Synthesis and Characterization of Nickel(II) Homogeneous and Supported Complexes for the Hydrogenation of Furfural to Furfuryl Alcohol
Abstract
1. Introduction
2. Results
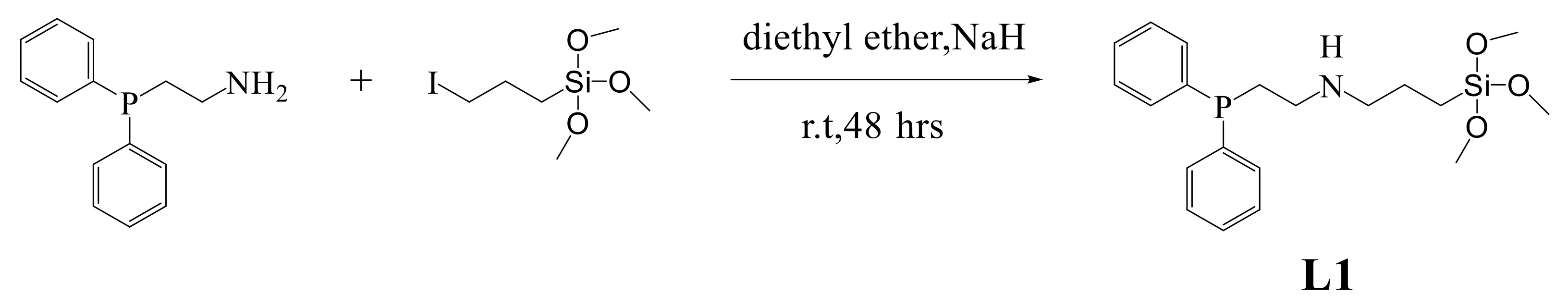
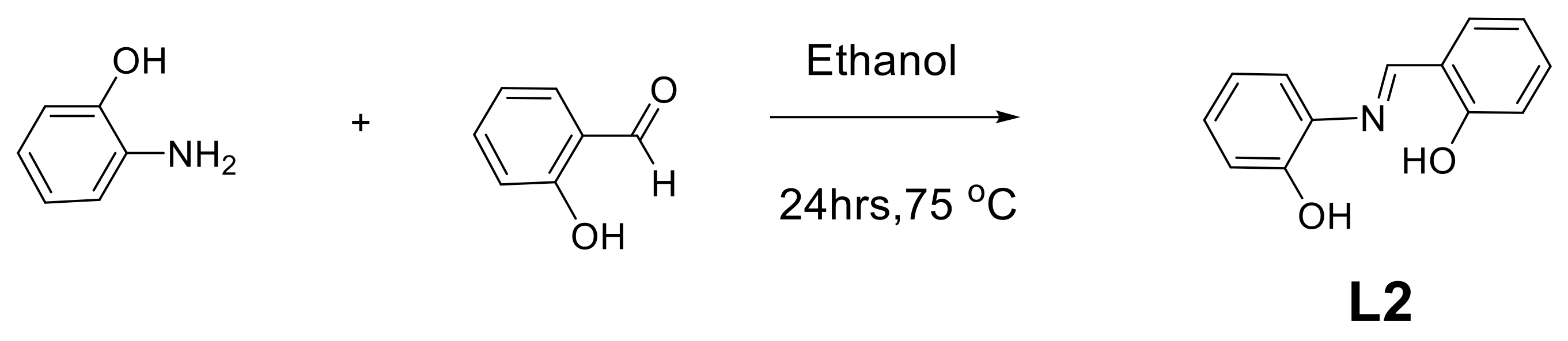
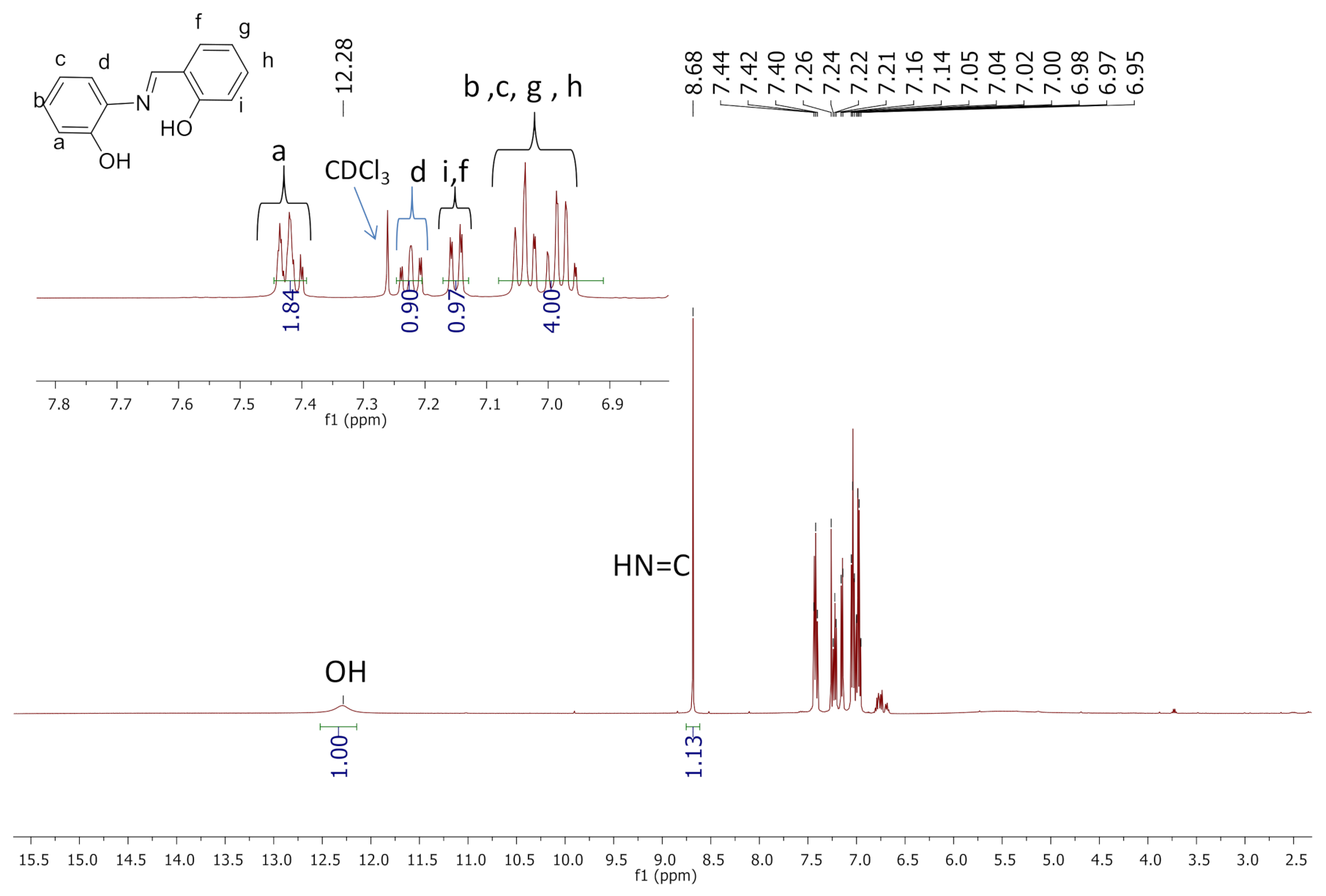
2.1. Synthesis and Characterization of Ligands (L1 and L2)
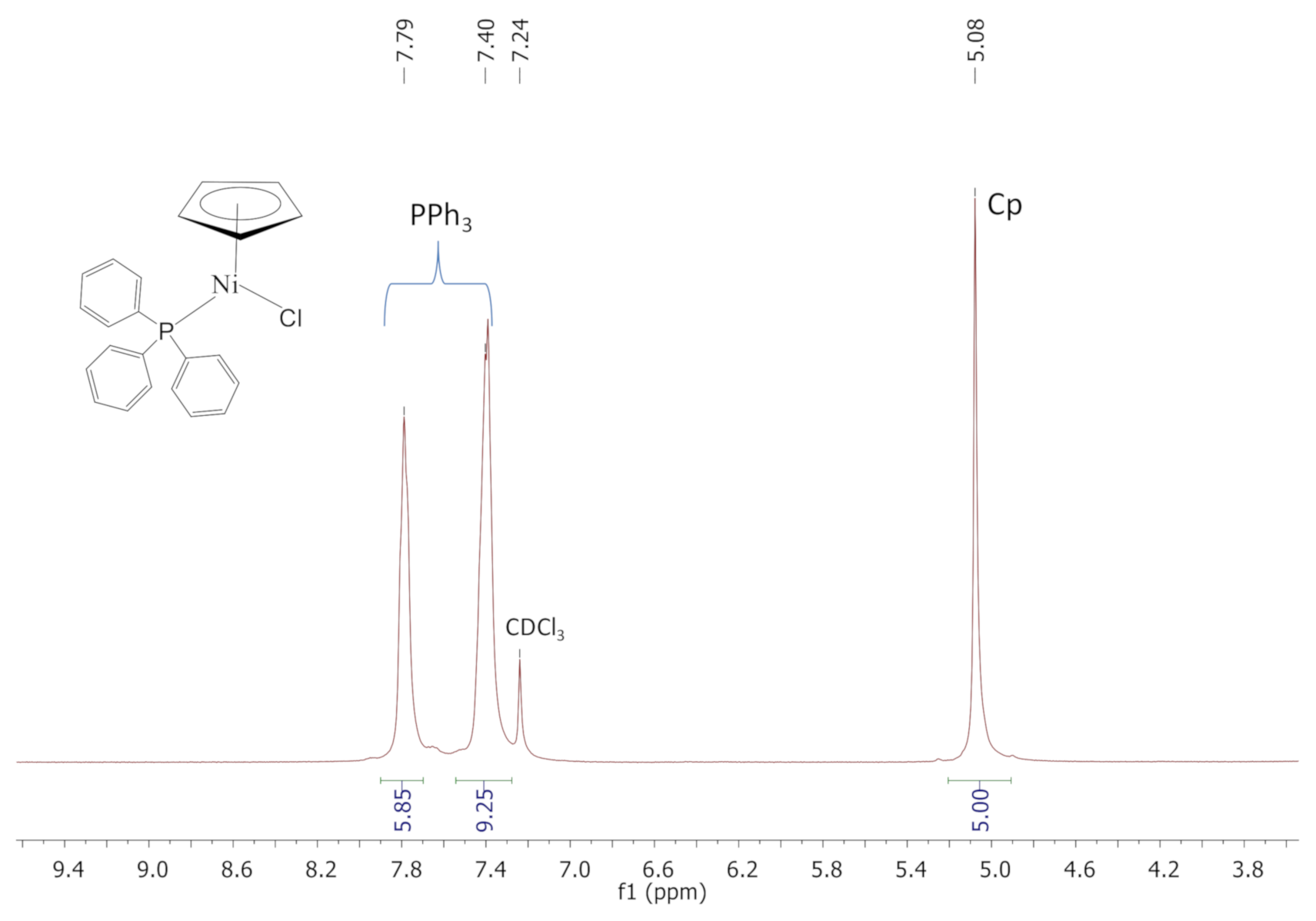
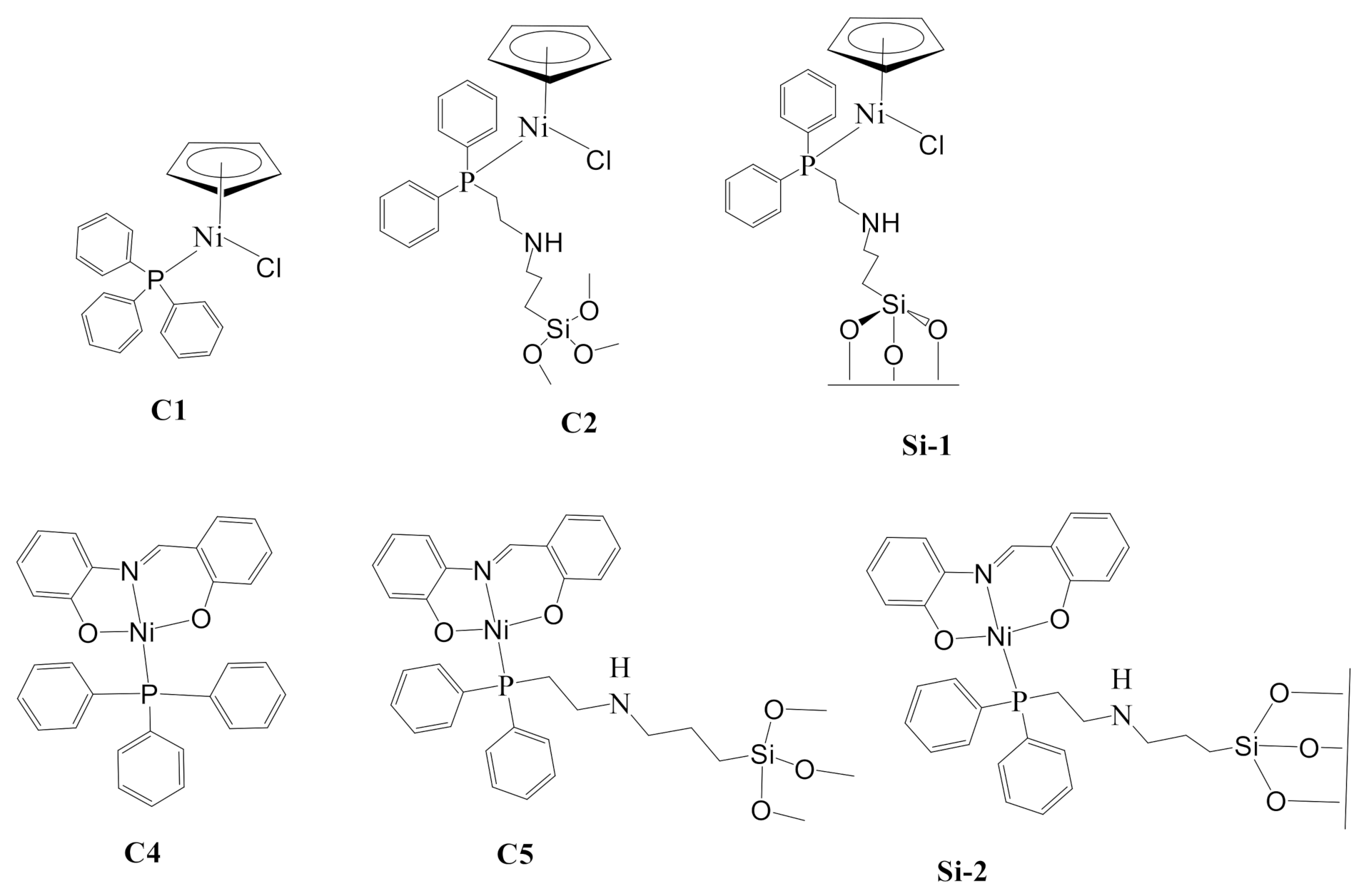
2.2. Synthesis and Characterization of Complexes C1, C2, and Si-1
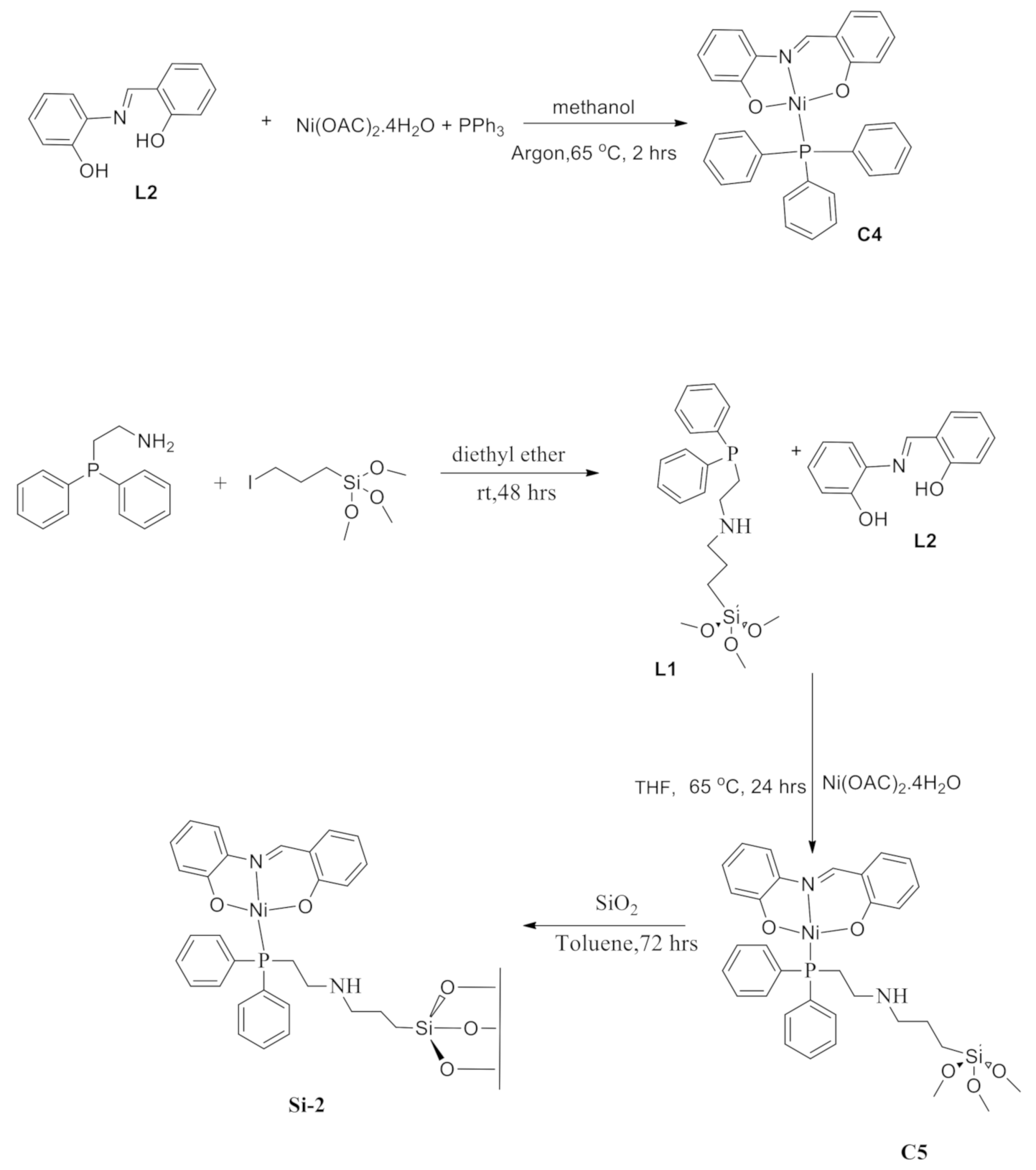
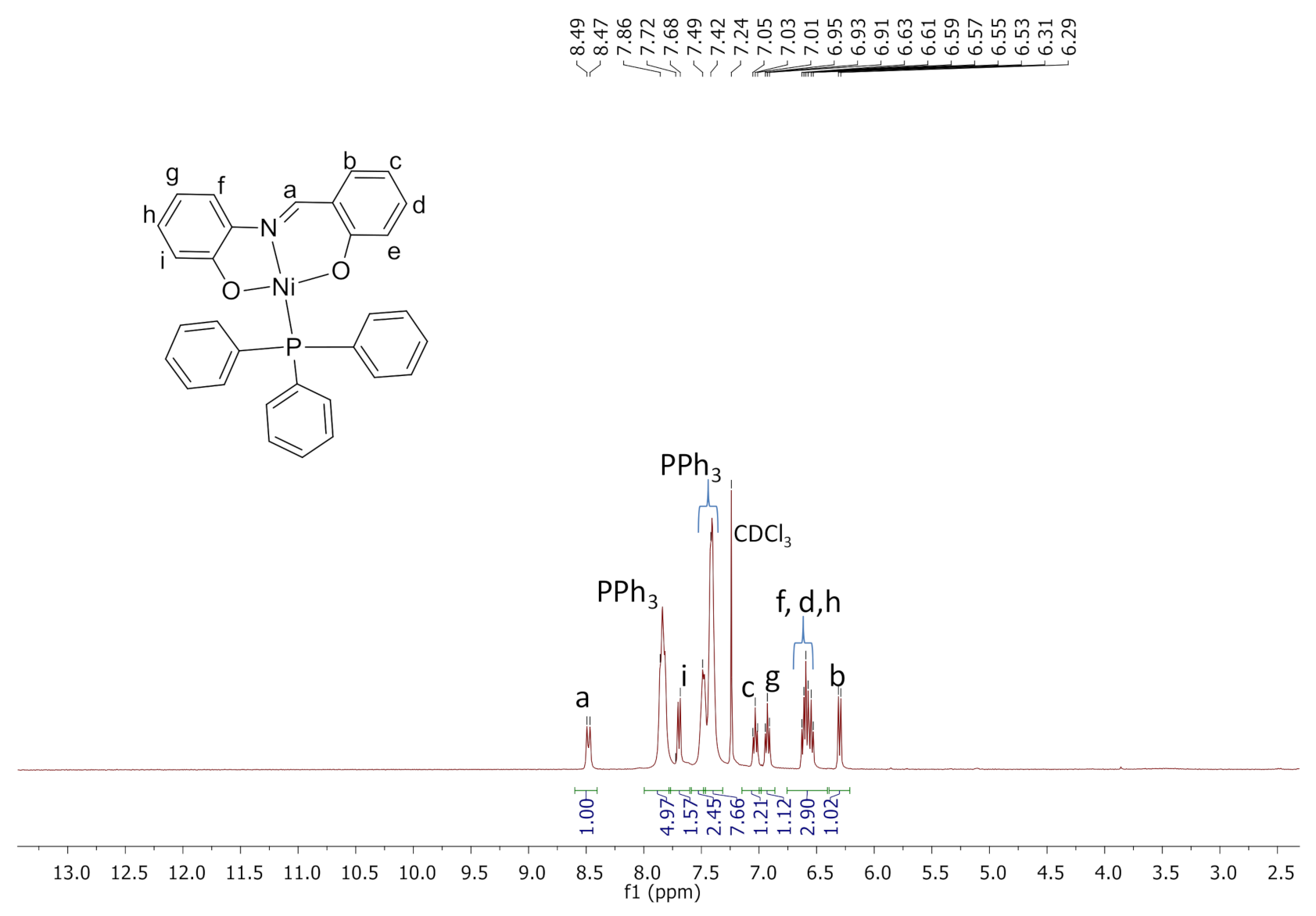
2.3. Synthesis and Characterization of Complexes C4, C5, and Si-2
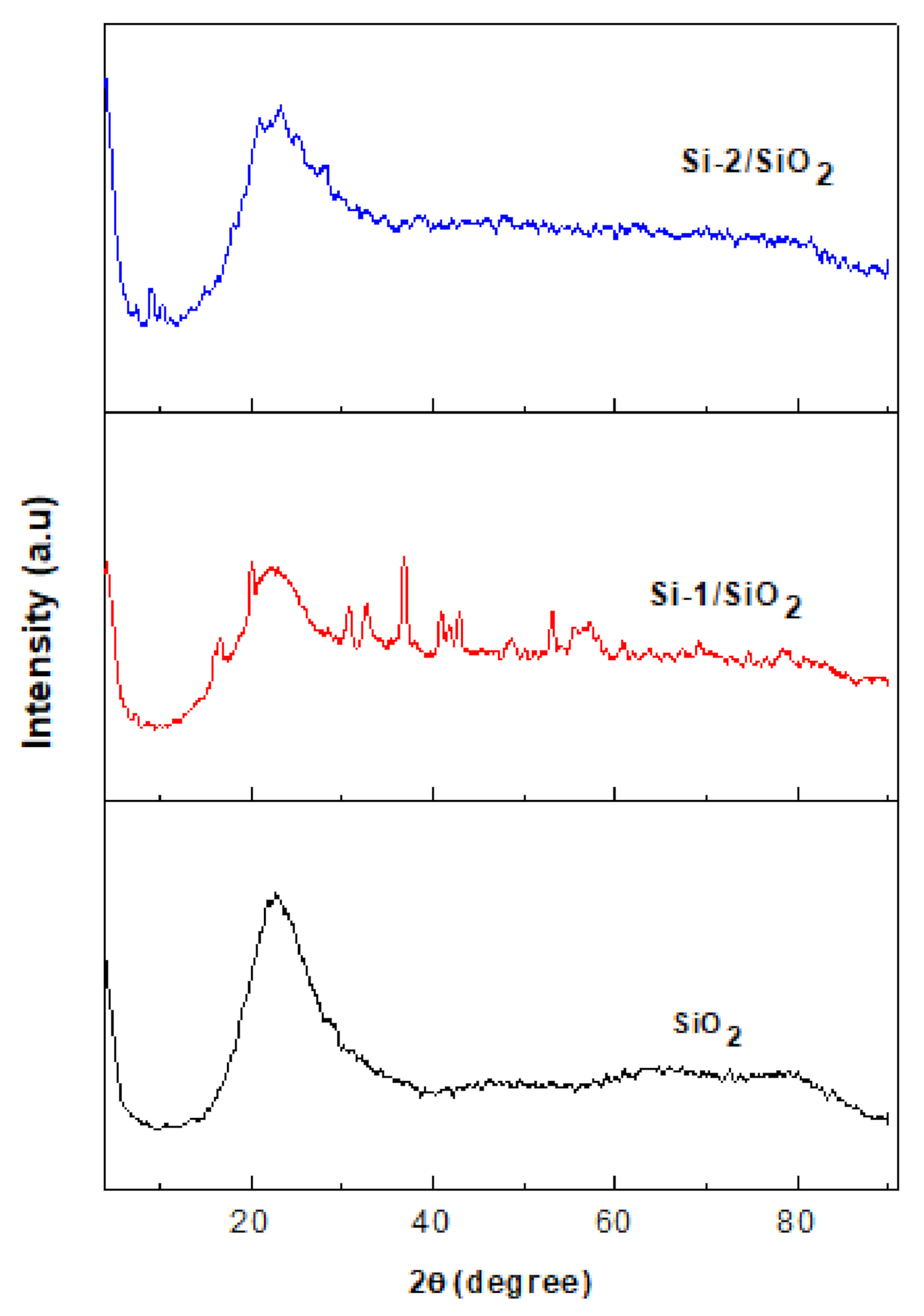
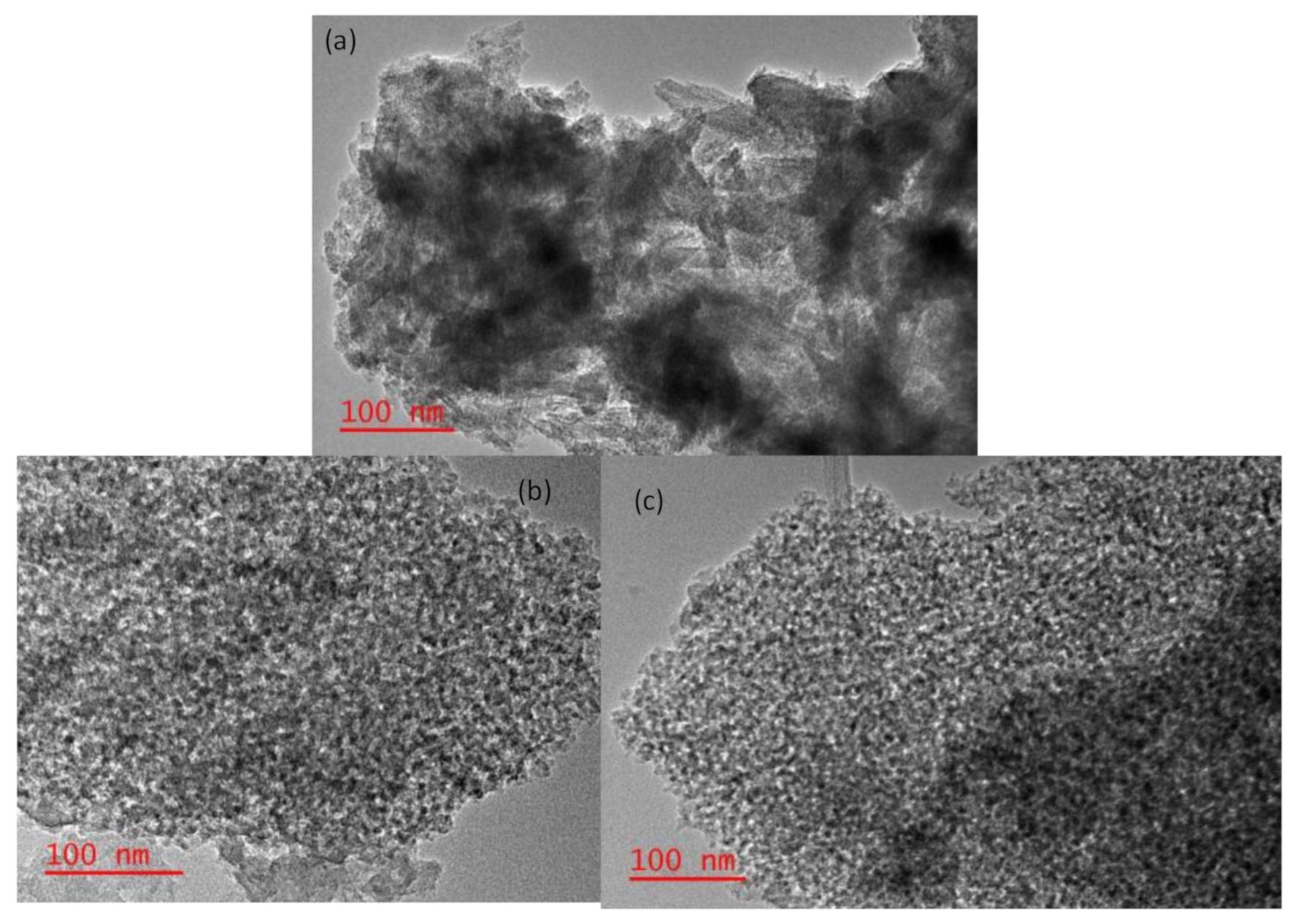
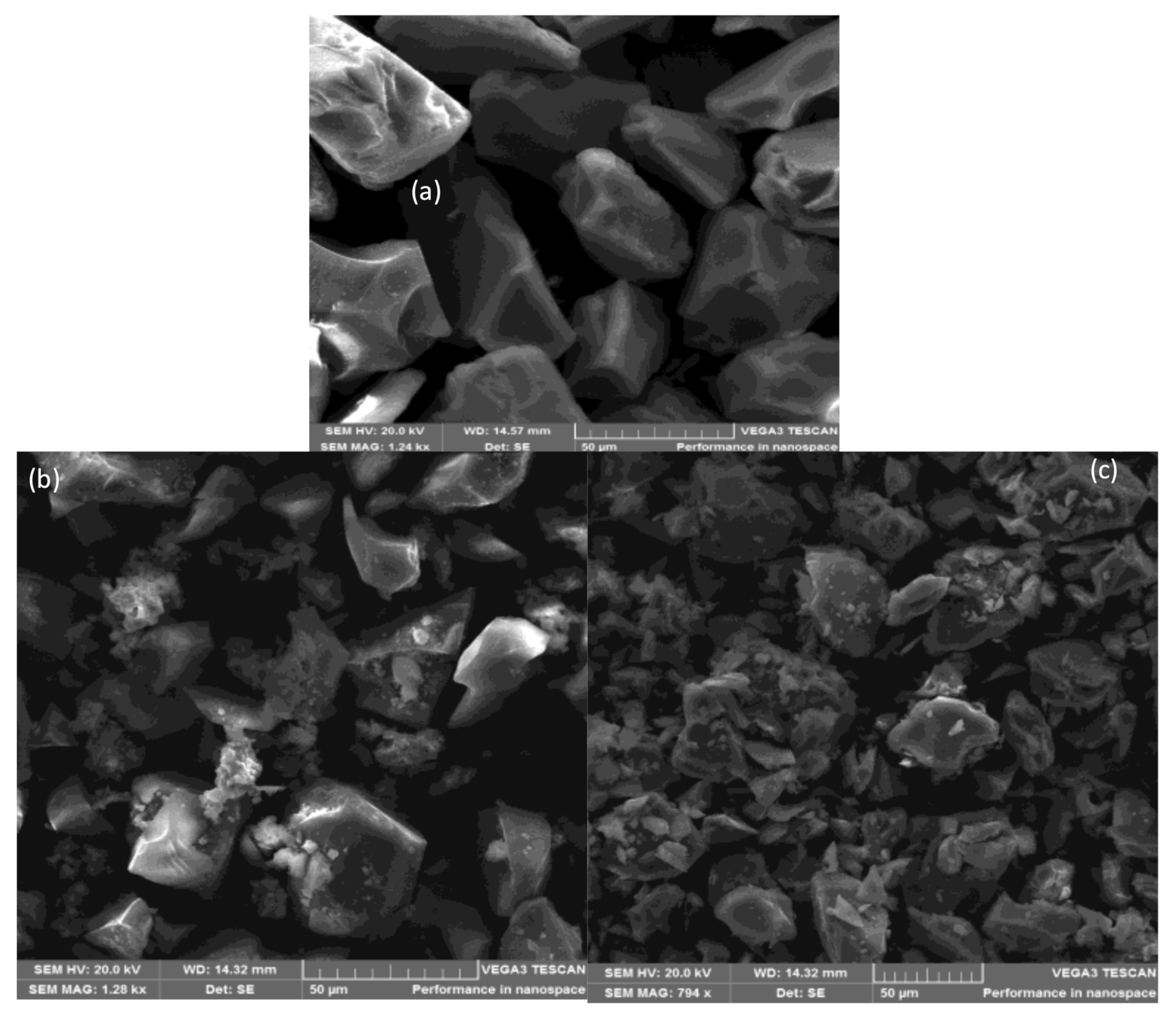
2.4. Surface Properties
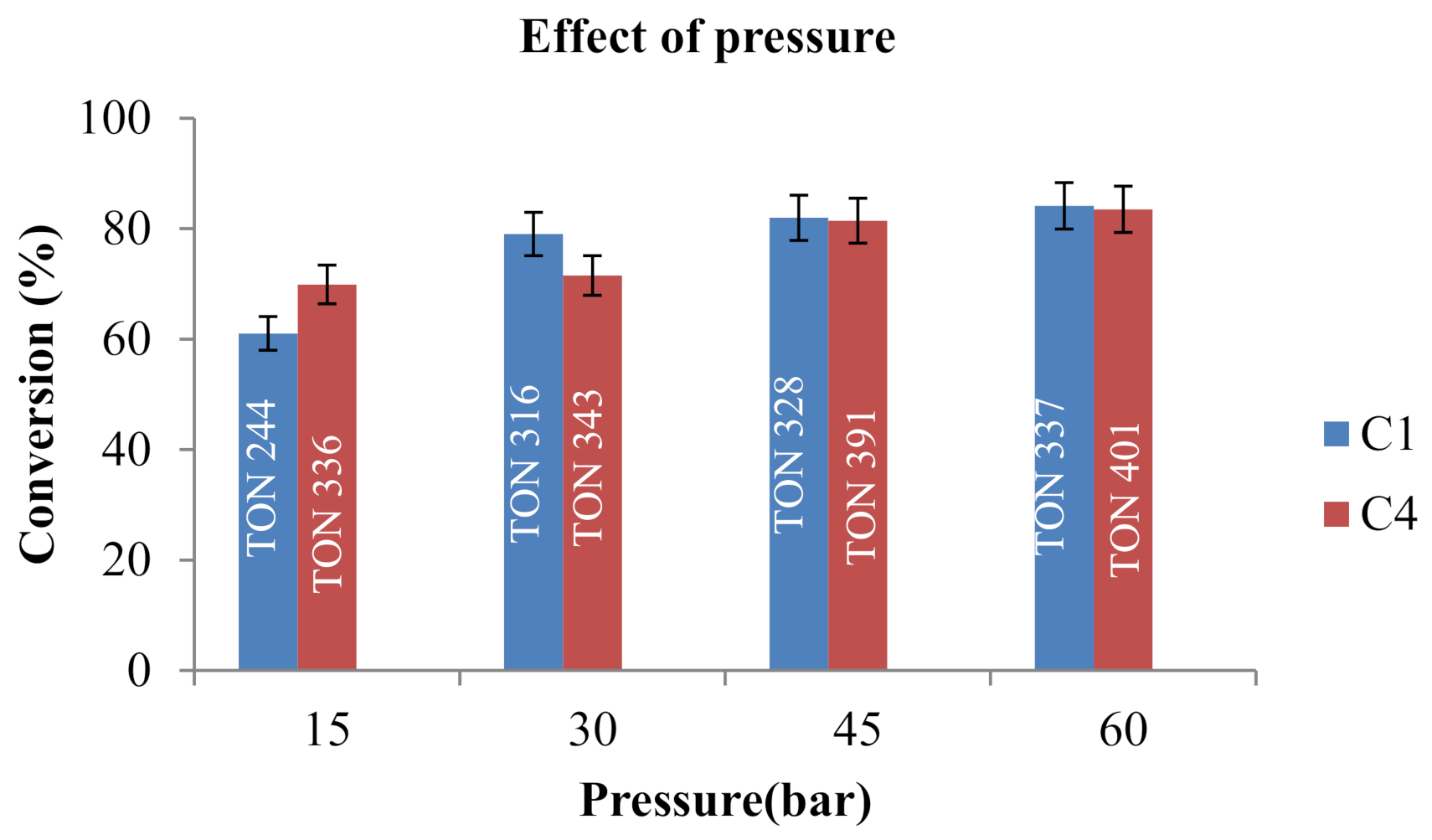
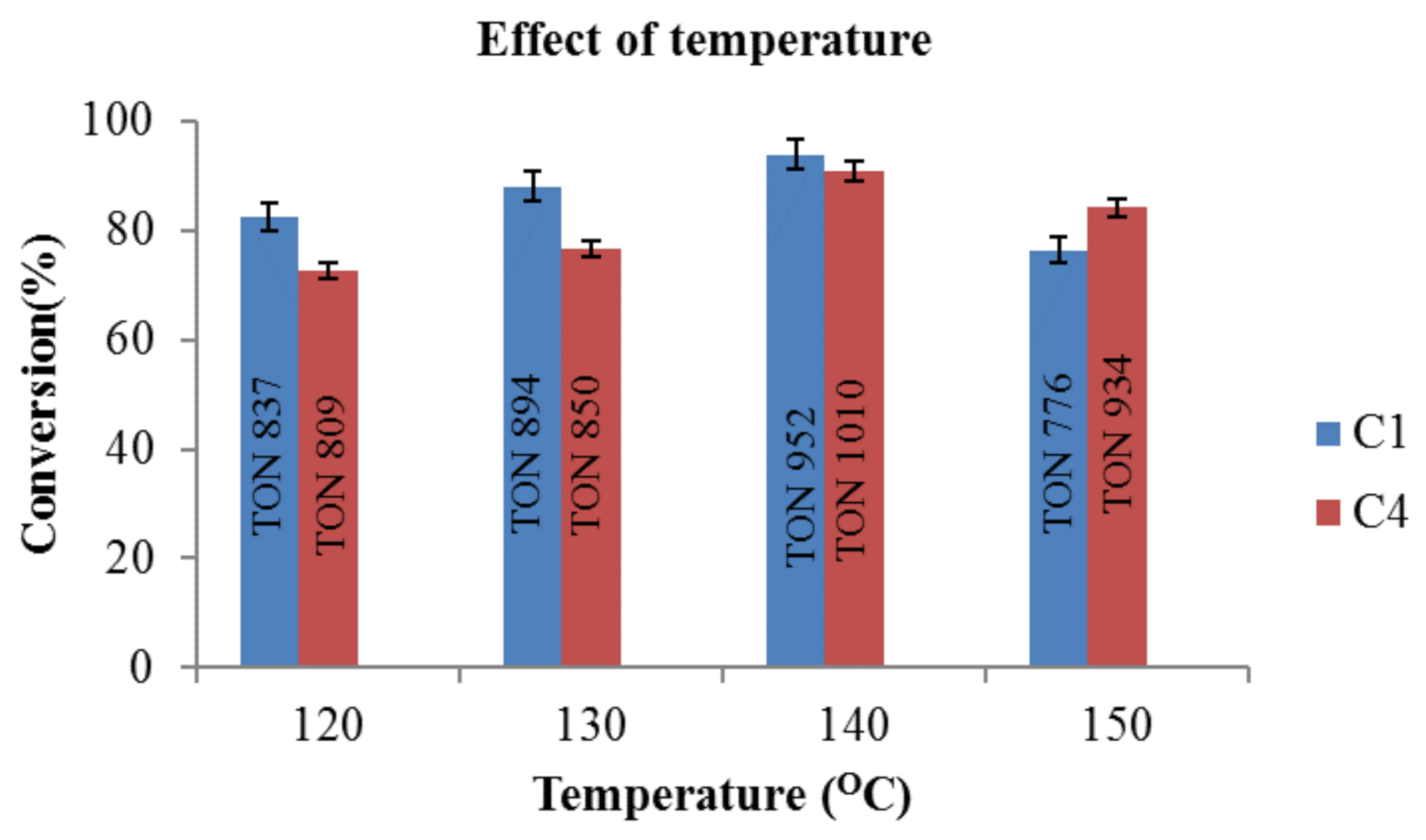
2.5. Optimization for Reduction of Furfural in Hydrogen Atmosphere
2.6. Hydrogenation of FF Using Different Metal Precursors
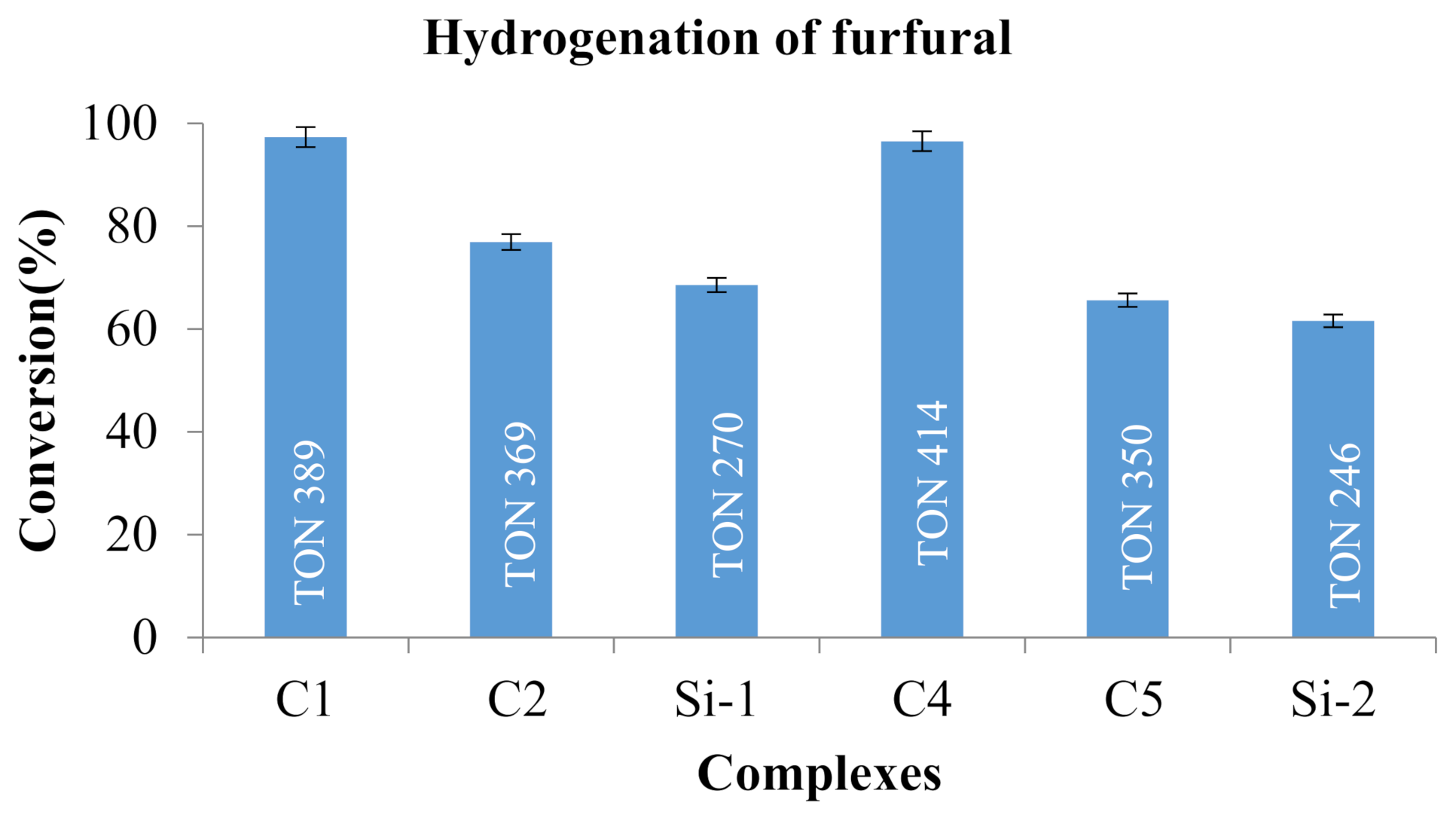
2.7. Catalyst Screening; Complexes C1, C2, Si-1, C4, C5 and Si-2
2.8. Homogeneity Test
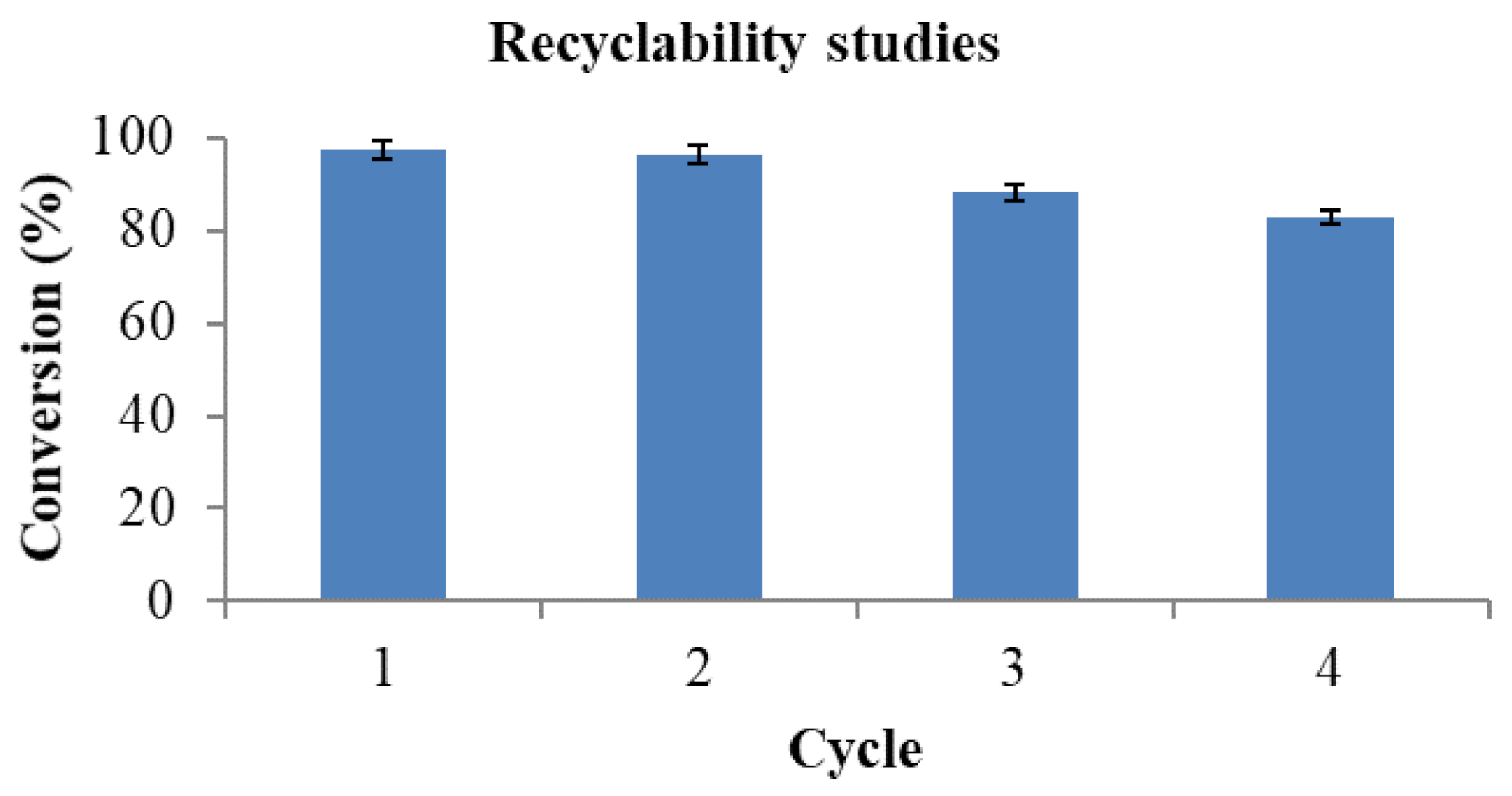
2.9. Recyclability Studies
2.10. Recyclability Studies of Supported Pre-Catalysts Si-1 and Si-2
2.11. Catalytic Transfer Hydrogenation of Furfural
2.12. Homogeneity Test
2.13. Recyclability Studies
2.14. Catalytic Transfer Hydrogenation of Other Substrates
