Catalytic Dehydrogenation of Ethane: A Mini Review of Recent Advances and Perspective of Chemical Looping Technology
Abstract
:1. Introduction
2. Steam Cracking Process
3. Catalytic Ethane Dehydrogenation
4. Oxidative Dehydrogenation in the Presence of Oxygen Gas
| Membrane Type | Temperature (°C) | Ethane Conversion (wt.%) | Ethylene Selectivity (%) | Reference |
|---|---|---|---|---|
| Ni-Mg | 600 | 69 | 53 | [46] |
| Ni-Nb | 400 | 45 | 76 | [40] |
| Carbon nanotubes | 400 | 20 | 60 | [47] |
| Ni-W-Ti | 330 | 40 | 70 | [48] |
| Mg-Dy-Li | 600 | 20–90 | 60–95 | [49] |
| Boron nitride | 590 | 11 | 95 | [44] |
| V-Mo-Al | 580 | 20–40 | 40–80 | [41] |
5. Oxidative Dehydrogenation of Ethane in the Presence of CO2
| Membrane Type | Temperature (°C) | Ethane Conversion (wt.%) | Ethylene Selectivity (%) | Reference |
|---|---|---|---|---|
| Cr-Ce | 700 | 55 | 96 | [57] |
| Cr-Zr | 700 | 65 | 87 | [58] |
| Fe-Cr | 650 | 54 | 93 | [59] |
| Cr2O3 | 650 | 27 | 87 | [60] |
| Cr-Si | 650 | 62 | 81 | [61] |
| Ga-Si | 650 | 25 | 92 | [62] |
| Ga-Si-Ti | 650 | 47 | 78 | [63] |
| Ga-Ti | 700 | 38 | 57 | [53] |
| Co-Ba | 650 | 48 | 92 | [64] |
| Co-Si | 700 | 46 | 85 | [65] |
| Mo2C | 600 | 2 | 60 | [66] |
6. The Membrane Oxidative Dehydrogenation Technology
7. Chemical Looping Oxidative Dehydrogenation
8. New Trends in the Ethane Conversion Process
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saito, H.; Sekine, Y. Catalytic conversion of ethane to valuable products through non-oxidative dehydrogenation and dehydroaromatization. RSC Adv. 2020, 10, 21427–21453. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef] [Green Version]
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New Trends in Olefin Production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Zhu, X.; Imtiaz, Q.; Donat, F.; Müller, C.R.; Li, F. Chemical looping beyond combustion-a perspective. Energy Environ. Sci. 2020, 13, 772–804. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Xu, R.; Li, X.; Li, Z.; Zhu, X.; Li, K. Chemical Looping Conversion of Gaseous and Liquid Fuels for Chemical Production: A Review. Energy Fuels 2020, 34, 5381–5413. [Google Scholar] [CrossRef]
- Gao, Y.; Neal, L.; Ding, D.; Wu, W.; Baroi, C.; Gaffney, A.M.; Li, F. Recent Advances in Intensified Ethylene Production—A Review. ACS Catal. 2019, 9, 8592–8621. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Goyal, R.; Sivakumar Konathala, L.N.; Pendem, C.; Sasaki, T.; Bal, R. MoO3 Nanoclusters Decorated on TiO2 Nanorods for Oxidative dehydrogenation of ethane to ethylene. Appl. Catal. B Environ. 2017, 217, 637–649. [Google Scholar] [CrossRef]
- Gong, S.; Shao, C.; Zhu, L. Energy efficiency evaluation in ethylene production process with respect to operation classification. Energy 2017, 118, 1370–1379. [Google Scholar] [CrossRef]
- Zhao, Z.; Chong, K.; Jiang, J.; Wilson, K.; Zhang, X.; Wang, F. Low-carbon roadmap of chemical production: A case study of ethylene in China. Renew. Sustain. Energy Rev. 2018, 97, 580–591. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.M.; Yu, B.; Wei, Y.M. Energy technology roadmap for ethylene industry in China. Appl. Energy 2018, 224, 160–174. [Google Scholar] [CrossRef]
- Propylene—Study: Market, Analysis, Trends|Ceresana. Available online: https://www.ceresana.com/en/market-studies/chemicals/propylene/ (accessed on 30 May 2021).
- Ethylene Uses and Market Data. Available online: http://www.icis.com/Articles/2007/11/05/9075777/ethylene-uses-and-market-data.html (accessed on 30 May 2021).
- Al-Douri, A.; Sengupta, D.; El-Halwagi, M.M. Shale gas monetization—A review of downstream processing to chemicals and fuels. J. Nat. Gas Sci. Eng. 2017, 45, 436–455. [Google Scholar] [CrossRef]
- Yang, M.; You, F. Comparative Techno-Economic and Environmental Analysis of Ethylene and Propylene Manufacturing from Wet Shale Gas and Naphtha. Ind. Eng. Chem. Res. 2017, 56, 4038–4051. [Google Scholar] [CrossRef]
- He, C.; You, F. Shale gas processing integrated with ethylene production: Novel process designs, exergy analysis, and techno-economic analysis. Ind. Eng. Chem. Res. 2014, 53, 11442–11459. [Google Scholar] [CrossRef]
- America’s New Energy Future: The Unconventional Oil & Gas Revolution and the US Economy. Available online: http://www.ihs.com/info/ecc/a/americas-new-energy-future-report-vol-3.aspx (accessed on 30 May 2021).
