Recent Progress in Low-Cost Catalysts for Pyrolysis of Plastic Waste to Fuels
Abstract
1. Introduction
2. Clay-Based Catalysts for Plastic Pyrolysis
3. Plastic Waste-to-Fuel over Zeolite Catalysts
4. Effect of Co-Feeding with Biomass Feedstock
| Type of Biomass/Polymer | Catalyst | Reaction Condition | Conversion | Selectivity to | Remark (Catalyst/Plastic Ratio) | Ref |
|---|---|---|---|---|---|---|
| Laminaria japonica/polypropylene | Pt/mesoporous MFI, Mesoporous MFI, HZSM-5 Al-SBA-16 | 500 °C, 1 atm | 60.50 59.11 58.15 52.44 | 20–40% of monoaromatic hydrocarbon, 5–20% of polyaromatic hydrocarbon | Biomass/plastic/catalyst ratio = 10:10:1 | [86] |
| Corn stalk/HDPE | ZSM-5 | 700 °C, 1 atm | 90% of hydrocarbon | 20–30% of aromatic hydrocarbon | Biomass/plastic/catalyst = 1:4:1 | [87] |
| pine sawdust/LDPE | Ni-CaO-C | - | 90% of gas product | 86.74% to H2 gas | Biomass/plastic/catalyst: 1:1:2 | [88] |
| Corn stalk/Polystyrene | ZSM-5 | 600 °C | 90% of liquid product | 78.89% of monoaromatic hydrocarbon | 4:1:0.1 | [89] |
| L. japonica/polypropylene | Al-SBA 15 | 500 | 30% of liquid product | 35% of liquid product is oxygenate | Seaweed/polypropylene/catalyst = 1: 1:1 | [90] |
| Rice husk/PE | Ni/γ-Al2O3 | Pyrolysis at 600 °C followed by catalytic reforming at 800 °C | 80% of H2 and CO | 45% of gaseous product is H2 | 50~75% PE proportion | [91] |
5. Recent Reports Bimetallic Catalysts for Pyrolysis of Plastics
6. Factor Affecting in Pyrolysis Process
7. Conclusions and Future Prospect
Funding
Data Availability Statement
Conflicts of Interest
References
- Miandad, R.; Barakat, M.A.; Rehan, M.; Aburiazaiza, A.S.; Ismail, I.M.I.; Nizami, A.S. Plastic waste to liquid oil through catalytic pyrolysis using natural and synthetic zeolite catalysts. Waste Manag. 2017, 69, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M.; Nizami, A.-S.; Asam, Z.-U.-Z.; Ouda, O.K.M.; Gardy, J.; Raza, G.; Naqvi, M.; Mohammad Ismail, I. Waste to Energy: A Case Study of Madinah City. Energy Procedia 2017, 142, 688–693. [Google Scholar] [CrossRef]
- Samun, I.; Saeed, R.; Abbas, M.; Rehan, M.; Nizami, A.-S.; Asam, Z.-U.-Z. Assessment of Bioenergy Production from Solid Waste. Energy Procedia 2017, 142, 655–660. [Google Scholar] [CrossRef]
- Banu, J.R.; Sharmila, V.G.; Ushani, U.; Amudha, V.; Kumar, G. Impervious and influence in the liquid fuel production from municipal plastic waste through thermo-chemical biomass conversion technologies—A review. Sci. Total Environ. 2020, 718, 137287. [Google Scholar] [CrossRef]
- Zakir Hossain, H.M.; Hasna Hossain, Q.; Uddin Monir, M.M.; Ahmed, M.T. Municipal solid waste (MSW) as a source of renewable energy in Bangladesh: Revisited. Renew. Sustain. Energy Rev. 2014, 39, 35–41. [Google Scholar] [CrossRef]
- Munir, D.; Irfan, M.F.; Usman, M.R. Hydrocracking of virgin and waste plastics: A detailed review. Renew. Sustain. Energy Rev. 2018, 90, 490–515. [Google Scholar] [CrossRef]
- Walendziewski, J. Continuous flow cracking of waste plastics. Fuel Process. Technol. 2005, 86, 1265–1278. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Zhan, H.; Zhuang, X.; Song, Y.; Liu, J.; Li, S.; Chang, G.; Yin, X.; Wu, C.; Wang, X. A review on evolution of nitrogen-containing species during selective pyrolysis of waste wood-based panels. Fuel 2019, 253, 1214–1228. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Olaremu, A.G.; Adedoyin, W.R.; Ore, O.T.; Adeola, A.O. Sustainable development and enhancement of cracking processes using metallic composites. Appl. Petrochem. Res. 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Saidan, M.; Hararah, M.; Rawajfeh, K.; Alkhasawneh, H.E.; Al-Shannag, M. Wastes and biomass materials as sustainable-renewable energy resources for Jordan. Renew. Sustain. Energy Rev. 2017, 67, 295–314. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Lei, T.; Gupta, A.K. Co-pyrolysis of waste plastic and solid biomass for synergistic production of biofuels and chemicals-A review. Prog. Energy Combust. Sci. 2021, 84, 100899. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Li, H.; Wang, Y.; Liu, Y.; Cobb, K.; Cheng, Y.; Lei, H.; Chen, P.; Ruan, R. Catalytic fast pyrolysis of low density polyethylene into naphtha with high selectivity by dual-catalyst tandem catalysis. Sci. Total Environ. 2021, 771, 144995. [Google Scholar] [CrossRef]
