Palladium Nanoparticles Supported on Smopex-234® as Valuable Catalysts for the Synthesis of Heterocycles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Morphology of the Catalysts
2.2. Catalytic Activity of Pd/Smopex®-111 and Pd/Smopex®-234
3. Materials and Methods
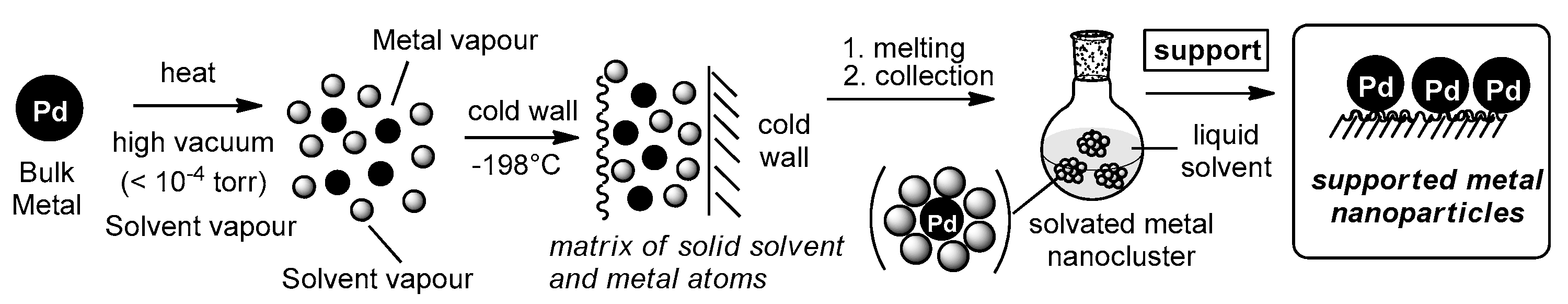
3.1. Preparation of Solvated Palladium Atoms Solutions
3.2. Preparation of Supported Palladium Catalysts
3.3. Synthesis of Phthalans: General Procedure
3.4. Synthesis of Isochromans: General Procedure
3.5. Synthesis of N-Heterocyclic Compounds: General Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Albano, G.; Aronica, L.A. From alkynes to heterocycles through metal-promoted silylformylation and silylcarbocyclization reactions. Catalysts 2020, 10, 1012. [Google Scholar] [CrossRef]
- Albano, G.; Aronica, L.A. Acyl sonogashira cross-coupling: State of the art and application to the synthesis of heterocyclic compounds. Catalysts 2020, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, R.; Pahari, P.; Mal, D. Phthalides and phthalans: Synthetic methodologies and their applications in the total synthesis. Chem. Rev. 2014, 114, 6213–6284. [Google Scholar] [CrossRef]
- Ilya, E.; Kulikova, L.; Van der Eycken, E.V.; Voskressensky, L. Recent advances in phthalan and coumaran chemistry. ChemistryOpen 2018, 7, 914–929. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.K.; Arif, A.M.; Ford, E.J.; Strobel, G.A.; Porco, J.A.; Tomer, D.P.; Oneill, K.L.; Heider, E.M.; Grant, D.M. Pestacin: A 1,3-dihydro isobenzofuran from Pestalotiopsis microspora possessing antioxidant and antimycotic activities. Tetrahedron 2003, 59, 2471–2476. [Google Scholar] [CrossRef]
- Sánchez, C.; Bøgesø, K.P.; Ebert, B.; Reines, E.H.; Braestrup, C. Escitalopram versus citalopram: The surprising role of the R-enantiomer. Psychopharmacology 2004, 174, 163–176. [Google Scholar] [CrossRef]
- Larsen, M.A.B.; Plenge, P.; Andersen, J.; Eildal, J.N.; Kristensen, A.S.; Bøgesø, K.P.; Gether, U.; Strømgaard, K.; Bang-Andersen, B.; Loland, C.J. Structure–activity relationship studies of citalopram derivatives: Examining substituents conferring selectivity for the allosteric site in the 5-HT transporter. Br. J. Pharmacol. 2016, 173, 925–936. [Google Scholar] [CrossRef] [Green Version]
- Waugh, J.; Goa, K.L. Escitalopram. CNS Drugs 2003, 17, 343–362. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Reines, E.H.; Guiton, C.; Weiller, E. Escitalopram therapy for major depression and anxiety disorders. Ann. Pharmacother. 2007, 41, 1583–1592. [Google Scholar] [CrossRef]
- Leonard, B.; Taylor, D. Review: Escitalopram—Translating molecular properties into clinical benefit: Reviewing the evidence in major depression. J. Psychopharmacol. 2010, 24, 1143–1152. [Google Scholar] [CrossRef] [Green Version]
- Eildal, J.N.N.; Andersen, J.; Kristensen, A.S.; Jørgensen, A.M.; Bang-Andersen, B.; Jørgensen, M.; Strømgaard, K. From the selective serotonin transporter inhibitor citalopram to the selective norepinephrine transporter inhibitor talopram: Synthesis and structure−activity relationship studies. J. Med. Chem. 2008, 51, 3045–3048. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, J.; Zhang, W.; Zhou, B.; Zhang, C.; Gerwick, W.H.; Cao, Z. Alkaloids from Corydalis decumbens suppress neuronal excitability in primary cultures of mouse neocortical neurons. Phytochemistry 2018, 150, 85–92. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Huang, Q.-L.; Chen, J.; Zhang, W.-J.; Jin, H.-X.; Wang, H.-B.; Naman, C.B.; Cao, Z.-Y. Phthalideisoquinoline hemiacetal alkaloids from corydalis decumbens that inhibit spontaneous calcium oscillations, including alkyl derivatives of (+)-egenine that are strikingly levorotatory. J. Nat. Prod. 2019, 82, 2713–2720. [Google Scholar] [CrossRef]
