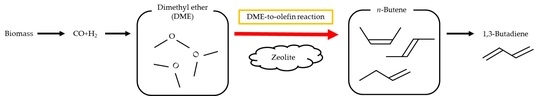
n-Butene Synthesis in the Dimethyl Ether-to-Olefin Reaction over Zeolites
Abstract
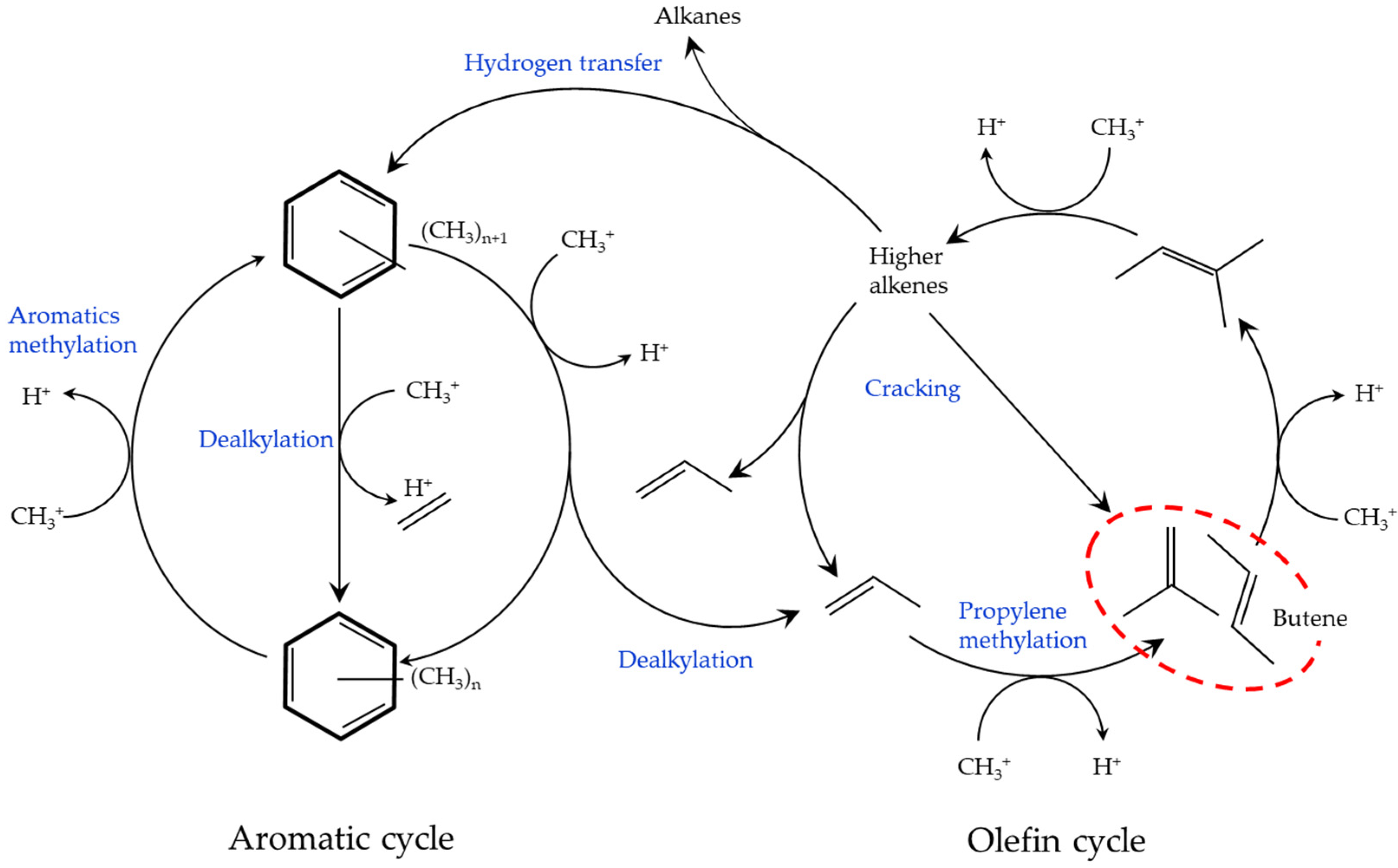
1. Introduction
2. Results and Discussion
2.1. Screening of Zeolites
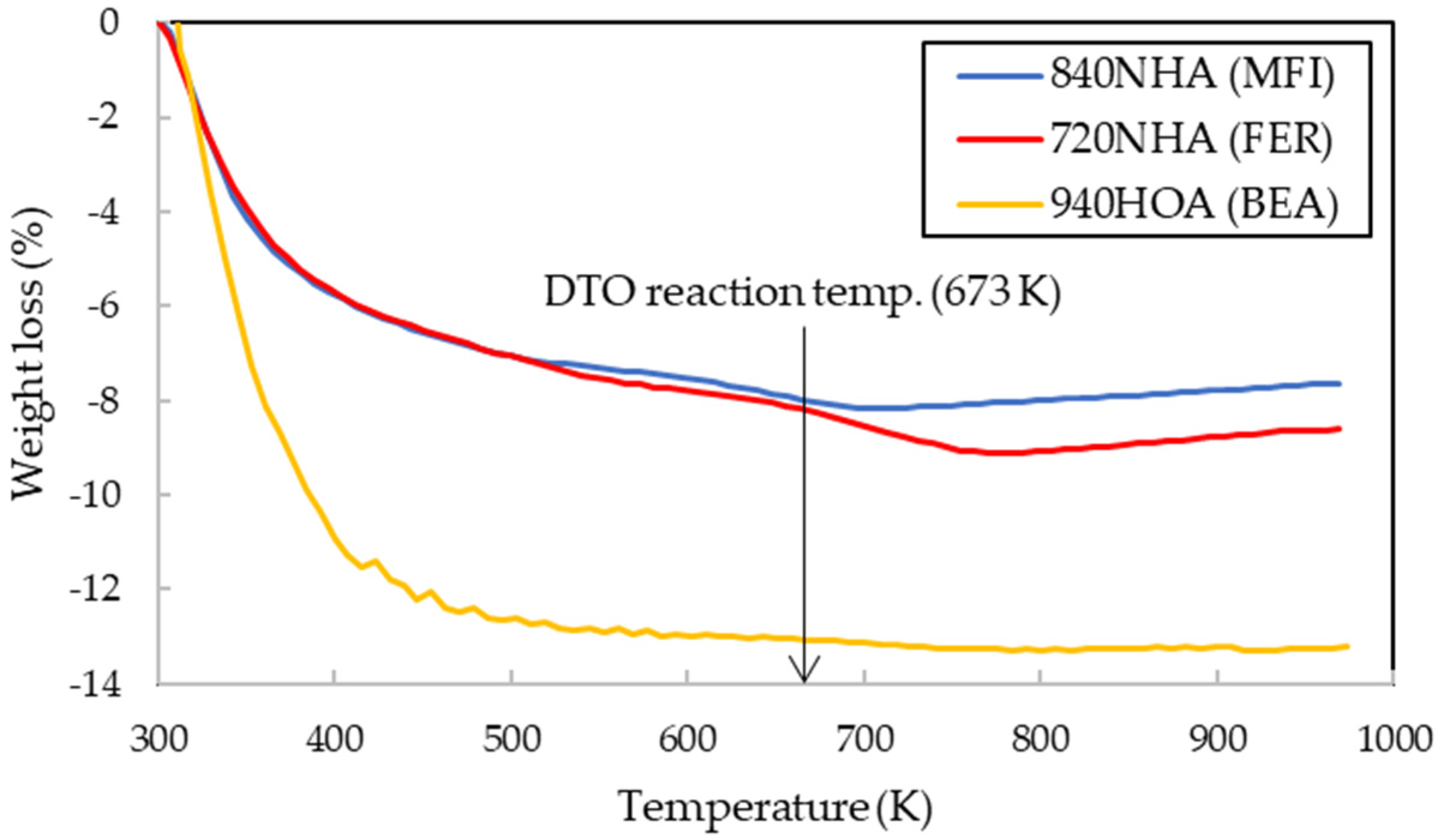
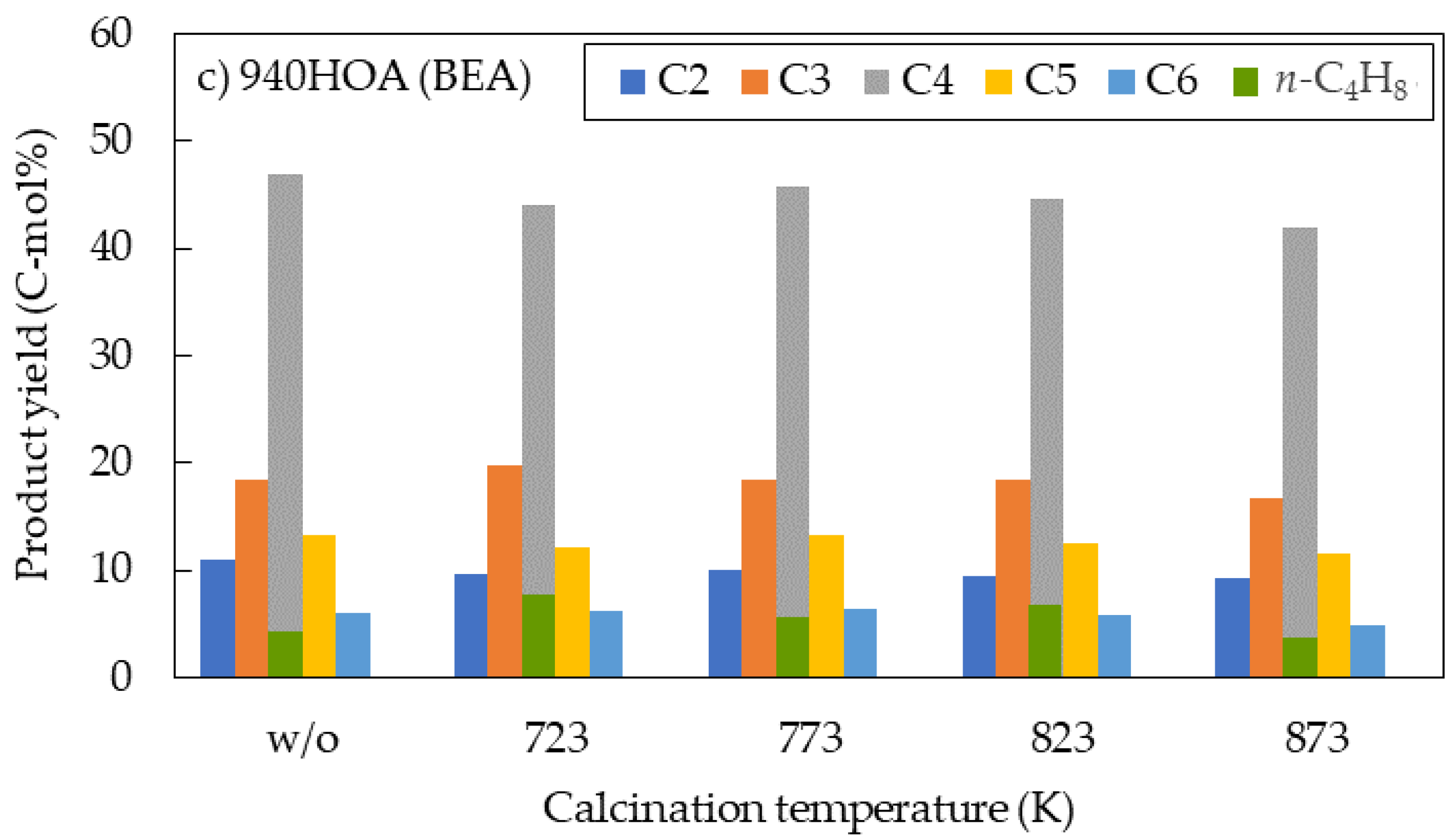
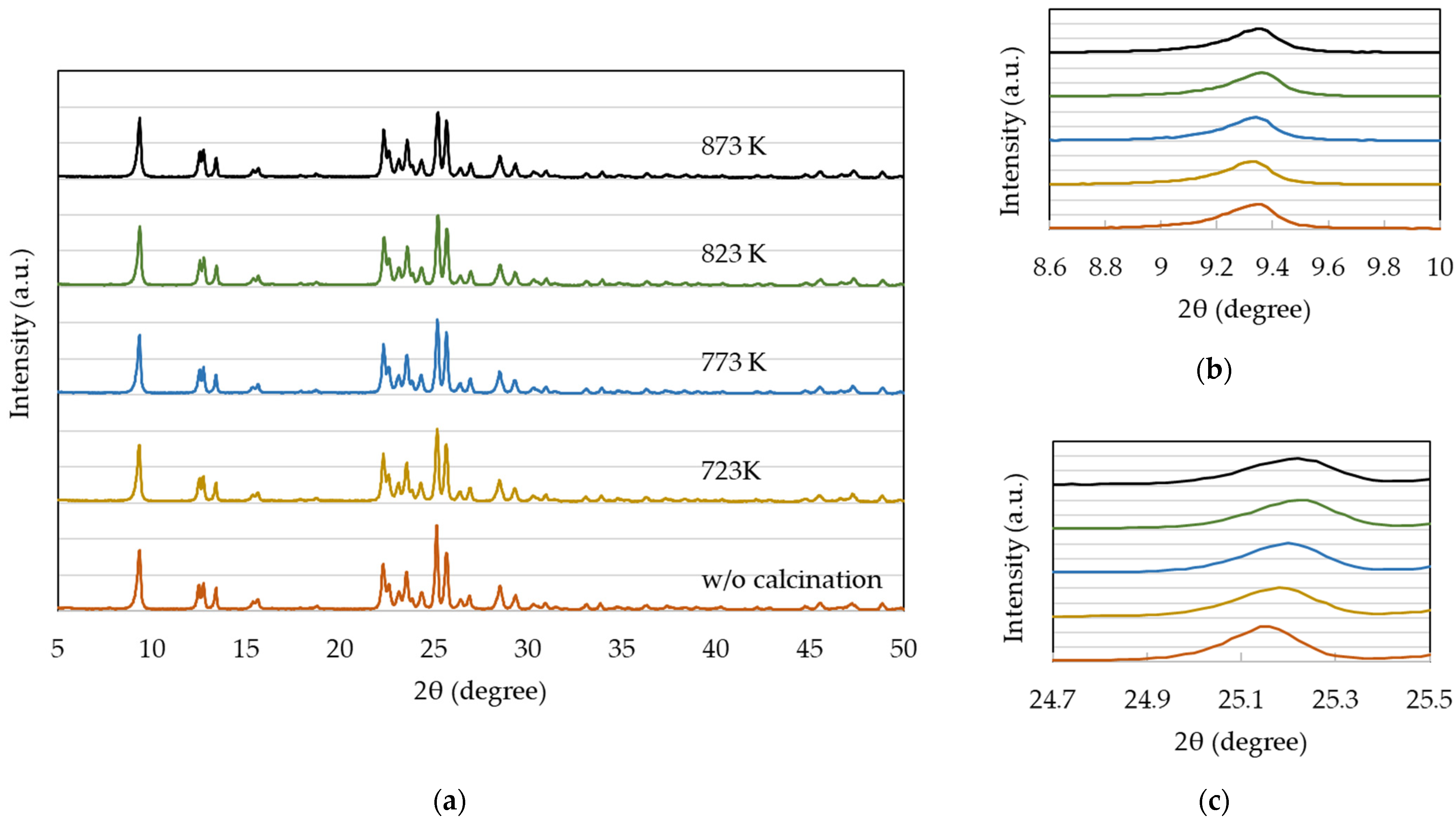
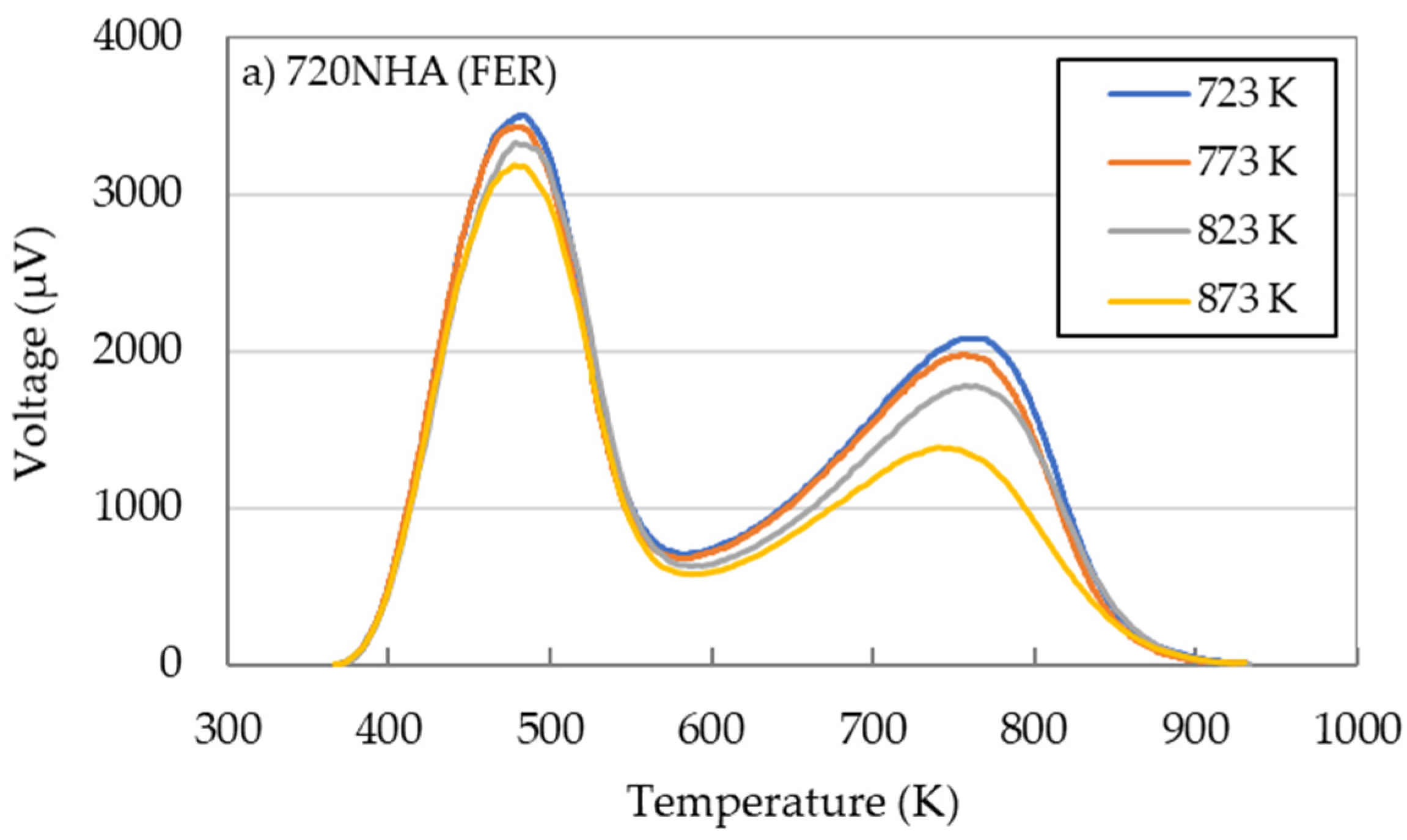