- Budsaereechai, S.; Hunt, A.J.; Ngernyen, Y. Catalytic pyrolysis of plastic waste for the production of liquid fuels for engines. RSC Adv. 2019, 9, 5844–5857. [Google Scholar] [CrossRef]
- Kumar, B.S.; Dhakshinamoorthy, A.; Pitchumani, K. K10 montmorillonite clays as environmentally benign catalysts for organic reactions. Catal. Sci. Technol. 2014, 4, 2378–2396. [Google Scholar] [CrossRef]
- Pienkoß, F.; Ochoa-Hernández, C.; Theyssen, N.; Leitner, W. Kaolin: A Natural Low-Cost Material as Catalyst for Isomerization of Glucose to Fructose. Acs Sustain. Chem. Eng. 2018, 6, 8782–8789. [Google Scholar] [CrossRef]
- Król, M. Natural vs. Synthetic Zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Influence of Metal-Oxide-Supported Bentonites on the Pyrolysis Behavior of Polypropylene and High-Density Polyethylene. J. Appl. Polym. Sci. 2014, 132, 1–19. [Google Scholar] [CrossRef]
- Patil, V.; Adhikari, S.; Cross, P. Co-pyrolysis of lignin and plastics using red clay as catalyst in a micro-pyrolyzer. Bioresour. Technol. 2018, 270, 311–319. [Google Scholar] [CrossRef]
- Fatimah, I.; Rubiyanto, D.; Prakoso, N.I.; Yahya, A.; Sim, Y.-L. Green conversion of citral and citronellal using tris(bipyridine)ruthenium(II)-supported saponite catalyst under microwave irradiation. Sustain. Chem. Pharm. 2019, 11, 61–70. [Google Scholar] [CrossRef]
- Tarach, K.A.; Góra-Marek, K.; Martinez-Triguero, J.; Melián-Cabrera, I. Acidity and accessibility studies of desilicated ZSM-5 zeolites in terms of their effectiveness as catalysts in acid-catalyzed cracking processes. Catal. Sci. Technol. 2017, 7, 858–873. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef]
- Geng, J.; Sun, Q. Effects of high temperature treatment on physical-thermal properties of clay. Thermochim. Acta 2018, 666, 148–155. [Google Scholar] [CrossRef]
- Ferrini, P.; Dijkmans, J.; De Clercq, R.; Van de Vyver, S.; Dusselier, M.; Jacobs, P.A.; Sels, B.F. Lewis acid catalysis on single site Sn centers incorporated into silica hosts. Coord. Chem. Rev. 2017, 343, 220–255. [Google Scholar] [CrossRef]
- Panda, A.K. Thermo-catalytic degradation of different plastics to drop in liquid fuel using calcium bentonite catalyst. Int. J. Ind. Chem. 2018, 9, 167–176. [Google Scholar] [CrossRef]
- Hakeem, I.G.; Aberuagba, F.; Musa, U. Catalytic pyrolysis of waste polypropylene using Ahoko kaolin from Nigeria. Appl. Petrochem. Res. 2018, 8, 203–210. [Google Scholar] [CrossRef]
- De Stefanis, A.; Cafarelli, P.; Gallese, F.; Borsella, E.; Nana, A.; Perez, G. Catalytic pyrolysis of polyethylene: A comparison between pillared and restructured clays. J. Anal. Appl. Pyrolysis 2013, 104, 479–484. [Google Scholar] [CrossRef]
- Li, K.; Lei, J.; Yuan, G.; Weerachanchai, P.; Wang, J.-Y.; Zhao, J.; Yang, Y. Fe-, Ti-, Zr- and Al-pillared clays for efficient catalytic pyrolysis of mixed plastics. Chem. Eng. J. 2017, 317, 800–809. [Google Scholar] [CrossRef]
- Faillace, J.G.; de Melo, C.F.; de Souza, S.P.L.; da Costa Marques, M.R. Production of light hydrocarbons from pyrolysis of heavy gas oil and high density polyethylene using pillared clays as catalysts. J. Anal. Appl. Pyrolysis 2017, 126, 70–76. [Google Scholar] [CrossRef]
- Attique, S.; Batool, M.; Yaqub, M.; Görke, O.; Gregory, D.; Shah, A. Highly efficient catalytic pyrolysis of polyethylene waste to derive fuel products by novel polyoxometalate/kaolin composites. Waste Manag. Res. 2020, 0734242X1989971. [Google Scholar] [CrossRef]
- Silva, A.O.S.; Souza, M.J.B.; Pedrosa, A.M.G.; Coriolano, A.C.F.; Fernandes, V.J.; Araujo, A.S. Development of HZSM-12 zeolite for catalytic degradation of high-density polyethylene. Microporous Mesoporous Mater. 2017, 244, 1–6. [Google Scholar] [CrossRef]
- Manos, G.; Yusof, I.Y.; Gangas, N.H.; Papayannakos, N. Tertiary Recycling of Polyethylene to Hydrocarbon Fuel by Catalytic Cracking over Aluminum Pillared Clays. Energy Fuels 2002, 16, 485–489. [Google Scholar] [CrossRef]
- Kassargy, C.; Awad, S.; Burnens, G.; Kahine, K.; Tazerout, M. Experimental study of catalytic pyrolysis of polyethylene and polypropylene over USY zeolite and separation to gasoline and diesel-like fuels. J. Anal. Appl. Pyrolysis 2017, 127, 31–37. [Google Scholar] [CrossRef]
