A Bio-Based Alginate Aerogel as an Ionic Liquid Support for the Efficient Synthesis of Cyclic Carbonates from CO2 and Epoxides
Abstract
1. Introduction
2. Results and Discussion
2.1. Infrared Spectroscopy Studies
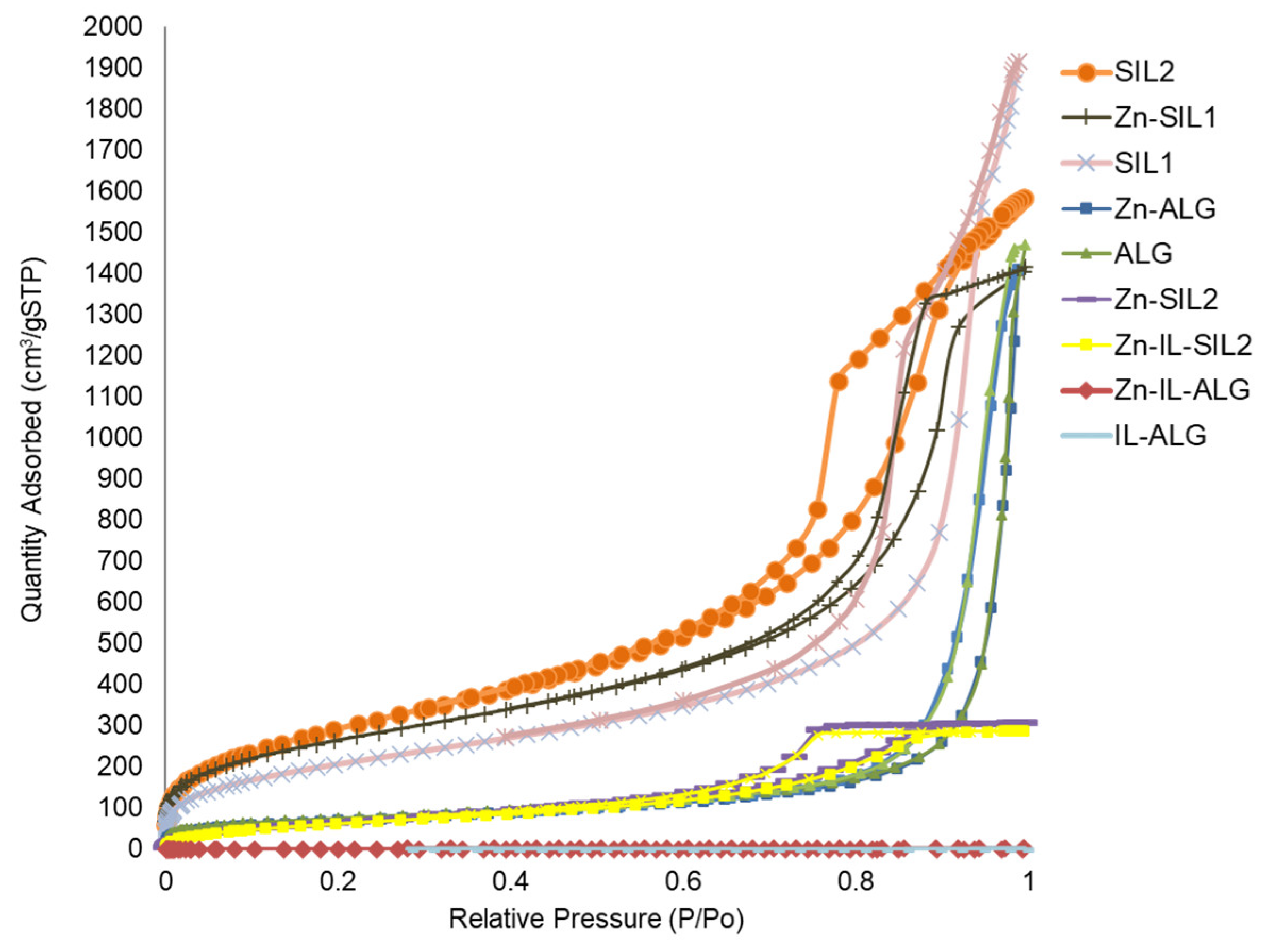
2.2. Nitrogen Physisorption Studies
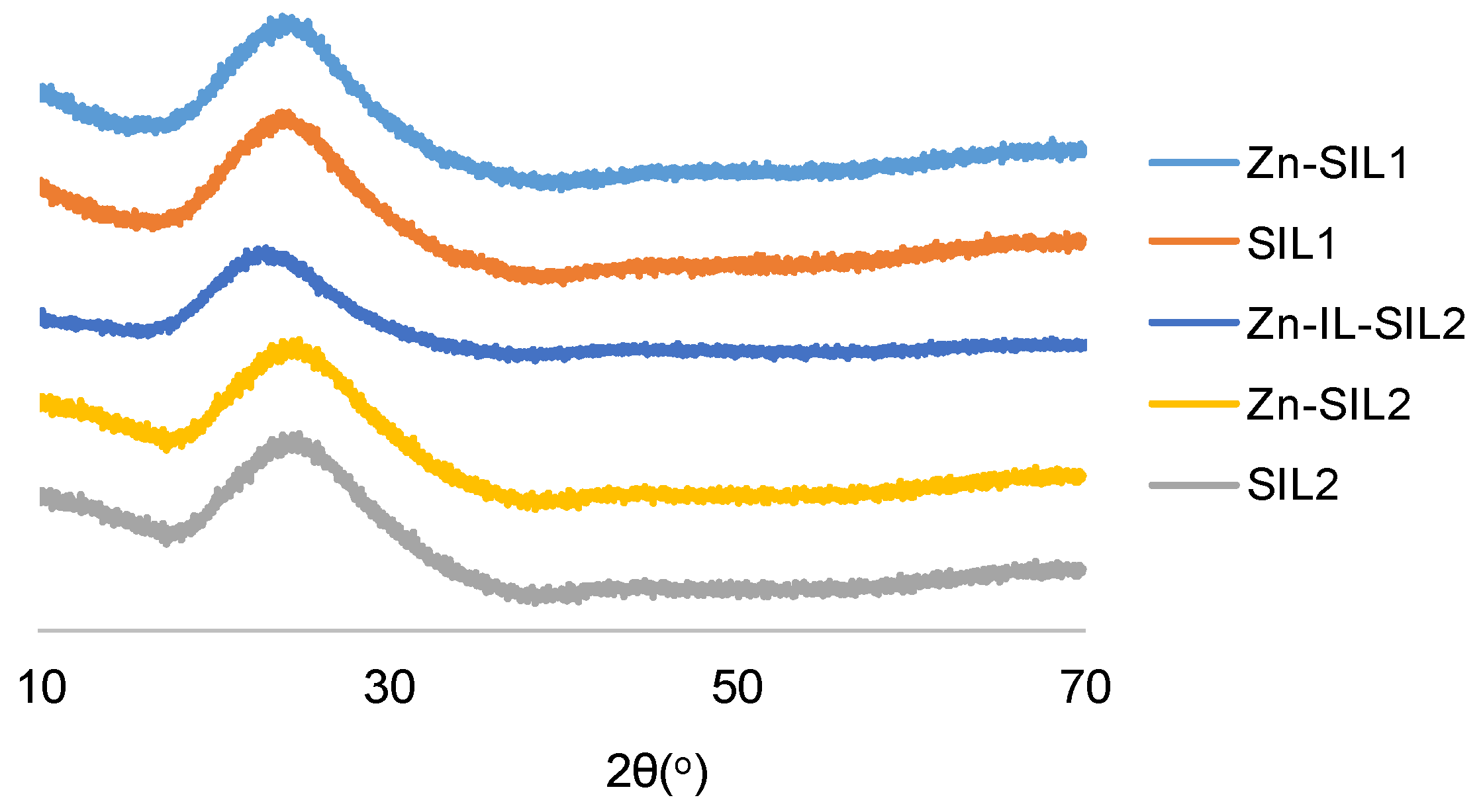
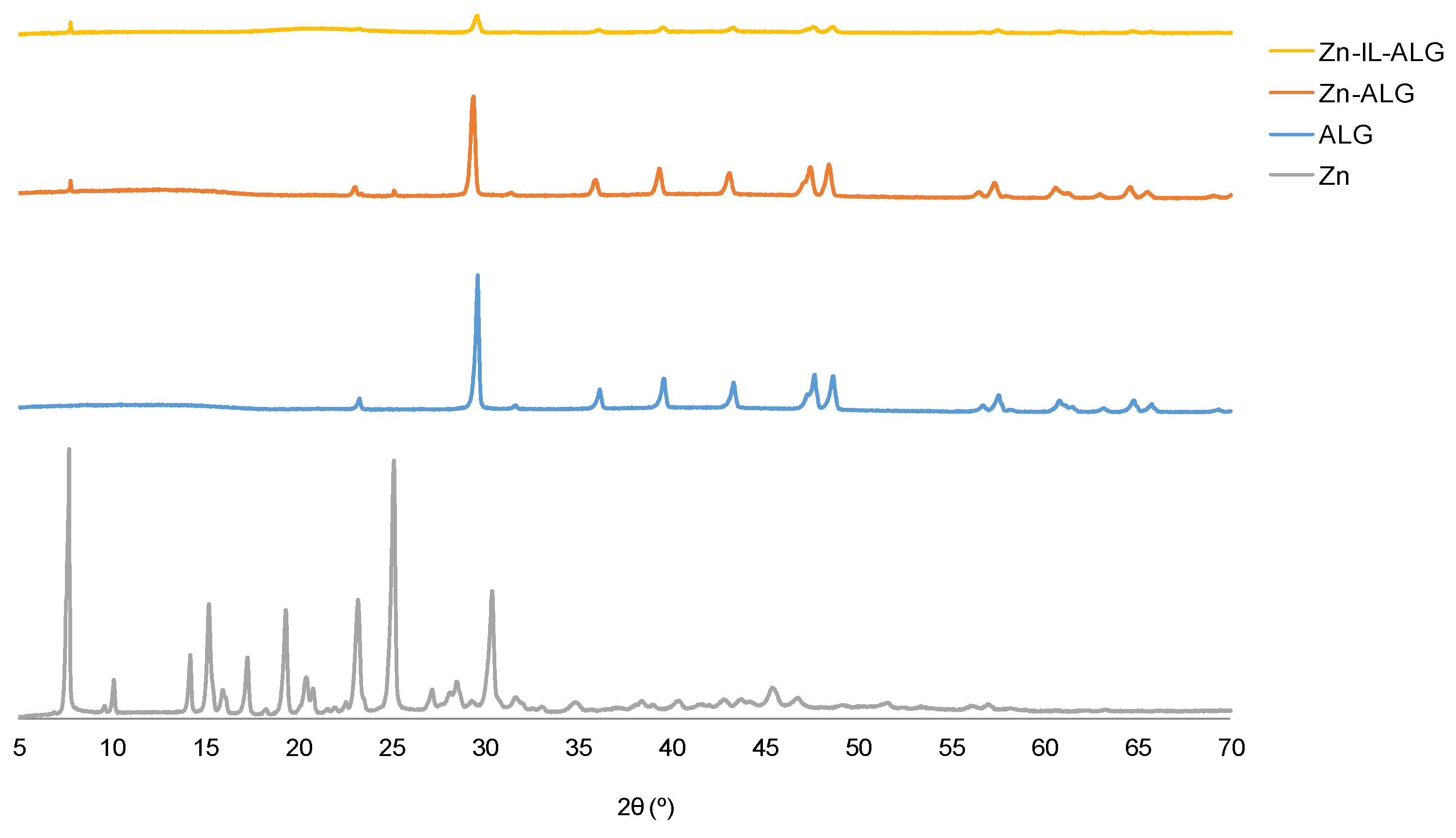
2.3. Powder X-ray Diffraction Studies
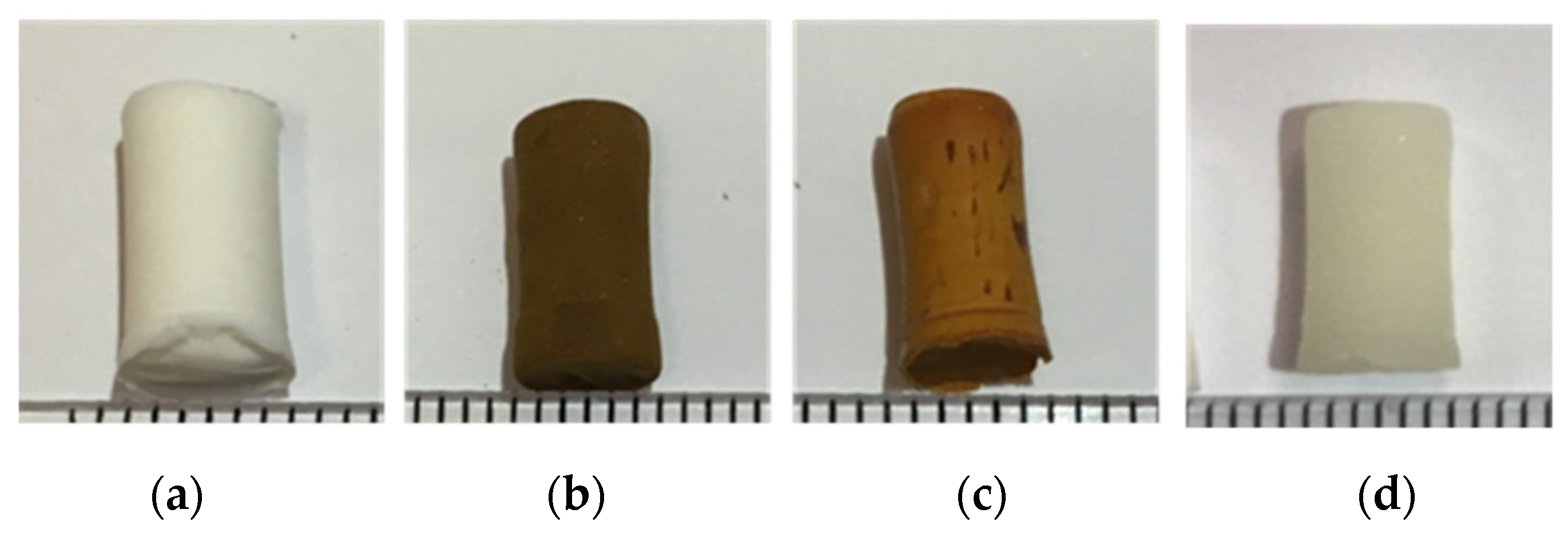
2.4. Aerogels Images
2.5. Scanning Electron Microscopy
2.6. Atomic Absorption Studies
2.7. Reactions Using Impregnated Aerogels as Catalysts
3. Experimental Section
3.1. Materials and Equipment
3.2. Preparation of Alcogels
3.2.1. Silica Alcogels Synthesis
3.2.2. Alginate Alcogels Synthesis
3.3. Aerogels Impregnation
3.4. Supercritical Drying of Alcogels
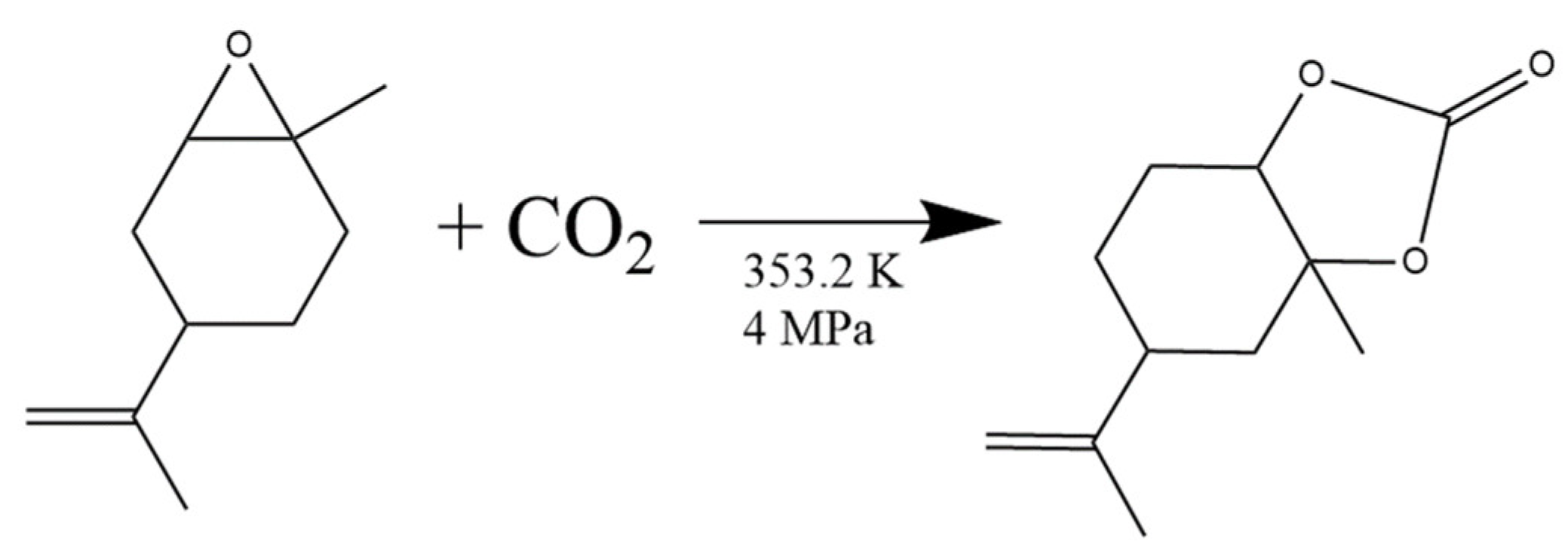
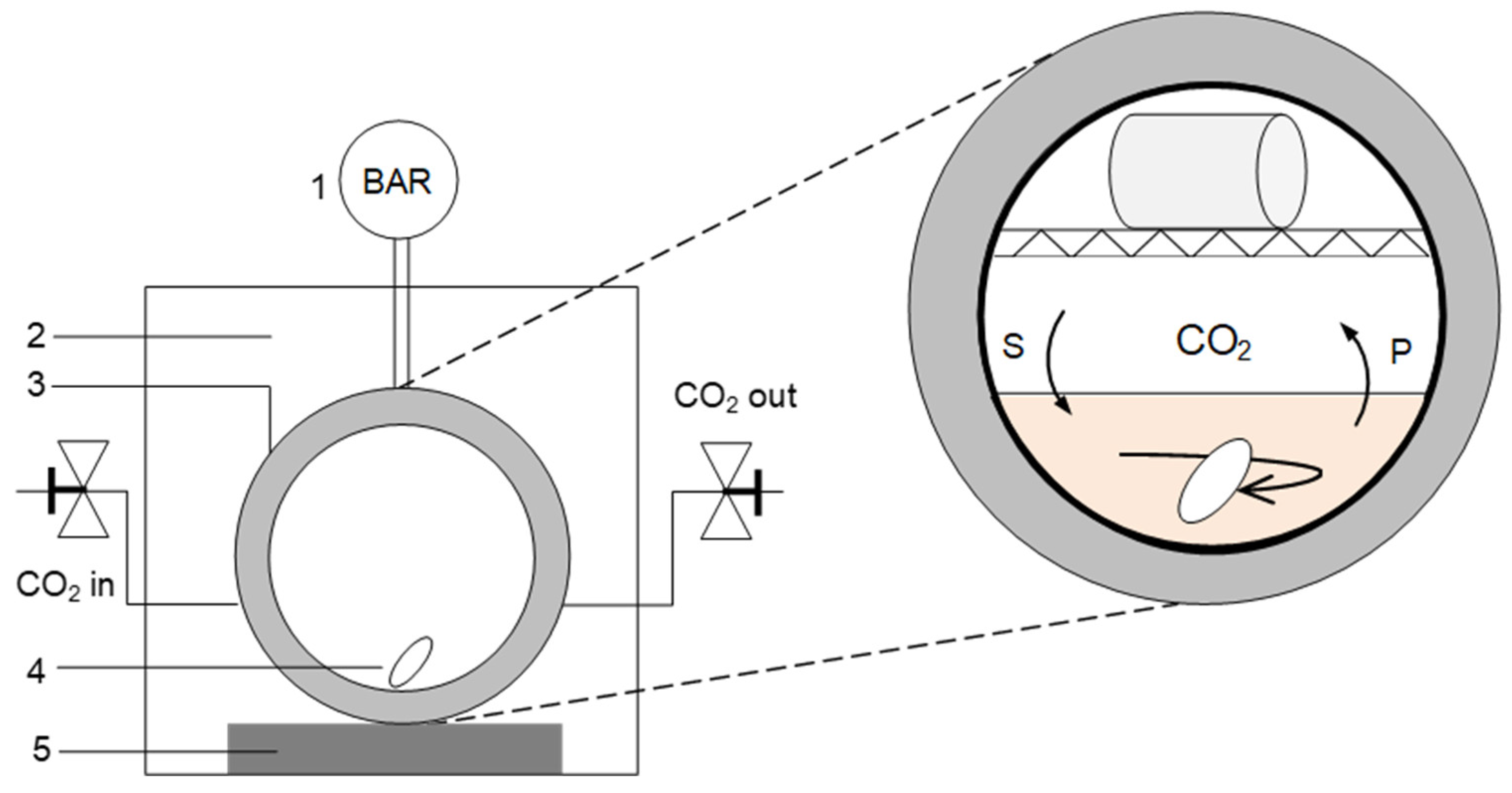
3.5. CO2 and Epoxides Coupling Reactions
3.6. Aerogels Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Machado, A.S.R.; Nunes, A.V.M.; Nunes da Ponte, M. Carbon dioxide utilization–Electrochemical reduction to fuels and synthesis of polycarbonates. J. Supercrit. Fluids 2018, 134, 150–156. [Google Scholar] [CrossRef]
- Kamphuis, A.J.; Picchioni, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef]
- Schäffner, B.; Schäffner, F.; Verevkin, S.P.; Börner, A. Organic carbonates as solvents in synthesis and catalysis. Chem. Rev. 2010, 110, 4554–4581. [Google Scholar] [CrossRef] [PubMed]
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- Martin, C.; Fiorani, G.; Kleij, A.W. Recent advances in the catalytic preparation of cyclic organic carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic Strategies for the cycloaddition of pure, diluted, and waste CO2 to epoxides under ambient conditions. ACS Catal. 2018, 8, 419–450. [Google Scholar] [CrossRef]
