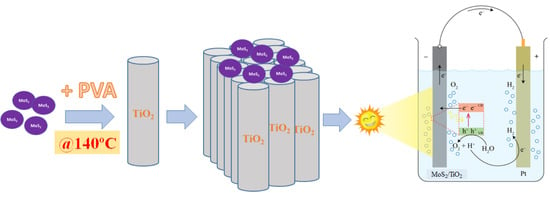
Thermal Treatment of Polyvinyl Alcohol for Coupling MoS2 and TiO2 Nanotube Arrays toward Enhancing Photoelectrochemical Water Splitting Performance
Abstract
1. Introduction
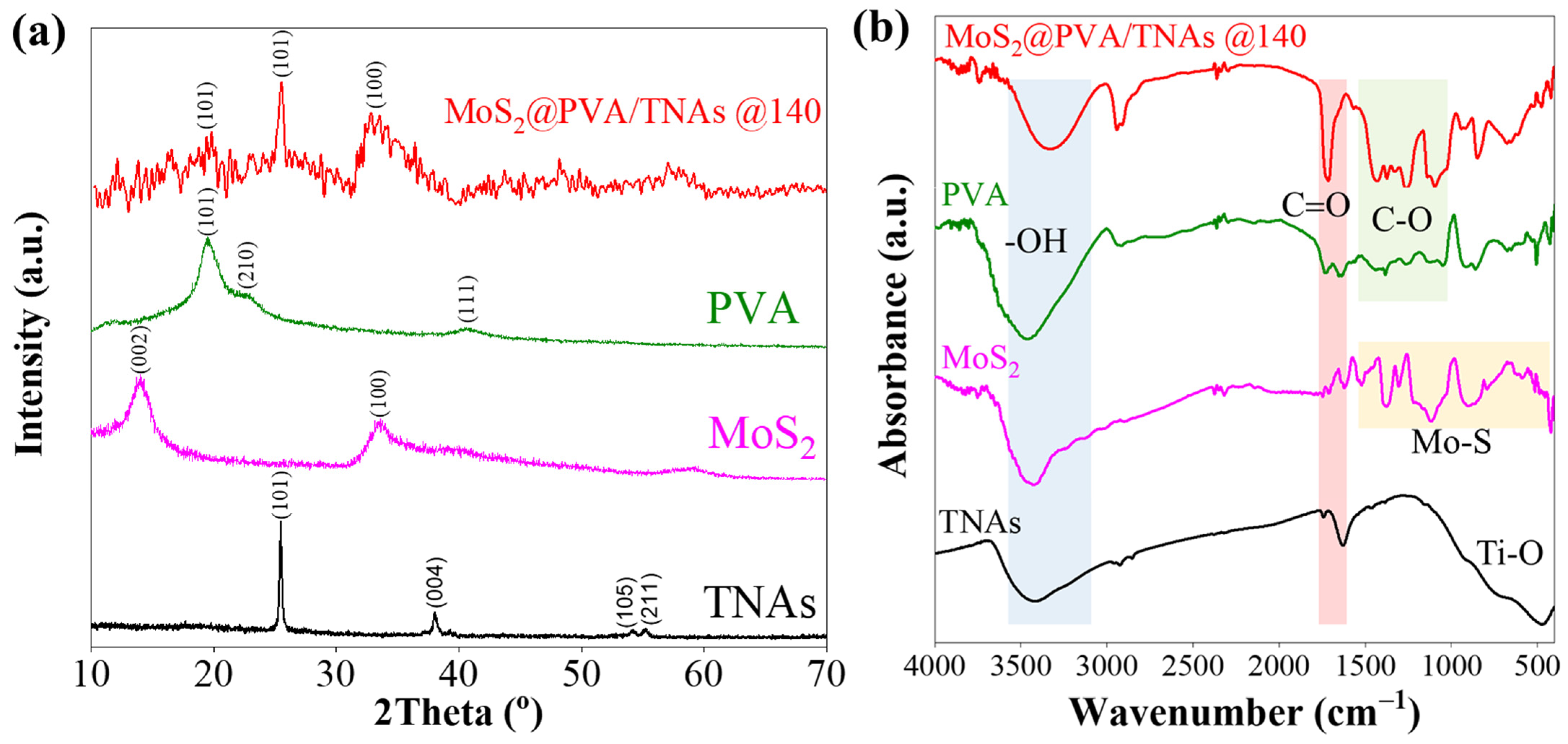
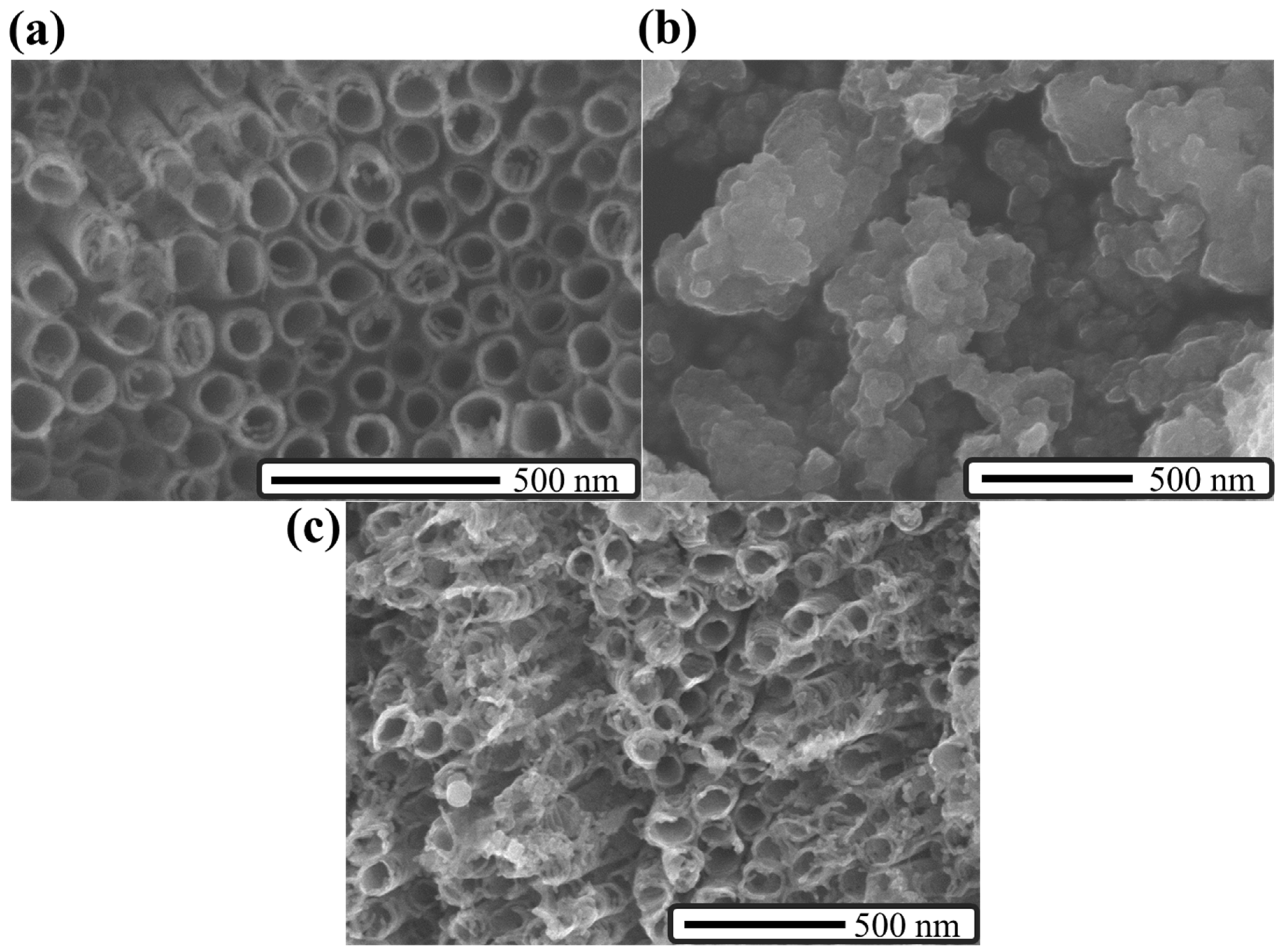
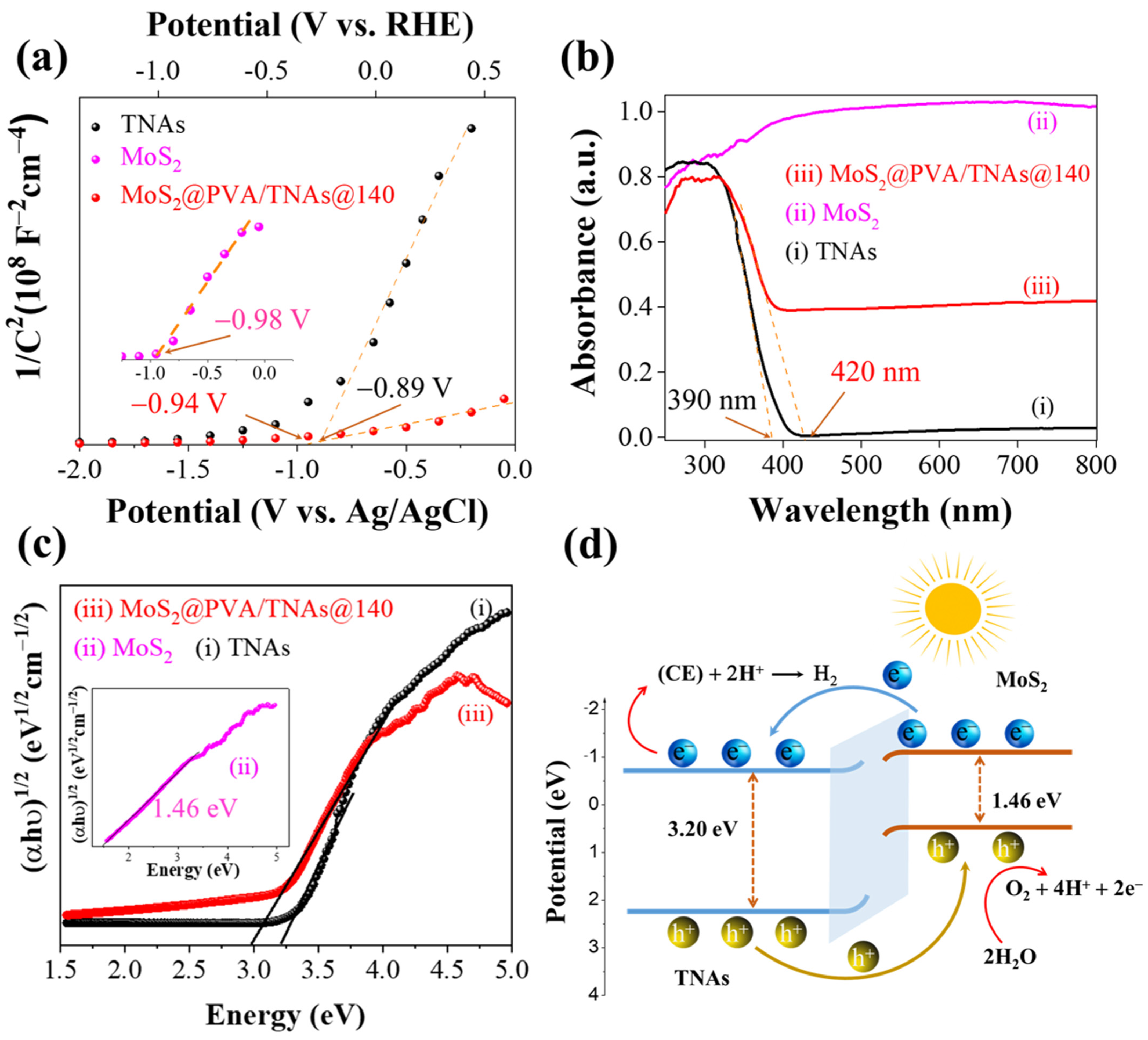
2. Results and Discussion
3. Experimental Section
3.1. Chemicals and Materials
3.2. Synthesis of TNAs by an Anodization Method
3.3. Synthesis of MoS2 by a Hydrothermal Method
3.4. Thermal Treatment of PVA for the Formation MoS2@PVA/TNAs Materials
3.5. Characterizations and PEC Evaluation of Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, L.; Shinde, A.; Guevarra, D.; Haber, J.A.; Persson, K.A.; Neaton, J.B.; Gregoire, J.M. Successes and Opportunities for Discovery of Metal Oxide Photoanodes for Solar Fuels Generators. ACS Energy Lett. 2020, 5, 1413–1421. [Google Scholar] [CrossRef]
- Sathre, R.; Greenblatt, J.B.; Walczak, K.; Sharp, I.D.; Stevens, J.C.; Ager, J.W.; Houle, F.A. Opportunities to improve the net energy performance of photoelectrochemical water-splitting technology. Energy Environ. Sci. 2016, 9, 803–819. [Google Scholar] [CrossRef]
- Han, H.S.; Park, W.; Hwang, S.W.; Kim, H.; Sim, Y.; Surendran, S.; Sim, U.; Cho, I.S. (0 2 0)-Textured tungsten trioxide nanostructure with enhanced photoelectrochemical activity. J. Catal. 2020, 389, 328–336. [Google Scholar] [CrossRef]
- Alam, K.; Sim, Y.; Yu, J.H.; Gnanaprakasam, J.; Choi, H.; Chae, Y.; Sim, U.; Cho, H. In-Situ Deposition of Graphene Oxide Catalyst for Efficient Photoelectrochemical Hydrogen Evolution Reaction Using Atmospheric Plasma. Materials (Basel) 2019, 13, 12. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at A Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis Under UV/visible Light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Xiu, Z.; Guo, M.; Zhao, T.; Pan, K.; Xing, Z.; Li, Z.; Zhou, W. Recent Advances in Ti3+ Self-Doped Nanostructured TiO2 Visible Light Photocatalysts for Environmental and Energy Applications. Chem. Eng. J. 2020, 382. [Google Scholar] [CrossRef]