- Gerzeliev, I.M.; Fairuzov, D.K.; Gerzelieva, Z.I.; Maksimov, A.L. Production of Ethylene from Ethane Fraction by a Method Alternative to Steam Cracking. Russ. J. Appl. Chem. 2019, 92, 1549–1557. [Google Scholar] [CrossRef]
- Gaffney, A.M.; Mason, O.M. Ethylene production via Oxidative Dehydrogenation of Ethane using M1 catalyst. Catal. Today 2017, 285, 159–165. [Google Scholar] [CrossRef]
- Muñoz Gandarillas, A.E.; Van Geem, K.M.; Reyniers, M.F.; Marin, G.B. Influence of the reactor material composition on coke formation during ethane steam cracking. Ind. Eng. Chem. Res. 2014, 53, 6358–6371. [Google Scholar] [CrossRef]
- Van Goethem, M.W.M.; Barendregt, S.; Grievink, J.; Moulijn, J.A.; Verheijen, P.J.T. Ideal chemical conversion concept for the industrial production of ethene from hydrocarbons. Ind. Eng. Chem. Res. 2007, 46, 4045–4062. [Google Scholar] [CrossRef]
- van Goethem, M.W.M.; Barendregt, S.; Grievink, J.; Verheijen, P.J.T.; Dente, M.; Ranzi, E. A kinetic modelling study of ethane cracking for optimal ethylene yield. Chem. Eng. Res. Des. 2013, 91, 1106–1110. [Google Scholar] [CrossRef]
- Galvita, V.; Siddiqi, G.; Sun, P.; Bell, A.T. Ethane dehydrogenation on Pt/Mg(Al)O and PtSn/Mg(Al)O catalysts. J. Catal. 2010, 271, 209–219. [Google Scholar] [CrossRef]
- Wegener, E.C.; Wu, Z.; Tseng, H.T.; Gallagher, J.R.; Ren, Y.; Diaz, R.E.; Ribeiro, F.H.; Miller, J.T. Structure and reactivity of Pt–In intermetallic alloy nanoparticles: Highly selective catalysts for ethane dehydrogenation. Catal. Today 2018, 299, 146–153. [Google Scholar] [CrossRef]
- Peng, G.; Gerceker, D.; Kumbhalkar, M.; Dumesic, J.A.; Mavrikakis, M. Ethane dehydrogenation on pristine and AlO: X decorated Pt stepped surfaces. Catal. Sci. Technol. 2018, 8, 2159–2174. [Google Scholar] [CrossRef]
- Wu, J.; Peng, Z.; Bell, A.T. Effects of composition and metal particle size on ethane dehydrogenation over PtxSn100-x/Mg(Al)O (70 ≤ x ≤ 100). J. Catal. 2014, 311, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, G.; Sun, P.; Galvita, V.; Bell, A.T. Catalyst performance of novel Pt/Mg(Ga)(Al)O catalysts for alkane dehydrogenation. J. Catal. 2010, 274, 200–206. [Google Scholar] [CrossRef]
- Xie, Q.; Lei, T.; Miao, C.; Hua, W.; Yue, Y.; Gao, Z. Au/TiO2 for Ethane Dehydrogenation: Effect of Silica Doping. Catal. Letters 2020, 150, 2013–2020. [Google Scholar] [CrossRef]
- Monoue, R.; Galiasso, R.; Giannetto, G. Transformation of LPG into Aromatic Hydrocarbons and Hydrogen over Zeolite Catalysts. Catal. Rev. 1994, 36, 271–304. [Google Scholar] [CrossRef]
- Bhan, A.; Delgass, W.N. Propane aromatization over HZSM-5 and Ga/HZSM-5 catalysts. Catal. Rev. Sci. Eng. 2008, 50, 19–151. [Google Scholar] [CrossRef]
- Rao, T.V.M.; Zahidi, E.M.; Sayari, A. Ethane dehydrogenation over pore-expanded mesoporous silica-supported chromium oxide: 2. Catalytic properties and nature of active sites. J. Mol. Catal. A Chem. 2009, 301, 159–165. [Google Scholar] [CrossRef]