- Ghaffar, N.; Johari, A.; Tuan Abdullah, T.A.; Ripin, A. Catalytic Cracking of High Density Polyethylene Pyrolysis Vapor over Zeolite ZSM-5 towards Production of Diesel. IOP Conf. Ser. Mater. Sci. Eng. 2020, 808, 012025. [Google Scholar] [CrossRef]
- Seddegi, Z.S.; Budrthumal, U.; Al-Arfaj, A.A.; Al-Amer, A.M.; Barri, S.A.I. Catalytic cracking of polyethylene over all-silica MCM-41 molecular sieve. Appl. Catal. A Gen. 2002, 225, 167–176. [Google Scholar] [CrossRef]
- Lok, C.M.; Van Doorn, J.; Aranda Almansa, G. Promoted ZSM-5 catalysts for the production of bio-aromatics, a review. Renew. Sustain. Energy Rev. 2019, 113, 109248. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Aguayo, A.T.; Atutxa, A.; Prieto, R.; Bilbao, J. Deactivation of a HZSM-5 Zeolite Catalyst in the Transformation of the Aqueous Fraction of Biomass Pyrolysis Oil into Hydrocarbons. Energy Fuels 2004, 18, 1640–1647. [Google Scholar] [CrossRef]
- Kang, Y.-H.; Wei, X.-Y.; Liu, G.-H.; Ma, X.-R.; Gao, Y.; Li, X.; Li, Y.-J.; Ma, Y.-J.; Yan, L.; Zong, Z.-M. Catalytic Hydroconversion of Ethanol-Soluble Portion from the Ethanolysis of Hecaogou Subbituminous Coal Extraction Residue to Clean Liquid Fuel over a Zeolite Y/ZSM-5 Composite Zeolite-Supported Nickel Catalyst. Energy Fuels 2020, 34, 4799–4807. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Song, J.; Li, W.; Li, P.; Xu, R.; Ma, H.; Tian, Z. High quality diesel-range alkanes production via a single-step hydrotreatment of vegetable oil over Ni/zeolite catalyst. Catal. Today 2014, 234, 153–160. [Google Scholar] [CrossRef]
- Hou, X.; Qiu, Y.; Zhang, X.; Liu, G. Analysis of reaction pathways for n-pentane cracking over zeolites to produce light olefins. Chem. Eng. J. 2017, 307, 372–381. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Muhammad, C.; Williams, P.T. Influence of catalyst bed temperature and properties of zeolite catalysts on pyrolysis-catalysis of a simulated mixed plastics sample for the production of upgraded fuels and chemicals. J. Energy Ins. 2019, 92, 1337–1347. [Google Scholar] [CrossRef]
- Kassargy, C.; Awad, S.; Burnens, G.; Kahine, K.; Tazerout, M. Gasoline and diesel-like fuel production by continuous catalytic pyrolysis of waste polyethylene and polypropylene mixtures over USY zeolite. Fuel 2018, 224, 764–773. [Google Scholar] [CrossRef]
- Miskolczi, N.; Juzsakova, T.; Sója, J. Preparation and application of metal loaded ZSM-5 and y-zeolite catalysts for thermo-catalytic pyrolysis of real end of life vehicle plastics waste. J. Energy Ins. 2019, 92, 118–127. [Google Scholar] [CrossRef]
- Ahmed, M.H.M.; Masuda, T.; Muraza, O. The role of acidity, side pocket, and steam on maximizing propylene yield from light naphtha cracking over one-dimensional zeolites: Case studies of EU–1 and disordered ZSM–48. Fuel 2019, 258, 116034. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Zeolite catalyst design for the conversion of glucose to furans and other renewable fuels. Fuel 2019, 258, 115851. [Google Scholar] [CrossRef]
- Ghorbannezhad, P.; Park, S.; Onwudili, J.A. Co-pyrolysis of biomass and plastic waste over zeolite- and sodium-based catalysts for enhanced yields of hydrocarbon products. Waste Manag. 2020, 102, 909–918. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Wang, Q. Evaluation of zeolite catalysts on product distribution and synergy during wood-plastic composite catalytic pyrolysis. Energy 2019, 189, 116174. [Google Scholar] [CrossRef]
- Kianfar, E.; Hajimirzaee, S.; Mousavian, S.; Mehr, A.S. Zeolite-based catalysts for methanol to gasoline process: A review. Microchem. J. 2020, 156, 104822. [Google Scholar] [CrossRef]
- Han, J.; Xiong, Z.; Zhang, Z.; Zhang, H.; Zhou, P.; Yu, F. The Influence of Texture on Co/SBA–15 Catalyst Performance for Fischer–Tropsch Synthesis. Catalysts 2018, 8, 661. [Google Scholar] [CrossRef]
- Susastriawan, A.A.P.; Purnomo; Sandria, A. Experimental study the influence of zeolite size on low-temperature pyrolysis of low-density polyethylene plastic waste. Therm. Sci. Eng. Prog. 2020, 17, 100497. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Heo, S.; Choi, J.W. Effects of phenolic hydroxyl functionality on lignin pyrolysis over zeolite catalyst. Fuel 2018, 232, 81–89. [Google Scholar] [CrossRef]