- Praveen, C.; Lyyappan, C.; Perumal, P.T.; Girija, K. AgOTf as an alternative catalyst for the regioselective cyclization of 2-(alkynyl)benzyl alcohols: Synthesis and biological evaluation of phthalans. Indian J. Chem. Sect B 2012, 51B, 498–507. [Google Scholar] [CrossRef]
- Nishihara, Y.; Tsujii, E.; Yamagishi, Y.; Sakamoto, K.; Tsurumi, Y.; Furukawa, S.; Ohtsu, R.; Kino, T.; Hino, M.; Yamashita, M.; et al. FR198248, a new anti-influenza agent isolated from aspergillus terreus No. 13830. J. Antibiot. 2001, 54, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Nishihara, Y.; Takase, S.; Tsujii, E.; Hatanaka, H.; Hashimoto, S. New anti-influenza agents, FR198248 and its derivatives II. characterization of FR198248, its related compounds and some derivatives. J. Antibiot. 2001, 54, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Araniti, F.; Mancuso, R.; Ziccarelli, I.; Sunseri, F.; Abenavoli, M.R.; Gabriele, B. 3-(Methoxycarbonylmethylene)isobenzofuran-1-imines as a new class of potential herbicides. Molecules 2014, 19, 8261–8275. [Google Scholar] [CrossRef] [Green Version]
- Mandali, P.K.; Pati, A.K.; Mishra, A.K.; Chand, D.K. Fluorescent 1-Arylidene-1,3-dihydroisobenzofuran: Ligand-Free palladium nanoparticles, catalyzed domino synthesis and photophysical studies. ChemistrySelect 2017, 2, 5259–5265. [Google Scholar] [CrossRef]
- Albano, G.; Aronica, L.A. Potentiality and synthesis of O- and N-heterocycles: Pd-catalyzed cyclocarbonylative sonogashira coupling as a valuable route to phthalans, isochromans, and isoindolines. Eur. J. Org. Chem. 2017, 2017, 7204–7221. [Google Scholar] [CrossRef]
- Larghi, E.L.; Kaufman, T.S. The oxa-pictet-spengler cyclization: Synthesis of isochromans and related pyran-type heterocycles. Synthesis 2006, 2006, 187–220. [Google Scholar] [CrossRef]
- Zhao, Z.; Kang, K.; Yue, J.; Ji, X.; Qiao, H.; Fan, P.; Zheng, X. Research progress in biological activities of isochroman derivatives. Eur. J. Med. Chem. 2021, 210, 113073. [Google Scholar] [CrossRef]
- Bianco, A.; Coccioli, F.; Guiso, M.; Marra, C. The occurrence in olive oil of a new class of phenolic compounds: Hydroxy-isochromans. Food Chem. 2002, 77, 405–411. [Google Scholar] [CrossRef]
- Guiso, M.; Marra, C.; Arcos, R.R. An investigation on dihydroxy-isochromans in extra virgin olive oil. Nat. Prod. Res. 2008, 22, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade alessandra. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Feng-Lin, H.; Jhy-Yih, C. Phenolics from tectaria subtriphylla. Phytochemistry 1993, 34, 1625–1627. [Google Scholar] [CrossRef]
- Malmstrøm, J.; Christophersen, C.; Frisvad, J.C. Secondary metabolites characteristic of Penicillium citrinum, Penicillium steckii and related species. Phytochemistry 2000, 54, 301–309. [Google Scholar] [CrossRef]
- Chen, G.; Lin, Y.; Vrijmoed, L.L.P.; Fong, W.-F. A new isochroman from the marine endophytic fungus 1893#. Chem. Nat. Compd. 2006, 42, 138–141. [Google Scholar]
- Khamthong, N.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Bioactive polyketides from the sea fan-derived fungus Penicillium citrinum PSU-F51. Tetrahedron 2012, 68, 8245–8250. [Google Scholar] [CrossRef]
