Towards Computer-Aided Graphene Covered TiO2-Cu/(CuxOy) Composite Design for the Purpose of Photoinduced Hydrogen Evolution
Abstract
:1. Introduction
2. Results
2.1. Morphology and Raman Spectra
2.2. XRD
2.3. XPS
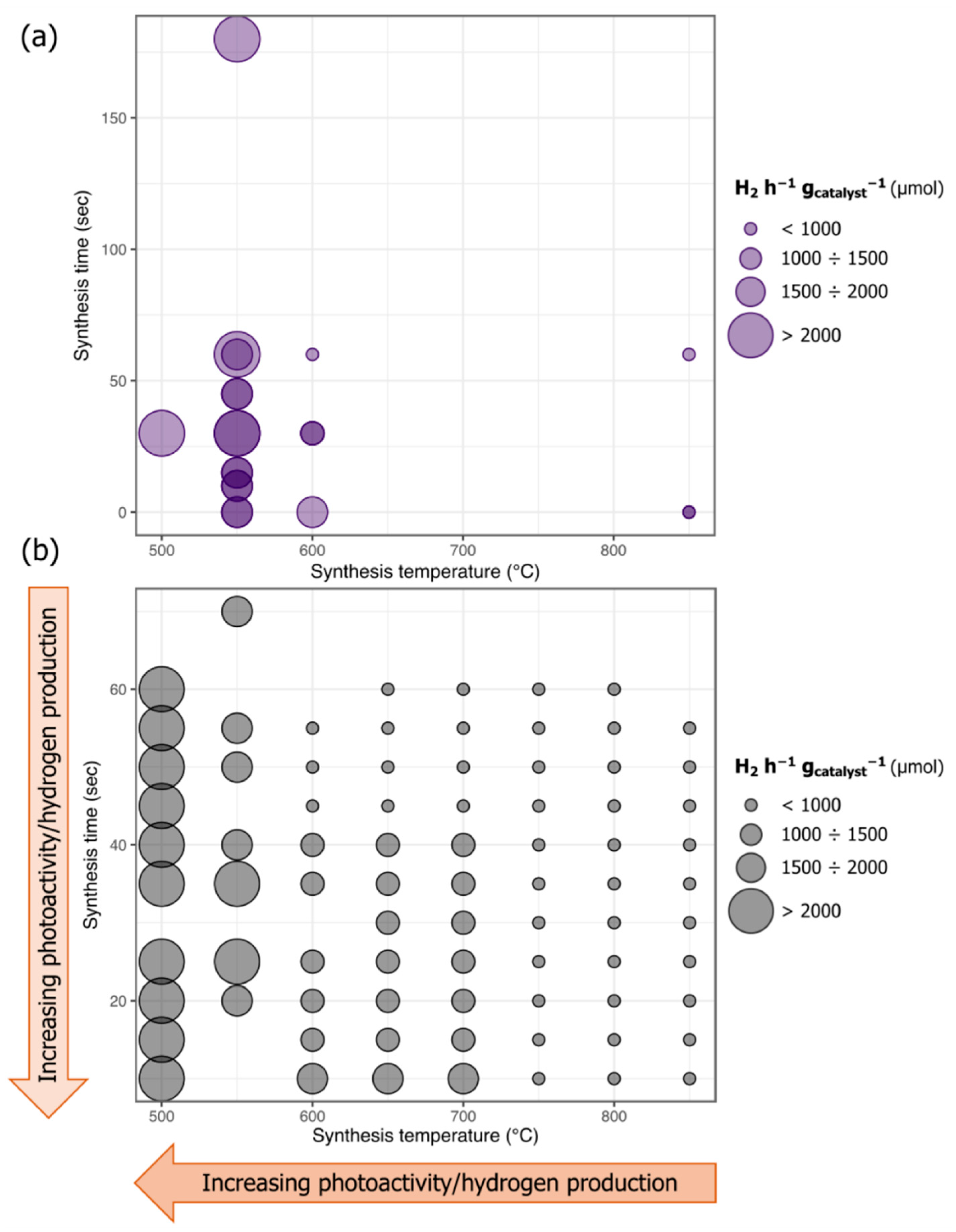
2.4. Hydrogen Evolution Results
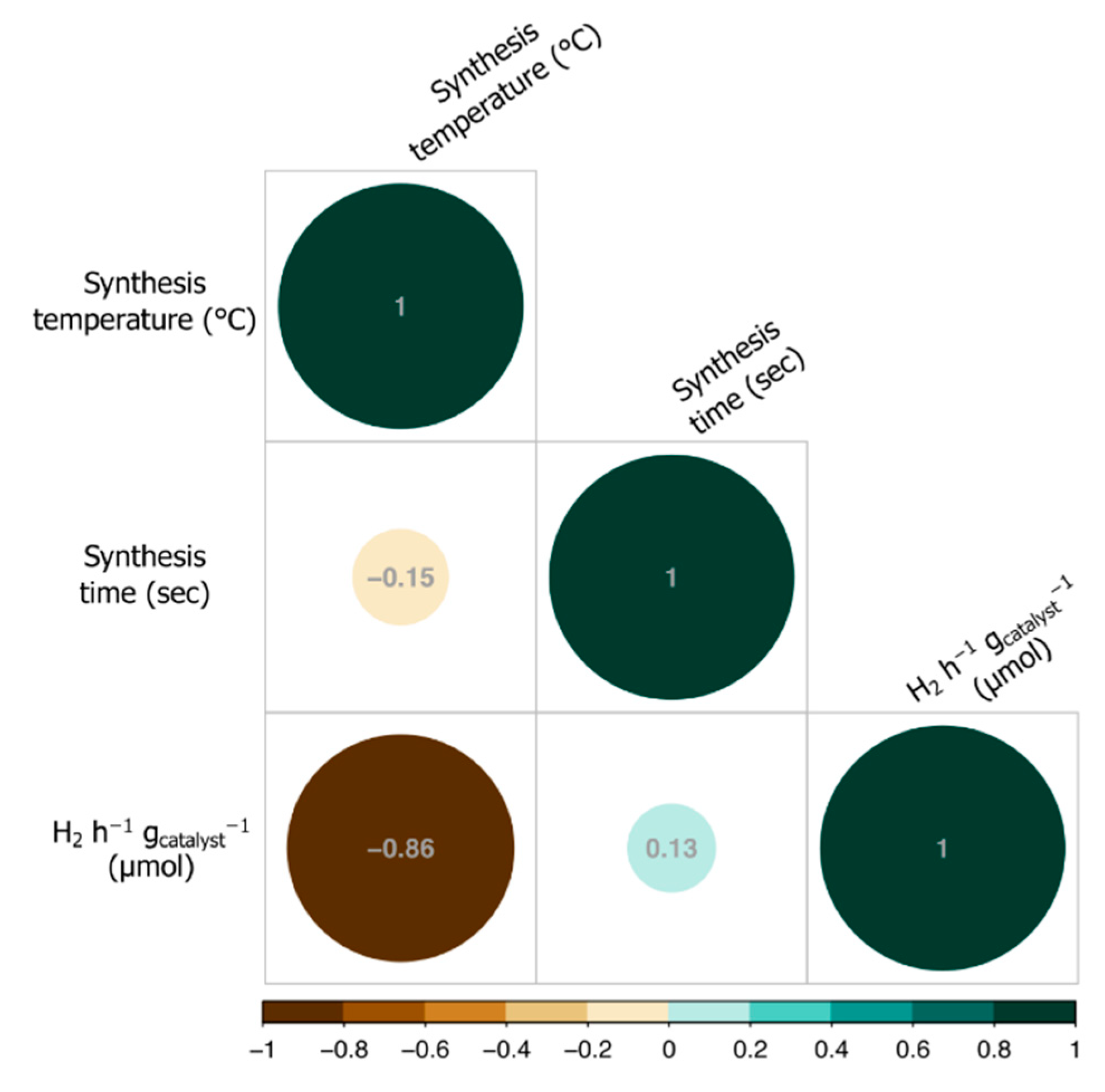
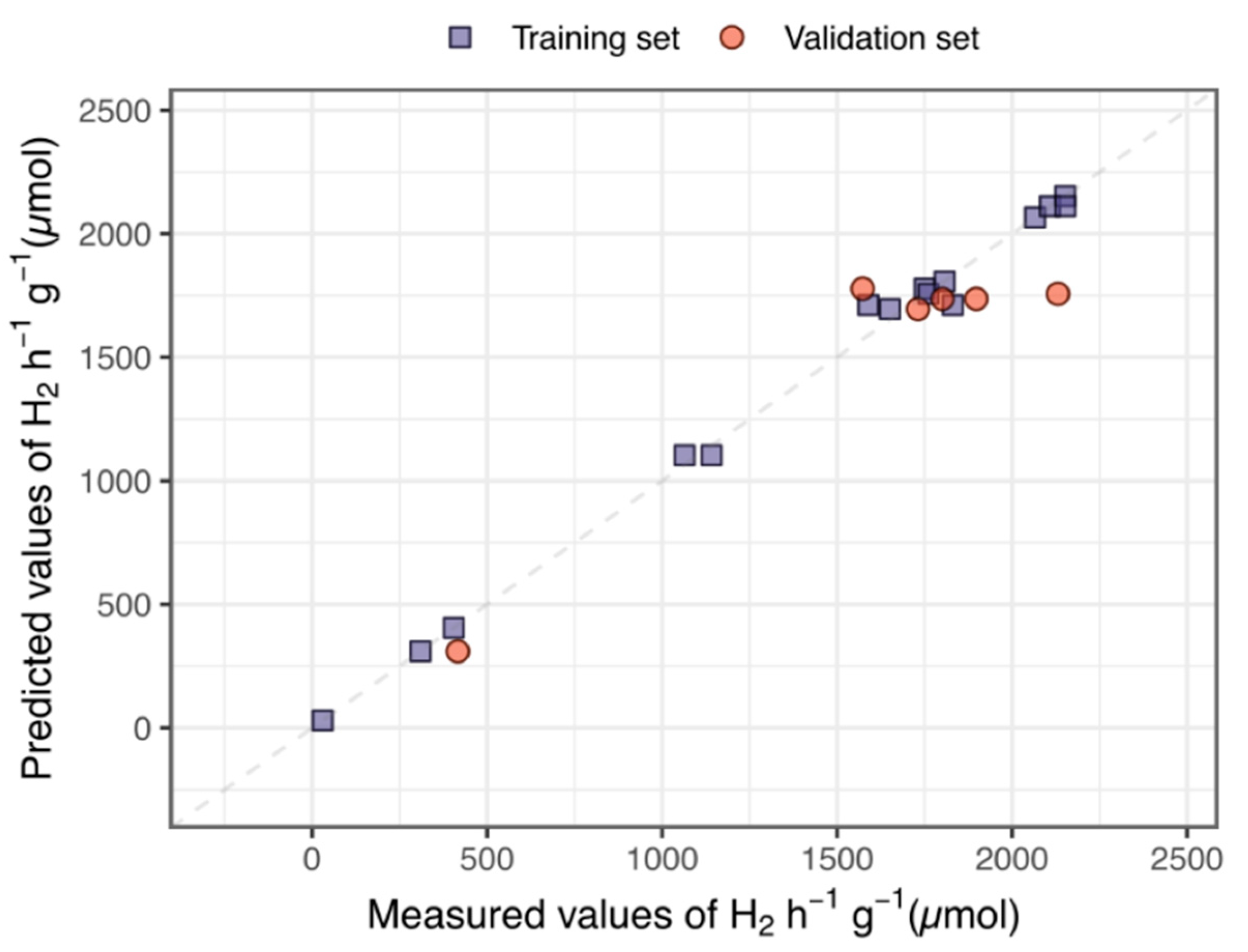
2.5. Computer-Aided Modelling
3. Discussion and Proposed Mechanism
4. Materials and Methods
4.1. Chemicals
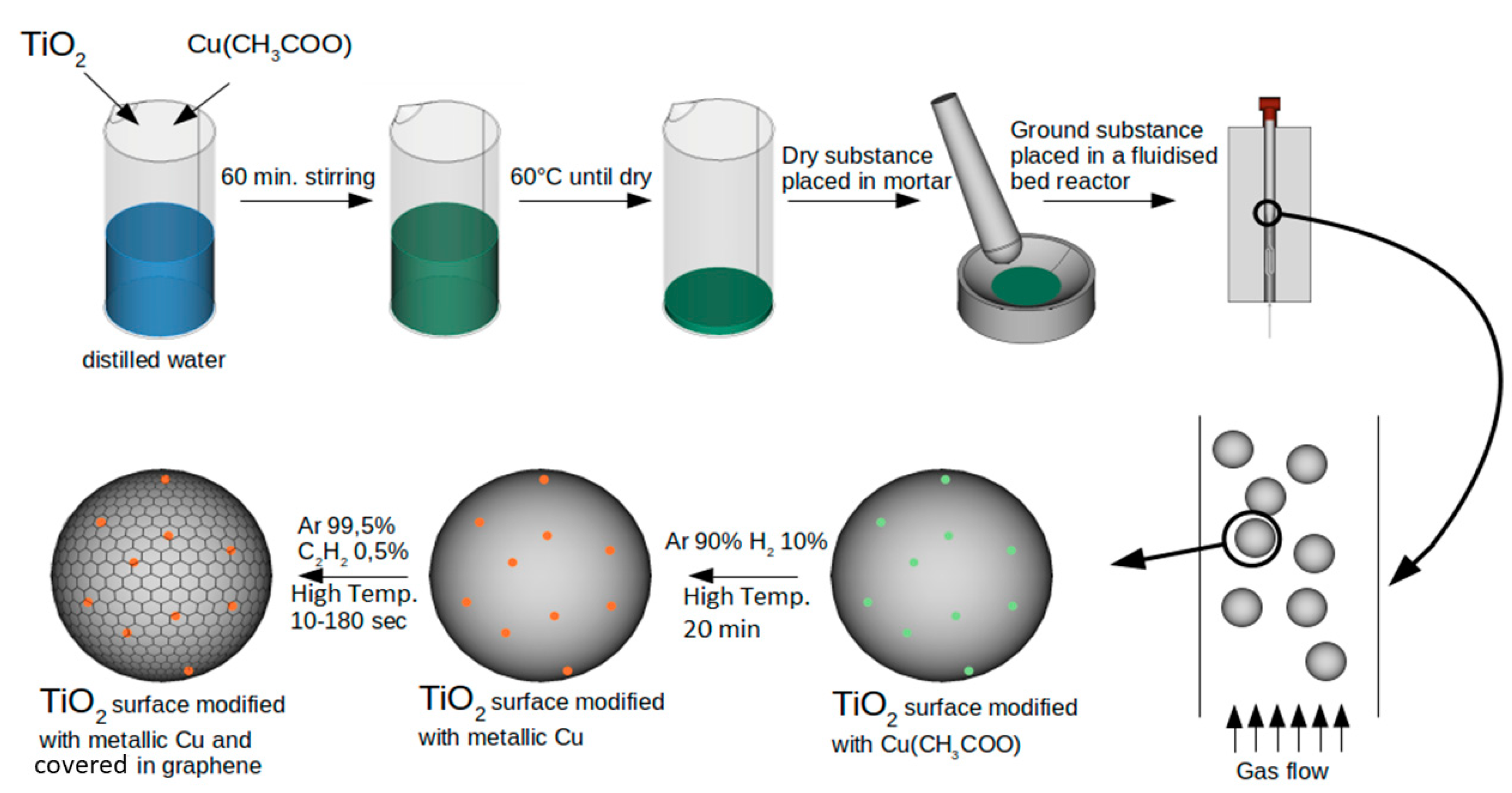
4.2. Photocatalyst Synthesis
4.2.1. Apparatus
4.2.2. Base Material Preparation
4.2.3. Modification of TiO2 with Metallic Copper
4.2.4. Graphene Cover Synthesis
4.3. Characterization
4.4. Photocatalytic Activity Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, S.; Hao, L.; Yoshida, H.; Itoi, T.; Cheng, Y.; Seki, S.; Nishina, Y.; Lu, Y. Enhanced photocatalytic activity and stability of TiO2/graphene oxide composites coatings by electrophoresis deposition. Mater. Lett. 2021, 286, 129258. [Google Scholar] [CrossRef]
- Park, S.; Dikin, D.A.; Nguyen, S.T.; Ruoff, R.S. Graphene Oxide Sheets Chemically Cross-Linked by Polyallylamine. J. Phys. Chem. C 2009, 113, 15801–15804. [Google Scholar] [CrossRef]
- Li, D.; Sun, J.; Shen, T.; Song, H.; Liu, J.; Wang, C.; Wang, X.; Zhao, R. Influence of morphology and interfacial interaction of TiO2-Graphene nanocomposites on the visible light photocatalytic performance. J. Solid State Chem. 2020, 286, 121301. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Zhu, W.; Zhang, L.; Zhao, B.; Ji, Y.; Li, G.; An, T. Superoxide radical enhanced photocatalytic performance of styrene alters its degradation mechanism and intermediate health risk on TiO2/graphene surface. Environ. Res. 2021, 195, 110747. [Google Scholar] [CrossRef] [PubMed]