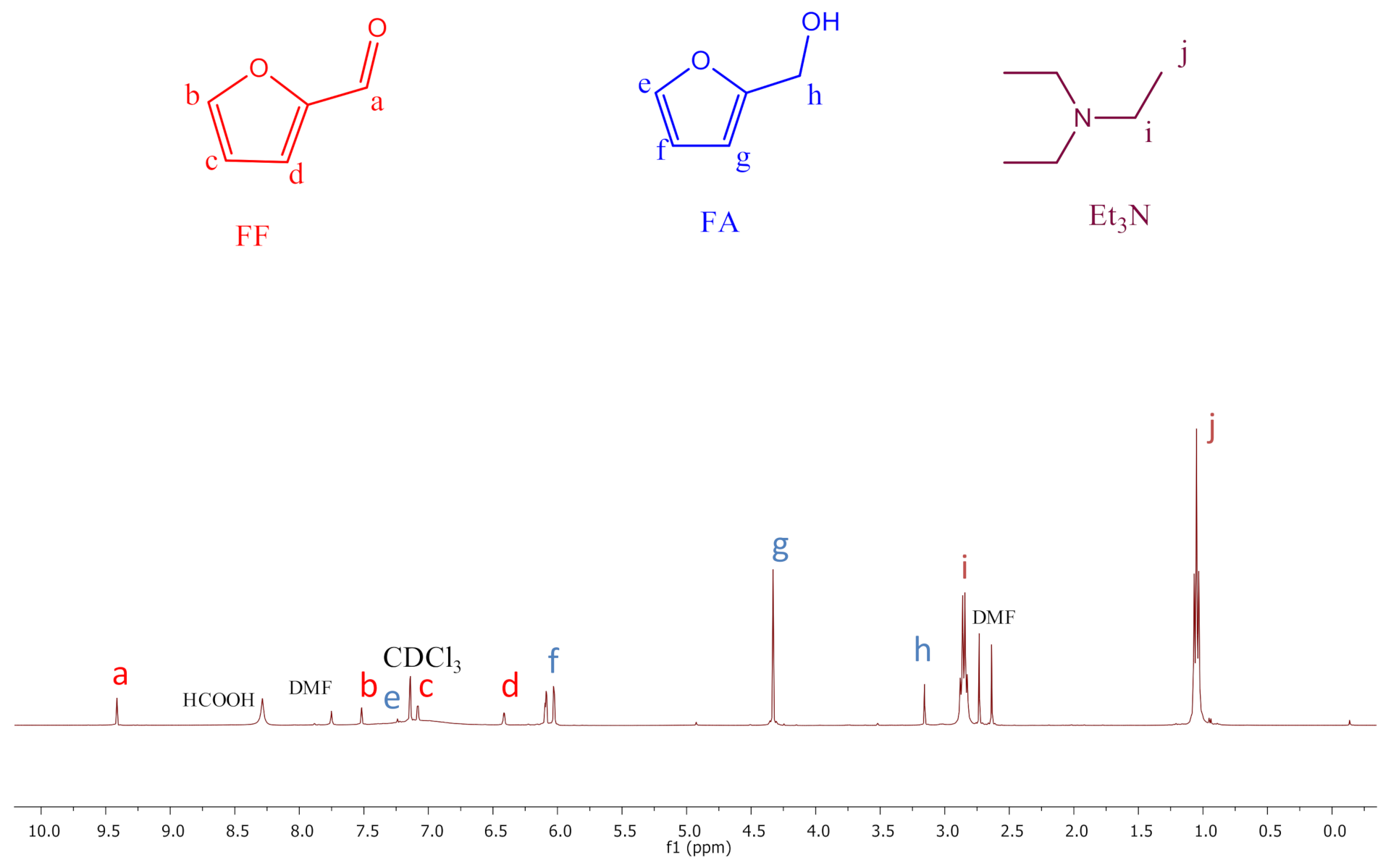
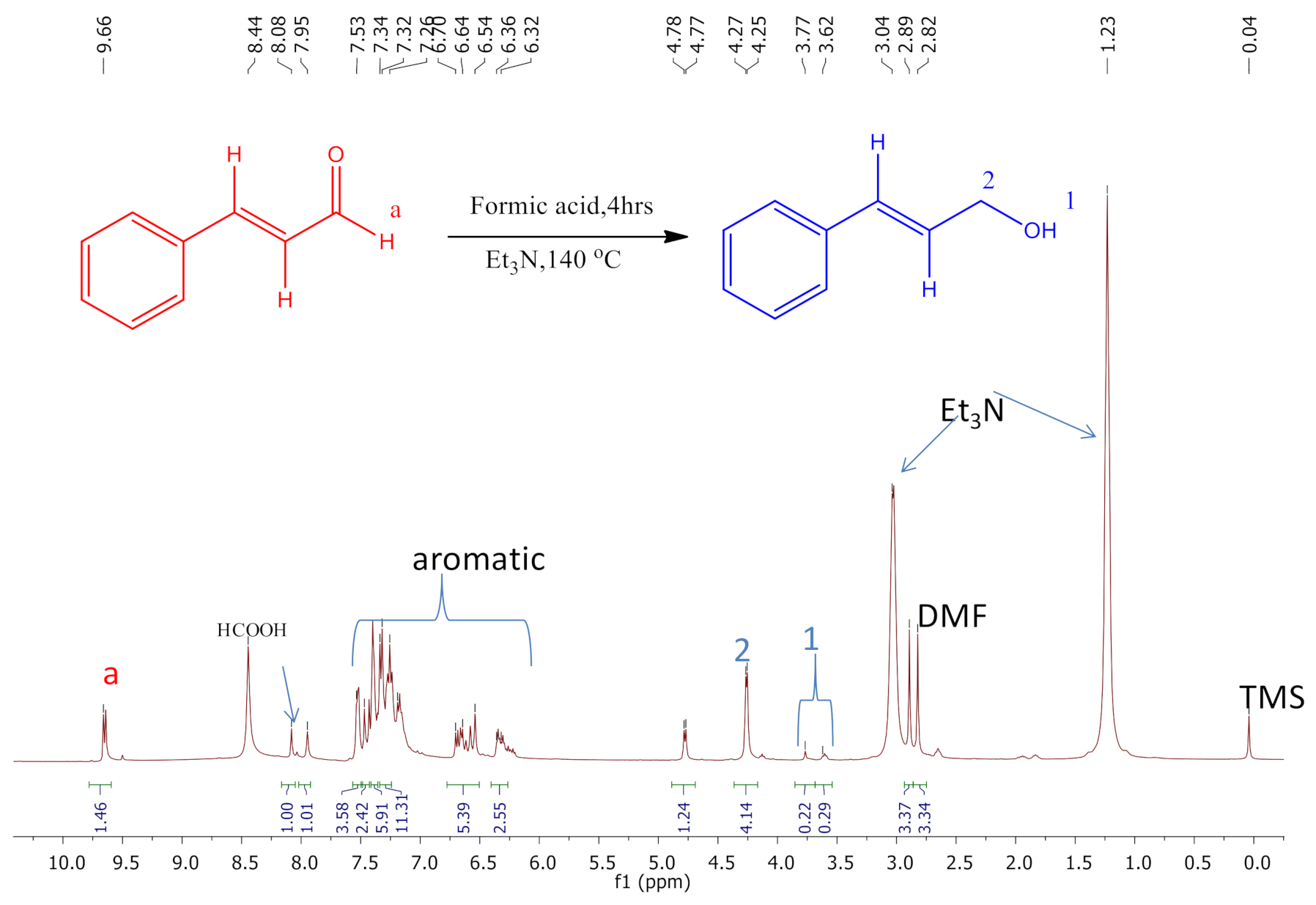
2.15. In Situ NMR Studies of the Catalytic Reactions
3. Materials and Methods
3.1. Synthesis and Characterization of L1
3.2. Synthesis and Characterization of L2
3.3. Synthesis of and Characterization of Complex C1
3.4. Synthesis and Characterization of Complexes C2 and Si-1
3.5. Synthesis and Characterization of Complex C4
3.6. Synthesis and Characterization of Complexes C5 and Si-2
3.7. Catalytic Tests
3.7.1. Hydrogenation of FF under Hydrogen Atmosphere
3.7.2. Transfer Hydrogenation Using a Hydrogen Carrier (Formic Acid)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Jong, W.; Marcotullio, G. Overview of Biorefineries based on Co-Production of Furfural, Existing Concepts and Novel Developments. Int. J. Chem. React. Eng. 2010, 8, 2–27. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural over Pt based and Pd based bimetallic catalysts supported on modified multiwalled carbon nanotubes (MWNT). Appl. Catal. A Gen. 2018, 550, 1–10. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516. [Google Scholar] [CrossRef]
- Liu, L.; Chang, H.; Jameel, H.; Park, S. Furfural production from biomass pretreatment hydrolysate using vapor-releasing reactor system. Bioresour. Technol. 2018, 252, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Christoph, R.; Schmidt, B.; Steinberner, U.; Dilla, W.; Karinen, R. Glycerol. Ullmann’s Encycl. Ind. Chem. 1998, 16, 67–82. [Google Scholar]