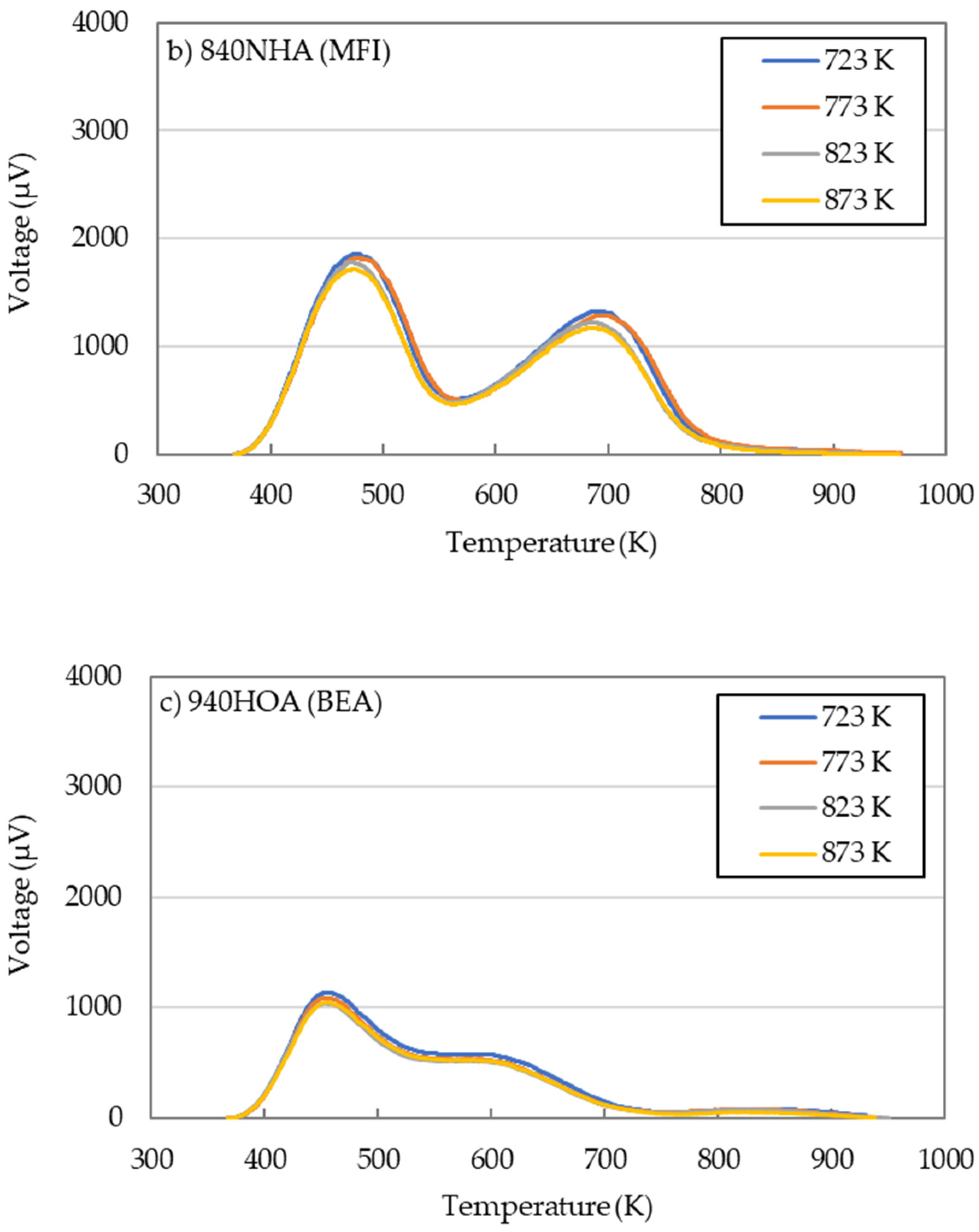
2.2. Effects of Calcination Temperature
2.3. Correlation of Observed Values to n-Butene Yield
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. DTO Reaction
/Total carbon number in feed gas [C-mol] × 100
4. Conclusions
- A screening study indicated that 720NHA (FER) showed the highest n-butene yield.
- The effects of calcination temperature on the catalytic performance in the DTO reaction were investigated for 720NHA (FER), 840NHA (MFI), and 940HOA (BEA). The 720NHA (FER) provided the highest n-butene yield at a calcination temperature of 773 K. In contrast, for 840NHA (MFI) and 940HOA (BEA), the n-butene yield decreased with an increase in the calcination temperature.
- Multiple regression analysis was performed on the six observed values and n-butene yield. The strong acid site and micropore volume were selected as statistically important explanatory variables. Strong acid sites should be dispersed, and the spatially narrow micropores should have a volume that provides an appropriate residence time in zeolites to suppress iso-butene production.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomu Data Book, 2020th ed.; Gomutimes Inc.: Tokyo, Japan, 2020; p. 9.