- Le, C.T.; Kim, J.; Ullah, F.; Nguyen, A.D.; Nguyen Tran, T.N.; Le, T.E.; Chung, K.H.; Cheong, H.; Jang, J.I.; Kim, Y.S. Effects of Interlayer Coupling and Band Offset on Second Harmonic Generation in Vertical MoS2/MoS2(1-x)Se2x Structures. ACS Nano 2020, 14, 4366–4373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, L.; Chen, T.; Zhou, W.; Lou, X.W.D. Surface Modulation of Hierarchical MoS2 Nanosheets by Ni Single Atoms for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Shen, Y.; Ren, X.; Qi, X.; Zhou, J.; Huang, Z.; Zhong, J. MoS2 Nanosheet Loaded with TiO2 Nanoparticles: An Efficient Electrocatalyst for Hydrogen Evolution Reaction. J. Electrochem. Soc. 2016, 163, H1087–H1090. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, P.; Wei, C. Fabrication of MoS2/TiO2 heterostructures with enhanced photocatalytic activity. CrystEngComm 2019, 21, 3439–3450. [Google Scholar] [CrossRef]
- Zhao, F.; Rong, Y.; Wan, J.; Hu, Z.; Peng, Z.; Wang, B. MoS2 quantum dots@TiO2 nanotube composites with enhanced photoexcited charge separation and high-efficiency visible-light driven photocatalysis. Nanotechnology 2018, 29, 105403. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Z.; Marcus, K.; Li, Z.; Luo, B.; Zhou, L.; Wang, X.; Du, Y.; Yang, Y. MoS2/TiO2 heterostructures as nonmetal plasmonic photocatalysts for highly efficient hydrogen evolution. Energy Environ. Sci. 2018, 11, 106–114. [Google Scholar] [CrossRef]
- Mawlong, L.P.L.; Paul, K.K.; Giri, P.K. Direct Chemical Vapor Deposition Growth of Monolayer MoS2 on TiO2 Nanorods and Evidence for Doping-Induced Strong Photoluminescence Enhancement. J. Phys. Chem. C 2018, 122, 15017–15025. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Deshmukh, R.R.; Pasha, S.K.K.; Bhagat, P.R.; Chidambaram, K. 3—Biopolymer Composites with High Dielectric Performance. In Interface Engineering. Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2017, p. 101. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Yang, H.; Xu, S.; Jiang, L.; Dan, Y. Preparation and visible light-induced photo-catalytic activity of H-PVA/TiO2 composite loaded on glass via sol–gel method. Appl. Surf. Sci. 2014, 292, 978–985. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Ghangrekar, M.M.; Pradhan, D.; Das, D. Graphene Oxide-Impregnated PVA–STA Composite Polymer Electrolyte Membrane Separator for Power Generation in a Single-Chambered Microbial Fuel Cell. Ind. Eng. Chem. Res. 2013, 52, 11597–11606. [Google Scholar] [CrossRef]
- Libby, R.D. Advanced Organic Chemistry, Part A: Structure and Mechanism, 4th Edition (Carey, Francis A.; Sundberg, Richard J.). J. Chem. Educ. 2001, 78. [Google Scholar] [CrossRef][Green Version]
- Cheeseman, J.R.; Carroll, M.T.; Bader, R.F.W. The mechanics of hydrogen bond formation in conjugated systems. Chem. Phys. Lett. 1988, 143, 450–458. [Google Scholar] [CrossRef]
- Gebben, B.; van den Berg, H.W.A.; Bargeman, D.; Smolders, C.A. Intramolecular crosslinking of poly(vinyl alcohol). Polymer 1985, 26, 1737–1740. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, W.-F.; Koshy, P.; Sorrell, C.C. Enhanced photocatalytic performance of nanostructured TiO2 thin films through combined effects of polymer conjugation and Mo-doping. J. Mater. Sci. 2019, 54, 5266–5279. [Google Scholar] [CrossRef]
- Yang, H.; Xu, S.; Jiang, L.; Dan, Y. Thermal Decomposition Behavior of Poly (Vinyl Alcohol) with Different Hydroxyl Content. J. Macromol. Sci. Part B 2011, 51, 464–480. [Google Scholar] [CrossRef]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A.P. Characterization of poly(vinyl alcohol)/poly(ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Prosanov, I.Y.; Matvienko, A.A. Study of PVA thermal destruction by means of IR and Raman spectroscopy. Phys. Solid State 2010, 52, 2203–2206. [Google Scholar] [CrossRef]