- de Jong, E.; Stichnothe, H.; Bell, G.; Jørgensen, H. IEA Bioenergy Task 42–Bio-Based Chemicals: A 2020 Update; IEA Bioenergy: Paris, France, 2020; pp. 110–123. [Google Scholar]
- Taylor, M.J.; Jiang, L.; Reichert, J.; Papageorgiou, A.C.; Beaumont, S.K.; Wilson, K.; Lee, A.F.; Barth, J.V.; Kyriakou, G. Catalytic Hydrogenation and Hydrodeoxygenation of Furfural over Pt(111): A Model System for the Rational Design and Operation of Practical Biomass Conversion Catalysts. J. Phys. Chem. C 2017, 121, 8490–8497. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Wang, Y.; Xu, S.; Zhang, Z.; Chen, Y.; Fan, J.; Yuan, H.; Gao, L.; Xiao, G. Efficient and Selective Ni/Al2O3–C Catalyst Derived from Metal–Organic Frameworks for the Hydrogenation of Furfural to Furfuryl Alcohol. Catal. Lett. 2019, 149, 2158–2168. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Niu, S.; Li, Y. A study of furfural decarbonylation on K-doped Pd/Al2O3 catalysts. J. Mol. Catal. A Chem. 2011, 335, 71–81. [Google Scholar] [CrossRef]
- Li, C.; Xu, G.; Liu, X.; Zhang, Y.; Fu, Y. Hydrogenation of Biomass-Derived Furfural to Tetrahydrofurfuryl Alcohol over Hydroxyapatite-Supported Pd Catalyst under Mild Conditions. Ind. Eng. Chem. Res. 2017, 56, 8843–8849. [Google Scholar] [CrossRef]
- Wang, S.; Vorotnikov, V.; Vlachos, D.G. Coverage-Induced Conformational Effects on Activity and Selectivity: Hydrogenation and Decarbonylation of Furfural on Pd(111). ACS Catal. 2015, 5, 104–112. [Google Scholar] [CrossRef]