- Tismanar, I.; Obreja, A.C.; Buiu, O.; Duta, A. VIS-active TiO2-graphene oxide composite thin films for photocatalytic applications. Appl. Surf. Sci. 2021, 538, 147833. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Li, S.; Liu, Y. Enhanced photocatalytic degradation of organic dyes by ultrasonic-assisted electrospray TiO2/graphene oxide on polyacrylonitrile/β-cyclodextrin nanofibrous membranes. Ultrason. Sonochemistry 2021, 70, 105343. [Google Scholar] [CrossRef] [PubMed]
- Ayoubi-Feiz, B.; Mashhadizadeh, M.H.; Sheydaei, M. Degradation of diazinon by new hybrid nanocomposites N-TiO2/Graphene/Au and N-TiO2/Graphene/Ag using visible light photo-electro catalysis and photo-electro catalytic ozonation: Optimization and comparative study by Taguchi method. Sep. Purif. Technol. 2019, 211, 704–714. [Google Scholar] [CrossRef]
- Ghasemi, S.; Esfandiar, A.; Setayesh, S.R.; Habibi-Yangjeh, A.; Gholami, M.R. Synthesis and characterization of TiO2–graphene nanocomposites modified with noble metals as a photocatalyst for degradation of pollutants. Appl. Catal. A Gen. 2013, 462, 82–90. [Google Scholar] [CrossRef]
- Ghasemi, S.; Hashemian, S.J.; Alamolhoda, A.A.; Gocheva, I.; Setayesh, S.R. Plasmon enhanced photocatalytic activity of Au@ TiO2-graphene nanocomposite under visible light for degradation of pollutants. Mater. Res. Bull. 2017, 87, 40–47. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Chen, W.J.; Wu, C.T. Pt-TiO2/graphene photocatalysts for degradation of AO7 dye under visible light. Appl. Surf. Sci. 2015, 340, 9–17. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M. Preparation and characterization of Pt, N-TiO2-graphene nanocomposites for hydrogen production. Ceram. Int. 2019, 45, 6058–6065. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, E.; Dai, H.; Kang, L.; Wu, H.; Fan, J.; Hu, X.; Liu, H. Photocatalytic activity of Ag–TiO2-graphene ternary nanocomposites and application in hydrogen evolution by water splitting. Int. J. Hydrogen Energy 2014, 39, 7664–7671. [Google Scholar] [CrossRef]
- Khalid, N.R.; Ahmed, E.; Hong, Z.; Ahmad, M.; Zhang, Y.; Khalid, S. Cu-doped TiO2 nanoparticles/graphene composites for efficient visible-light photocatalysis. Ceram. Int. 2013, 39, 7107–7113. [Google Scholar] [CrossRef]