- Yang, X.; Wei, T.; Chi, B.; Pu, J.; Li, J. Lanthanum manganite-based perovskite as a catalyst for co-production of ethylene and hydrogen by ethane dehydrogenation. J. Catal. 2019, 377, 629–637. [Google Scholar] [CrossRef]
- Toko, K.; Ito, K.; Saito, H.; Hosono, Y.; Murakami, K.; Misaki, S.; Higo, T.; Ogo, S.; Tsuneki, H.; Maeda, S.; et al. Catalytic Dehydrogenation of Ethane over Doped Perovskite via the Mars-van Krevelen Mechanism. J. Phys. Chem. C 2020, 124, 10462–10469. [Google Scholar] [CrossRef]
- Saito, H.; Seki, H.; Hosono, Y.; Higo, T.; Seo, J.G.; Maeda, S.; Hashimoto, K.; Ogo, S.; Sekine, Y. Dehydrogenation of Ethane via the Mars-van Krevelen Mechanism over La0.8Ba0.2MnO3-δ Perovskites under Anaerobic Conditions. J. Phys. Chem. C 2019, 123, 26272–26281. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kajita, C.; Ide, Y.; Okamura, M.; Kato, S.; Kasuya, H.; Ikenaga, N.O.; Kobayashiand, T.; Suzuki, T. Promoting effect of carbon dioxide on the dehydrogenation and aromatization of ethane over gallium-loaded catalysts. Catal. Lett. 2000, 64, 215–221. [Google Scholar] [CrossRef]
- Wang, L.C.; Zhang, Y.; Xu, J.; Diao, W.; Karakalos, S.; Liu, B.; Song, X.; Wu, W.; He, T.; Ding, D. Non-oxidative dehydrogenation of ethane to ethylene over ZSM-5 zeolite supported iron catalysts. Appl. Catal. B Environ. 2019, 256, 117816. [Google Scholar] [CrossRef]
- Olsbye, U.; Virnovskaia, A.; Prytz, O.; Tinnemans, S.J.; Weckhuysen, B.M. Mechanistic insight in the ethane dehydrogenation reaction over Cr/Al2O3 catalysts. Catal. Lett. 2005, 103, 143–148. [Google Scholar] [CrossRef]
- Yokoyama, C.; Bharadwaj, S.S.; Schmidt, L.D. Platinum-Tin and Platinum-Copper Catalysts for Autothermal Oxidative Dehydrogenation of Ethane to Ethylene. 1996, 38, 181–188.
- Donsì, F.; Williams, K.A.; Schmidt, L.D. A multistep surface mechanism for ethane oxidative dehydrogenation on Pt- And Pt/Sn-coated monoliths. Ind. Eng. Chem. Res. 2005, 44, 3453–3470. [Google Scholar] [CrossRef]
- Skoufa, Z.; Heracleous, E.; Lemonidou, A.A. Investigation of engineering aspects in ethane ODH over highly selective Ni0.85Nb0.15Ox catalyst. Chem. Eng. Sci. 2012, 84, 48–56. [Google Scholar] [CrossRef]
- Solsona, B.; Dejoz, A.; Garcia, T.; Concepción, P.; Nieto, J.M.L.; Vázquez, M.I.; Navarro, M.T. Molybdenum-vanadium supported on mesoporous alumina catalysts for the oxidative dehydrogenation of ethane. Catal. Today 2006, 117, 228–233. [Google Scholar] [CrossRef]
- Cavani, F.; Ballarini, N.; Cericola, A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation? Catal. Today 2007, 127, 113–131. [Google Scholar] [CrossRef]
- Wang, H.; Cong, Y.; Yang, W. Continuous oxygen ion transfer medium as a catalyst for high selective oxidative dehydrogenation of ethane. Catal. Lett. 2002, 84, 101–106. [Google Scholar] [CrossRef]
- Shi, L.; Yan, B.; Shao, D.; Jiang, F.; Wang, D.; Lu, A.H. Selective oxidative dehydrogenation of ethane to ethylene over a hydroxylated boron nitride catalyst. Cuihua Xuebao/Chin. J. Catal. 2017, 38, 389–395. [Google Scholar] [CrossRef]