- Li, T.; Ma, H.; Wu, S.; Yin, Y. Effect of highly selective oxypropylation of phenolic hydroxyl groups on subsequent lignin pyrolysis: Toward the lignin valorization. Energy Convers. Manag. 2020, 207, 112551. [Google Scholar] [CrossRef]
- Ma, H.; Li, T.; Wu, S.; Zhang, X. Effect of the interaction of phenolic hydroxyl with the benzene rings on lignin pyrolysis. Bioresour. Technol. 2020, 309, 123351. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 1–17. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Chen, H.; Williams, P.T. Investigation of nickel-impregnated zeolite catalysts for hydrogen/syngas production from the catalytic reforming of waste polyethylene. Appl. Catal. B Environ. 2018, 227, 477–487. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts. J. Energy Inst. 2019, 92, 195–202. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Adrados, A.; Laresgoiti, M.F. Deactivation and regeneration of ZSM-5 zeolite in catalytic pyrolysis of plastic wastes. Waste Manag. 2011, 31, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A.; Aranzabal, A. Catalytic pyrolysis of plastic wastes with two different types of catalysts: ZSM-5 zeolite and Red Mud. Appl. Catal. B Environ. 2011, 104, 211–219. [Google Scholar] [CrossRef]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass pyrolysis—A review of modelling, process parameters and catalytic studies. Renew. Sustain. Energy Rev. 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Ha, J.-M.; Hwang, K.-R.; Kim, Y.-M.; Jae, J.; Kim, K.H.; Lee, H.W.; Kim, J.-Y.; Park, Y.-K. Recent progress in the thermal and catalytic conversion of lignin. Renew. Sustain. Energy Rev. 2019, 111, 422–441. [Google Scholar] [CrossRef]
- Hossain, Z.; Johnson, E.N.; Wang, L.; Blackshaw, R.E.; Cutforth, H.; Gan, Y. Plant establishment, yield and yield components of Brassicaceae oilseeds as potential biofuel feedstock. Ind. Crop. Prod. 2019, 141, 111800. [Google Scholar] [CrossRef]
- Umrigar, V.R.; Chakraborty, M.; Parikh, P. Catalytic activity of zeolite Hβ for the preparation of fuels’ additives: Its product distribution and scale up calculation for the biofuel formation in a microwave assisted batch reactor. J. Environ. Chem. Eng. 2018, 6, 6816–6827. [Google Scholar] [CrossRef]
- Ibn Ferjani, A.; Jeguirim, M.; Jellali, S.; Limousy, L.; Courson, C.; Akrout, H.; Thevenin, N.; Ruidavets, L.; Muller, A.; Bennici, S. The use of exhausted grape marc to produce biofuels and biofertilizers: Effect of pyrolysis temperatures on biochars properties. Renew. Sustain. Energy Rev. 2019, 107, 425–433. [Google Scholar] [CrossRef]
- Kumar, M.; Olajire Oyedun, A.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Brigagão, G.V.; de Queiroz Fernandes Araújo, O.; de Medeiros, J.L.; Mikulcic, H.; Duic, N. A techno-economic analysis of thermochemical pathways for corncob-to-energy: Fast pyrolysis to bio-oil, gasification to methanol and combustion to electricity. Fuel Process. Technol. 2019, 193, 102–113. [Google Scholar] [CrossRef]
- Akyurek, Z. Sustainable Valorization of Animal Manure and Recycled Polyester: Co-pyrolysis Synergy. Sustainability 2019, 11, 2280. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, Y.-T.; Vispute, T.; Xiao, R.; Huber, G. Catalytic Conversion of Biomass-derived Feedstocks into Olefins and Aromatics with ZSM-5: The Hydrogen to Carbon Effective Ratio. Energy Environ. Sci. 2011, 4, 2297–2307. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, P.; Wang, J.; Jiang, P.; Wu, X.; Xue, H.; Liu, J.; Zhou, X.; Li, Q. Production of jet and diesel biofuels from renewable lignocellulosic biomass. Appl. Energy 2015, 150, 128–137. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, Z.; Xia, S.; Lu, Q.; Walters, K.B. Catalytic Pyrolysis of Biomass and Polymer Wastes. Catalysts 2018, 8, 659. [Google Scholar] [CrossRef]