- Lv, X.-J.; Zhou, S.-X.; Zhang, C.; Chang, H.-X.; Chen, Y.; Fu, W.-F. Synergetic effect of Cu and graphene as cocatalyst on TiO2 for enhanced photocatalytic hydrogen evolution from solar water splitting. J. Mater. Chem. 2012, 22, 18542–18549. [Google Scholar] [CrossRef]
- Jo, W.-K.; Jin, Y.-J. 2D graphene-assisted low-cost metal (Ag, Cu, Fe, or Ni)-doped TiO2 nanowire architectures for enhanced hydrogen generation. J. Alloy. Compd. 2018, 765, 106–112. [Google Scholar] [CrossRef]
- Fan, W.; Yu, X.; Lu, H.-C.; Bai, H.; Zhang, C.; Shi, W. Fabrication of TiO2/RGO/Cu2O heterostructure for photoelectrochemical hydrogen production. Appl. Catal. B Environ. 2015, 7, 32. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Lajaunie, L.; Dvoranová, D.; Brezová, V.; Figueiredo, B.; Seabra, M.P.; Calvino, J.J.; Labrincha, J.A. Cooperative and fully reversible color switching activation in hybrid graphene decorated nanocages and copper-TiO2 nanoparticles. Mater. Today Energy 2020, 17, 100460. [Google Scholar] [CrossRef]
- Lewandowski, Ł.; Zaleska-Medynska, A. Method for Production of Carbon Layers Containing Graphene on Nanoparticles, Especially Quantum Dots, Containing at Least One Semiconductor Material, and a Reaction Vessel for Production of Carbon Layers Containing Graphene on Nanoparticles, Especially Quantum Dots, Containing at Least One Semiconductor Material. Patent Application P.430994, 29 August 2019. (In Polish). [Google Scholar]
- Wang, B.; Sun, Q.; Liu, S.; Li, Y. Synergetic catalysis of CuO and graphene additives on TiO2 for photocatalytic water splitting. Int. J. Hydrogen Energy 2013, 38, 7232–7240. [Google Scholar] [CrossRef]
- Schuepfer, D.B.; Badaczewski, F.; Guerra-Castro, J.M.; Hofmann, D.M.; Heiliger, C.; Smarsly, B.; Klar, P.J. Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon 2020, 161, 359–372. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.