- Baroi, C.; Gaffney, A.M.; Fushimi, R. Process economics and safety considerations for the oxidative dehydrogenation of ethane using the M1 catalyst. Catal. Today 2017, 298, 138–144. [Google Scholar] [CrossRef]
- Nakamura, K.I.; Miyake, T.; Konishi, T.; Suzuki, T. Oxidative dehydrogenation of ethane to ethylene over NiO loaded on high surface area MgO. J. Mol. Catal. A Chem. 2006, 260, 144–151. [Google Scholar] [CrossRef]
- Frank, B.; Morassutto, M.; Schomäcker, R.; Schlögl, R.; Su, D.S. Oxidative dehydrogenation of ethane over multiwalled carbon nanotubes. ChemCatChem 2010, 2, 644–648. [Google Scholar] [CrossRef]
- Zhu, H.; Dong, H.; Laveille, P.; Saih, Y.; Caps, V.; Basset, J.M. Metal oxides modified NiO catalysts for oxidative dehydrogenation of ethane to ethylene. Catal. Today 2014, 228, 58–64. [Google Scholar] [CrossRef]
- Gärtner, C.A.; Van Veen, A.C.; Lercher, J.A. Oxidative dehydrogenation of ethane on dynamically rearranging supported chloride catalysts. J. Am. Chem. Soc. 2014, 136, 12691–12701. [Google Scholar] [CrossRef]
- Maffia, G.J.; Gaffney, A.M.; Mason, O.M. Techno-Economic Analysis of Oxidative Dehydrogenation Options. Top. Catal. 2016, 59, 1573–1579. [Google Scholar] [CrossRef]
- Wang, S.; Murata, K.; Hayakawa, T.; Hamakawa, S.; Suzuki, K. Oxidative dehydrogenation of ethane by carbon dioxide over sulfate-modified Cr2O3/SiO2 catalysts. Catal. Lett. 1999, 63, 59–64. [Google Scholar] [CrossRef]
- Shi, X.; Ji, S.; Li, C. Oxidative dehydrogenation of ethane with CO2 over novel Cr/SBA-15 /Al2O3/FeCrAl monolithic catalysts. Energy Fuels 2008, 22, 3631–3638. [Google Scholar] [CrossRef]
- Koirala, R.; Buechel, R.; Krumeich, F.; Pratsinis, S.E.; Baiker, A. Oxidative dehydrogenation of ethane with CO2 over flame-made Ga-loaded TiO2. ACS Catal. 2015, 5, 690–702. [Google Scholar] [CrossRef]
- Yabe, T.; Sekine, Y. Methane conversion using carbon dioxide as an oxidizing agent: A review. Fuel Process. Technol. 2018, 181, 187–198. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.H. Catalytic conversion of alkanes to olefins by carbon dioxide oxidative dehydrogenation—A review. Energy Fuels 2004, 18, 1126–1139. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- Shi, X.; Ji, S.; Wang, K. Oxidative dehydrogenation of ethane to ethylene with carbon dioxide over Cr-Ce/SBA-15 catalysts. Catal. Lett. 2008, 125, 331–339. [Google Scholar] [CrossRef]
- Rahmani, F.; Haghighi, M.; Mohammadkhani, B. Enhanced dispersion of Cr nanoparticles over nanostructured ZrO2-doped ZSM-5 used in CO2-oxydehydrogenation of ethane. Microporous Mesoporous Mater. 2017, 242, 34–49. [Google Scholar] [CrossRef]
- Deng, S.; Li, H.; Li, S.; Zhang, Y. Activity and characterization of modified Cr2O3/ZrO2 nano-composite catalysts for oxidative dehydrogenation of ethane to ethylene with CO2. J. Mol. Catal. A Chem. 2007, 268, 169–175. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kajita, C.; Ikenaga, N.O.; Suzuki, T.; Kobayashi, T.; Nishitani-Gamo, M.; Ando, T. The role of chemisorbed oxygen on diamond surfaces for the dehydrogenation of ethane in the presence of carbon dioxide. J. Phys. Chem. B 2003, 107, 4048–4056. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X. Oxidative dehydrogenation of ethane to ethylene by carbon dioxide over Cr/TS-1 catalysts. Catal. Commun. 2006, 7, 633–638. [Google Scholar] [CrossRef]