- Zacharopoulou, V.; Lemonidou, A.A. Olefins from biomass intermediates: A review. Catalysts 2018, 8, 2. [Google Scholar] [CrossRef]
- Camacho, C.E.C.; Alonso-Fariñas, B.; Perales, A.L.V.; Vidal-Barrero, F.; Ollero, F. Techno-economic and life-cycle assessment of one-step production of 1,3-butadiene from bioethanol using reaction data under industrial operating conditions. ACS Sustain. Chem. Eng. 2020, 8, 10201–10211. [Google Scholar] [CrossRef]
- Hanaoka, T.; Fujimoto, S.; Yoshida, M. Efficiency estimation and improvement of the 1,3-butadiene production process from lignin via syngas through process simulation. Energy Fuel. 2017, 31, 12965–12976. [Google Scholar] [CrossRef]
- Hanaoka, T.; Fujimoto, S.; Kihara, H. Improvement of the 1,3-butadiene production process from lignin—A comparison with the gasification power generation process. Renew. Energy 2019, 135, 1303–1313. [Google Scholar] [CrossRef]
- Hanaoka, T.; Fujimoto, S.; Kihara, H. Evaluation of n-butene synthesis from dimethyl ether in the production of 1,3-butadiene from lignin: A techno-economic analysis. Renew. Energy 2021, 163, 964–973. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Aguayo, A.T.; Bilbao, J. Reactor-regenerator system for the dimethyl ether-to-olefins process over HZSM-5 catalysts: Conceptual development and analysis of the process variables. Ind. Eng. Chem. Res. 2020, 59, 14689–14702. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Li, Z.; Wang, Y.; Yu, J. Synthesis of AEI/CHA intergrowth zeolites by dual templates and their catalytic performance for dimethyl ether to olefins. Chem. Eng. J. 2017, 323, 295–303. [Google Scholar] [CrossRef]
- Hirota, Y.; Yamada, M.; Uchida, Y.; Sakamoto, Y.; Yokoi, T.; Nishiyama, N. Synthesis of SAPO-18 with low acidic strength and its application in conversion of dimethylether to olefins. Micropor. Mesopor. Mater. 2016, 232, 65–69. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Li, Z.; Wang, Y.; Yu, J. Synthesis of SAPO-18/34 intergrowth zeolites and their enhanced stability for dimethyl ether to olefins. RSC Adv. 2017, 7, 939–946. [Google Scholar] [CrossRef]
- Hirota, Y.; Murata, K.; Miyamoto, M.; Egashira, Y.; Nishiyama, N. Light olefins synthesis from methanol and dimethylether over SAPO-34 nanocrystals. Catal. Lett. 2010, 140, 22–26. [Google Scholar] [CrossRef]
- Lee, S.-G.; Kim, H.-S.; Kim, Y.-H.; Kang, E.-J.; Lee, D.-H.; Park, C.-S. Dimethyl ether conversion to light olefins over the SAPO-34/ZrO2 composite catalysts with high lifetime. J. Ind. Eng. Chem. 2014, 20, 61–67. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Wang, D.; Wei, F.; Wang, Y. Differences in the methanol-to-olefins reaction catalyzed by SAPO-34 with dimethyl ether as reactant. J. Catal. 2014, 311, 281–287. [Google Scholar] [CrossRef]
- Péres-Uriarte, P.; Ateka, A.T.; Aguayo, A.T.; Bilbao, J. Kinetic model for the reaction of DME to olefins over a HZSM-5 zeolite catalyst. Chem. Eng. J. 2016, 302, 801–810. [Google Scholar] [CrossRef]
- Péres-Uriarte, P.; Ateka, A.; Gamero, M.; Aguayo, A.T.; Bilbao, J. Effect of the operating conditions over a HZSM-5 zeolite catalyst. Ind. Eng. Chem. Res. 2016, 55, 6569–6578. [Google Scholar] [CrossRef]
- Péres-Uriarte, P.; Ateka, A.; Aguayo, A.T.; Bilbao, J. Comparison of HZSM-5 zeolite and SAPO- (-18 and -34) based catalysts for the production of light olefins from DME. Cata. Lett. 2016, 146, 1892–1902. [Google Scholar] [CrossRef]
- Ibáñez, M.; Péres-Uriarte, P.; Sánchez-Contador, M.; Cordero-Lanzac, T.; Aguayo, A.T.; Bilbao, J.; Castaño, P. Nature and location of carbonaceous species in a composite HZSM-5 zeolite catalyst during the conversion of dimethyl ether into light olefins. Catalysts 2017, 7, 254. [Google Scholar] [CrossRef]
- Péres-Uriarte, P.; Ateka, A.; Gayubo, A.G.; Cordero-Lanzac, T.; Aguayo, A.T.; Bilbao, J. Deactivation kinetics for the conversion of dimethyl ether to olefins over a HZSM-5 zeolite catalyst. Chem Eng. J. 2017, 311, 367–377. [Google Scholar] [CrossRef]
- Park, S.; Watanabe, Y.; Nishita, Y.; Fukuoka, T.; Inagaki, S.; Kubota, Y. Catalytic conversion of dimethyl ether into propylene over MCM-68 zeolite. J. Catal. 2014, 319, 265–273. [Google Scholar] [CrossRef]
- Kubota, Y.; Inagaki, S. High-performance catalysts with MSE-type zeolite framework. Top. Catal. 2015, 58, 480–493. [Google Scholar] [CrossRef]