-X.; Wang, J.; Lim, A.; Wang, S.; Loh, K.P. One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano 2009, 3, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cui, J.; Tao, C.A.; Wu, Y.; Li, Z.; Ma, L.; Wen, Y.; Li, G. A strategy for producing pure single-layer graphene sheets based on a confined self-assembly approach. Angew. Chem. 2009, 121, 5978–5982. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, Q.; Tang, L.A.L.; Zhong, Y.; Loh, K.P. Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem. Mater. 2009, 21, 2950–2956. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman spectrum with the chemical composition of graphene oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Cuong, T.V.; Pham, V.H.; Tran, Q.T.; Hahn, S.H.; Chung, J.S.; Shin, E.W.; Kim, E.J. Photoluminescence and Raman studies of graphene thin films prepared by reduction of graphene oxide. Mater. Lett. 2010, 64, 399–401. [Google Scholar] [CrossRef]
- Admanabhan, N.T.; Ganguly, P.; Pillai, S.C.; John, H. Morphology engineered spatial charge separation in superhydrophilic TiO2/graphene hybrids for hydrogen production. Mater. Today Energy 2020, 17, 100447. [Google Scholar] [CrossRef]
- Muthirulan, P.; Devi, C.N.; Sundaram, M.M. TiO2 wrapped graphene as a high performance photocatalyst for acid orange 7 dye degradation under solar/UV light irradiations. Ceram. Int. 2014, 40, 5945–5957. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Kočí, K.; Edelmannová, M.; Lajaunie, L.; Figueiredo, B.; Calvino, J.J.; Seabra, M.P.; Labrincha, J.A. CuxO and carbon–modified TiO2–based hybrid materials for photocatalytically assisted H2 generation. Mater. Today Energy 2021, 19, 100607. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corp. 1992, 40, 221. [Google Scholar]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Rudd, J.A.; Jones, D.R.; Dunnill, C.W.; Andreoli, E. Study of copper (II) oxide and copper (II) acetate on multiwalled carbon nanotubes by XPS. Surf. Sci. Spectra 2019, 26, 014013. [Google Scholar] [CrossRef]