- Cheng, Y.; Lei, T.; Miao, C.; Hua, W.; Yue, Y.; Gao, Z. Ga2O3/NaZSM-5 for C2H6 dehydrogenation in the presence of CO2: Conjugated effect of silanol. Microporous Mesoporous Mater. 2018, 268, 235–242. [Google Scholar] [CrossRef]
- Lei, T.Q.; Cheng, Y.H.; Miao, C.X.; Hua, W.M.; Yue, Y.H.; Gao, Z. Silica-doped TiO2 as support of gallium oxide for dehydrogenation of ethane with CO2. Fuel Process. Technol. 2018, 177, 246–254. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, Q.; Xu, B.; He, D. Oxidative dehydrogenation of ethane over Co-BaCO3 catalysts using CO2 as oxidant: Effects of Co promoter. Catal. Lett. 2007, 117, 140–145. [Google Scholar] [CrossRef]
- Koirala, R.; Safonova, O.V.; Pratsinis, S.E.; Baiker, A. Effect of cobalt loading on structure and catalytic behavior of CoOx/SiO2 in CO2-assisted dehydrogenation of ethane. Appl. Catal. A Gen. 2018, 552, 77–85. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Myint, M.N.Z.; Kattel, S.; Xie, Z.; Gomez, E.; Liu, P.; Chen, J.G. Identifying Different Types of Catalysts for CO2 Reduction by Ethane through Dry Reforming and Oxidative Dehydrogenation. Angew. Chem. 2015, 127, 15721–15725. [Google Scholar] [CrossRef]
- Gärtner, C.A.; VanVeen, A.C.; Lercher, J.A. Oxidative dehydrogenation of ethane: Common principles and mechanistic aspects. ChemCatChem 2013, 5, 3196–3217. [Google Scholar] [CrossRef]
- Lobera, M.P.; Escolástico, S.; Serra, J.M. High ethylene production through oxidative dehydrogenation of ethane membrane reactors based on fast oxygen-ion conductors. ChemCatChem 2011, 3, 1503–1508. [Google Scholar] [CrossRef]
- Rebeilleau-Dassonneville, M.; Rosini, S.; Van Veen, A.C.; Farrusseng, D.; Mirodatos, C. Oxidative activation of ethane on catalytic modified dense ionic oxygen conducting membranes. Catal. Today 2005, 104, 131–137. [Google Scholar] [CrossRef]
- Akin, F.T.; Lin, Y.S. Selective oxidation of ethane to ethylene in a dense tubular membrane reactor. J. Memb. Sci. 2002, 209, 457–467. [Google Scholar] [CrossRef]
- Champagnie, A.M.; Tsotsis, T.T.; Minet, R.G.; Webster, A.I. A high temperature catalytic membrane reactor for ethane dehydrogenation. Chem. Eng. Sci. 1990, 45, 2423–2429. [Google Scholar] [CrossRef]
- Tonkovich, A.L.Y.; Zilka, J.L.; Jimenez, D.M.; Roberts, G.L.; Cox, J.L. Experimental investigations of inorganic membrane reactors: A distributed feed approach for partial oxidation reactions. Chem. Eng. Sci. 1996, 51, 789–806. [Google Scholar] [CrossRef]
- Wang, H.; Tablet, C.; Schiestel, T.; Caro, J. Hollow fiber membrane reactors for the oxidative activation of ethane. Catal. Today 2006, 118, 98–103. [Google Scholar] [CrossRef]