- Park, S.; Inagaki, S.; Kubota, Y. Selective formation of light olefins from dimethyl ether over MCM-68 modified with phosphate species. Catal. Today 2016, 265, 218–224. [Google Scholar] [CrossRef]
- Han, Q.; Enoeda, K.; Inagaki, S.; Kubota, Y. Catalytic performance of Ce-modified MCM-68 zeolite in the dimethyl ether-to-olefin reaction: Impact of high calcination temperature. Chem. Lett. 2017, 46, 1434–1437. [Google Scholar] [CrossRef]
- Ahmed, M.H.M.; Muraza, O.; Al Amer, A.M.; Sugiura, Y.; Nishiyama, N. Development of desilicated EU-1 zeolite and its application in conversion of dimethyl ether to olefins. Micropor. Mesopor. Mater. 2015, 207, 9–16. [Google Scholar] [CrossRef]
- Liu, Q.; Yoshida, Y.; Nakazawa, N.; Inagaki, S.; Kubota, Y. The synthesis of YNU-5 zeolite and its application to the catalysis in the dimethyl ether-to-olefin reaction. Materials 2020, 13, 2030. [Google Scholar] [CrossRef] [PubMed]
- Al-Dughaither, A.S.; de Lasa, H. Neat dimethyl ether conversion to olefins (DTO) over HZSM-5: Effect of SiO2/Al2O3 on porosity, surface chemistry, and reactivity. Fuel 2014, 138, 52–64. [Google Scholar] [CrossRef]
- Badmaev, S.D.; Belyaev, V.D.; Volkova, G.G.; Paukshtis, E.A.; Sobyanin, V.A. Hydration of dimethyl ether to methanol over solid acids. React. Kinet. Catal. Lett. 2007, 90, 197–204. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, D.; Fan, F.; Zhang, Q.; Zhu, Z. A novel monolith catalyst of plate-type anodic alumina for the hydrolysis of dimethyl ether. Catal. Commun. 2013, 34, 64–68. [Google Scholar] [CrossRef]
- Magomedova, M.; Galanova, E.; Davidov, I.; Afokin, M.; Maximov, A. Dimethyl Ether to Olefins over Modified ZSM-5 Based Catalysts Stabilized by Hydrothermal Treatment. Catalysts 2019, 9, 485. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Yang, Y.; Cheng, K.; Kang, J.; Zhang, L.; Zhang, G.; Min, X.; Zhang, Q.; Wang, Y. Design of efficient bifunctional catalysts for direct conversion of syngas into lower olefins via methanol/dimethyl ether intermediates. Chem. Sci. 2018, 9, 4708–4718. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Jang, H.-G.; Lee, K.-Y.; Cho, S.J. Improved methanol-to-olefin reaction selectivity and catalyst life by CeO2 coating of ferrierite zeolite. Micropor. Mesopor. Mat. 2018, 256, 155–164. [Google Scholar] [CrossRef]
- Teketel, S.; Skistad, W.; Benard, S.; Olsbye, U.; Lillerud, K.P.; Beato, P.; Svelle, S. Shape selectivity in the conversion of methanol to hydrocarbons: The catalytic performance of one-dimensional 10-ring zeolites: ZSM-22, ZSM-23, ZSM-48, and EU-1. ACS Catal. 2012, 2, 26–37. [Google Scholar] [CrossRef]
- Teketel, S.; Svelle, S.; Lillerud, K.P.; Olsbye, U. Shape-selective conversion of methanol to hydrocarbons over 10-ring unidirectional-channel acidic H-ZSM-22. ChemCatChem 2009, 1, 78–81. [Google Scholar] [CrossRef]
- Baek, S.-C.; Lee, Y.-J.; Jun, K.-W.; Hong, S.B. Influence of catalytic functionalities of zeolites on product selectivities in methanol conversion. Energy Fuel. 2009, 23, 593–598. [Google Scholar] [CrossRef]
- Rackwalik, R.; Hunger, M.; Sulikowski, B. Transformations of monoterpene hydrocarbons on ferrierite type zeolites. Appl. Catal. A Gen. 2012, 427–428, 98–105. [Google Scholar] [CrossRef]
- Święs, A.; Kowalczyk, A.; Rutkowska, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Ferrierite and its delaminated and silica-intercalated forms modified with copper as effective catalysts for NH3-SCR process. Catalysts 2020, 10, 734. [Google Scholar] [CrossRef]
- Jang, H.-G.; Ha, K.; Kim, J.-H.; Sugi, Y.; Seo, G. Ceria and lanthana as blocking modifiers for the external surface of MFI zeolite. Appl. Catal. A Gen. 2014, 476, 175–185. [Google Scholar] [CrossRef]
- Huang, S.; Liu, S.; Zhu, Q.; Zhu, X.; Xin, W.; Liu, H.; Feng, Z.; Li, C.; Xie, S.; Wang, Q.; et al. The effect of calcination time on the activity of WO3/Al2O3/HY catalysts for the metathesis reaction between ethene and 2-butene. Appl. Catal. A Gen. 2007, 323, 94–103. [Google Scholar] [CrossRef]
- Ndlela, S.S.; Friedrich, H.B.; Cele, M.N. Effects of Framework Disruption of Ga and Ba Containing Zeolitic Materials by Thermal Treatment. Catalysts 2020, 10, 975. [Google Scholar] [CrossRef]
- Kanezashi, M.; Yamamoto, A.; Yoshioka, T.; Tsuru, T. Characteristics of Ammonia Permeation Through Porous Silica Membranes. AIChE J. 2010, 56, 1204–1212. [Google Scholar] [CrossRef]
- Martínez, S.L.; Romero, R.; Natividad, R.; González, J. Optimization of biodiesel production from sunflower oil by transesterification using Na2O/NaX and methanol. Catal. Today 2014, 220–222, 12–20. [Google Scholar] [CrossRef]