- McMullin, D.R.; Nsiama, T.K.; Miller, J.D. Secondary metabolites from Penicillium corylophilum isolated from damp buildings. Mycologia 2014, 106, 621–628. [Google Scholar] [CrossRef]
- Kock, I.; Draeger, S.; Schulz, B.; Elsässer, B.; Kurtán, T.; Kenéz, Á.; Antus, S.; Pescitelli, G.; Salvadori, P.; Speakman, J.-B.; et al. Pseudoanguillosporin A and B: Two new isochromans isolated from the endophytic fungus pseudoanguillospora sp. Eur. J. Org. Chem. 2009, 2009, 1427–1434. [Google Scholar] [CrossRef]
- Trefiletti, G.; Rita Togna, A.; Latina, V.; Marra, C.; Guiso, M.; Togna, G.I. 1-Phenyl-6,7-dihydroxy-isochroman suppresses lipopolysaccharide-induced pro-inflammatory mediator production in human monocytes. Br. J. Nutr. 2011, 106, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Togna, A.R.; Latina, V.; Trefiletti, G.; Guiso, M.; Moschini, S.; Togna, G.I. 1-Phenil-6,7-dihydroxy-isochroman inhibits inflammatory activation of microglia. Brain Res. Bull. 2013, 95, 33–39. [Google Scholar] [CrossRef]
- Li, W.; Lee, C.; Bang, S.H.; Ma, J.Y.; Kim, S.; Koh, Y.-S.; Shim, S.H. Isochromans and related constituents from the endophytic fungus annulohypoxylon truncatum of zizania caduciflora and their anti-inflammatory effects. J. Nat. Prod. 2017, 80, 205–209. [Google Scholar] [CrossRef]
- Trisuwan, K.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Furo[3,2-h]isochroman, furo[3,2-h]isoquinoline, isochroman, phenol, pyranone, and pyrone derivatives from the sea fan-derived fungus Penicillium sp. PSU-F40. Tetrahedron 2010, 66, 4484–4489. [Google Scholar] [CrossRef]
- Niaz, S.-I.; Zhang, P.; Shen, H.; Li, J.; Chen, B.; Chen, S.; Liu, L.; He, J. Two new isochromane derivatives penisochromanes A and B from ascidian-derived fungus Penicillium sp. 4829. Nat. Prod. Res. 2019, 33, 1262–1268. [Google Scholar] [CrossRef]
- He, G.; Matsuura, H.; Takushi, T.; Kawano, S.; Yoshihara, T. A new antifungal metabolite from penicillium expansum. J. Nat. Prod. 2004, 67, 1084–1087. [Google Scholar] [CrossRef]
- Togna, G.I.; Togna, A.R.; Franconi, M.; Marra, C.; Guiso, M. Olive oil isochromans inhibit human platelet reactivity. J. Nutr. 2003, 133, 2532–2536. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Madrona, A.; Pereira-Caro, G.; Domínguez, V.; Cert, R.M.; Parrado, J.; Sarriá, B.; Bravo, L.; Espartero, J.L. Synthesis and antioxidant evaluation of isochroman-derivatives of hydroxytyrosol: Structure–activity relationship. Food Chem. 2015, 173, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, K.; Tsubaki, K.; Kuriyama, I.; Mizushina, Y.; Yoshida, H.; Takeuchi, T.; Kamisuki, S.; Sugawara, F.; Kobayashi, S. Synthesis, structure, and cytotoxicity studies of some fungal isochromanes. J. Nat. Prod. 2013, 76, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Tobe, M.; Tashiro, T.; Sasaki, M.; Takikawa, H. A concise synthesis of (±)-pseudodeflectusin, an antitumor isochroman derivative isolated from Aspergillus sp. Tetrahedron 2007, 63, 9333–9337. [Google Scholar] [CrossRef]
- Ennis, M.D.; Ghazal, N.B.; Hoffman, R.L.; Smith, M.W.; Schlachter, S.K.; Lawson, C.F.; Im, W.B.; Pregenzer, J.F.; Svensson, K.A.; Lewis, R.A.; et al. Isochroman-6-carboxamides as highly selective 5-HT1D agonists: Potential new treatment for migraine without cardiovascular side effects. J. Med. Chem. 1998, 41, 2180–2183. [Google Scholar] [CrossRef] [PubMed]
- Fráter, G.; Müller, U.; Kraft, P. Preparation and olfactory characterization of the enantiomerically pure isomers of the perfumery synthetic Galaxolide®. Helv. Chim. Acta 1999, 82, 1656–1665. [Google Scholar] [CrossRef]