- Moyo, P.S.; Matsinha, L.C.; Makhubela, B.C.E. Pd(II) and Pt(II) catalysed selective synthesis of furfuryl alcohol: Solvent effects and insights into the mechanism. J. Organomet. Chem. 2020, 922, 121362. [Google Scholar] [CrossRef]
- Guerrero-Torres, A.; Jiménez-Gómez, C.P.; Cecilia, J.A.; García-Sancho, C.; Franco, F.; Quirante-Sánchez, J.J.; Maireles-Torres, P. Ni supported on sepiolite catalysts for the hydrogenation of furfural to value-added chemicals: Influence of the synthesis method on the catalytic performance. Top. Catal. 2019, 62, 535–550. [Google Scholar] [CrossRef]
- MacIntosh, K.L.; Beaumont, S.K. Nickel-Catalysed Vapour-Phase Hydrogenation of Furfural, Insights into Reactivity and Deactivation. Top. Catal. 2020, 23, 122–151. [Google Scholar]
- Giorgianni, G.; Abate, S.; Centi, G.; Perathoner, S.; van Beuzekom, S.; Soo-Tang, S.H.; van der Waal, J.C. Effect of the Solvent in Enhancing the Selectivity to Furan Derivatives in the Catalytic Hydrogenation of Furfural. ACS Sustain. Chem. Eng. 2018, 6, 16235–16247. [Google Scholar] [CrossRef]
- Jia, P.; Lan, X.; Li, X.; Wang, T. Highly Active and Selective NiFe/SiO2 Bimetallic Catalyst with Optimized Solvent Effect for the Liquid-Phase Hydrogenation of Furfural to Furfuryl Alcohol. ACS Sustain. Chem. Eng. 2018, 6, 13287–13295. [Google Scholar] [CrossRef]
