Green and High Effective Scale Inhibitor Based on Ring-Opening Graft Modification of Polyaspartic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ring-Opening Graft Modification of PASP
2.2. Chemical Characterization of the Modified PASP
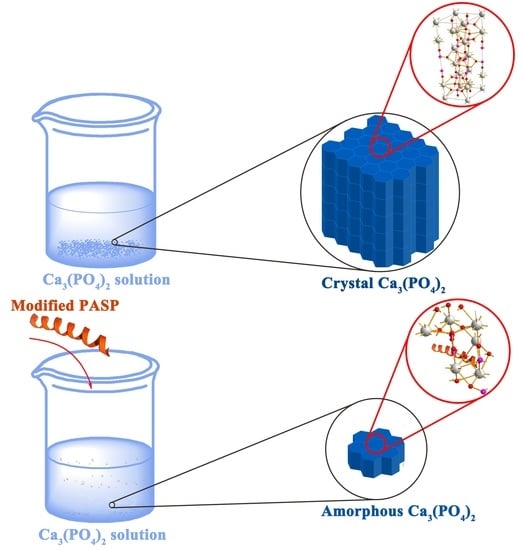
2.3. Scale Inhibition Performance of the Modified PASP
2.4. Biodegradation Performance of the Modified PASP
3. Experiment
3.1. Materials
3.2. Characterization
3.3. Synthesis of Polyaspartic Acid (PASP) in Ionic Liquid
3.4. Ring-Opening Graft Modification of PASP
3.5. Measurement of the Efficiency of Static Scale Inhibition
3.6. Biodegradation of the Modified PASP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antony, A.; Low, J.H.; Gray, S.; Childress, A.E.; Le-Clech, P.; Leslie, G. Scale formation and control in high pressure membrane water treatment systems: A review. J. Membr. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Wolowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of heavy metals and metalloids from water using drinking water treatment residuals as adsorbents: A review. Minerals 2019, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.R.; Hu, Y.; Shi, K.; Bilan, Y. Valuation of water resource green efficiency based on SBM-TOBIT panel model: Case study from henan province, China. Sustainability 2020, 12, 6944. [Google Scholar] [CrossRef]
- Zhang, S.P.; Qu, H.J.; Yang, Z.; Fu, C.E.; Tian, Z.Q.; Yang, W.B. Scale inhibition performance and mechanism of sulfamic/amino acids modified polyaspartic acid against calcium sulfate. Desalination 2017, 419, 152–159. [Google Scholar] [CrossRef]
- Yu, W.; Song, D.; Chen, W.; Yang, H. Antiscalants in RO membrane scaling control. Water Res. 2020, 183, 115985. [Google Scholar] [CrossRef]
- Yin, Y.M.; Jeong, N.; Minjarez, R.; Robbins, C.A.; Carlson, K.H.; Tong, T.Z. Contrasting behaviors between gypsum and silica scaling in the presence of antiscalants during membrane distillation. Environ. Sci. Technol. 2021, 55, 5335–5346. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Y.; Gao, L.J.; Wen, R.Z.; Deng, Y.Y.; Wu, X.J.; Deng, S.L. Fluorescent polyaspartic acid with an enhanced inhibition performance against calcium phosphate. Desalination 2014, 345, 72–76. [Google Scholar] [CrossRef]
- Chen, J.X.; Chen, F.J.; Han, J.; Su, M.; Li, Y.H. Evaluation of scale and corrosion inhibition of modified polyaspartic acid. Chem. Eng. Technol. 2020, 43, 1048–1058. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Zhou, Y.M.; Yao, Q.Z.; Nan, Q.L.; Zhang, M.J.; Sun, W. Synthesis of modified polyepoxysuccinic acid and evaluation of its scale inhibition on CaCO3, CaSO4, and Ca3(PO4)2 precipitation for industrial recycling water. Desalin. Water Treat. 2019, 152, 16–25. [Google Scholar] [CrossRef]
- Hasson, D.; Shemer, H.; Sher, A. State of the art of friendly “green” scale control inhibitors: A review article. Ind. Eng. Chem. Res. 2011, 50, 7601–7607. [Google Scholar] [CrossRef]
- Li, H.S.; Liu, W.; Qi, X.J. Evaluation of a novel CaSO4 scale inhibitor for a reverse osmosis system. Desalination 2007, 214, 193–199. [Google Scholar] [CrossRef]
- Zhu, T.Z.; Wang, L.D.; Sun, W.; Yang, Z.Q.; Wang, S.L.; Zhao, L.Q.; Xiao, G.J.; Wang, G.Z.; Liu, Z.M.; Shu, X.Q.; et al. Local scaling of CaCO3 on carbon steel surface with different corrosion types. Powder Technol. 2019, 356, 990–1000. [Google Scholar] [CrossRef]
- Mady, M.F.; Bayat, P.; Kelland, M.A. Environmentally friendly phosphonated polyetheramine scale inhibitors-excellent calcium compatibility for oilfield applications. Ind. Eng. Chem. Res. 2020, 59, 9808–9818. [Google Scholar] [CrossRef]