- Lobera, M.P.; Escolástico, S.; Garcia-Fayos, J.; Serra, J.M. Ethylene production by ODHE in catalytically modified Ba0.5Sr0.5Co0.8Fe0.2O3-γ membrane reactors. ChemSusChem 2012, 5, 1587–1596. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.; He, G.; Jia, L.; Jiang, H. Cobalt-free dual-phase oxygen transporting membrane reactor for the oxidative dehydrogenation of ethane. Sep. Purif. Technol. 2019, 211, 966–971. [Google Scholar] [CrossRef]
- Neal, L.M.; Yusuf, S.; Sofranko, J.A.; Li, F. Oxidative Dehydrogenation of Ethane: A Chemical Looping Approach. Energy Technol. 2016, 4, 1200–1208. [Google Scholar] [CrossRef]
- Hu, J.; Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Catalyst-assisted chemical looping auto-thermal dry reforming: Spatial structuring effects on process efficiency. Appl. Catal. B Environ. 2018, 231, 123–136. [Google Scholar] [CrossRef]
- Hu, J.; Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Pressure-induced deactivation of core-shell nanomaterials for catalyst-assisted chemical looping. Appl. Catal. B Environ. 2019, 247, 86–99. [Google Scholar] [CrossRef]
- Poelman, H.; Galvita, V. V Intensification of Chemical Looping Processes by Catalyst Assistance and Combination. Catalysts 2021, 11, 266. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, Z.; Fan, J.A.; Fan, L.S.; Gong, J. Metal oxide redox chemistry for chemical looping processes. Nat. Rev. Chem. 2018, 2, 349–364. [Google Scholar] [CrossRef]
- Tian, X.; Zheng, C.; Li, F.; Zhao, H. Co and Mo Co-doped Fe2O3 for Selective Ethylene Production via Chemical Looping Oxidative Dehydrogenation. ACS Sustain. Chem. Eng. 2021, 9, 8002–8011. [Google Scholar] [CrossRef]
- Yusuf, S.; Neal, L.; Bao, Z.; Wu, Z.; Li, F. Effects of Sodium and Tungsten Promoters on Mg6MnO8-Based Core-Shell Redox Catalysts for Chemical Looping—Oxidative Dehydrogenation of Ethane. ACS Catal. 2019, 9, 3174–3186. [Google Scholar] [CrossRef]
- Burger, C.M.; Zhu, W.; Ma, G.; Zhao, H.; van Duin, A.C.T.; Ju, Y. Experimental and computational investigations of ethane and ethylene kinetics with copper oxide particles for Chemical Looping Combustion. Proc. Combust. Inst. 2021, 38, 5249–5257. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, L.; Mu, R.; Xiong, C.; Zhao, Z.J.; Zhao, C.; Pei, C.; Peng, L.; Luo, J.; Fan, L.S.; et al. Modulating Lattice Oxygen in Dual-Functional Mo-V-O Mixed Oxides for Chemical Looping Oxidative Dehydrogenation. J. Am. Chem. Soc. 2019, 141, 18653–18657. [Google Scholar] [CrossRef]
- Zhao, K.; He, F.; Huang, Z.; Wei, G.; Zheng, A.; Li, H.; Zhao, Z. Perovskite-type oxides LaFe1-xCoxO3 for chemical looping steam methane reforming to syngas and hydrogen co-production. Appl. Energy 2016, 168, 193–203. [Google Scholar] [CrossRef]
- Novotný, P.; Yusuf, S.; Li, F.; Lamb, H.H. Oxidative dehydrogenation of ethane using MoO3/Fe2O3 catalysts in a cyclic redox mode. Catal. Today 2018, 317, 50–55. [Google Scholar] [CrossRef]