- Muzakir, M.M.; Zainal, Z.; Lim, H.N.; Abdullah, A.H.; Bahrudin, N.N.; Sarif@Mohd Ali, M. Electrochemically Reduced Titania Nanotube Synthesized from Glycerol-Based Electrolyte as Supercapacitor Electrode. Energies 2020, 13, 2767. [Google Scholar] [CrossRef]
- Nischk, M.; Mazierski, P.; Wei, Z.; Siuzdak, K.; Kouame, N.A.; Kowalska, E.; Remita, H.; Zaleska-Medynska, A. Enhanced photocatalytic, electrochemical and photoelectrochemical properties of TiO2 nanotubes arrays modified with Cu, AgCu and Bi nanoparticles obtained via radiolytic reduction. Appl. Surf. Sci. 2016, 387, 89–102. [Google Scholar] [CrossRef]
- Peighambardoust, N.S.; Khameneh Asl, S.; Mohammadpour, R.; Asl, S.K. Band-gap narrowing and electrochemical properties in N-doped and reduced anodic TiO2 nanotube arrays. Electrochim. Acta 2018, 270, 245–255. [Google Scholar] [CrossRef]
- Prass, S.; St-Pierre, J.; Klingele, M.; Friedrich, K.A.; Zamel, N. Hydrogen Oxidation Artifact During Platinum Oxide Reduction in Cyclic Voltammetry Analysis of Low-Loaded PEMFC Electrodes. Electrocatalysis 2020, 12, 45–55. [Google Scholar] [CrossRef]
- Bisquert, J.; Fabregat-Santiago, F.; Mora-Seró, I.; Garcia-Belmonte, G.; Barea, E.M.; Palomares, E. A review of recent results on electrochemical determination of the density of electronic states of nanostructured metal-oxide semiconductors and organic hole conductors. Inorg. Chim. Acta 2008, 361, 684–698. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Yu, J.; Parkin, I.P.; Fujishima, A.; Nakata, K. Intrinsic intermediate gap states of TiO2 materials and their roles in charge carrier kinetics. J. Photochem. Photobiol. C Photochem. Rev. 2019, 39, 1–57. [Google Scholar] [CrossRef]
- Li, C.; Hou, T.; Vongsvivut, J.; Li, Y.; She, X.; She, F.; Gao, W.; Kong, L. Simultaneous crystallization and decomposition of PVA/MMT composites during non-isothermal process. Thermochim. Acta 2015, 618, 26–35. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Yi, M.; Ge, J.; Yin, G.; Li, X. Preparation and Physicochemical Properties of Blend Films of Feather Keratin and Poly(vinyl alcohol) Compatibilized by Tris(hydroxymethyl)aminomethane. Polymers (Basel) 2018, 10, 54. [Google Scholar] [CrossRef]