- Can, H.K.; Uner, G. Water-soluble anhydride containing alternating copolymers as scale inhibitors. Desalination 2015, 355, 225–232. [Google Scholar] [CrossRef]
- Martinod, A.; Neville, A.; Euvrad, M.; Sorbie, K. Electrodeposition of a calcareous layer: Effects of green inhibitors. Chem. Eng. Sci. 2009, 64, 2413–2421. [Google Scholar] [CrossRef]
- Mazumder, M.A.J. A review of green scale inhibitors: Process, types, mechanism and properties. Coatings 2020, 10, 928. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Lu, M.L.; Liu, J.; Chen, H.L.; Chen, Q.L.; Wang, B. Fluorescent-tagged hyper-branched polyester for inhibition of CaSO4 scale and the scale inhibition mechanism. Mater. Today Commun. 2020, 25, 101359. [Google Scholar] [CrossRef]
- Migahed, M.A.; Rashwan, S.M.; Kamel, M.M.; Habib, R.E. Synthesis, characterization of polyaspartic acid-glycine adduct and evaluation of their performance as scale and corrosion inhibitor in desalination water plants. J. Mol. Liq. 2016, 224, 849–858. [Google Scholar] [CrossRef]
- Ketrane, R.; Saidani, B.; Gil, O.; Leleyter, L.; Baraud, F. Efficiency of five scale inhibitors on calcium carbonate precipitation from hard water: Effect of temperature and concentration. Desalination 2009, 249, 1397–1404. [Google Scholar] [CrossRef]
- Ling, L.; Zhou, Y.M.; Huang, J.Y.; Yao, Q.Z.; Liu, G.Q.; Zhang, P.X.; Sun, W.; Wu, W.D. Carboxylate-terminated double-hydrophilic block copolymer as an effective and environmental inhibitor in cooling water systems. Desalination 2012, 304, 33–40. [Google Scholar] [CrossRef]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.J.; de With, G.; Sommerdijk, N. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemethy, A.; Solti, K.; Kiss, L.; Gyarmati, B.; Deli, M.A.; Csanyi, E.; Szilagyi, A. pH- and temperature-responsive poly(aspartic acid)-l-poly (N-isopropylacrylamide) conetwork hydrogel. Eur. Polym. J. 2013, 49, 2392–2403. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.N.; Zhao, L.L.; Cui, Y.C. Synthesis of polyaspartic acid-aminobenzenesulfonic acid grafted copolymer and its scale inhibition performance and dispersion capacity. Water Sci. Technol. 2011, 64, 423–430. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Wang, B.S.; Qin, L.; Mu, T.C.; Xue, Z.M.; Gao, G.H. Are ionic liquids chemically stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef]
- Reddy, P.N.; Padmaja, P.; Reddy, B.V.S.; Rambabu, G. Ionic liquid/water mixture promoted organic transformations. RSC Adv. 2015, 5, 51035–51054. [Google Scholar] [CrossRef]
- Coxon, J.M.; Townsend, M.A.E. Computational study on the ring-opening reaction of protonated oxirane and methylpropene. Tetrahedron 2007, 63, 5665–5668. [Google Scholar] [CrossRef]
- Quan, Z.H.; Chen, Y.C.; Wang, X.R.; Shi, C.; Liu, Y.J.; Ma, C.F. Experimental study on scale inhibition performance of a green scale inhibitor polyaspartic acid. Sci. China Ser. B Chem. 2008, 51, 695–699. [Google Scholar] [CrossRef]
- Tang, Y.M.; Yang, W.Z.; Yin, X.S.; Liu, Y.; Yin, P.W.; Wang, J.T. Investigation of CaCO3 scale inhibition by PAA, ATMP and PAPEMP. Desalination 2008, 228, 55–60. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Golovesov, V.; Ryabova, A.; Frolova, S.; Tkachenko, S.; Kamagurov, S.; Rudakova, G.; Popov, K. Synthesis and visualization of a novel fluorescent-taggedpolymeric antiscalant during gypsum crystallization in combination with bisphosphonate fluorophore. Crystals 2020, 10, 992. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Golovesov, V.; Ryabova, A.; Tkachenko, S.; Redchuk, A.; Ronkkomaki, H.; Rudakova, G.; Pervov, A.; Popov, K. Visualization of a novel fluorescent-tagged bisphosphonate behavior during reverse osmosis desalination of water with high sulfate content. Sep. Purif. Technol. 2021, 255, 117382. [Google Scholar] [CrossRef]
