liquid–water mixture and its photocatalytic performance. Solid State Sci. 2009, 11, 1655–1660. [Google Scholar] [CrossRef]
- Paszkiewicz-Gawron, M.; Makurat, S.; Rak, J.; Zdrowowicz, M.; Lisowski, W.; Zaleska-Medynska, A.; Kowalska, E.; Mazierski, P.; Łuczak, J. Theoretical and Experimental Studies on the Visible Light Activity of TiO2 Modified with Halide-Based Ionic Liquids. Catalysts 2020, 10, 371. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Li, M.; Zhang, Q.; Wang, Z.; Dai, Y.; Zhang, X.; Liu, Y.; Whangbo, M.-H.; Huang, B. Adsorption of gaseous ethylene via induced polarization on plasmonic photocatalyst Ag/AgCl/TiO2 and subsequent photodegradation. Appl. Catal. B Environ. 2018, 220, 356–361. [Google Scholar] [CrossRef]
- Pinto, I.; Buss, A. ζ Potential as a Measure of Asphalt Emulsion Stability. Energy Fuels 2020, 34, 2143–2151. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-Gap Energy Estimation from Diffuse Reflectance Measurements on Sol–Gel and Commercial TiO2: A Comparative Study. J. Sol-Gel Sci. Technol. 2011, 61, 1–7. [Google Scholar] [CrossRef]
- He, F.; Ma, F.; Li, J.; Li, T.; Li, G. Effect of calcination temperature on the structural properties and photocatalytic activities of solvothermal synthesized TiO2 hollow nanoparticles. Ceram. Int. 2014, 40, 6441–6446. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Liu, B.; Dong, L. A facile synthesis of brown anatase TiO2 rich in oxygen vacancies and its visible light photocatalytic property. Solid State Ion. 2021, 361, 115564. [Google Scholar] [CrossRef]
- Yu, J.; Yu, J.C.; Ho, W.; Jiang, Z. Effects of calcination temperature on the photocatalytic activity and photo-induced super-hydrophilicity of mesoporous TiO2 thin films. New J. Chem. 2002, 26, 607–613. [Google Scholar] [CrossRef]
- Ma, G.Q.; Liu, F.S.; Wang, S.; Dang, Z.C.; Zhang, J.W.; Fu, X.J.; Hou, M.S. Preparation and characterization of Bi2S3/3DOM-TiO2 for efficient photocatalytic degradation of rhodamine B. Mater. Sci. Semicond. Process. 2019, 100, 61–72. [Google Scholar] [CrossRef]
- Guillard, C.; Lachheb, H.; Houas, A.; Ksibi, M.; Elaloui, E.; Herrmann, J.-M. Influence of chemical structure of dyes, of pH and of inorganic salts on their photocatalytic degradation by TiO2 comparison of the efficiency of powder and supported TiO2. J. Photochem. Photobiol. A Chem. 2003, 158, 27–36. [Google Scholar] [CrossRef]
- Lin, W.D.; Liao, C.T.; Chang, T.C.; Chen, S.H.; Wu, R.J. Humidity sensing properties of novel graphene/TiO2 composites by sol–gel process. Sens. Actuators B Chem. 2015, 209, 555–561. [Google Scholar] [CrossRef]







| Dye | Chemical Formula | Mw (g/mol) | λmax (nm) |
|---|---|---|---|
| Rh B |  | 479.01 | 566 |
| Methyl blue |  | 799.80 | 662 |
| Methyl orange |  | 327.33 | 505 |
| Linear Range (min) | k (min−1) | R2 |
|---|---|---|
| 0~100 | 0.00704 | 0.9885 |
| 100~300 | 0.01674 | 0.9422 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Li, Y. Preparation of TiO2/Ag[BMIM]Cl Composites and Their Visible Light Photocatalytic Properties for the Degradation of Rhodamine B. Catalysts 2021, 11, 661. https://doi.org/10.3390/catal11060661
Lin X, Li Y. Preparation of TiO2/Ag[BMIM]Cl Composites and Their Visible Light Photocatalytic Properties for the Degradation of Rhodamine B. Catalysts. 2021; 11(6):661. https://doi.org/10.3390/catal11060661
Chicago/Turabian StyleLin, Xi, and Yanxia Li. 2021. "Preparation of TiO2/Ag[BMIM]Cl Composites and Their Visible Light Photocatalytic Properties for the Degradation of Rhodamine B" Catalysts 11, no. 6: 661. https://doi.org/10.3390/catal11060661
APA StyleLin, X., & Li, Y. (2021). Preparation of TiO2/Ag[BMIM]Cl Composites and Their Visible Light Photocatalytic Properties for the Degradation of Rhodamine B. Catalysts, 11(6), 661. https://doi.org/10.3390/catal11060661