- Büttner, H.; Longwitz, L.; Steinbauer, J.; Wulf, C.; Werner, T. Recent developments in the synthesis of cyclic carbonates from epoxides and CO2. Top. Curr. Chem. 2017, 375, 50–106. [Google Scholar] [CrossRef]
- Cokoja, M.; Wilhelm, M.E.; Anthofer, M.H.; Herrmann, W.A.; Kühn, F.E. Synthesis of cyclic carbonates from epoxides and carbon dioxide by using organocatalysts. ChemSusChem 2015, 8, 2436–2454. [Google Scholar] [CrossRef]
- Bobbink, F.D.; Dyson, P.J. Synthesis of carbonates and related compounds incorporating CO2 using ionic liquid-type catalysts: State-of-the-art and beyond. J. Catal. 2016, 343, 52–61. [Google Scholar] [CrossRef]
- Alves, M.; Grignard, B.; Mereau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. Organocatalyzed coupling of carbon dioxide with epoxides for the synthesis of cyclic carbonates: Catalyst design and mechanistic studies. Catal. Sci. Technol. 2017, 7, 2651–2684. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Conversion of CO2 to value-added products mediated by ionic liquids. Green Chem. 2019, 21, 2544–2574. [Google Scholar] [CrossRef]
- Paninho, A.B.; Ventura, A.; Branco, L.C.; Pombeiro, A.J.L.; Guedes da Silva, M.F.C.; Nunes da Ponte, M.; Mahmudov, K.T.; Nunes, A.V.M. CO2 + ionic liquid biphasic system for reaction/product separation in the synthesis of cyclic carbonates. J. Supercrit. Fluids 2018, 132, 71–75. [Google Scholar] [CrossRef]
- Serbanovic, A.; Branco, L.C.; Nunes da Ponte, M.; Afonso, C.A.M. Osmium catalyzed asymmetric dihydroxylation of methyl trans-cinnamate in ionic liquids, followed by supercritical CO2 product recovery. J. Organomet. Chem. 2005, 690, 3600–3608. [Google Scholar] [CrossRef]