- Chai, C.X.; Xu, Y.H.; Li, D.Y.; Zhao, X.W.; Xu, Y.; Zhang, L.; Wu, Y.F. Cysteamine modified polyaspartic acid as a new class of green corrosion inhibitor for mild steel in sulfuric acid medium: Synthesis, electrochemical, surface study and theoretical calculation. Prog. Org. Coat. 2019, 129, 159–170. [Google Scholar] [CrossRef]
- Liu, D.; Dong, W.B.; Li, F.T.; Hui, F.; Ledion, J. Comparative performance of polyepoxysuccinic acid and polyaspartic acid on scaling inhibition by static and rapid controlled precipitation methods. Desalination 2012, 304, 1–10. [Google Scholar] [CrossRef]
- Wang, H.C.; Zhou, Y.M.; Yao, Q.Z.; Ma, S.S.; Wu, W.D.; Sun, W. Synthesis of fluorescent-tagged scale inhibitor and evaluation of its calcium carbonate precipitation performance. Desalination 2014, 340, 1–10. [Google Scholar] [CrossRef]
- Naciri, Y.; Hsini, A.; Ajmal, Z.; Bouddouch, A.; Bakiz, B.; Navio, J.A.; Albourine, A.; Valmalette, J.C.; Ezahri, M.; Benlhachemi, A. Influence of Sr-doping on structural, optical and photocatalytic properties of synthesized Ca3(PO4)2. J. Colloid Interface Sci. 2020, 572, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Nakato, T.; Tomida, M.; Suwa, M.; Morishima, Y.; Kusuno, A.; Kakuchi, T. Preparation and characterization of dodecylamine-modified poly(aspartic acid) as a biodegradable water-soluble polymeric material. Polym. Bull. 2000, 44, 385–391. [Google Scholar] [CrossRef]
- Juriga, D.; Nagy, K.; Jedlovszky-Hajdu, A.; Perczel-Kovach, K.; Chen, Y.M.; Varga, G.; Zrinyi, M. Biodegradation and osteosarcoma cell cultivation on poly(aspartic acid) based hydrogels. ACS Appl. Mater. Interfaces 2016, 8, 23463–23476. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Kong, L. Preparation and characterization of poly(aspartic acid) derivatives as biodegradable water treatment agents. Asian J. Chem. 2013, 25, 10233–10237. [Google Scholar] [CrossRef]
- Yang, J.H.; Liu, T.; Liu, H.B.; Zhang, D.; Zhai, L.M.; Liu, J.; Wang, M.; Chen, Y.X.; Chen, B.Y.; Wang, H.Y. Biodegradable PASP can effectively inhibit nitrification, moderate NH3 emission, and promote crop yield. Arch. Agron. Soil Sci. 2019, 65, 1273–1286. [Google Scholar] [CrossRef]
- Dong, F.; Jun, L.; Zhou, X.L.; Liu, Z.L. Mannich reaction in water using acidic ionic liquid as recoverable and reusable catalyst. Catal. Lett. 2007, 116, 76–80. [Google Scholar] [CrossRef]












| PSI-ASP-MEA | Mn (kDa) | Mw (kDa) | Mw/Mn |
|---|---|---|---|
| 1:0:0 | 4.87 | 7.93 | 1.63 |
| 1:1:0 | 4.96 | 8.09 | 1.63 |
| 1:0.8:0.2 | 4.94 | 8.19 | 1.66 |
| 1:0.5:0.5 | 4.90 | 7.89 | 1.61 |
| 1:0.2:0.8 | 4.91 | 7.80 | 1.59 |
| 1:0:1 | 4.92 | 7.92 | 1.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, J.; Fang, Y. Green and High Effective Scale Inhibitor Based on Ring-Opening Graft Modification of Polyaspartic Acid. Catalysts 2021, 11, 802. https://doi.org/10.3390/catal11070802
Zhou Y, Wang J, Fang Y. Green and High Effective Scale Inhibitor Based on Ring-Opening Graft Modification of Polyaspartic Acid. Catalysts. 2021; 11(7):802. https://doi.org/10.3390/catal11070802
Chicago/Turabian StyleZhou, Yongsheng, Jie Wang, and Yan Fang. 2021. "Green and High Effective Scale Inhibitor Based on Ring-Opening Graft Modification of Polyaspartic Acid" Catalysts 11, no. 7: 802. https://doi.org/10.3390/catal11070802
APA StyleZhou, Y., Wang, J., & Fang, Y. (2021). Green and High Effective Scale Inhibitor Based on Ring-Opening Graft Modification of Polyaspartic Acid. Catalysts, 11(7), 802. https://doi.org/10.3390/catal11070802