- Barnett, K.W. Nickel complexes with organic and phosphorus ligands: An integrated set of inorganic experiments. J. Chem. Educ. 1974, 51, 300–422. [Google Scholar] [CrossRef]
- Sobola, A.O.; Watkins, G.M. Antimicrobial activity and Cu(II) complexes of Schiff bases derived from ortho-aminophenol and salicylaldehyde derivatives. J. Chem. Pharm. Res. 2013, 5, 147–154. [Google Scholar]
- Kursunlu, A.N.; Guler, E.; Dumrul, H.; Kocyigit, O.; Gubbuk, I.H. Chemical modification of silica gel with synthesized new Schiff base derivatives and sorption studies of cobalt (II) and nickel (II). Appl. Surf. Sci. 2009, 255, 8798–8803. [Google Scholar] [CrossRef]
- Kianfar, A.H.; Farrokhpour, H.; Dehghani, P.; Khavasi, H.R. Experimental and theoretical spectroscopic study and structural determination of nickel(II) tridentate Schiff base complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 220–229. [Google Scholar] [CrossRef]
- Kianfar, A.H.; Ebrahimi, M. Synthesis, characterization and structural determination of some nickel(II) complexes containing imido Schiff bases and substituted phosphine ligands. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 725–729. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Shi, D.; Yang, Q.; Peterson, C.; Lamic-Humblot, A.-F.; Girardon, J.-S.; Griboval-Constant, A.; Stievano, L.; Sougrati, M.T.; Briois, V.; Bagot, P.A.J.; et al. Bimetallic Fe-Ni/SiO2 catalysts for furfural hydrogenation: Identification of the interplay between Fe and Ni during deposition-precipitation and thermal treatments. Catal. Today 2019, 334, 162–172. [Google Scholar] [CrossRef]
