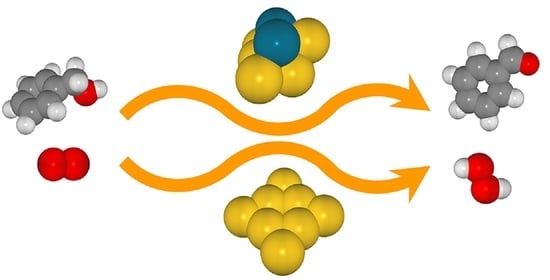
Catalytic Oxidation of Benzyl Alcohol to Benzaldehyde on Au8 and Au6Pd2 Clusters: A DFT Study on the Reaction Mechanism
Abstract
Share and Cite
Kodchasee, J.; Chanloi, C.; Khemthong, P.; Uapipatanakul, B.; Ehara, M.; Bobuatong, K. Catalytic Oxidation of Benzyl Alcohol to Benzaldehyde on Au8 and Au6Pd2 Clusters: A DFT Study on the Reaction Mechanism. Catalysts 2021, 11, 720. https://doi.org/10.3390/catal11060720
Kodchasee J, Chanloi C, Khemthong P, Uapipatanakul B, Ehara M, Bobuatong K. Catalytic Oxidation of Benzyl Alcohol to Benzaldehyde on Au8 and Au6Pd2 Clusters: A DFT Study on the Reaction Mechanism. Catalysts. 2021; 11(6):720. https://doi.org/10.3390/catal11060720
Chicago/Turabian StyleKodchasee, Jitlada, Chanon Chanloi, Pongtanawat Khemthong, Boontida Uapipatanakul, Masahiro Ehara, and Karan Bobuatong. 2021. "Catalytic Oxidation of Benzyl Alcohol to Benzaldehyde on Au8 and Au6Pd2 Clusters: A DFT Study on the Reaction Mechanism" Catalysts 11, no. 6: 720. https://doi.org/10.3390/catal11060720
APA StyleKodchasee, J., Chanloi, C., Khemthong, P., Uapipatanakul, B., Ehara, M., & Bobuatong, K. (2021). Catalytic Oxidation of Benzyl Alcohol to Benzaldehyde on Au8 and Au6Pd2 Clusters: A DFT Study on the Reaction Mechanism. Catalysts, 11(6), 720. https://doi.org/10.3390/catal11060720