- Franciò, G.; Hintermair, U.; Leitner, W. Unlocking the potential of supported liquid phase catalysts with supercritical fluids: Low temperature continuous flow catalysis with integrated product separation. Philos. Trans. R. Soc. A 2015, 373, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Giacalone, F.; Aprile, C. Hybrid catalysts for CO2 conversion into cyclic carbonates. Catalysts 2019, 9, 325. [Google Scholar] [CrossRef]
- Mehnert, C.P.; Cook, R.A.; Dispenziere, N.C.; Afeworki, M. Supported ionic liquid catalysis–A new concept for homogeneous hydroformylation catalysis. J. Am. Chem. Soc. 2002, 124, 12932–12933. [Google Scholar] [CrossRef] [PubMed]
- Riisager, A.; Fehrmann, R.; Haumann, M.; Wasserscheid, P. Supported ionic liquid phase (SILP) catalysis: An innovative concept for homogeneous catalysis in continuous fixed-bed reactors. Eur. J. Inorg. Chem. 2006, 4, 695–706. [Google Scholar] [CrossRef]
- Romanovsky, B.V.; Tarkhanova, I.G. Supported ionic liquids in catalysis. Russ. Chem. Rev. 2017, 86, 444–458. [Google Scholar] [CrossRef]
- Kaur, P.; Chopra, H.K. Recent advances in applications of supported ionic liquids. Curr. Org. Chem. 2019, 23, 2881–2915. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Yin, L. Progress in the heterogeneous catalytic cyclization of CO2 with epoxides using immobilized ionic liquids. Catal. Lett. 2019, 149, 985–997. [Google Scholar] [CrossRef]
- Lemus, J.; Palomar, J.; Gilarranz, M.A. Characterization of supported ionic liquid phase (SILP) materials prepared from different supports. J. Intern. Adsorp. Soc. 2011, 17, 561–571. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Gu, Y.; Xue, B.; Li, Y. A new and efficient method of graphene oxide immobilized with ionic liquids: Promoted catalytic activity for CO2 cycloaddition. Mater. Chem. Phys. 2018, 208, 68–76. [Google Scholar] [CrossRef]