- Bhoi, P.R.; Ouedraogo, A.S.; Soloiu, V.; Quirino, R. Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew. Sustain. Energy Rev. 2020, 121, 109676. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, G.; Song, W.; Zhao, Y.; Miao, Z.; Yao, R.; Gao, J. Catalytic microwave pyrolysis of orange peel: Effects of acid and base catalysts mixture on products distribution. J. Energy Inst. 2021. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Investigation of the Effect of Polystyrene (PS) Waste Washing Process and Pyrolysis Temperature on (PS) Pyrolysis Product Quality. Energy Procedia 2017, 118, 189–194. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, Y.; Yang, H.; Xin, S.; Zhang, X.; Wang, X.; Chen, H. Effect of volatiles interaction during pyrolysis of cellulose, hemicellulose, and lignin at different temperatures. Fuel 2019, 248, 1–7. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Xu, S.; Cao, B.; Uzoejinwa, B.B.; Odey, E.A.; Wang, S.; Shang, H.; Li, C.; Hu, Y.; Wang, Q.; Nwakaire, J.N. Synergistic effects of catalytic co-pyrolysis of macroalgae with waste plastics. Process Saf. Environ. Prot. 2020, 137, 34–48. [Google Scholar] [CrossRef]
- Adnan, M.A.; Xiong, Q.; Muraza, O.; Hossain, M.M. Gasification of wet microalgae to produce H2-rich syngas and electricity: A thermodynamic study considering exergy analysis. Renew. Energy 2020, 147, 2195–2205. [Google Scholar] [CrossRef]
- Kositkanawuth, K.; Sattler, M.L.; Dennis, B. Pyrolysis of Macroalgae and Polysytrene: A Review. Curr. Sustain. Renew. Energy 2014, 1, 121–128. [Google Scholar] [CrossRef][Green Version]
- Milledge, J.; Benjamin, S.; Dyer, P.; Harvey, P. Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Li, F.; Srivatsa, S.C.; Bhattacharya, S. A review on catalytic pyrolysis of microalgae to high-quality bio-oil with low oxygeneous and nitrogenous compounds. Renew. Sustain. Energy Rev. 2019, 108, 41–497. [Google Scholar] [CrossRef]
- Qari, H.; Rehan, M.; Nizami, A.-S. Key Issues in Microalgae Biofuels: A Short Review. Energy Procedia 2017, 142, 898–903. [Google Scholar] [CrossRef]
- Chen, W.; Lu, J.; Zhang, C.; Xie, Y.; Wang, Y.; Wang, J.; Zhang, R. Aromatic hydrocarbons production and synergistic effect of plastics and biomass via one-pot catalytic co-hydropyrolysis on HZSM-5. J. Anal. Appl. Pyrolysis 2020, 147, 104800. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, H.W.; Choi, S.J.; Jeon, J.-K.; Park, S.H.; Jung, S.-C.; Kim, S.C.; Park, Y.-K. Catalytic co-pyrolysis of polypropylene and Laminaria japonica over zeolitic materials. Int. J. Hydrogen Energy 2017, 42, 18434–18441. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Z.; Ding, K.; Song, Z. Production of aromatic hydrocarbons from catalytic co-pyrolysis of biomass and high density polyethylene: Analytical Py–GC/MS study. Fuel 2015, 139, 622–628. [Google Scholar] [CrossRef]
- Chai, Y.; Gao, N.; Wang, M.; Wu, C. H2 production from co-pyrolysis/gasification of waste plastics and biomass under novel catalyst Ni-CaO-C. Chem. Eng. J. 2020, 382, 122947. [Google Scholar] [CrossRef]
- Muneer, B.; Zeeshan, M.; Qaisar, S.; Razzaq, M.; Iftikhar, H. Influence of in-situ and ex-situ HZSM-5 catalyst on co-pyrolysis of corn stalk and polystyrene with a focus on liquid yield and quality. J. Clean. Prod. 2019, 237, 117762. [Google Scholar] [CrossRef]
- Lee, H.; Choi, S.; Park, S.; Jeon, J.-K.; Jung, S.-C.; Kim, S.; Park, Y.-K. Pyrolysis and co-pyrolysis of Laminaria japonica and polypropylene over mesoporous Al-SBA-15 catalyst. Nanoscale Res. Lett. 2014, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xiong, Y.; Ye, J.; Su, Y.; Dong, Q.; Zhang, S. Performances of syngas production and deposited coke regulation during co-gasification of biomass and plastic wastes over Ni/γ-Al2O3 catalyst: Role of biomass to plastic ratio in feedstock. Chem. Eng. J. 2020, 392, 123728. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, Z.; Sun, J.; Wang, Q. Recent progress of catalytic pyrolysis of biomass by HZSM-5. Chin. J. Catal. 2013, 34, 641–650. [Google Scholar] [CrossRef]
- Balasundram, V.; Ibrahim, N.; Kasmani, R.M.; Isha, R.; Hamid, M.K.A.; Hasbullah, H.; Ali, R.R. Catalytic upgrading of sugarcane bagasse pyrolysis vapours over rare earth metal (Ce) loaded HZSM-5: Effect of catalyst to biomass ratio on the organic compounds in pyrolysis oil. Appl. Energy 2018, 220, 787–799. [Google Scholar] [CrossRef]
- Wen, X.; Chen, X.; Tian, N.; Gong, J.; Liu, J.; Rümmeli, M.H.; Chu, P.K.; Mijiwska, E.; Tang, T. Nanosized Carbon Black Combined with Ni2O3 as “Universal” Catalysts for Synergistically Catalyzing Carbonization of Polyolefin Wastes to Synthesize Carbon Nanotubes and Application for Supercapacitors. Environ. Sci. Technol. 2014, 48, 4048–4055. [Google Scholar] [CrossRef] [PubMed]