- Wijanarko, T.A.W.; Kusumaatmaja, A.; Chotimah, R.; Triyana, K. Effect of heat treatment on morphology and crystallinity of electrospun Poly(vinyl alcohol) nanofibers. AIP Conf. Proc. 2016, 1755, 150010. [Google Scholar] [CrossRef]
- Feng, W.; Chen, L.; Qin, M.; Zhou, X.; Zhang, Q.; Miao, Y.; Qiu, K.; Zhang, Y.; He, C. Flower-Like PEGylated MoS2 Nanoflakes for Near-Infrared Photothermal Cancer Therapy. Sci. Rep. 2015, 5, 17422. [Google Scholar] [CrossRef]
- Ritika, M.K.; Umar, A.; Mehta, S.K.; Singh, S.; Kansal, S.K.; Fouad, H.; Alothman, O.Y. Rapid Solar-Light Driven Superior Photocatalytic Degradation of Methylene Blue Using MoS(2)-ZnO Heterostructure Nanorods Photocatalyst. Materials (Basel) 2018, 11, 2254. [Google Scholar] [CrossRef]
- Sonker, A.K.; Rathore, K.; Nagarale, R.K.; Verma, V. Crosslinking of Polyvinyl Alcohol (PVA) and Effect of Crosslinker Shape (Aliphatic and Aromatic) Thereof. J. Polym. Environ. 2017, 26, 1782–1794. [Google Scholar] [CrossRef]
- Premathilake, D.; Outlaw, R.A.; Parler, S.G.; Butler, S.M.; Miller, J.R. Electric double layer capacitors for ac filtering made from vertically oriented graphene nanosheets on aluminum. Carbon 2017, 111, 231–237. [Google Scholar] [CrossRef]
- Gurung, A.; Pokharel, J.; Baniya, A.; Pathak, R.; Chen, K.; Lamsal, B.S.; Ghimire, N.; Zhang, W.-H.; Zhou, Y.; Qiao, Q. A review on strategies addressing interface incompatibilities in inorganic all-solid-state lithium batteries. Sustain. Energy Fuels 2019, 3, 3279–3309. [Google Scholar] [CrossRef]
- Komba, N.; Zhang, G.; Pu, Z.; Wu, M.; Rosei, F.; Sun, S. MoS2-supported on free-standing TiO2-nanotubes for efficient hydrogen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 4468–4480. [Google Scholar] [CrossRef]