- Terzyk, A.P. The influence of activated carbon surface chemical composition on the adsorption of acetaminophen (paracetamol) in vitro: Part II. TG, FTIR, and XPS analysis of carbons and the temperature dependence of adsorption kinetics at the neutral pH. Colloids Surf. A Physicochem. Eng. Asp. 2001, 177, 23–45. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, W. Design of copper and titanium dioxide nanoparticles doped with reduced graphene oxide for hydrogen evolution by water splitting. Russ. J. Phys. Chem. A 2018, 92, 968–975. [Google Scholar] [CrossRef]
- Loader, C. Local Regression and Likelihoo; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- De Brabanter, K.; De Brabanter, J.; De Moor, B.; Gijbels, I. Derivative Estimation with Local Polynomial Fitting. J. Mach. Learn. Res. 2013, 14, 281–301. [Google Scholar]
- Fan, J.; Gijbels, I. Local Polynomial Modelling and Its Applications: Monographs on Statistics and Applied Probability 66; CRC Press: Boca Raton, FL, USA, 1996; Volume 66. [Google Scholar]
- Gajewicz-Skretna, A.; Kar, S.; Piotrowska, M.; Leszczynski, J. The kernel-weighted local polynomial regression (KwLPR) approach: An efficient, novel tool for development of QSAR/QSAAR toxicity extrapolation models. J. Cheminformatics 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Gramatica, P. Principles of QSAR models validation: Internal and external. QSAR Comb. Sci. 2007, 26, 694–701. [Google Scholar] [CrossRef]
- Tropsha, A. Best practices for QSAR model development, validation, and exploitation. Mol. Inform. 2010, 29, 476–488. [Google Scholar] [CrossRef]
- Chirico, N.; Gramatica, P. Real external predictivity of QSAR models: How to evaluate it? Comparison of different validation criteria and proposal of using the concordance correlation coefficient. J. Chem. Inf. Modeling 2011, 51, 2320–2335. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Das, R.N.; Ambure, P.; Aher, R.B. Be aware of error measures. Further studies on validation of predictive QSAR models. Chemom. Intell. Lab. Syst. 2016, 152, 18–33. [Google Scholar] [CrossRef]