- Kraft, P.; Fráter, G. Enantioselectivity of the musk odor sensation. Chirality 2001, 13, 388–394. [Google Scholar] [CrossRef] [PubMed]
- David, O.R.P. A chemical history of polycyclic musks. Chem. Eur. J. 2020, 26, 7537–7555. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thokchom Prasanta, S.; Okram Mukherjee, S. Recent progress in biological activities of indole and indole alkaloids. Mini Rev. Med. Chem. 2018, 18, 9–25. [Google Scholar]
- Sravanthi, T.V.; Manju, S.L. Indoles—A promising scaffold for drug development. Eur. J. Pharm. Sci. 2016, 91, 1–10. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical importance of indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef]
- Mancuso, R.; Dalpozzo, R. Recent progress in the transition metal catalyzed synthesis of indoles. Catalysts 2018, 8, 458. [Google Scholar] [CrossRef] [Green Version]
- Speck, K.; Magauer, T. The chemistry of isoindole natural products. Beilstein J. Org. Chem. 2013, 9, 2048–2078. [Google Scholar] [CrossRef]
- Csende, F.; Porkoláb, A. Antiviral activity of isoindole derivatives. J. Med. Chem. Sci. 2020, 3, 254–285. [Google Scholar]
- Albano, G.; Aronica, L.A. Cyclization reactions for the synthesis of phthalans and isoindolines. Synthesis 2018, 50, 1209–1227. [Google Scholar]
- Richa Kaur, B. Isoindole derivatives: Propitious anticancer structural motifs. Curr. Top. Med. Chem. 2017, 17, 189–207. [Google Scholar]
- Csonka, R.; Speier, G.; Kaizer, J. Isoindoline-derived ligands and applications. RSC Adv. 2015, 5, 18401–18419. [Google Scholar] [CrossRef]
- Radtke, V.; Erk, P.; Sens, B. Isoindoline pigments. In High Performance Pigments; Smith, H.M., Ed.; Wiley-VCH: Weinheim, Germany, 2002; pp. 211–230. [Google Scholar]
- Dell’Acqua, M.; Facoetti, D.; Abbiati, G.; Rossi, E. From domino to multicomponent: Synthesis of dihydroisobenzofurans. Tetrahedron 2011, 67, 1552–1556. [Google Scholar] [CrossRef]
- Ammann, S.E.; Rice, G.T.; White, M.C. Terminal olefins to chromans, isochromans, and pyrans via allylic C–H oxidation. J. Am. Chem. Soc. 2014, 136, 10834–10837. [Google Scholar] [CrossRef] [Green Version]
- Petrone, D.A.; Malik, H.A.; Clemenceau, A.; Lautens, M. Functionalized chromans and isochromans via a diastereoselective Pd(0)-catalyzed carboiodination. Org. Lett. 2012, 14, 4806–4809. [Google Scholar] [CrossRef]
- Zanardi, A.; Mata, J.A.; Peris, E. Domino approach to benzofurans by the sequential sonogashira/hydroalkoxylation couplings catalyzed by new n-heterocyclic-carbene-palladium complexes. Organometallics 2009, 28, 4335–4339. [Google Scholar] [CrossRef]
- Buxaderas, E.; Alonso, D.A.; Nájera, C. Synthesis of dihydroisobenzofurans via palladium-catalyzed sequential alkynylation/annulation of 2-bromobenzyl and 2-chlorobenzyl alcohols under microwave irradiation. Adv. Synth. Catal. 2014, 356, 3415–3421. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, D.-H.; Engle, K.M.; Yu, J.-Q. Pd(II)-catalyzed hydroxyl-directed c−h olefination enabled by monoprotected amino acid ligands. J. Am. Chem. Soc. 2010, 132, 5916–5921. [Google Scholar] [CrossRef] [Green Version]
- Ammann, S.E.; Liu, W.; White, M.C. Enantioselective allylic C−H oxidation of terminal olefins to isochromans by palladium(ii)/chiral sulfoxide catalysis. Angew. Chem. Int. Ed. 2016, 55, 9571–9575. [Google Scholar] [CrossRef] [Green Version]