- Kaya, B.; Irmak, S.; Hasanoğlu, A.; Erbatur, O. Developing Pt based bimetallic and trimetallic carbon supported catalysts for aqueous-phase reforming of biomass-derived compounds. Int. J. Hydrog. Energy 2015, 40, 3849–3858. [Google Scholar] [CrossRef]
- Yao, D.; Wu, C.; Yang, H.; Zhang, Y.; Nahil, M.A.; Chen, Y.; Williams, P.T.; Chen, H. Co-production of hydrogen and carbon nanotubes from catalytic pyrolysis of waste plastics on Ni-Fe bimetallic catalyst. Energy Convers. Manag. 2017, 148, 692–700. [Google Scholar] [CrossRef]
- Chen, T.; Yu, J.; Ma, C.; Bikane, K.; Sun, L. Catalytic performance and debromination of Fe–Ni bimetallic MCM-41 catalyst for the two-stage pyrolysis of waste computer casing plastic. Chemosphere 2020, 248, 125964. [Google Scholar] [CrossRef]
- Yao, D.; Wang, C.-H. Pyrolysis and in-line catalytic decomposition of polypropylene to carbon nanomaterials and hydrogen over Fe- and Ni-based catalysts. Appl. Energy 2020, 265, 114819. [Google Scholar] [CrossRef]
- Li, J.; Xia, H.; Wu, Q.; Hu, Z.; Hao, Z.; Zhu, Z. Hydrocracking of the crude oil from thermal pyrolysis of municipal wastes over bi-functional Mo–Ni catalyst. Catal. Today 2016, 271, 172–178. [Google Scholar] [CrossRef]
- Sridhar, A.; Rahman, M.; Infantes-Molina, A.; Wylie, B.J.; Borcik, C.G.; Khatib, S.J. Bimetallic Mo-Co/ZSM-5 and Mo-Ni/ZSM-5 catalysts for methane dehydroaromatization: A study of the effect of pretreatment and metal loadings on the catalytic behavior. Appl. Catal. A Gen. 2020, 589, 117247. [Google Scholar] [CrossRef]
- Upare, D.P.; Park, S.; Kim, M.S.; Jeon, Y.P.; Kim, J.; Lee, D.; Lee, J.; Chang, H.; Choi, S.; Choi, W.; et al. Selective hydrocracking of pyrolysis fuel oil into benzene, toluene and xylene over CoMo/beta zeolite catalyst. J. Ind. Eng. Chem. 2017, 46, 356–363. [Google Scholar] [CrossRef]
- Hamid, S.; Niaz, Y.; Bae, S.; Lee, W. Support induced influence on the reactivity and selectivity of nitrate reduction by Sn-Pd bimetallic catalysts. J. Environ. Chem. Eng. 2020, 8, 103754. [Google Scholar] [CrossRef]
- Cai, N.; Yang, H.; Zhang, X.; Xia, S.; Yao, D.; Bartocci, P.; Fantozzi, F.; Chen, Y.; Chen, H.; Williams, P.T. Bimetallic carbon nanotube encapsulated Fe-Ni catalysts from fast pyrolysis of waste plastics and their oxygen reduction properties. Waste Manag. 2020, 109, 119–126. [Google Scholar] [CrossRef]
- Kasar, P.; Sharma, D.K.; Ahmaruzzaman, M. Thermal and catalytic decomposition of waste plastics and its co-processing with petroleum residue through pyrolysis process. J. Clean. Prod. 2020, 265, 121639. [Google Scholar] [CrossRef]
- Gao, Z.; Li, N.; Wang, Y.; Niu, W.; Yi, W. Pyrolysis behavior of xylan-based hemicellulose in a fixed bed reactor. J. Anal. Appl. Pyrolysis 2020, 146, 104772. [Google Scholar] [CrossRef]
- Lai, C.; Wang, J.; Lei, W.; Xuan, C.; Xiao, W.; Zhao, T.; Huang, T.; Chen, L.; Zhu, Y.; Wang, D. Restricting Growth of Ni3Fe Nanoparticles on Heteroatom-Doped Carbon Nanotube/Graphene Nanosheets as Air-Electrode Electrocatalyst for Zn–Air Battery. Acs Appl. Mater. Interfaces 2018, 10, 38093–38100. [Google Scholar] [CrossRef]
- Zhou, H.; Saad, J.M.; Li, Q.; Xu, Y. Steam reforming of polystyrene at a low temperature for high H2/CO gas with bimetallic Ni-Fe/ZrO2 catalyst. Waste Manag. 2020, 104, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Baamran, K.S.; Tahir, M. Ni-embedded TiO2-ZnTiO3 reducible perovskite composite with synergistic effect of metal/support towards enhanced H2 production via phenol steam reforming. Energy Convers. Manag. 2019, 200, 112064. [Google Scholar] [CrossRef]
- Kharaji, A.G.; Shariati, A.; Takassi, M.A. A Novel γ-Alumina Supported Fe-Mo Bimetallic Catalyst for Reverse Water Gas Shift Reaction. Chin. J. Chem. Eng. 2013, 21, 1007–1014. [Google Scholar] [CrossRef]
- Wu, P.; Sun, J.; Abbas, M.; Wang, P.; Chen, Y.; Chen, J. Hydrophobic SiO2 supported Fe-Ni bimetallic catalyst for the production of high-calorie synthetic natural gas. Appl. Catal. A Gen. 2020, 590, 117302. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Awadallah, A.E. Production of nanostructured carbon materials using Fe–Mo/MgO catalysts via mild catalytic pyrolysis of polyethylene waste. Chem. Eng. J. 2018, 354, 802–816. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Awadallah, A.E. Production of nanostructure carbon materials via non-oxidative thermal degradation of real polypropylene waste plastic using La2O3 supported Ni and Ni-Cu catalysts. Polym. Degrad. Stab. 2019, 167, 157–169. [Google Scholar] [CrossRef]