- Lignier, P.; Bellabarba, R.; Tooze, R.P. Scalable strategies for the synthesis of well-defined copper metal and oxide nanocrystals. Chem. Soc. Rev. 2012, 41, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Huang, J.; Li, G.; Chen, J.; Zhang, Z.; Deng, Z.; Jiang, Y.; Guo, W.; Cao, Y. Effective water splitting using CuOx/TiO2 composite films: Role of Cu species and content in hydrogen generation. Appl. Surf. Sci. 2016, 369, 201–206. [Google Scholar] [CrossRef]


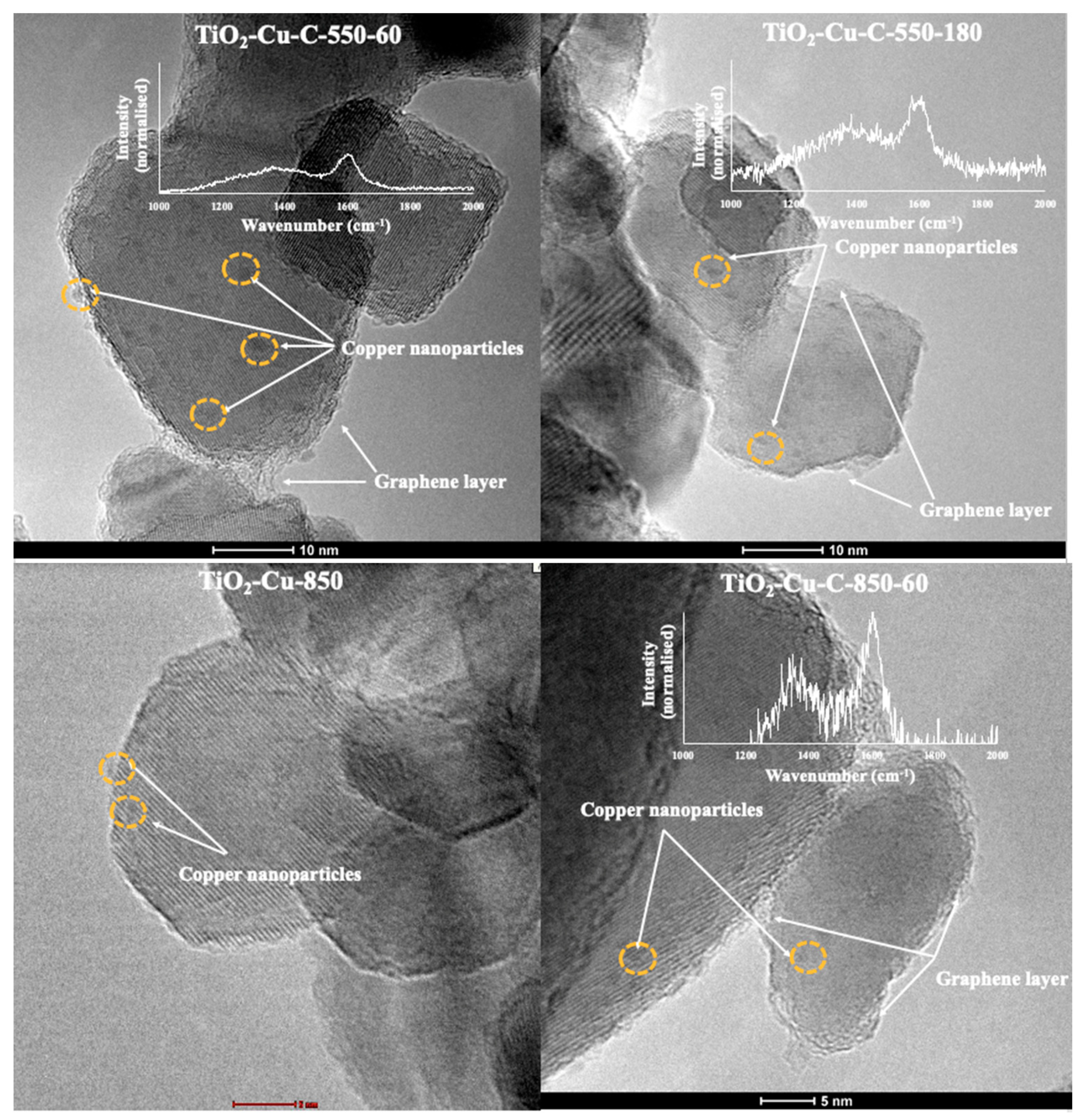
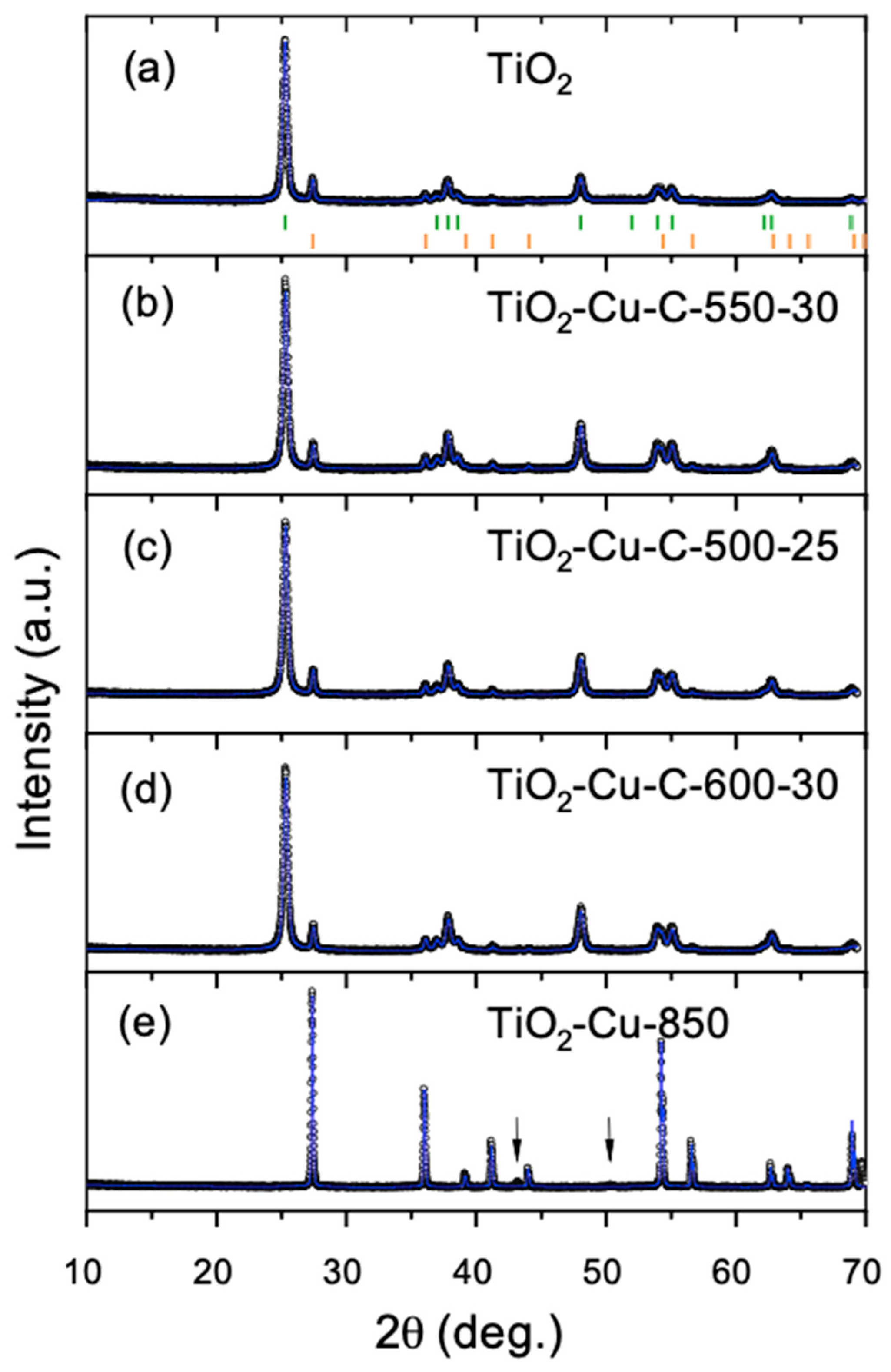
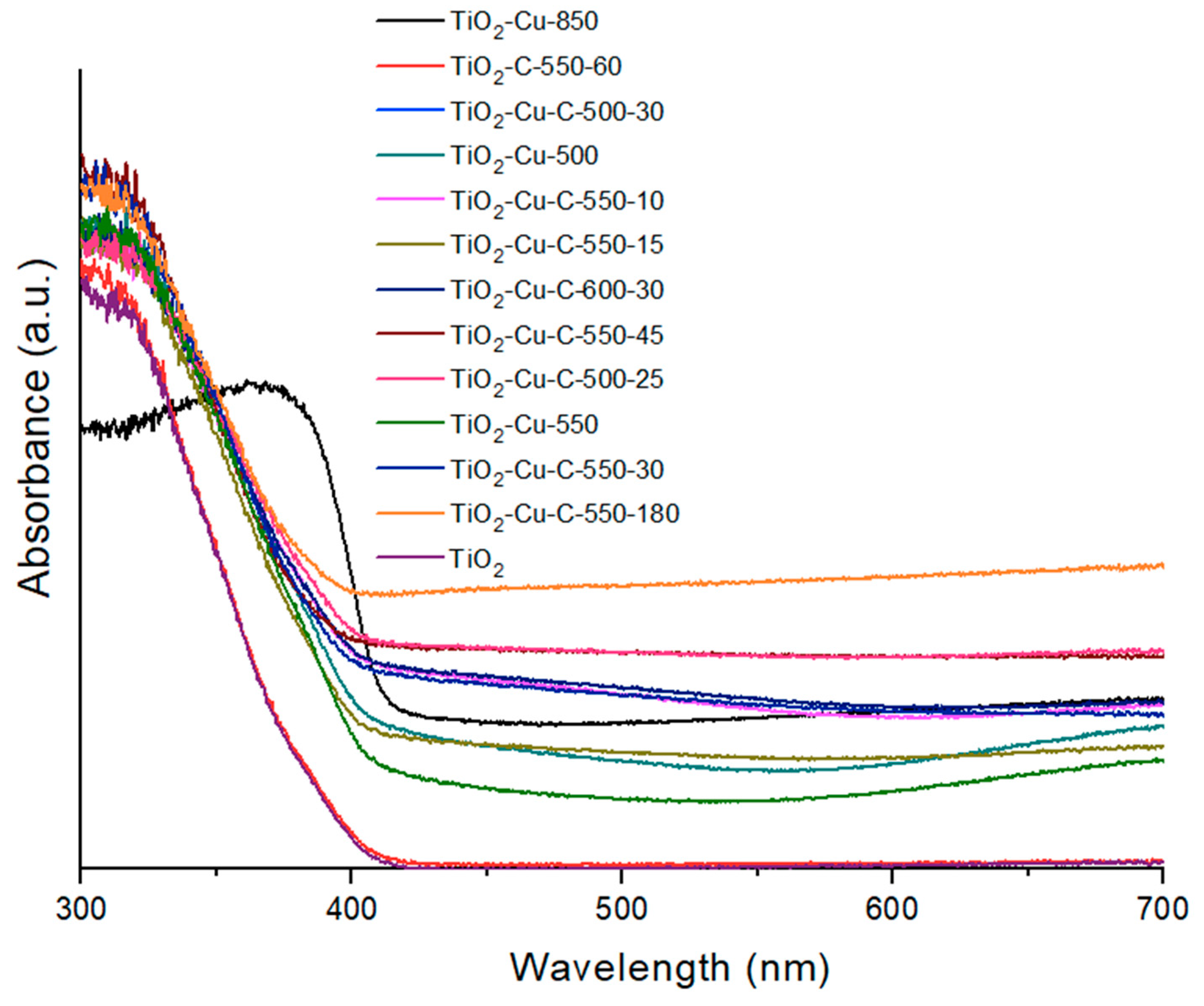



| Sample ID | Graphene Synthesis Time (s) | Graphene Synthesis Temperature (°C) | G/D Band Ratio | Size of Cu Nanoparticles * (nm) | Thickness of Graphene Layer ** (nm) |
|---|---|---|---|---|---|
| TiO2-Cu-550 | 0 | 550 | - | 1.7 | - |
| TiO2-Cu-850 | 0 | 850 | - | 1.6 | - |
| TiO2-Cu-C-550-10 | 10 | 550 | 1.42 | 1.5 | 1.2 |
| TiO2-Cu-C-550-15 | 15 | 550 | 1.44 | 1.7 | 1.4 |
| TiO2-Cu-C-550-30 | 30 | 550 | 1.77 | 1.6 | 1.8 |
| TiO2-Cu-C-550-60 | 60 | 550 | 0.6 | 2.1 | 1.1 |
| TiO2-Cu-C-550-180 | 180 | 550 | 1.35 | 1.3 | 1.4 |
| TiO2-Cu-C-850-60 | 60 | 850 | 1.41 | 1.5 | 1.5 |
| Sample ID | Anatase | Rutile | ||||
|---|---|---|---|---|---|---|
| a | c | d | a | c | ||
| TiO2 | 3.7841(2) | 9.5036(7) | 190 | 4.5925(5) | 2.9565(5) | 20% |
| TiO2-Cu-C-550-30 | 3.7864(3) | 9.5077(9) | 190 | 4.5964(5) | 2.9587(4) | 17% |
| TiO2-Cu-C-500-25 | 3.7858(3) | 9.5065(7) | 200 | 4.5955(4) | 2.9582(3) | 18% |
| TiO2-Cu-C-600-30 | 3.7859(3) | 9.5058(8) | 200 | 4.5948(6) | 2.9587(3) | 15% |
| TiO2-Cu-850 | - | - | - | 4.5924(1) | 2.9598(1) | 100% |
| Sample Label | H2 h−1 gcat−1 (µmol) | Synthesis Temperature (°C) | Graphene Synthesis Time (s) |
|---|---|---|---|
| 10% methanol | 0.00 | - | 0 |
| TiO2 | 86.56 | - | 0 |
| TiO2-Cu-850 | 309.70 | 850 | 0 |
| TiO2-Cu-C-850-60 | 29.92 | 850 | 60 |
| TiO2-Cu-600 | 1807.05 | 600 | 0 |
| TiO2-Cu-C-600-60 | 404.70 | 600 | 60 |
| TiO2-Cu-C-600-30 | 1141.38 | 600 | 30 |
| TiO2-Cu-550 | 1589.50 | 550 | 0 |
| TiO2-Cu-C-550-180 | 2065.62 | 550 | 180 |
| TiO2-Cu-C-550-60 | 2131.11 | 550 | 60 |
| TiO2-Cu-C-550-45 | 1731.25 | 550 | 45 |