- He, F.; Chen, J.; Liu, S.; Huang, Z.; Wei, G.; Wang, G.; Cao, Y.; Zhao, K. La1-xSrxFeO3 perovskite-type oxides for chemical-looping steam methane reforming: Identification of the surface elements and redox cyclic performance. Int. J. Hydrog. Energy 2019, 44, 10265–10276. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Chen, D.; Wei, G.; He, F.; Zhao, K.; Zheng, A.; Zhao, Z.; Li, H. Exploration of Reaction Mechanisms on Hydrogen Production through Chemical Looping Steam Reforming Using NiFe2O4 Oxygen Carrier. ACS Sustain. Chem. Eng. 2019, 7, 11621–11632. [Google Scholar] [CrossRef]
- Gao, Y.; Haeri, F.; He, F.; Li, F. Alkali Metal-Promoted LaxSr2-xFeO4-δ Redox Catalysts for Chemical Looping Oxidative Dehydrogenation of Ethane. ACS Catal. 2018, 8, 1757–1766. [Google Scholar] [CrossRef]
- Yusuf, S.; Neal, L.; Haribal, V.; Baldwin, M.; Lamb, H.H.; Li, F. Manganese silicate based redox catalysts for greener ethylene production via chemical looping—Oxidative dehydrogenation of ethane. Appl. Catal. B Environ. 2018, 232, 77–85. [Google Scholar] [CrossRef]
- Yusuf, S.; Neal, L.M.; Li, F. Effect of Promoters on Manganese-Containing Mixed Metal Oxides for Oxidative Dehydrogenation of Ethane via a Cyclic Redox Scheme. ACS Catal. 2017, 7, 5163–5173. [Google Scholar] [CrossRef]
- Yusuf, S.; Haribal, V.; Jackson, D.; Neal, L.; Li, F. Mixed iron-manganese oxides as redox catalysts for chemical looping–oxidative dehydrogenation of ethane with tailorable heat of reactions. Appl. Catal. B Environ. 2019, 257, 117885. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, S.; Wang, C.; Hu, Y.H. Photocatalytic conversion of ethane: Status and perspective. Int. J. Energy Res. 2020, 44, 708–717. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, H.; Tang, S.; Liu, C.; Dong, F.; Yue, H.; Liang, B. Photocatalytic Oxidative Dehydrogenation of Ethane Using CO2 as a Soft Oxidant over Pd/TiO2 Catalysts to C2H4 and Syngas. ACS Catal. 2018, 8, 9280–9286. [Google Scholar] [CrossRef]
- Han, B.; Wei, W.; Li, M.; Sun, K.; Hu, Y.H. A thermo-photo hybrid process for steam reforming of methane: Highly efficient visible light photocatalysis. Chem. Commun. 2019, 55, 7816–7819. [Google Scholar] [CrossRef]
- Wang, S.; Luo, J.L.; Sanger, A.R.; Chuang, K.T. Performance of ethane/oxygen fuel cells using yttrium-doped barium cerate as electrolyte at intermediate temperatures. J. Phys. Chem. C 2007, 111, 5069–5074. [Google Scholar] [CrossRef]
- Ding, D.; Zhang, Y.; Wu, W.; Chen, D.; Liu, M.; He, T. A novel low-thermal-budget approach for the co-production of ethylene and hydrogen via the electrochemical non-oxidative deprotonation of ethane. Energy Environ. Sci. 2018, 11, 1710–1716. [Google Scholar] [CrossRef]
- Lin, J.Y.; Shao, L.; Si, F.Z.; Liu, S.B.; Fu, X.Z.; Luo, J.L. Co2CrO4 Nanopowders as an Anode Catalyst for Simultaneous Conversion of Ethane to Ethylene and Power in Proton-Conducting Fuel Cell Reactors. J. Phys. Chem. C 2018, 122, 4165–4171. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Q.; Fu, X.Z.; Luo, J.L. Cogeneration of ethylene and energy in protonic fuel cell with an efficient and stable anode anchored with in-situ exsolved functional metal nanoparticles. Appl. Catal. B Environ. 2018, 220, 283–289. [Google Scholar] [CrossRef]