- Guo, L.; Deng, L.; Jin, X.; Wang, Y.; Wang, H. Catalytic conversion of CO2 into propylene carbonate in a continuous fixed bed reactor by immobilized ionic liquids. RSC Adv. 2018, 8, 26554–26562. [Google Scholar] [CrossRef]
- Hu, Y.L.; Zhang, R.L.; Fang, D. Quaternary phosphonium cationic ionic liquid/porous metal–organic framework as an efficient catalytic system for cycloaddition of carbon dioxide into cyclic carbonates. Environ. Chem. Lett. 2019, 17, 501–508. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W.; Lyu, Y.; Chen, X.; Li, C.; Zhang, Y.; Song, X.; Ding, Y. Highly recyclable polymer supported ionic liquids as efficient heterogeneous catalysts for batch and flow conversion of CO2 to cyclic carbonates. RSC Adv. 2017, 7, 2836–2841. [Google Scholar] [CrossRef]
- Sang, Y.; Huang, J. Benzimidazole-based hyper-cross-linked poly(ionic liquid)s for efficient CO2 capture and conversion. Chem. Eng. J. 2020, 1385, 123973–123982. [Google Scholar] [CrossRef]
- Li, J.; Jia, D.; Guo, Z.; Liu, Y.; Lyu, Y.; Zhou, Y.; Wang, J. Imidazolinium based porous hypercrosslinked ionic polymers for efficient CO2 capture and fixation with epoxides. Green Chem. 2017, 19, 2675–2686. [Google Scholar] [CrossRef]
- Martínez-Ferraté, O.; Chacón, G.; Bernardi, F.; Grehl, T.; Brüner, F.; Dupont, J. Cycloaddition of carbon dioxide to epoxides catalysed by supported ionic liquids. Catal. Sci. Technol. 2018, 8, 3081–3089. [Google Scholar] [CrossRef]
- Martinez, A.S.; Hauzenberger, C.; Sahoo, A.R.; Csendes, Z.; Hoffmann, H.; Bica, K. Continuous conversion of carbon dioxide to propylene carbonate with supported ionic liquids. ACS Sustain. Chem. Eng. 2018, 6, 13131–13139. [Google Scholar]
- Kolle, J.M.; Sayari, A. Substrate dependence on the fixation of CO2 to cyclic carbonates over reusable porous hybrid solids. J. CO2 Util. 2018, 26, 564–574. [Google Scholar] [CrossRef]