- Nandakumar, A.; Balakrishnan, K.; Perumal, P.T. Palladium-catalyzed intramolecular hydroarylation of 2-bromobenzyl propargyl ethers: A new access to exocyclic isochromans. Synlett 2011, 2011, 2733–2739. [Google Scholar] [CrossRef]
- Shen, R.-W.; Yang, J.-J.; Zhang, L.-X. Facile synthesis of phthalan derivatives via a Pd-catalyzed tandem hydroalkynylation, isomerization, Diels–Alder cycloaddition and aromatization reaction. Chin. Chem. Lett. 2015, 26, 73–76. [Google Scholar] [CrossRef]
- Ghosh, M.; Singha, R.; Dhara, S.; Ray, J.K. Synthesis of 4,5,6-trisubstituted-1,3-dihydroisobenzofurans by virtue of palladium-catalyzed domino carbopalladation of bromoenynes and internal alkynes. RSC Adv. 2015, 5, 85911–85914. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nagata, A.; Nagata, H.; Ando, Y.; Arikawa, Y.; Tatsumi, K.; Itoh, K. Palladium(0)-catalyzed intramolecular [2+2+2] alkyne cyclotrimerizations with electron-deficient diynes and triynes. Chem. Eur. J. 2003, 9, 2469–2483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zheng, M.; Jiang, H.; Li, X.; Qi, C. An aerobic [2 + 2 + 2] cyclization via chloropalladation: From 1,6-diynes and acrylates to substituted aromatic carbocycles. J. Org. Chem. 2011, 76, 4759–4763. [Google Scholar] [CrossRef]
- Tsubakiyama, M.; Sato, Y.; Mori, M. Synthesis of bicyclic heterocycles from propargyl esters using a palladium catalyst bearing a bidentate ligand. Heterocycles 2004, 64, 27–31. [Google Scholar] [CrossRef]
- Solé, D.; Serrano, O. Selective synthesis of either isoindole- or isoindoline-1-carboxylic acid esters by Pd(0)-catalyzed enolate arylation. J. Org. Chem. 2010, 75, 6267–6270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.-X.; Luo, J.-Y.; Zhao, L.-B.; Ye, Y.-Y.; Liang, Y.-M. Palladium-catalyzed insertion of N-tosylhydrazones for the synthesis of isoindolines. Chem. Commun. 2013, 49, 3254–3256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, W.; Fu, C.; Huang, X.; Ma, S. Benzene construction via Pd-catalyzed cyclization of 2,7-alkadiynylic carbonates in the presence of alkynes. Chem. Sci. 2019, 10, 2228–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, F.J.; Jarvo, E.R. Palladium-catalyzed cascade reaction for the synthesis of substituted isoindolines. Angew. Chem. Int. Ed. 2011, 50, 4459–4462. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Fazio, A.; Pittelli, R. Versatile synthesis of (Z)-1-alkylidene-1,3-dihydroisobenzofurans and 1H-isochromenes by palladium-catalyzed cycloisomerization of 2-alkynylbenzyl alcohols. Tetrahedron 2003, 59, 6251–6259. [Google Scholar] [CrossRef]
- Bacchi, A.; Costa, M.; Della Cà, N.; Fabbricatore, M.; Fazio, A.; Gabriele, B.; Nasi, C.; Salerno, G. Synthesis of 1-(Alkoxycarbonyl)methylene-1,3-dihydroisobenzofurans and 4-(Alkoxycarbonyl)benzo[c]pyrans by palladium-catalysed oxidative carbonylation of 2-Alkynylbenzyl alcohols, 2-Alkynylbenzaldehydes and 2-Alkynylphenyl Ketones. Eur. J. Org. Chem. 2004, 2004, 574–585. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Costa, M. PdI2-Catalyzed synthesis of heterocycles. Synlett 2004, 2004, 2468–2483. [Google Scholar] [CrossRef]
- Della Ca’, N.; Campanini, F.; Gabriele, B.; Salerno, G.; Massera, C.; Costa, M. Cascade reactions: Catalytic synthesis of functionalized 1,3-dihydroisobenzofuran and tetrahydrofuran derivatives by sequential nucleophilic ring opening–heterocyclization–oxidative carbonylation of alkynyloxiranes. Adv. Synth. Catal. 2009, 351, 2423–2432. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Ziccarelli, I.; Salerno, G. A new approach to isoindolinone derivatives by sequential palladium iodide-catalyzed oxidative aminocarbonylation–heterocyclization of 2-ethynylbenzamides. Tetrahedron Lett. 2012, 53, 6694–6696. [Google Scholar] [CrossRef]