- Resasco, J.; Zhang, H.; Kornienko, N.; Becknell, N.; Lee, H.; Guo, J.; Briseno, A.L.; Yang, P. TiO2/BiVO4 Nanowire Heterostructure Photoanodes Based on Type II Band Alignment. ACS Cent. Sci. 2016, 2, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Hankin, A.; Bedoya-Lora, F.E.; Alexander, J.C.; Regoutz, A.; Kelsall, G.H. Flat band potential determination: Avoiding the pitfalls. J. Mater. Chem. A 2019, 7, 26162–26176. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Pawar, S.A.; Potphode, D.; Moon, C.-K.; Suh, J.M.; Kim, C.; Choi, S.; Patil, D.S.; Kim, J.-J.; Shin, J.C.; et al. Substantially Enhanced Photoelectrochemical Performance of TiO2 nanorods/CdS Nanocrystals Heterojunction Photoanode Decorated with MoS2 Nanosheets. Appl. Catal. B Environ. 2019, 259. [Google Scholar] [CrossRef]
- Nan, F.; Li, P.; Li, J.; Cai, T.; Ju, S.; Fang, L. Experimental and Theoretical Evidence of Enhanced Visible Light Photoelectrochemical and Photocatalytic Properties in MoS2/TiO2 Nanohole Arrays. J. Phys. Chem. C 2018, 122, 15055–15062. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shifa, T.A.; Zhan, X.; Huang, Y.; Liu, K.; Cheng, Z.; Jiang, C.; He, J. Recent advances in transition-metal dichalcogenide based nanomaterials for water splitting. Nanoscale 2015, 7, 19764–19788. [Google Scholar] [CrossRef]
- Tho, N.T.; Thi, C.M.; Van Hieu, L.; Van Viet, P. Visible-light-driven photocatalysis for methylene blue degradation and hydrogen evolution reaction: A case of black TiO2 nanotube arrays. J. Aust. Ceram. Soc. 2019, 56, 849–857. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Zhang, J.; Zhang, H.; Tian, W.; Li, X.; Tade, M.O.; Sun, H.; Wang, S. Flower-like MoS2 on graphitic carbon nitride for enhanced photocatalytic and electrochemical hydrogen evolutions. Appl. Catal. B Environ. 2018, 239, 334–344. [Google Scholar] [CrossRef]
- Ritika, M.K.; Umar, A.; Mehta, S.K.; Kansal, S.K.; Khan, M.A.; Algarni, H. Enhanced solar light-mediated photocatalytic degradation of brilliant green dye in aqueous phase using BiPO4 nanospindles and MoS2/BiPO4 nanorods. J. Mater. Sci. Mater. Electron. 2019, 30, 20741–20750. [Google Scholar] [CrossRef]