- Ramalingam, R.J.; Appaturi, J.N. Synthesis, characterization and catalytic activity of ionic liquid mimic halides modified MCM-41 for solvent free synthesis of phenyl glycidyl carbonate. Mater. Chem. Phys. 2019, 233, 79–88. [Google Scholar] [CrossRef]
- Hu, Y.L.; Wang, H.B.; Chen, Z.W.; Li, X.G. Titanium incorporated mesoporous silica immobilized functional ionic liquid as an efficient reusable catalyst for cycloaddition of carbon dioxide to epoxides. Chem. Select 2018, 3, 5087–5091. [Google Scholar] [CrossRef]
- Takahashi, T.; Watahiki, T.; Kitazume, S.; Yasuda, H.; Sakakura, T. Synergistic hybrid catalyst for cyclic carbonate synthesis: Remarkable acceleration caused by immobilization of homogeneous catalyst on silica. Chem. Commun. 2006, 1, 1664–1666. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Heidari, Y.; Kozehgary, G. Silica grafted ammonium salts based on DABCO as heterogeneous catalyst for cyclic carbonate synthesis from carbon dioxide and epoxides. RSC Adv. 2015, 5, 22373–22379. [Google Scholar] [CrossRef]
- Sakai, T.; Tsutsumi, Y.; Ema, T. Highly active and robust organic-inorganic hybrid catalyst for the synthesis of cyclic carbonates from carbon dioxide and epoxides. Green Chem. 2008, 10, 337–341. [Google Scholar] [CrossRef]
- Roshan, K.R.; Mathai, G.; Kim, J.; Tharun, J.; Parka, G.A.; Park, D.W. A biopolymer mediated efficient synthesis of cyclic carbonates from epoxides and carbon dioxide. Green Chem. 2012, 14, 2933–2940. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Cheng, W.; Zhang, J.; Li, X.; Zhang, S.; Sheb, Y. Chitosan functionalized ionic liquid as a recyclable biopolymer-supported catalyst for cycloaddition of CO2. Green Chem. 2012, 14, 654–660. [Google Scholar] [CrossRef]