- Akinnawo, C.A.; Bingwa, N.; Meijboom, R. Surface properties vs activity of meso-ZrO2 catalyst in chemoselective Meerwein-Ponndorf-Verley reduction of citral: Effect of calcination temperature. Microporous Mesoporous Mater. 2021, 311, 110693–110721. [Google Scholar] [CrossRef]
- Dias, J.A.; Caliman, E.; Dias, S.C.L.; Paulo, M.; de Souza, A.T.C. Preparation and characterization of supported H3PW12O40 on silica gel: A potential catalyst for green chemistry processes. Catal. Today 2003, 85, 39–48. [Google Scholar] [CrossRef]
- Park, Y.; Kang, T.; Cho, Y.; Kim, P.; Park, J.; Yi, J. Finely-dispersed Ni/Cu catalysts supported on mesoporous silica for the hydrodechlorination of chlorinated hydrocarbons. Stud. Surf. Sci. Catal. 2003, 146, 637–640. [Google Scholar]
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural to tetrahydrofurfuryl alcohol over Ni/CNTs and bimetallic Cu-Ni/CNTs catalysts. Int. J. Hydrog. Energy 2016, 41, 14721–14731. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Kim, K.; Ahn, W.-S. Catalytic transfer hydrogenation of bio-based furfural by palladium supported on nitrogen-doped porous carbon. Catal. Today 2019, 324, 49–58. [Google Scholar] [CrossRef]