- Bajad, G.S.; Tiwari, S.K.; Vijayakumar, R.P. Synthesis and characterization of CNTs using polypropylene waste as precursor. Mater. Sci. Eng. B 2015, 194, 68–77. [Google Scholar] [CrossRef]
- Shen, Y.; Gong, W.; Zheng, B.; Gao, L. Ni–Al bimetallic catalysts for preparation of multiwalled carbon nanotubes from polypropylene: Influence of the ratio of Ni/Al. Appl. Catal. B Environ. 2016, 181, 769–778. [Google Scholar] [CrossRef]
- Nahil, M.A.; Wu, C.; Williams, P.T. Influence of metal addition to Ni-based catalysts for the co-production of carbon nanotubes and hydrogen from the thermal processing of waste polypropylene. Fuel Process. Technol. 2015, 130, 46–53. [Google Scholar] [CrossRef]
- Wu, S.-L.; Kuo, J.-H.; Wey, M.-Y. Thermal degradation of waste plastics in a two-stage pyrolysis-catalysis reactor over core-shell type catalyst. J. Anal. Appl. Pyrolysis 2019, 104641. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Awadallah, A.E. Impact of Co/Mo ratio on the activity of CoMo/MgO catalyst for production of high-quality multi-walled carbon nanotubes from polyethylene waste. Mater. Chem. Phys. 2019, 238, 121879. [Google Scholar] [CrossRef]
- Wang, J.; Shen, B.; Lan, M.; Kang, D.; Wu, C. Carbon nanotubes (CNTs) production from catalytic pyrolysis of waste plastics: The influence of catalyst and reaction pressure. Catalysis Today 2019, 351, 50–57. [Google Scholar] [CrossRef]
- Wu, C.; Nahil, M.A.; Miskolczi, N.; Huang, J.; Williams, P.T. Processing Real-World Waste Plastics by Pyrolysis-Reforming for Hydrogen and High-Value Carbon Nanotubes. Environ. Sci. Technol. 2014, 48, 819–826. [Google Scholar] [CrossRef]
- Saravanan, A.; Hemavathy, R.V.; Sundararaman, T.R.; Jeevanantham, S.; Kumar, P.S.; Yaashikaa, P.R. 1—Solid waste biorefineries. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 3–17. [Google Scholar]
- Xue, Y.; Zhou, S.; Brown, R.C.; Kelkar, A.; Bai, X. Fast pyrolysis of biomass and waste plastic in a fluidized bed reactor. Fuel 2015, 156, 40–46. [Google Scholar] [CrossRef]
- Orozco, S.; Alvarez, J.; Lopez, G.; Artetxe, M.; Bilbao, J.; Olazar, M. Pyrolysis of plastic wastes in a fountain confined conical spouted bed reactor: Determination of stable operating conditions. Energy Convers. Manag. 2021, 229, 113768. [Google Scholar] [CrossRef]
- Khlewee, M.; Gunukula, S.; Wheeler, M.C.; DeSisto, W.J. Hydrotreating of reduced oxygen content bio-oils produced by formate-assisted pyrolysis. Fuel 2019, 254, 115570. [Google Scholar] [CrossRef]
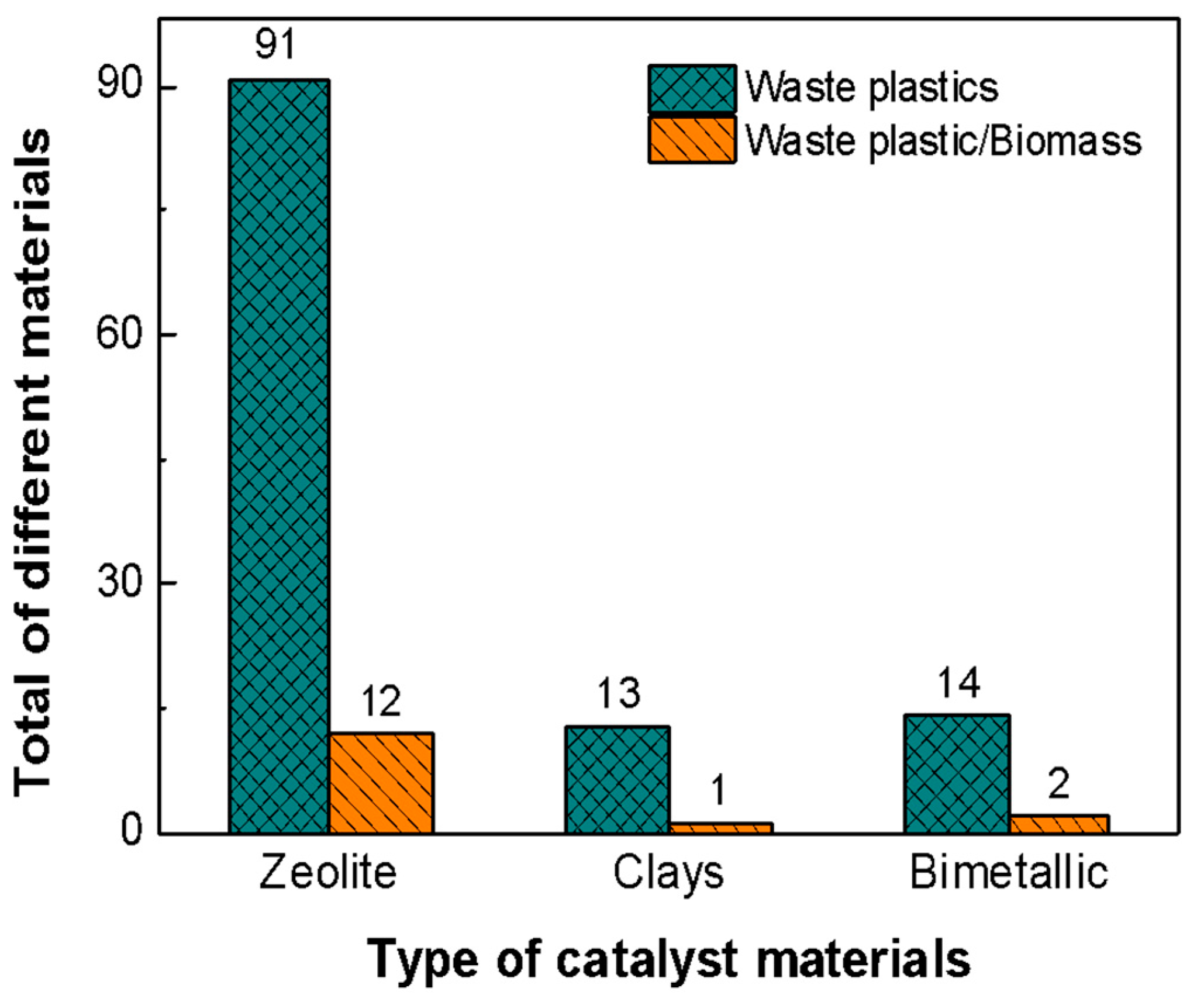
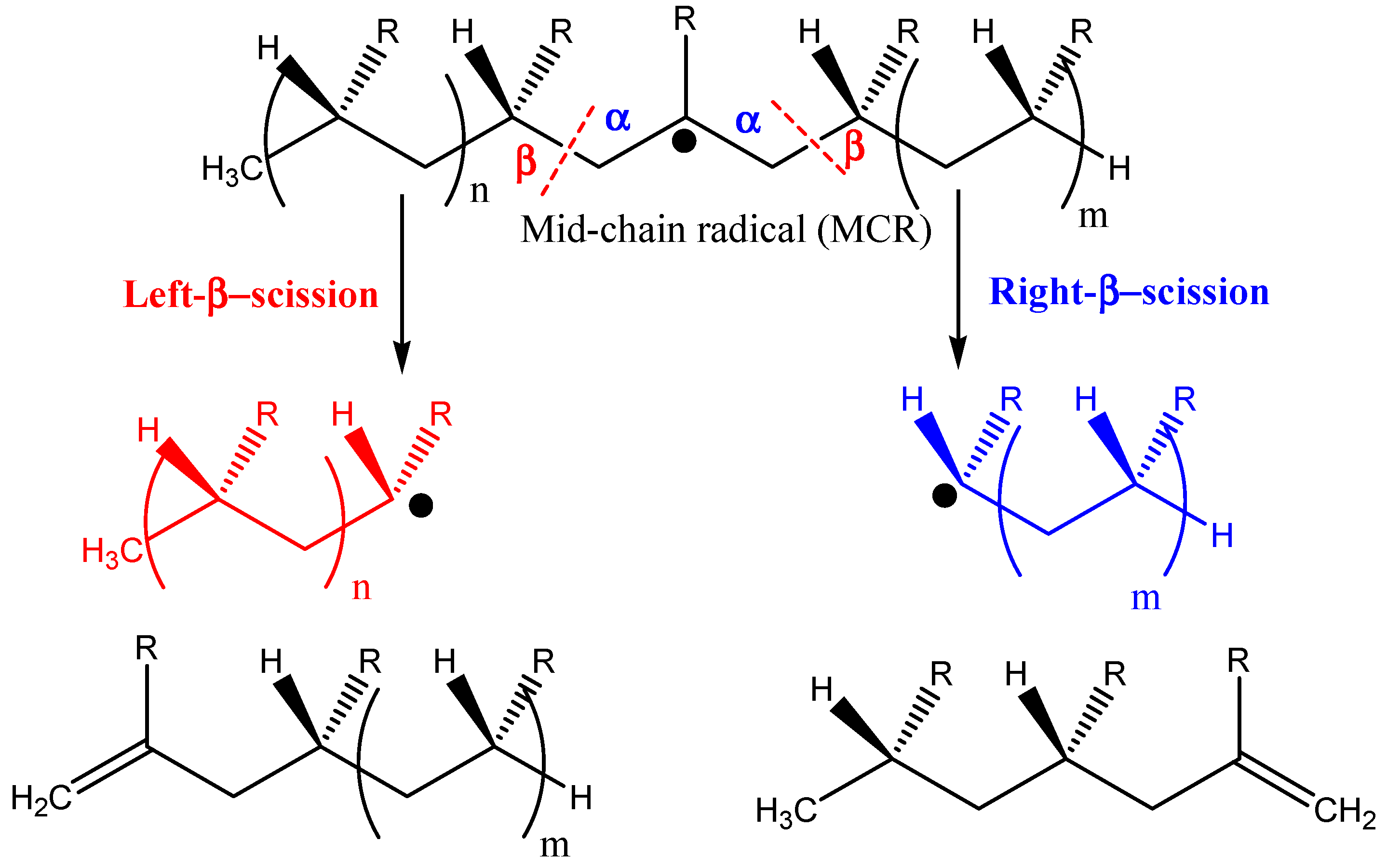
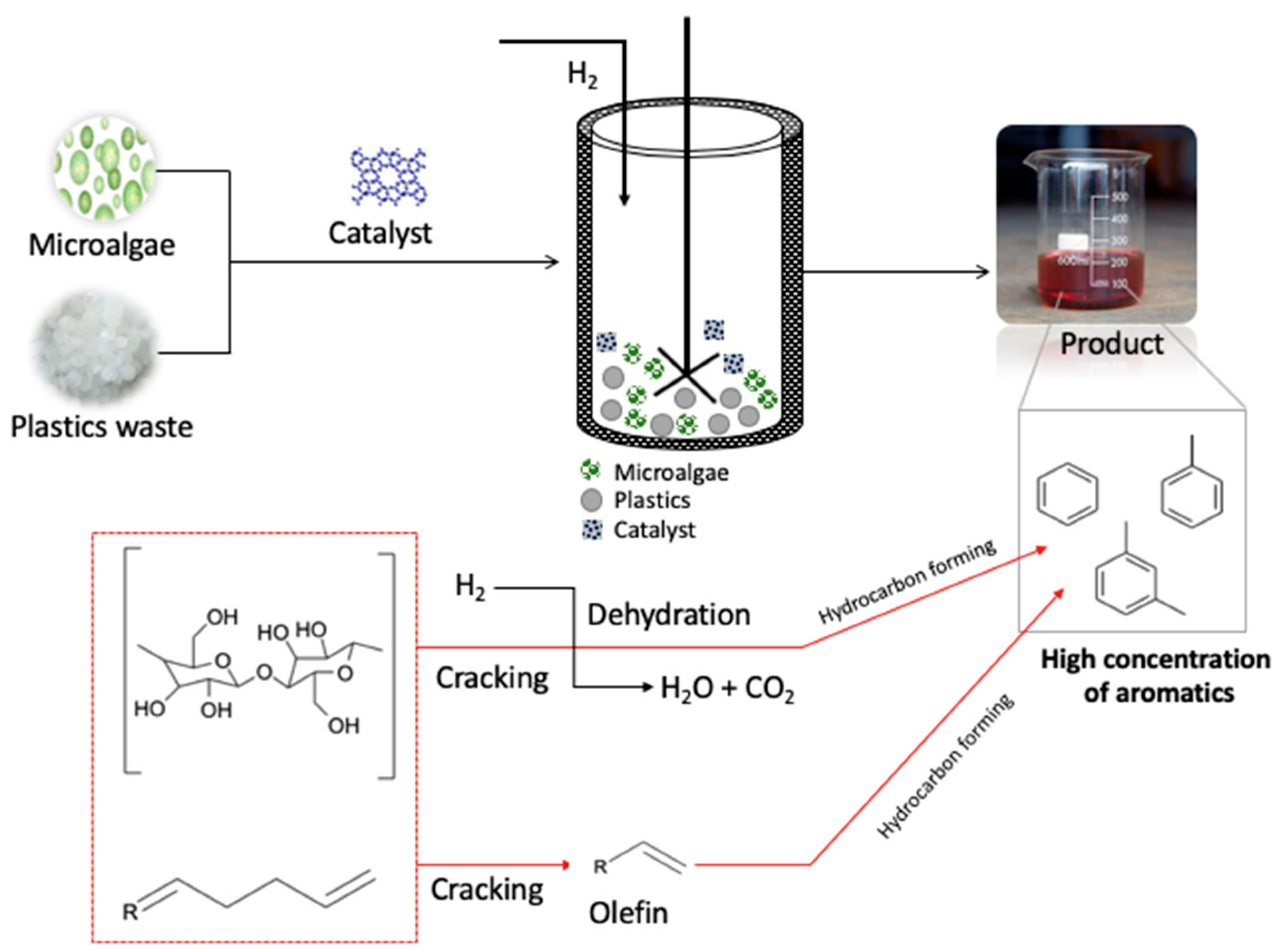
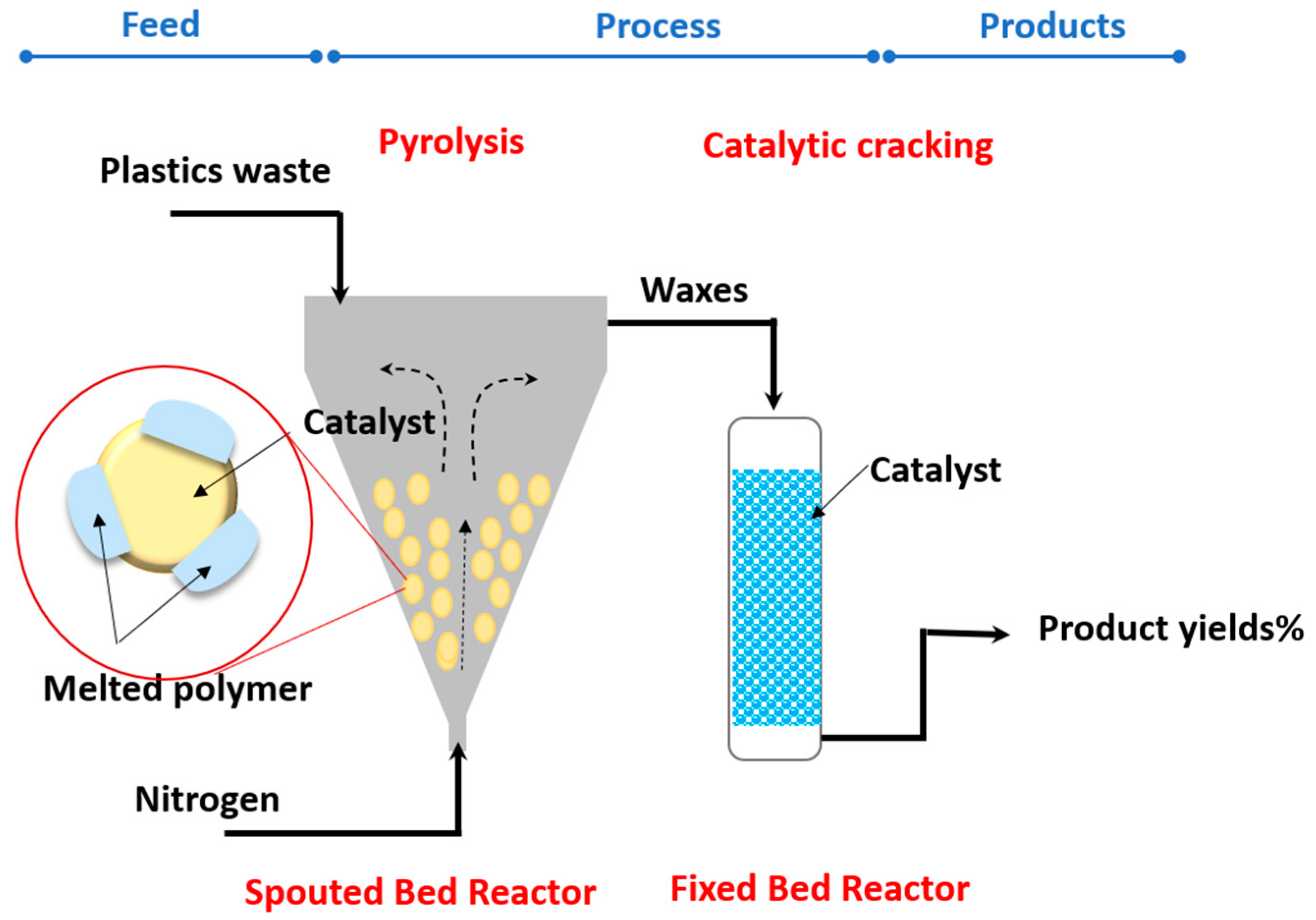
| No | Catalyst | Plastic Type | Result | Ref |
|---|---|---|---|---|
| 1 | Calcium Bentonite | Polypropylene (PP), low-density polyethylene (LDPE), and high-density polyethylene (HDPE) | The yield was influenced by temperature and ratio of effect to catalyst. The major product as condensable fraction was in the temperature range 400–550 °C and the optimum condition was at 500 °C at the catalyst to plastics ratio of 1:3 | [27] |
| 2 | Kaolin | PP | Ahoko kaolin exhibited as effective as a low-cost catalyst for producing gasoline/diesel grade fuel with the PP as waste sources. The yield was influenced by catalyst to plastic ratio | [28] |
| 3 | Restructured and pillared clay | polyolefin | Restructured and pillared clay showed good selectivity towards aliphatic, produced more liquid | [29] |
| 5 | Fe, Ti, Zr- pillared clay | HDPE, polystyrene [PS], PP | Fe-pillared clay showed excellent yield of diesel fraction in liquid product and H2 | [30] |
| 6 | Fe-pillared clay | Heavy gas oil(HGO)/HDPE | The presence of HGO improved the oil yield from both thermal and catalytic pyrolysis of HDPE | [31] |
| 7 | Tungstophosphoric acid (TPA)/kaolin | Low-density polyethylene (LDPE) | TPA loaded kaolin (5-TPA-K) produced higher percentages of gasoline-like hydrocarbons (C11–C14) | [32] |
| 8 | Co, Fe, Mn, Zn impregnated acid-washed bentonite clay (AWBC) | PP and HDPE | Metal oxide impregnation on acid-washed bentonite clay not only improves conversion but also yield reduce coke formation | [20] |
| Manos |