- Taheri, M.; Ghiaci, M.; Shchukarev, A. Cross-linked chitosan with a dicationic ionic liquid as a recyclable biopolymer-supported catalyst for cycloaddition of carbon dioxide with epoxides into cyclic carbonates. New J. Chem. 2018, 42, 587–597. [Google Scholar] [CrossRef]
- Paninho, A.B. Sustainable intensification strategies for the production of cyclic carbonates from CO2. Ph.D. Dissertation, Universidade Nova de Lisboa—Faculdade de Ciências e Tecnologia, Caparika, Portugal, 2018. [Google Scholar]
- Cruz-Martínez, F.; Buchaca, M.M.S.; Martínez, J.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Rodríguez-Diéguez, A.; Castro-Osma, J.A.; Lara-Sánchez, A. Synthesis of Bio-Derived Cyclic Carbonates from Renewable Resources. ACS Sustain. Chem. Eng. 2019, 7, 20126–20138. [Google Scholar] [CrossRef]
- Aomchad, V.; Cristòfol, A.; Monica, F.D.; Limburg, B.; D’Elia, V.; Kleij, A.W. Recent progress in the catalytic transformation of carbon dioxide into biosourced organic carbonates. Green Chem. 2021, 23, 1077–1113. [Google Scholar] [CrossRef]
- Rehman, A.; Saleem, F.; Javed, F.; Ikhlaq, A.; Ahmad, S.W.; Harvey, A. Recent advances in the synthesis of cyclic carbonates via CO2 cycloaddition to epoxides. J. Environ. Chem. Eng. 2021, 9, 105113. [Google Scholar] [CrossRef]
- Fiorani, G.; Stuck, M.; Martin, C.; Belmonte, M.M.; Martin, E.; Escudero-Adan, E.C.; Kleij, A.W. Catalytic Coupling of Carbon Dioxide with Terpene Scaffolds: Access to Challenging Bio-Based Organic Carbonates. Chem. Sus. Chem. 2016, 9, 1304–1311. [Google Scholar] [CrossRef]
- Rehman, A.; Fernández, A.M.L.; Resul, M.F.M.G.; Harvey, A.P. Highly selective, sustainable synthesis of limonene cyclic carbonate from bio-based limonene oxide and CO2: A kinetic study. J. CO2 Util. 2019, 29, 126–133. [Google Scholar] [CrossRef]