- Crabtree, R.H. Resolving Heterogeneity Problems and Impurity Artifacts in Operationally Homogeneous Transition Metal Catalysts. Chem. Rev. 2012, 112, 1536–1554. [Google Scholar] [CrossRef]
- Villaverde, M.M.; Garetto, T.F.; Marchi, A.J. Liquid-phase transfer hydrogenation of furfural to furfuryl alcohol on Cu–Mg–Al catalysts. Catal. Commun. 2015, 58, 6–10. [Google Scholar] [CrossRef]
- Santos, F.; Tafur, J.P.; Abad, J.; Romero, A.J.F. Structural modifications and ionic transport of PVA-KOH hydrogels applied in Zn/Air batteries. J. Electroanal. Chem. 2019, 850, 113380–114392. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Lin, W.; Song, W.; Li, S. High efficient conversion of furfural to 2-methylfuran over Ni-Cu/Al2O3 catalyst with formic acid as a hydrogen donor. Appl. Catal. A Gen. 2017, 547, 248–255. [Google Scholar] [CrossRef]


| Entry | Catalyst Precursor | Solvent | Conv. (%) | TON | TOF | FA Sel. (%) |
|---|---|---|---|---|---|---|
| 1 | C1/C4 | Water | 0 | - | - | - |
| 2 | C1/C4 | Toluene | 0 | - | - | - |
| 3 | C1 | Ethanol | 97.31 | 468 | 19 | 100 |
| 4 | C4 | Ethanol | 96.51 | 386 | 18 | 100 |
| 5 | C1 | Isopropanol | 75.24 | 301 | 13 | 100 |
| 6 | C4 | Isopropanol | 74.2 | 297 | 12 | 100 |
| Entry | Nickel Catalyst | Conv. (%) | TON | TOF |
|---|---|---|---|---|
| 1 | NiCl2·6H2O | 26.07 | 52 | 2 |
| 2 | Ni(OAc)2·4H2O | 38.49 | 77 | 3 |
| 3 | NiCl2(PPh3)2 | 71.51 | 74 | 3 |
| 4 | 2[PPh2ClNHSiO3]Ni | 67.48 | 270 | 11 |
| Entry | Complex | Substrate | Conv. (%) | TON | TOF | Selectivity |