- Mancuso, R.; Ziccarelli, I.; Armentano, D.; Marino, N.; Giofrè, S.V.; Gabriele, B. Divergent palladium iodide catalyzed multicomponent carbonylative approaches to functionalized isoindolinone and isobenzofuranimine derivatives. J. Org. Chem. 2014, 79, 3506–3518. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Della Ca’, N.; Veltri, L.; Ziccarelli, I.; Gabriele, B. PdI2-Based catalysis for carbonylation reactions: A personal account. Catalysts 2019, 9, 610. [Google Scholar] [CrossRef] [Green Version]
- Gabriele, B.; Mancuso, R.; Veltri, L.; Ziccarelli, I.; Della Ca, N. Palladium-catalyzed double cyclization processes leading to polycyclic heterocycles: Recent advances. Eur. J. Org. Chem. 2019, 2019, 5073–5092. [Google Scholar] [CrossRef]
- Mancuso, R.; Ziccarelli, I.; Brindisi, M.; Altomare, C.D.; Frattaruolo, L.; Falcicchio, A.; Della Ca’, N.; Cappello, A.R.; Gabriele, B. A stereoselective, multicomponent catalytic carbonylative approach to a new class of α,β-Unsaturated γ-Lactam derivatives. Catalysts 2021, 11, 227. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Salerno, G. Oxidative carbonylation as a powerful tool for the direct synthesis of carbonylated heterocycles. Eur. J. Org. Chem. 2012, 2012, 6825–6839. [Google Scholar] [CrossRef]
- Aronica, L.A.; Giannotti, L.; Giuntini, S.; Caporusso, A.M. Synthesis of 2-Alkylideneisochromans by cyclocarbonylative sonogashira reactions. Eur. J. Org. Chem. 2014, 2014, 6858–6862. [Google Scholar] [CrossRef] [Green Version]
- Aronica, L.A.; Giannotti, L.; Tuci, G.; Zinna, F. Cyclocarbonylative sonogashira reactions of 1-ethynylbenzyl alcohols: Synthesis of 1-Carbonylmethylene-1,3-Dihydroisobenzofurans. Eur. J. Org. Chem. 2015, 2015, 4944–4949. [Google Scholar] [CrossRef]
- Aronica, L.A.; Albano, G.; Giannotti, L.; Meucci, E. Synthesis of N-heteroaromatic compounds through cyclocarbonylative sonogashira reactions. Eur. J. Org. Chem. 2017, 2017, 955–963. [Google Scholar] [CrossRef]
- Albano, G.; Morelli, M.; Aronica, L.A. Synthesis of functionalised 3-Isochromanones by silylcarbocyclisation/desilylation reactions. Eur. J. Org. Chem. 2017, 2017, 3473–3480. [Google Scholar] [CrossRef]
- Albano, G.; Morelli, M.; Lissia, M.; Aronica, L.A. Synthesis of functionalised indoline and isoquinoline derivatives through a silylcarbocyclisation/desilylation sequence. ChemistrySelect 2019, 4, 2505–2511. [Google Scholar] [CrossRef]
- Albano, G.; Giuntini, S.; Aronica, L.A. Synthesis of 3-Alkylideneisoindolin-1-ones via sonogashira cyclocarbonylative reactions of 2-Ethynylbenzamides. J. Org. Chem. 2020, 85, 10022–10034. [Google Scholar] [CrossRef]
- Jiang, X.; Sclafani, J.; Prasad, K.; Repič, O.; Blacklock, T.J. Pd−Smopex-111: A new catalyst for heck and suzuki cross-coupling reactions. Org. Process. Res. Dev. 2007, 11, 769–772. [Google Scholar] [CrossRef]
- Colacot, T.J. Palladium based FibreCat and SMOPEX® as supported homogenous catalyst systems for simple to challenging carbon–carbon coupling reactions. Top. Catal. 2008, 48, 91–98. [Google Scholar] [CrossRef]
- Frankham, J. The use of metal scavengers for recovery of precious, base and heavy metals from waste streams. Platin. Metals Rev. 2010, 54, 200–202. [Google Scholar] [CrossRef]