- Khotimchenko, Y.S.; Kovalev, V.V.; Savchenko, O.V.; Ziganshina, O.A. Physical–Chemical Properties, Physiological Activity, and Usage of Alginates, the Polysaccharides of Brown Algae. Russ. J. Mar. Biol. 2001, 27, 53–64. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Mahmudov, K.T.; Pombeiro, A.J.L. Synthesis, structure and electrochemical behaviour of Na, MgII, MnII, ZnII, CdII and NiII complexes of 3-(2-carboxyphenylhydrazone)pentane-2,4-dione. Polyhedron 2013, 50, 374–382. [Google Scholar] [CrossRef][Green Version]
- Kopylovich, M.N.; Leod, T.C.O.M.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Zinc(II) ortho-hydroxyphenylhydrazo-β-diketonate complexes and their catalytic ability towards diastereoselective nitroaldol (Henry) reaction. Dalton Trans. 2011, 40, 5352–5361. [Google Scholar] [CrossRef] [PubMed]
- Mustapa, A.N.; Martin, Á.; Sanz-Moral, L.M.; Rueda, M.; Cocero, M.J. Impregnation of medicinal plant phytochemical compounds into silica and alginate aerogels. J. Supercrit. Fluids 2016, 116, 251–263. [Google Scholar] [CrossRef]
- Naz, G.; Othaman, Z.; Shamsuddin, M.; Ghoshal, S.K. Aliquat 336 stabilized multi-faceted gold nanoparticles with minimal ligand density. Appl. Surf. Sci. 2016, 363, 74–82. [Google Scholar] [CrossRef]
- Patel, R.P.; Purohit, N.S.; Suthar, A.M. An overview of silica aerogels. Int. J. Chemtech Res. 2009, 1, 1052–1057. [Google Scholar]
- Fan, L.; Du, Y.; Wang, X.; Huang, R.; Zhang, L.; Hu, L. Preparation and characterization of alginate/poly(vinyl alcohol) blend fibers. J. Macromol. Sci. Pure Appl. Chem. 2005, 42, 41–50. [Google Scholar]
- Yang, G.; Zhang, L.; Penga, T.; Zhong, W. Effects of Ca2+ bridge cross-linking on structure and pervaporation of cellulose/alginate blend membranes. J. Membr. Sci. 2000, 175, 53–60. [Google Scholar] [CrossRef]