- Liu, S.; Chuang, K.T.; Luo, J.L. Double-Layered Perovskite Anode with in Situ Exsolution of a Co-Fe Alloy to Cogenerate Ethylene and Electricity in a Proton-Conducting Ethane Fuel Cell. ACS Catal. 2016, 6, 760–768. [Google Scholar] [CrossRef]


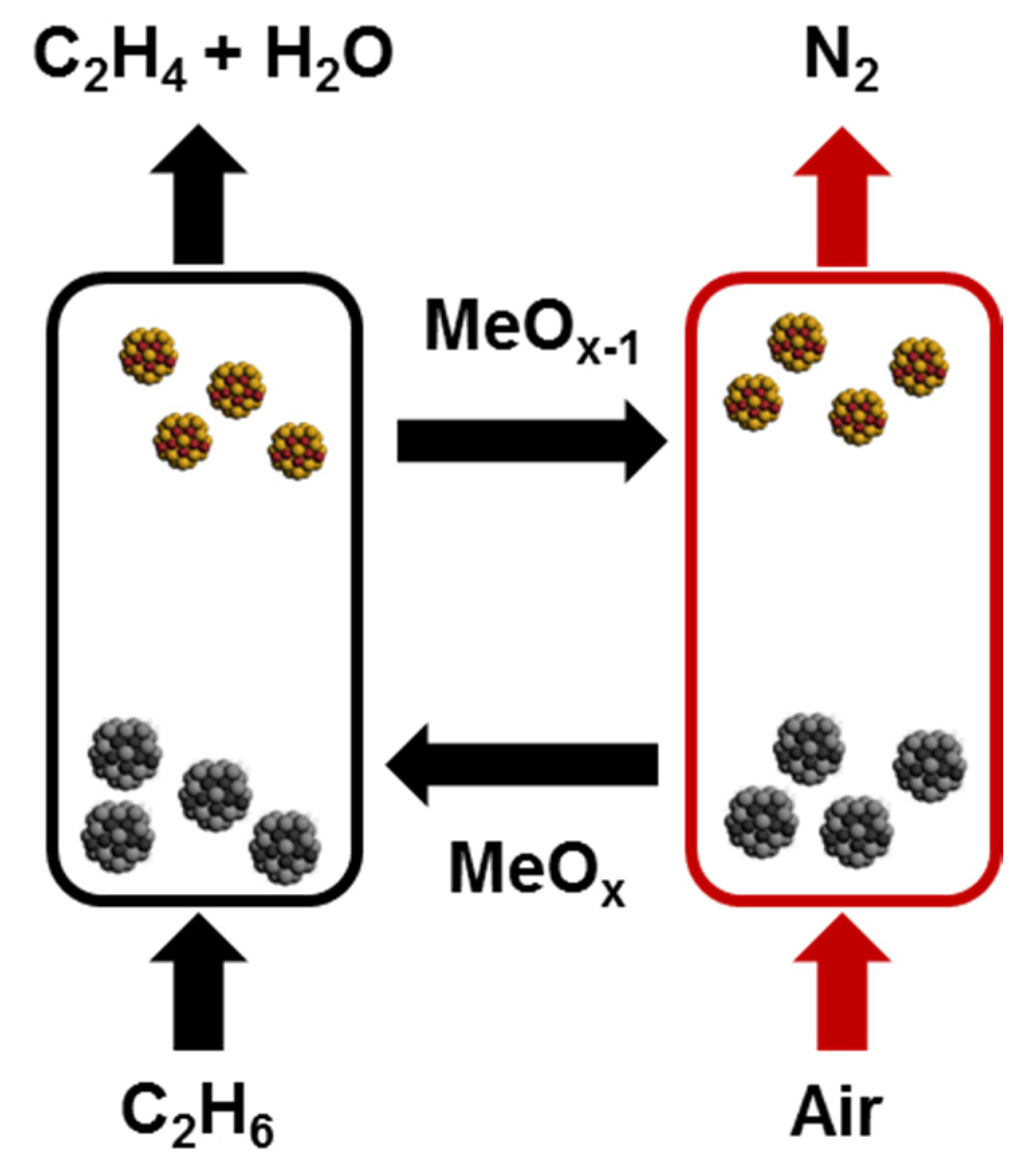
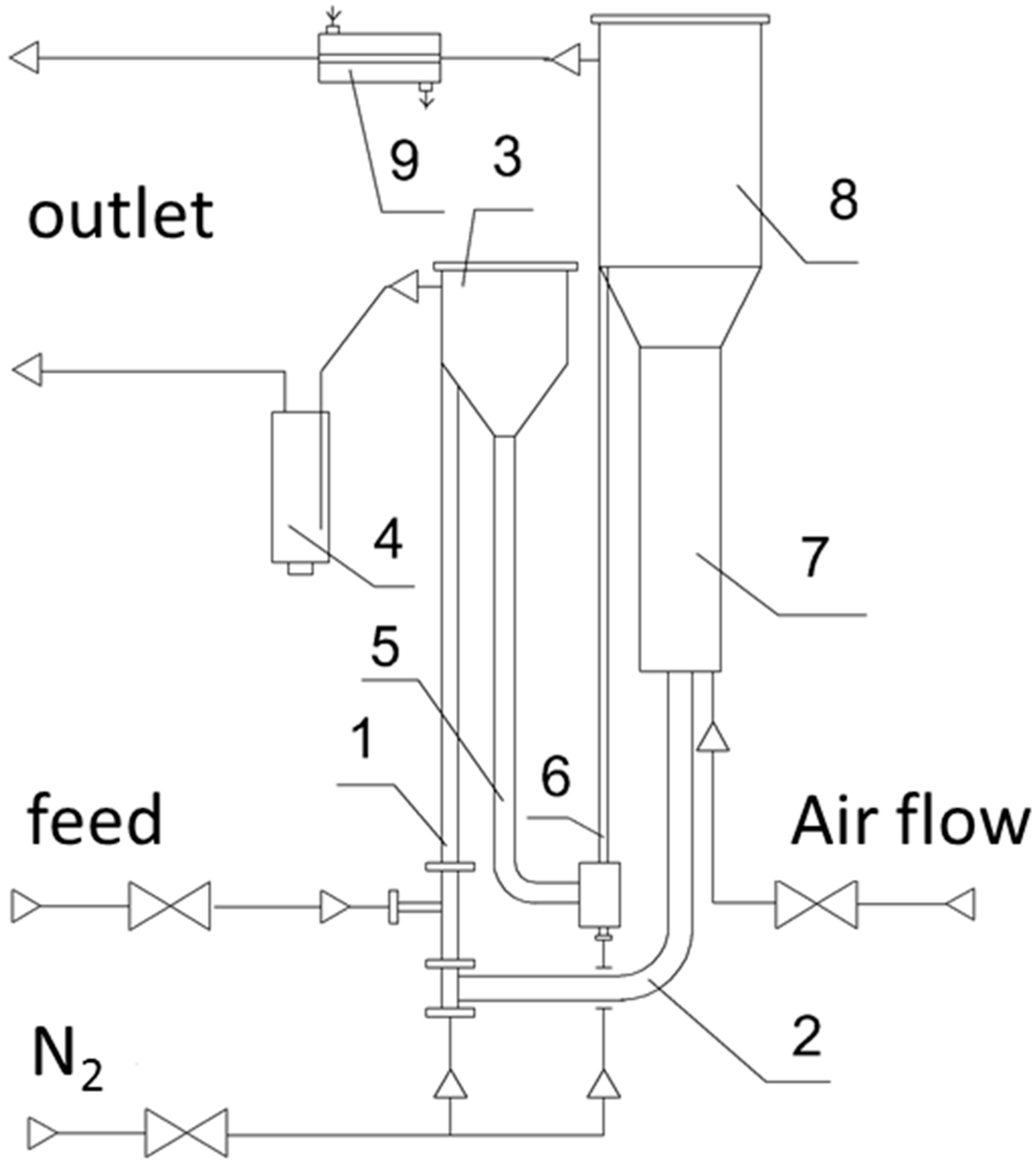
| Process | Oxidative Dehydrogenation of Ethane | Oxidative Dehydrogenation of Ethane with CO2 | Nonoxidative Dehydrogenation of Ethane |
|---|---|---|---|
| ΔH | −103 | 178 | 142 |
| Membrane Type | Temperature (°C) | Ethane Conversion (wt.%) | Ethylene Selectivity (%) | Reference |
|---|---|---|---|---|
| Bi-Y-Sm | 875 | 75 | 75 | [70] |
| Pt-Al2O3 | 600 | 46 | 96 | [71] |
| Li-Mg-Sm | 600 | 95 | 53 | [72] |
| Ba-Sr-Co-Fe | 850 | 90 | 65 | [43] |
| Ba-Sr-Co-Fe-Pd | 850 | 90 | 60 | [69] |
| Ba-Co-Fe-Zr | 850 | 83 | 25 | [73] |
| Ba-Sr-Co-Fe | 850 | 90 | 90 | [74] |
| Ba-Fe-Mg-Ce-Gd | 750 | 85 | 65 | [75] |
| Catalyst Type | Temperature (°C) | Conversion (wt.%) | Selectivity (%) | Reference |
|---|---|---|---|---|
| Mo-V/Al2O3 | 500 | 36 | 89 | [84] |
| Mg-Mn-O-Na | 850 | 92 | 12 | [91] |
| Mg-Mn-O-Na-P | 850 | 87 | 51 | [91] |
| Mg-Mn-O-Na-W | 850 | 78 | 89 | [91] |
| Mn-Si-O | 850 | 81 | 57 | [90] |
| Mn-Si-O-W | 850 | 67 | 87 | [90] |
| Fe-Mn-O | 850 | 79 | 43 | [92] |
| Fe-O | 600 | 33 | 42 | [86] |
| Mo-Fe-O | 600 | 8 | 57 | [86] |
| La-Sr-Fe-O-Na | 700 | 60 | 86 | [89] |
| Mn-Mg-O | 850 | 85 | 18 | [76] |
| Product | Pyrolysis in EP-300 Furnaces | Autothermal Pyrolysis | CL-ODH |
|---|---|---|---|
| CH4 | 5.9 | 5.5 | 2.8 |
| C2H2 | 0.4 | 0.2 | 0 |
| C2H4 | 77.9 | 79.7 | 89.2 |
| ∑C3H6 | 2.9 | 4.3 | 0.2 |
| ∑C4H8 | 4.4 | 0.5 | 0.1 |
| C3H4 | 0.7 | 0 | 0 |
| C5+ | 7.5 | 0 | 0 |
| Cox | 0.3 | 9.8 | 7.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fairuzov, D.; Gerzeliev, I.; Maximov, A.; Naranov, E. Catalytic Dehydrogenation of Ethane: A Mini Review of Recent Advances and Perspective of Chemical Looping Technology. Catalysts 2021, 11, 833. https://doi.org/10.3390/catal11070833
Fairuzov D, Gerzeliev I, Maximov A, Naranov E. Catalytic Dehydrogenation of Ethane: A Mini Review of Recent Advances and Perspective of Chemical Looping Technology. Catalysts. 2021; 11(7):833. https://doi.org/10.3390/catal11070833
Chicago/Turabian StyleFairuzov, Danis, Ilias Gerzeliev, Anton Maximov, and Evgeny Naranov. 2021. "Catalytic Dehydrogenation of Ethane: A Mini Review of Recent Advances and Perspective of Chemical Looping Technology" Catalysts 11, no. 7: 833. https://doi.org/10.3390/catal11070833
APA StyleFairuzov, D., Gerzeliev, I., Maximov, A., & Naranov, E. (2021). Catalytic Dehydrogenation of Ethane: A Mini Review of Recent Advances and Perspective of Chemical Looping Technology. Catalysts, 11(7), 833. https://doi.org/10.3390/catal11070833