- Phillips, S. The use of metal scavengers for recovery of palladium catalyst from solution. Platin. Metals Rev. 2010, 54, 69–70. [Google Scholar] [CrossRef]
- Caporusso, A.M.; Innocenti, P.; Aronica, L.A.; Vitulli, G.; Gallina, R.; Biffis, A.; Zecca, M.; Corain, B. Functional resins in palladium catalysis: Promising materials for Heck reaction in aprotic polar solvents. J. Catal. 2005, 234, 1–13. [Google Scholar] [CrossRef]
- Albano, G.; Evangelisti, C.; Aronica, L.A. Hydrogenolysis of benzyl protected phenols and aniline promoted by supported palladium nanoparticles. ChemistrySelect 2017, 2, 384–388. [Google Scholar] [CrossRef]
- Aronica, L.A.; Caporusso, A.M.; Tuci, G.; Evangelisti, C.; Manzoli, M.; Botavina, M.; Martra, G. Palladium nanoparticles supported on Smopex® metal scavengers as catalyst for carbonylative Sonogashira reactions: Synthesis of α,β-alkynyl ketones. Appl. Catal. A 2014, 480, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Albano, G.; Interlandi, S.; Evangelisti, C.; Aronica, L.A. Polyvinylpyridine-supported palladium nanoparticles: A valuable catalyst for the synthesis of alkynyl ketones via acyl sonogashira reactions. Catal. Lett. 2020, 150, 652–659. [Google Scholar] [CrossRef]
- Evangelisti, C.; Panziera, N.; D’Alessio, A.; Bertinetti, L.; Botavina, M.; Vitulli, G. New monodispersed palladium nanoparticles stabilized by poly-(N-vinyl-2-pyrrolidone): Preparation, structural study and catalytic properties. J. Catal. 2010, 272, 246–252. [Google Scholar] [CrossRef]
- Evangelisti, C.; Balerna, A.; Psaro, R.; Fusini, G.; Carpita, A.; Benfatto, M. Characterization of a Poly-4-vinylpyridine- supported cupd bimetallic catalyst for sonogashira coupling reactions. ChemPhysChem 2017, 18, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Fusini, G.; Rizzo, F.; Angelici, G.; Pitzalis, E.; Evangelisti, C.; Carpita, A. Polyvinylpyridine-supported palladium nanoparticles: An efficient catalyst for suzuki–miyaura coupling reactions. Catalysts 2020, 10, 330. [Google Scholar] [CrossRef] [Green Version]
- Oberhauser, W.; Evangelisti, C.; Jumde, R.P.; Petrucci, G.; Bartoli, M.; Frediani, M.; Mannini, M.; Capozzoli, L.; Passaglia, E.; Rosi, L. Palladium-nanoparticles on end-functionalized poly(lactic acid)-based stereocomplexes for the chemoselective cinnamaldehyde hydrogenation: Effect of the end-group. J. Catal. 2015, 330, 187–196. [Google Scholar] [CrossRef]
- Borodziński, A.; Bonarowska, M. Relation between crystallite size and dispersion on supported metal catalysts. Langmuir 1997, 13, 5613–5620. [Google Scholar] [CrossRef]
- Chai, Z.; Xie, Z.-F.; Liu, X.-Y.; Zhao, G.; Wang, J.-D. Tandem addition/cyclization reaction of organozinc reagents to 2-Alkynyl aldehydes: Highly efficient regio- and enantioselective synthesis of 1,3-dihydroisobenzofurans and tetrasubstituted furans. J. Org. Chem. 2008, 73, 2947–2950. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zhou, Y.; Li, Y.; Yan, S.; Gong, Y. Copper(II)-catalyzed asymmetric henry reaction of o-alkynylbenzaldehydes followed by Gold(I)-mediated cycloisomerization: An enantioselective route to chiral 1H-isochromenes and 1,3-dihydroisobenzofurans. J. Org. Chem. 2011, 76, 8869–8878. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Cress, K.; Waynant, K.; Ramos-Miranda, E.; Herndon, J.W. Synthesis of alkylidenephthalans through fluoride-induced cyclization of electron-deficient 2-siloxymethylphenylacetylene derivatives. Tetrahedron 2007, 63, 2959–2965. [Google Scholar] [CrossRef] [Green Version]