| TiO2-Cu-C-550-30 | 2152.17 | 550 | 30 |
| TiO2-Cu-C-550-15 | 1750.19 | 550 | 15 |
| TiO2-Cu-C-550-10 | 1897.72 | 550 | 10 |
| TiO2-Cu-500 | 1825.26 | 500 | 0 |
| TiO2-Cu-C-500-30 | 2151.66 | 500 | 30 |
| TiO2-Cu-C-500-25 | 2296.27 | 500 | 25 |
| No. | Catalyst (As Reported) | Hydrogen Evolution Result (As Reported in the Original Paper) | Hydrogen Evolution Normalized to µmol H2 h−1 g−1catalyst | Light Source | Ref. |
|---|---|---|---|---|---|
| 1 | TiO2 + reduced graphene oxide + 1.5% copper | 11 mmol H2/8 h | 68,750.00 | Xenon Lamp | [14] |
| 2 | TiO2 + 0.5 mol% copper + 0.5 weight% GNP’s | 268 µmol H2/3 h/gcatalyst | 89.30 | Mercury Lamp | [17] |
| 3 | TiO2/RGO/Cu2O | 631.6 μmol/h·m2 | N/A | Xenon Lamp | [19] |
| 4 | CuO/TiO2-GR-0.5 weight% | 2905.60 mmol/(h·gcatalyst) | 2905.60 | Xenon Lamp | [16] |
| 5 | Cu-TiO2-graphene | 2.3 mmol/gcatalyst/6 h | 383.30 | Xenon Lamp | [34] |
| 6 | TiO2-Cu-C-500-25 | - | 2296.27 | Xenon Lamp | current work |
| 7 | Cu-2D_graphene-TiO2 | 22 mmol H2/5 h | 220,000.0 | Xenon Lamp | [15] |
| Quality Metrics | Minimum Threshold | |
|---|---|---|
| Goodness-of-fit | ||
| R2 | 0.98 | >0.7 |
| RMSET | 47.86 | as low as possible |
| Predictive power | ||
| Q2F1 | 0.88 | >0.6 |
| Q2F2 | 0.88 | >0.6 |
| Q2F3 | 0.92 | >0.6 |
| CCC | 0.91 | >0.7 |
| RMSEExt | 193.68 | as low as possible |
| Sample ID | Synthesis Temperature (°C) | Synthesis Time (s) | Measured H2 h−1 gcat−1 (µmol) | Set * | Predicted H2 h−1 gcat−1 (µmol) |
|---|---|---|---|---|---|
| TiO2-Cu-C-550-30s | 550 | 30 | 2152.17 | T | 2117.96 |
| TiO2-Cu-C-500-30s | 500 | 30 | 2151.66 | T | 2151.66 |
| TiO2-Cu-C-550-60s | 550 | 60 | 2131.11 | V | 1756.58 |
| TiO2-Cu-C-550-30s | 550 | 30 | 2108.27 | T | 2117.96 |
| TiO2-Cu-C-550-180s | 550 | 180 | 2065.62 | T | 2065.62 |
| TiO2-Cu-C-550-10s | 550 | 10 | 1897.72 | V | 1736.10 |
| TiO2-Cu-550-0s | 550 | 0 | 1830.22 | T | 1710.44 |
| TiO2-Cu-C-600-0s | 600 | 0 | 1807.05 | T | 1807.05 |
| TiO2-Cu-C-550-10s | 550 | 10 | 1801.03 | V | 1736.10 |
| TiO2-Cu-C-550-60s | 550 | 60 | 1761.61 | T | 1758.44 |