- Azarhoosh, M.J.; Halladj, R.; Askari, S.; Aghaeinejad-Meybodi, A. Performance analysis of ultrasound-synthesized nano-hierachical SAPO-34 catalyst in the methanol-to-lights-olefins process via artificial intelligence methods. Ultrason. Sonochem. 2019, 58, 104646. [Google Scholar] [CrossRef]
- Malik, A.A.; Yang, W.; Ma, Z.; Sun, W.-H. The Catalytic Activities of Carbocyclic Fused Pyridineimine Nickel Complexes Analogues in Ethylene Polymerization by Modeling Study. Catalysts 2019, 9, 520. [Google Scholar] [CrossRef]
- Ilias, S.; Bhan, A. Tuning the selectivity of methanol-to-hydrocarbons conversion on H-ZSM-5 by co-processing olefin or aromatic compounds. J. Catal. 2012, 290, 186–192. [Google Scholar] [CrossRef]
- Olsbye, U.; Svelle, S.; Bjørgen, M.; Beato, P.; Janssens, T.V.W.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of Methanol to Hydrocarbons: How Zeolite Cavity and Pore Size Controls Product Selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810–5831. [Google Scholar] [CrossRef] [PubMed]
- Ilias, S.; Bhan, A. Mechanism of the Catalytic Conversion of Methanol to Hydrocarbons. ACS Catal. 2013, 3, 18–31. [Google Scholar] [CrossRef]
- Hill, I.M.; Hashimi, S.A.; Bhan, A. Kinetics and mechanism of olefin methylation reactions on zeolites. J. Catal. 2012, 285, 115–123. [Google Scholar] [CrossRef]
- Menges, M.; Kraushaar-Czarnetzki, B. Kinetics of methanol to olefins over AlPO4-bound ZSM-5 extrudates in a two-stage unit with dimethyl ether pre-reactor. Micropor. Mesopor. Mater. 2012, 164, 172–181. [Google Scholar] [CrossRef]
- Ortega, C.; Hessel, V.; Kolb, G. Dimethyl ether to hydrocarbons over ZSM-5: Kinetic study in an external recycle reactor. Chem. Eng. J. 2018, 354, 21–34. [Google Scholar] [CrossRef]
- Hill, I.M.; Ng, Y.S.; Bhan, A. Kinetics of butene isomer methylation with dimethyl ether over zeolite catalysts. ACS Catal. 2012, 2, 1742–1748. [Google Scholar] [CrossRef]
- Khitev, Y.P.; Ivanova, I.I.; Kolyagin, Y.G.; Ponomareva, O.A. Skeletal isomerization of 1-butene over micro/mesoporous materials based on FER zeolite. Appl. Catal. A Gen. 2012, 441–442, 124–135. [Google Scholar] [CrossRef]
- Suh, Y.-W.; Lee, J.-W.; Rhee, H.-K. Skeletal isomerization of 1-butene over sulfate-promoted zirconia with large surface area prepared by an atrane route. Appl. Catal. A Gen. 2004, 274, 159–165. [Google Scholar] [CrossRef]
- Laredo, G.C.; Castillo, J.J.; Navarrete-Bolaños, J.; Perez-Romo, P.; Lagos, F.A. Benzene reduction in gasoline by alkylation with olefins: Comparison of Beta and MCM-22 catalysts. Appl. Catal. A Gen. 2012, 413–414, 140–148. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Wei, F.; Wang, D.; Wang, Z. In situ synthesis of SAPO-34 crystals grown onto α-Al2O3 sphere supports as the catalyst for the fluidized bed conversion of dimethyl ether to olefins. Appl. Catal. A Gen. 2008, 341, 112–118. [Google Scholar] [CrossRef]




| Material | Topology | SiO2/Al2O3 (mol/mol) | DME Conv. (%) | Product Yield (C-mol%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1–C3 | C2= | C3= | MeOH | C4 1 | i-C4= | n-C4= 2 | C5 | C6≤ | ||||
| 720NHA | FER | 18.5 | 98.7 | 5.7 | 6.0 | 12.3 | 2.2 | 0.2 | 8.6 | 27.6 | 29.1 | 6.7 |
| 720KOA | 18.5 | 0.6 | 0.2 | 0.0 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 820NHA | MFI | 22.5 | 100.0 | 14.2 | 11.0 | 21.9 | 0.1 | 15.8 | 5.9 | 12.7 | 7.2 | 11.1 |
| 822HOA | 23.9 | 100.0 | 14.6 | 8.8 | 17.9 | 0.0 | 17.6 | 5.8 | 12.1 | 8.1 | 15.0 | |
| 840NHA | 39.0 | 100.0 | 13.6 | 7.4 | 13.7 | 0.0 | 14.8 | 7.1 | 13.4 | 9.2 | 20.7 | |
| 840HOA | 37.0 | 100.0 | 12.8 | 13.2 | 18.7 | 0.0 | 13.1 | 6.0 | 12.9 | 7.1 | 16.1 | |
| 890HOA | 2120 | 71.4 | 0.3 | 0.8 | 17.1 | 19.2 | 0.2 | 4.3 | 11.7 | 5.8 | 11.8 | |
| 940HOA | BEA | 41.6 | 99.6 | 6.9 | 9.8 | 12.5 | 2.5 | 38.4 | 1.6 | 5.7 | 13.2 | 9.0 |
| 640HOA | MOR | 18.0 | 30.0 | 7.0 | 8.3 | 5.0 | 5.8 | 0.7 | 0.2 | 2.3 | 0.4 | 0.1 |
| 385HUA | FAU | 110 | 3.9 | 1.2 | 0.6 | 0.4 | 0.2 | 0.7 | 0.0 | 0.2 | 0.3 | 0.1 |
| Material | Topology | Calcination Temp. (K) | DME Conv. (%) | Product Yield (C-mol%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1–C3 | C2= | C3= | MeOH | C4 1 | i-C4= | n-C4= 2 | C5 | C6≤ | ||||
| 720NHA | FER | N/A | 4.6 | 1.3 | 0.1 | 0.1 | 2.4 | 0.0 | 0.0 | 0.4 | 0.2 | 0.1 |
| 723 | 95.4 | 4.6 | 6.7 | 17.7 | 0.1 | 0.4 | 10.8 | 22.3 | 27.0 | 5.7 | ||