|---|---|---|---|---|---|---|
| 1 | C1 | cinnamaldehyde | 85.46 | 1709 | 427 | Cinnamyl alcohol |
| 2 | C4 | cinnamaldehyde | 79.52 | 1590 | 398 | Cinnamyl alcohol |
| 3 | C1 | 2-thiophenecarboxaldehyde | 68.59 | 1371 | 343 | 2-thiophenemethanol |
| 4 | C4 | 2-thiophenecarboxaldehyde | 60.22 | 1204 | 301 | 2-thiophenemethanol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalumpha, M.; Matsinha, L.C.; Makhubela, B.C.E. Synthesis and Characterization of Nickel(II) Homogeneous and Supported Complexes for the Hydrogenation of Furfural to Furfuryl Alcohol. Catalysts 2021, 11, 684. https://doi.org/10.3390/catal11060684
Kalumpha M, Matsinha LC, Makhubela BCE. Synthesis and Characterization of Nickel(II) Homogeneous and Supported Complexes for the Hydrogenation of Furfural to Furfuryl Alcohol. Catalysts. 2021; 11(6):684. https://doi.org/10.3390/catal11060684
Chicago/Turabian StyleKalumpha, Menala, Leah Charlie Matsinha, and Banothile C. E. Makhubela. 2021. "Synthesis and Characterization of Nickel(II) Homogeneous and Supported Complexes for the Hydrogenation of Furfural to Furfuryl Alcohol" Catalysts 11, no. 6: 684. https://doi.org/10.3390/catal11060684
APA StyleKalumpha, M., Matsinha, L. C., & Makhubela, B. C. E. (2021). Synthesis and Characterization of Nickel(II) Homogeneous and Supported Complexes for the Hydrogenation of Furfural to Furfuryl Alcohol. Catalysts, 11(6), 684. https://doi.org/10.3390/catal11060684