| TiO2-Cu-C-550-15s | 550 | 15 | 1750.19 | T | 1768.04 |
| TiO2-Cu-C-550-45s | 550 | 45 | 1731.25 | V | 1695.10 |
| TiO2-Cu-C-550-45s | 550 | 45 | 1650.60 | T | 1679.48 |
| TiO2-Cu-550-0s | 550 | 0 | 1589.50 | T | 1710.44 |
| TiO2-Cu-C-550-15s | 550 | 15 | 1573.01 | V | 1777.31 |
| TiO2-Cu-C-600-30s | 600 | 30 | 1141.38 | T | 1102.93 |
| TiO2-Cu-C-600-30s | 600 | 30 | 1064.48 | T | 1102.93 |
| TiO2-Cu-850-0s | 850 | 0 | 416.46 | V | 309.70 |
| TiO2-Cu-C-600-60s | 600 | 60 | 404.70 | T | 404.70 |
| TiO2-Cu-850-0s | 850 | 0 | 309.70 | T | 309.70 |
| TiO2-Cu-C-850-60s | 850 | 60 | 29.92 | T | 29.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowski, Ł.; Gajewicz-Skretna, A.; Klimczuk, T.; Trykowski, G.; Nikiforow, K.; Lisowski, W.; Gołąbiewska, A.; Zaleska-Medynska, A. Towards Computer-Aided Graphene Covered TiO2-Cu/(CuxOy) Composite Design for the Purpose of Photoinduced Hydrogen Evolution. Catalysts 2021, 11, 698. https://doi.org/10.3390/catal11060698
Lewandowski Ł, Gajewicz-Skretna A, Klimczuk T, Trykowski G, Nikiforow K, Lisowski W, Gołąbiewska A, Zaleska-Medynska A. Towards Computer-Aided Graphene Covered TiO2-Cu/(CuxOy) Composite Design for the Purpose of Photoinduced Hydrogen Evolution. Catalysts. 2021; 11(6):698. https://doi.org/10.3390/catal11060698
Chicago/Turabian StyleLewandowski, Łukasz, Agnieszka Gajewicz-Skretna, Tomasz Klimczuk, Grzegorz Trykowski, Kostiantyn Nikiforow, Wojciech Lisowski, Anna Gołąbiewska, and Adriana Zaleska-Medynska. 2021. "Towards Computer-Aided Graphene Covered TiO2-Cu/(CuxOy) Composite Design for the Purpose of Photoinduced Hydrogen Evolution" Catalysts 11, no. 6: 698. https://doi.org/10.3390/catal11060698
APA StyleLewandowski, Ł., Gajewicz-Skretna, A., Klimczuk, T., Trykowski, G., Nikiforow, K., Lisowski, W., Gołąbiewska, A., & Zaleska-Medynska, A. (2021). Towards Computer-Aided Graphene Covered TiO2-Cu/(CuxOy) Composite Design for the Purpose of Photoinduced Hydrogen Evolution. Catalysts, 11(6), 698. https://doi.org/10.3390/catal11060698