| 773 | 98.7 | 5.7 | 6.0 | 12.3 | 2.2 | 0.2 | 8.6 | 27.6 | 29.1 | 6.7 | ||
| 823 | 91.2 | 6.6 | 7.1 | 14.2 | 1.0 | 0.6 | 8.6 | 26.7 | 21.8 | 4.5 | ||
| 873 | 31.1 | 5.4 | 3.3 | 6.5 | 0.3 | 0.6 | 3.4 | 6.8 | 3.5 | 1.2 | ||
| 840NHA | MFI | N/A | 97.3 | 8.1 | 12.7 | 20.6 | 4.4 | 5.4 | 5.2 | 18.8 | 9.9 | 11.8 |
| 723 | 92.6 | 5.8 | 9.5 | 24.6 | 0.2 | 6.0 | 8.8 | 18.7 | 7.9 | 11.1 | ||
| 773 | 100.0 | 13.6 | 7.4 | 13.7 | 0.0 | 14.8 | 7.1 | 13.4 | 9.2 | 20.7 | ||
| 823 | 100.0 | 14.2 | 7.0 | 13.6 | 0.0 | 19.3 | 6.0 | 13.0 | 9.9 | 17.0 | ||
| 873 | 60.9 | 13.5 | 3.1 | 5.4 | 0.4 | 12.4 | 2.0 | 4.2 | 4.4 | 15.5 | ||
| 940HOA | BEA | N/A | 99.7 | 9.0 | 10.7 | 10.6 | 0.7 | 41.3 | 1.3 | 4.3 | 13.3 | 8.5 |
| 723 | 99.3 | 6.1 | 9.3 | 14.6 | 3.2 | 33.4 | 2.8 | 7.8 | 12.2 | 9.7 | ||
| 773 | 99.6 | 6.9 | 9.8 | 12.5 | 2.5 | 38.4 | 1.6 | 5.7 | 13.2 | 9.0 | ||
| 823 | 98.5 | 7.1 | 9.2 | 12.3 | 4.4 | 35.0 | 2.5 | 6.9 | 12.4 | 8.5 | ||
| 873 | 88.8 | 8.6 | 8.9 | 9.7 | 0.9 | 37.3 | 1.0 | 3.7 | 11.6 | 7.1 | ||
| Material | Topology | Calcination Temp. (K) | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | |||
|---|---|---|---|---|---|---|---|
| Total | Micro 1 | External 1 | Total 2 | Micro 1 | |||
| 720NHA | FER | N/A | 35 | 10 | 25 | 0.21 | 0.01 |
| 723 | 381 | 353 | 28 | 0.37 | 0.11 | ||
| 773 | 487 | 460 | 27 | 0.37 | 0.14 | ||
| 823 | 462 | 435 | 27 | 0.33 | 0.13 | ||
| 873 | 503 | 474 | 29 | 0.38 | 0.14 | ||
| 840NHA | MFI | N/A | 471 | 469 | 2 | 0.15 | 0.14 |
| 723 | 522 | 519 | 3 | 0.17 | 0.17 | ||
| 773 | 529 | 525 | 4 | 0.18 | 0.17 | ||
| 823 | 461 | 458 | 3 | 0.16 | 0.16 | ||
| 873 | 503 | 495 | 8 | 0.17 | 0.16 | ||
| 940HOA | BEA | N/A | 757 | 741 | 16 | 0.30 | 0.24 |
| 723 | 773 | 758 | 15 | 0.30 | 0.25 | ||
| 773 | 806 | 789 | 16 | 0.33 | 0.26 | ||
| 823 | 786 | 768 | 18 | 0.32 | 0.25 | ||
| 873 | 823 | 804 | 19 | 0.34 | 0.26 | ||
| Material | Topology | Calcination Temp. (K) | Weak 1 (mmol/g) | Strong 2 (mmol/g) | Total (mmol/g) |
|---|---|---|---|---|---|
| 720NHA | FER | 723 | 1.358 | 1.369 | 2.727 |
| 773 | 1.406 | 1.373 | 2.779 | ||
| 823 | 1.386 | 1.288 | 2.674 | ||
| 873 | 1.297 | 0.999 | 2.296 | ||
| 840NHA | MFI | 723 | 0.774 | 0.749 | 1.523 |
| 773 | 0.774 | 0.747 | 1.521 | ||
| 823 | 0.760 | 0.713 | 1.473 | ||
| 873 | 0.735 | 0.675 | 1.410 | ||
| 940HOA | BEA | 723 | 0.498 | 0.268 | 0.766 |
| 773 | 0.486 | 0.255 | 0.741 | ||
| 823 | 0.463 | 0.243 | 0.706 | ||
| 873 | 0.469 | 0.231 | 0.700 |
| Selected Explanatory Variables | Tolerance |
|---|---|
| x1 − x2 | 0.981 |
| x1 − x3 | 0.984 |
| x1 − x4 | 0.014 |
| x1 − x5 | 0.301 |
| x1 − x6 | 0.175 |
| x2 − x3 | 0.092 |
| x2 − x4 | 0.949 |
| x2 − x5 | 0.618 |
| x2 − x6 | 0.784 |
| x3 − x4 | 0.998 |
| x3 − x5 | 0.844 |
| x3 − x6 | 0.948 |
| x4 − x5 | 0.213 |
| x4 − x6 | 0.117 |
| x5 − x6 | 0.052 |
| Trial | Explanatory Variable | Residual Sum of Squares (RSS) | F-Ratio | Regression Equation | Contribution Rate (%) |
|---|---|---|---|---|---|
| 1 | None | 2894 | |||
| 2 | x6 | 253 | 104.40 | y = 0.74 + 16.61x6 | 69.9 |
| 3 | x6, x4 | 201 | 2.34 | y = −30.04 + 113.43x4 + 30.22x6 | 76.1 |
| 4 | x6, x1 | 219 | 1.39 | y = −20.46 + 25.26x6 + 0.03x1 | 74.0 |
| Material | Topology | Cation Type | Crystalline Size 1 (µm) | Particle Size 1 (µm) |
|---|---|---|---|---|
| 720NHA | FER | NH4+ | ≤1 | 6 |
| 720KOA | K+ | ≤1 | 20 | |
| 820NHA | MFI | NH4+ | 0.1 × 0.5 | 5 |
| 840NHA | NH4+ | 2 × 4 | 10 | |
| 822HOA | H+ | 0.1 × 0.5 | 5 | |
| 840HOA | H+ | 2 × 4 | 10 | |
| 890HOA | H+ | 2 × 5 | 10 | |
| 940HOA | BEA | H+ | 0.5–1 | 4 |
| 640HOA | MOR | H+ | 0.1 × 0.5 | 12 |
| 385HUA | FAU | H+ | 0.7–1.0 | 2–3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanaoka, T.; Aoyagi, M.; Edashige, Y. n-Butene Synthesis in the Dimethyl Ether-to-Olefin Reaction over Zeolites. Catalysts 2021, 11, 743. https://doi.org/10.3390/catal11060743
Hanaoka T, Aoyagi M, Edashige Y. n-Butene Synthesis in the Dimethyl Ether-to-Olefin Reaction over Zeolites. Catalysts. 2021; 11(6):743. https://doi.org/10.3390/catal11060743
Chicago/Turabian StyleHanaoka, Toshiaki, Masaru Aoyagi, and Yusuke Edashige. 2021. "n-Butene Synthesis in the Dimethyl Ether-to-Olefin Reaction over Zeolites" Catalysts 11, no. 6: 743. https://doi.org/10.3390/catal11060743
APA StyleHanaoka, T., Aoyagi, M., & Edashige, Y. (2021). n-Butene Synthesis in the Dimethyl Ether-to-Olefin Reaction over Zeolites. Catalysts, 11(6), 743. https://doi.org/10.3390/catal11060743