- Paul, K.K.; Sreekanth, N.; Biroju, R.K.; Narayanan, T.N.; Giri, P.K. Solar light driven photoelectrocatalytic hydrogen evolution and dye degradation by metal-free few-layer MoS2 nanoflower/TiO2(B) nanobelts heterostructure. Sol. Energy Mater. Sol. Cells 2018, 185, 364–374. [Google Scholar] [CrossRef]
- Steinhoff, A.; Kim, J.H.; Jahnke, F.; Rosner, M.; Kim, D.S.; Lee, C.; Han, G.H.; Jeong, M.S.; Wehling, T.O.; Gies, C. Efficient Excitonic Photoluminescence in Direct and Indirect Band Gap Monolayer MoS2. Nano Lett. 2015, 15, 6841–6847. [Google Scholar] [CrossRef] [PubMed]
- Yazici, B. Investigation of suitable cathodes for the production of hydrogen gas by electrolysis. Int. J. Hydrogen Energy 1995, 20, 957–965. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Cao, T.M.; Balayeva, N.O.; Pham, V.V. Thermal Treatment of Polyvinyl Alcohol for Coupling MoS2 and TiO2 Nanotube Arrays toward Enhancing Photoelectrochemical Water Splitting Performance. Catalysts 2021, 11, 857. https://doi.org/10.3390/catal11070857
Nguyen TT, Cao TM, Balayeva NO, Pham VV. Thermal Treatment of Polyvinyl Alcohol for Coupling MoS2 and TiO2 Nanotube Arrays toward Enhancing Photoelectrochemical Water Splitting Performance. Catalysts. 2021; 11(7):857. https://doi.org/10.3390/catal11070857
Chicago/Turabian StyleNguyen, Tho Truong, Thi Minh Cao, Narmina O. Balayeva, and Viet Van Pham. 2021. "Thermal Treatment of Polyvinyl Alcohol for Coupling MoS2 and TiO2 Nanotube Arrays toward Enhancing Photoelectrochemical Water Splitting Performance" Catalysts 11, no. 7: 857. https://doi.org/10.3390/catal11070857
APA StyleNguyen, T. T., Cao, T. M., Balayeva, N. O., & Pham, V. V. (2021). Thermal Treatment of Polyvinyl Alcohol for Coupling MoS2 and TiO2 Nanotube Arrays toward Enhancing Photoelectrochemical Water Splitting Performance. Catalysts, 11(7), 857. https://doi.org/10.3390/catal11070857