| Type of Plastic | Catalyst | Reaction Condition | Conversion | Selectivity to | Remark (Catalyst/Plastic Ratio) | Ref |
|---|---|---|---|---|---|---|
| Polystyrene and polyolefeins (PS/PO) | Y-zeolite | 600 °C for 30 min under N2 gas | High yield valuable aromatics such as benzene and toluene | 90% of the aromatic content | 2 g of catalyst and 2 g of plastic | [43] |
| Polyethylene and polypropylene (PE/PP) | USY-zeolite | 500 °C | Liquid fractions are dominated by hydrocarbon (C5–C7), C3 and C4 for gaseous products. | 80% of liquid production | Catalyst/plastics ratio of 1:10 | [44] |
| Polystyrene (PS) | Natural/Synthetic zeolite | 450 °C for 75 min | 60.8% conversion to ethylbenzene and 38.3% convertsion to alpha-methylstyrene for natural and synthetic zeolite respectively | 54% and 50% of liquids products for natural and synthetic zeolite, respectively | Catalys/PS ratio of 0.1 kg:1 kg | [1] |
| High density Polyethylene (HDPE)) | Co-Y–zeolite | 600 °C for 30 min | 40% of gas yield | 68% of hydrogen production | Catalys/HDPE ratio of 2:1 | [58] |
| Plastic mixtures (HDPE/PP/PS/PET/PVC) | Regenerated ZSM-5 | 440 °C for 30 min | Almost 60% of plastic waste conversion to liquids phase | 97.4% of aromatics with 23% of styrene as major composition | Catalyst/plastic waste ratio of 1:10 | [59] |
| Plastic mixtures (PE/PP/PS/PET/PVC) | ZSM-5 | 500 °C for 30 min | 58.4% conversion to gases phase | 50.7% of C3–C4 types and 27.9% of styrene | Catalyst/plastic waste ratio of 1:10 | [60] |
| Type of Plastic | 1st Metal | 2nd Metal | Condition, Pressure (atm), Temperature (°C) | Conversion | Selectivity to | Remark (Catalyst/Plastic Ratio) | Ref |
|---|---|---|---|---|---|---|---|
| Low density polyethylene (LDPE) | Mo-MgO | Fe | Atmospheric pressure, 750 and 400 °C | LDPE waste plastic to carbon nanotubes | High quality carbon nanostructures materials | 0.5 g:15 g plastics | [111] |
| Polypropylene (PP) | La2O3 | Ni-Cu | 500 °C, 700 °C for 2.5 h | PP to Carbon nanotubes and carbon nanofibers | Carbon yields of 1458% produced | 0.5 g:15 g plastics | [112] |
| Polypropylene (PP) | MgO | Ni/Mo | 800 °C, 10 min | PP to CNT | 394% of carbon product | 0.15 g:5 g polymer | [113] |
| Polypropylene (PP) | Ni- | Al | 800 °C | PP to MWCNTs | 85% | Dependence on the ratio Ni/Al and the amount of Ni-Al catalyst | [114] |
| Polypropylene (PP) | Ni-Al | Zn, Mg, Ca, Ce, Mn | 500 °C | PP to CNTs | The highest carbon deposition 62% and hydrogen 86.4% to Ni-Mn-Al | 1 g:2 g waste polypropylene | [115] |
| Polyehtylene (PE) | Ni | Ce (Ni-Ce core by silica) | 800 °C | PE to hydrogen | Hydrogen concentration 60% | Weight ratio Catalyst:plastic 1.0 | [116] |
| LDPE | Ni | Fe | 800 °C | Carbon nanotubes (CNTs) | Maximum hydrogen concentration and hydrogen yield 73.93% and 84.72 mg.g−1 | 0.5 g:1 g waste plastic | [96] |
| Low-density polyethylene (LDPE) waste | MgO | Co/Mo | 400 °C | High quality multi-walled Carbon Nanotubes and hydrogen | Optimum CNTs 1040% wtCoMo(6.5) MgO | 0.75 g:15 g plastics | [117] |
| Polypropylene (PP) | Ni | Fe | 500 °C | CNTs | 93% filamentous carbon nanotubes | 0.5 catalyst:1 g PP | [118] |
| HDPE | Ni | Mn-Al | 800 °C | Hydrogen and carbon nanotubes | 48% total carbon (with no steam), hydrogen yield 94,4% (with steam) | 0.5 g catalyst:1 g waste plastic (HDPE) | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadillah, G.; Fatimah, I.; Sahroni, I.; Musawwa, M.M.; Mahlia, T.M.I.; Muraza, O. Recent Progress in Low-Cost Catalysts for Pyrolysis of Plastic Waste to Fuels. Catalysts 2021, 11, 837. https://doi.org/10.3390/catal11070837
Fadillah G, Fatimah I, Sahroni I, Musawwa MM, Mahlia TMI, Muraza O. Recent Progress in Low-Cost Catalysts for Pyrolysis of Plastic Waste to Fuels. Catalysts. 2021; 11(7):837. https://doi.org/10.3390/catal11070837
Chicago/Turabian StyleFadillah, Ganjar, Is Fatimah, Imam Sahroni, Muhammad Miqdam Musawwa, Teuku Meurah Indra Mahlia, and Oki Muraza. 2021. "Recent Progress in Low-Cost Catalysts for Pyrolysis of Plastic Waste to Fuels" Catalysts 11, no. 7: 837. https://doi.org/10.3390/catal11070837
APA StyleFadillah, G., Fatimah, I., Sahroni, I., Musawwa, M. M., Mahlia, T. M. I., & Muraza, O. (2021). Recent Progress in Low-Cost Catalysts for Pyrolysis of Plastic Waste to Fuels. Catalysts, 11(7), 837. https://doi.org/10.3390/catal11070837