- Danyliuk, I.Y.; Vas’kevich, R.I.; Vas’kevich, A.I.; Vovk, M.V. Hydrogenated benzazepines: Recent advances in the synthesis and study of biological activity. Chem. Heterocycl. Compd. 2019, 55, 802–814. [Google Scholar] [CrossRef]
- Kawase, M.; Saito, S.; Motohashi, N. Chemistry and biological activity of new 3-benzazepines. Int. J. Antimicrob. Agents 2000, 14, 193–201. [Google Scholar] [CrossRef]
- Hamlin, J.E.; Hirai, K.; Millan, A.; Maitlis, P.M. A Simple Practical Test for Distinguishing a Heterogeneous Component in an Homogeneously Catalysed Reaction. J. Mol. Catal. A Chem. 1980, 7, 543–544. [Google Scholar]
- Rizzo, G.; Albano, G.; Lo Presti, M.; Milella, A.; Omenetto, F.G.; Farinola, G.M. Palladium supported on silk fibroin for suzuki–miyaura cross-coupling reactions. Eur. J. Org. Chem. 2020, 2020, 6992–6996. [Google Scholar] [CrossRef]
- Evangelisti, C.; Schiavi, E.; Aronica, L.A.; Psaro, R.; Balerna, A.; Martra, G. Solvated metal atoms in the preparation of supported gold catalysts. In Gold Catalysis, Preparation, Characterization, and Applications; Prati, L., Villa, A., Eds.; Jenny Stanford Publishing: New York, NY, USA, 2015; pp. 73–97. [Google Scholar]



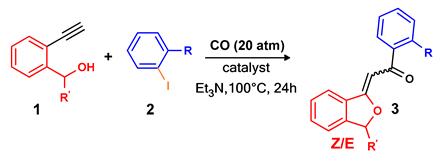
| Entry 1 | 1 | R’ | 2 | R | Catalyst 2 | Pd Loading (mol%) | Conversion (%) 3 | 3 | Selectivity (%) 3,4 | |
|---|---|---|---|---|---|---|---|---|---|---|
| (Z) | (E) | |||||||||
| 1 | a | H | a | H | PdCl2(PPh3)2 | 0.2 | 100 | aa | 65 (68) | 35 (25) |
| 2 | a | H | a | H | Pd/Smopex®-111 | 0.2 | 0 | aa | / | / |
| 3 | a | H | a | H | Pd/Smopex®-234 | 0.2 | 100 | aa | 75 | 25 |
| 4 | a | H | b | o-Me | Pd/Smopex®-234 | 0.2 | 100 | ab | 79 (70) | 21 (18) |
| 5 | a | H | c | o-CN | Pd/Smopex®-234 | 0.2 | 100 | ac | 100 (82) | / |
| 6 | b | t-Bu | a | H | PdCl2(PPh3)2 | 0.2 | 28 | ba | 22 | 78 |
| 7 | b | t-Bu | a | H | PdCl2(PPh3)2 | 0.5 | 100 | ba | 24 (15) | 76 (57) |
| 8 | b | t-Bu | a | H | Pd/Smopex®-234 | 0.2 | 16 | ba | 22 | 78 |
| 9 | b | t-Bu | a | H | Pd/Smopex®-234 | 1 | 82 | ba | 21 | 79 |
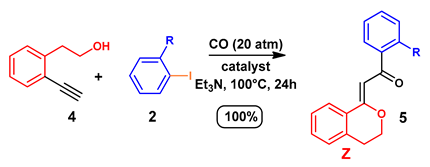
| Entry 1 | R | 2 | Catalyst 2 | Yield (%) 3 | R | 5 |
|---|---|---|---|---|---|---|
| 1 | H | a | PdCl2(PPh3)2 | 90 | H | a |
| 2 | H | a | Pd/Smopex®-234 | 93 | H | a |
| 3 | o-Me | b | Pd/Smopex®-234 | 95 | o-Me | b |
| 4 | o-CN | c | Pd/Smopex®-234 | 91 | o-CN | c |
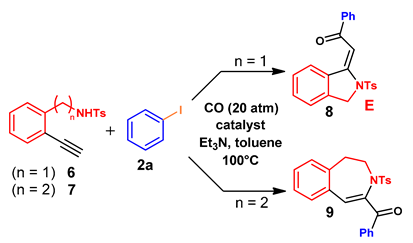
| Entry 1 | n | Tosylamide | Catalyst 2 | Pd Loading (mol%) | Reaction Time (h) | Conversion (%) 3 | Product 4 |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 6 | PdCl2(PPh3)2 | 0.2 | 4 | 100 | 8 (75%) |
| 2 | 1 | 6 | Pd/Smopex®-234 | 0.2 | 4 | 18 | 8 |
| 3 | 1 | 6 | Pd/Smopex®-234 | 0.2 | 24 | 69 | 8 |
| 4 | 1 | 6 | Pd/Smopex®-234 | 0.4 | 24 | 100 | 8 |
| 5 | 2 | 7 | Pd/Smopex®-234 | 0.4 | 24 | 92 | 9 (63%) 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, G.; Evangelisti, C.; Aronica, L.A. Palladium Nanoparticles Supported on Smopex-234® as Valuable Catalysts for the Synthesis of Heterocycles. Catalysts 2021, 11, 706. https://doi.org/10.3390/catal11060706
Albano G, Evangelisti C, Aronica LA. Palladium Nanoparticles Supported on Smopex-234® as Valuable Catalysts for the Synthesis of Heterocycles. Catalysts. 2021; 11(6):706. https://doi.org/10.3390/catal11060706
Chicago/Turabian StyleAlbano, Gianluigi, Claudio Evangelisti, and Laura Antonella Aronica. 2021. "Palladium Nanoparticles Supported on Smopex-234® as Valuable Catalysts for the Synthesis of Heterocycles" Catalysts 11, no. 6: 706. https://doi.org/10.3390/catal11060706