- Montoya, C.A.; Paninho, A.B.; Felix, P.M.; Zakrzewska, M.E.; Vital, J.; Najdanovic-Visak, V.; Nunes, A.V.M. Styrene carbonate synthesis from CO2 using tetrabutylammonium bromide as a non-supported heterogeneous catalyst phase. J. Supercrit. Fluids 2015, 100, 155–159. [Google Scholar] [CrossRef]
- Montoya, C.A.; Gómez, C.F.; Paninho, A.B.; Nunes, A.V.M.; Mahmudov, K.T.; Najdanovic-Visak, V.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Nunes da Ponte, M.; Pombeiro, A.J.L. Cyclic carbonate synthesis from CO2 and epoxides using zinc(II) complexes of arylhydrazones of β-diketones. J. Catal. 2016, 335, 135–140. [Google Scholar] [CrossRef]
- Novak, Z.; Knez, Ž. Diffusion of methanol–liquid CO2 and methanol–supercritical CO2 in silica aerogels. J. Non Cryst. Solids 1997, 221, 163–169. [Google Scholar] [CrossRef]
- Smirnova, I.; Arlt, W. Synthesis of silica aerogels: Influence of the supercritical CO2 on the Sol-Gel process. J. Solgel Sci. Techn. 2003, 28, 175–184. [Google Scholar] [CrossRef]
- Valentin, R.; Horga, R.; Bonelli, B.; Garrone, E.; Renzo, F.D.; Quignard, F. Acidity of alginate aerogels studied by FTIR spectroscopy of probe molecules. Macromol. Symp. 2005, 230, 71–77. [Google Scholar] [CrossRef]
- Mehling, T.; Smirnova, I.; Guenther, U.; Neubert, R.H.H. Polysaccharide-based aerogels as drug carriers. J. Non Cryst. Solids 2009, 355, 2472–2479. [Google Scholar] [CrossRef]
- Sanz-Moral, L.M.; Rueda, M.; Nieto, A.; Novak, Z.; Knez, Ž.; Martín, Á. Gradual hydrophobic surface functionalization of dry silica aerogels by reaction with silane precursors dissolved in supercritical carbon dioxide. J. Supercrit. Fluids 2013, 84, 74–79. [Google Scholar] [CrossRef]
- Carrodeguas, L.P.; González-Fabra, J.; Castro-Gómez, F.; Bo, C.; Kleij, A.W. AlIII-catalysed formation of poly(limonene)carbonate: DFT analysis of the origin of stereoregularity. Chem. Eur. J. 2015, 21, 6115–6124. [Google Scholar]

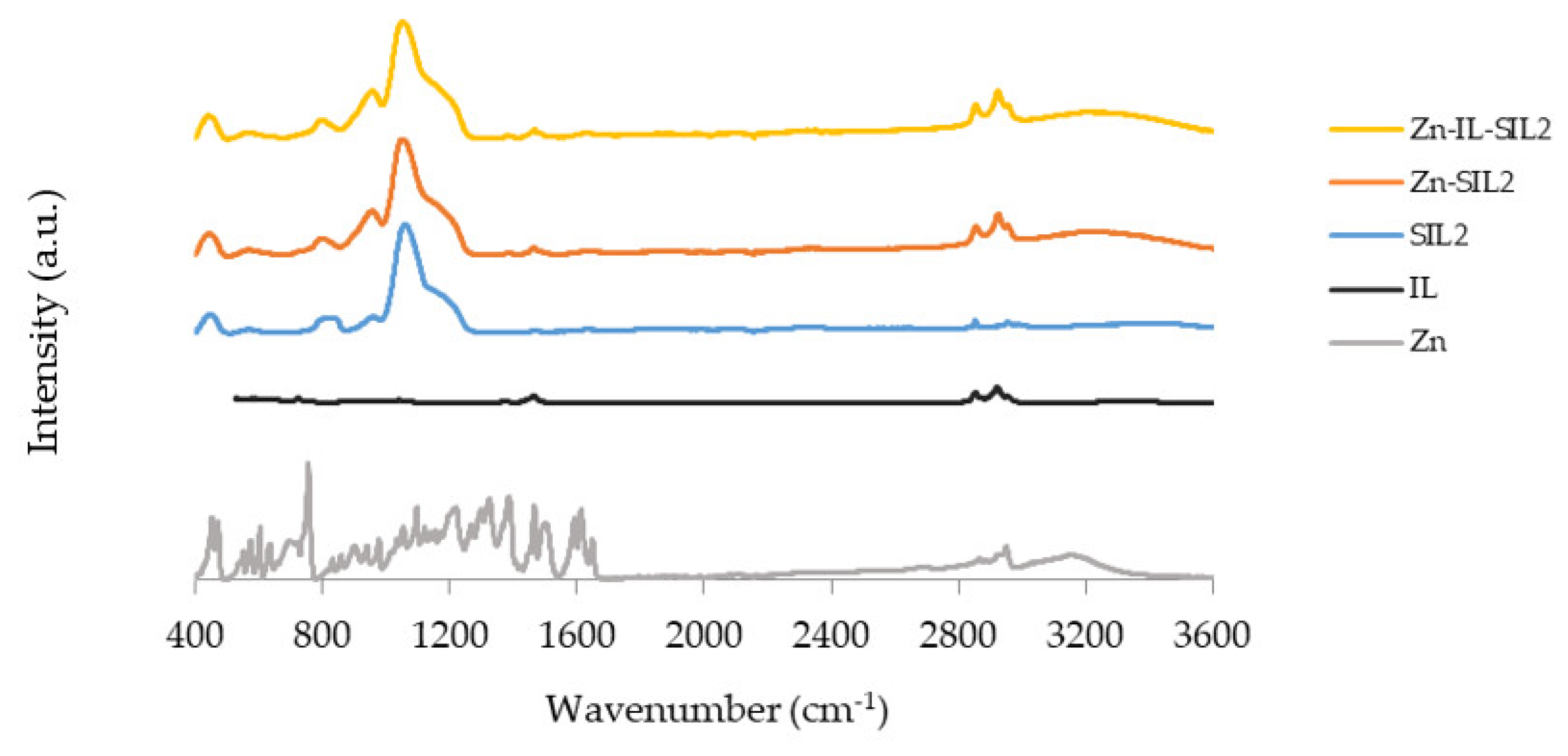
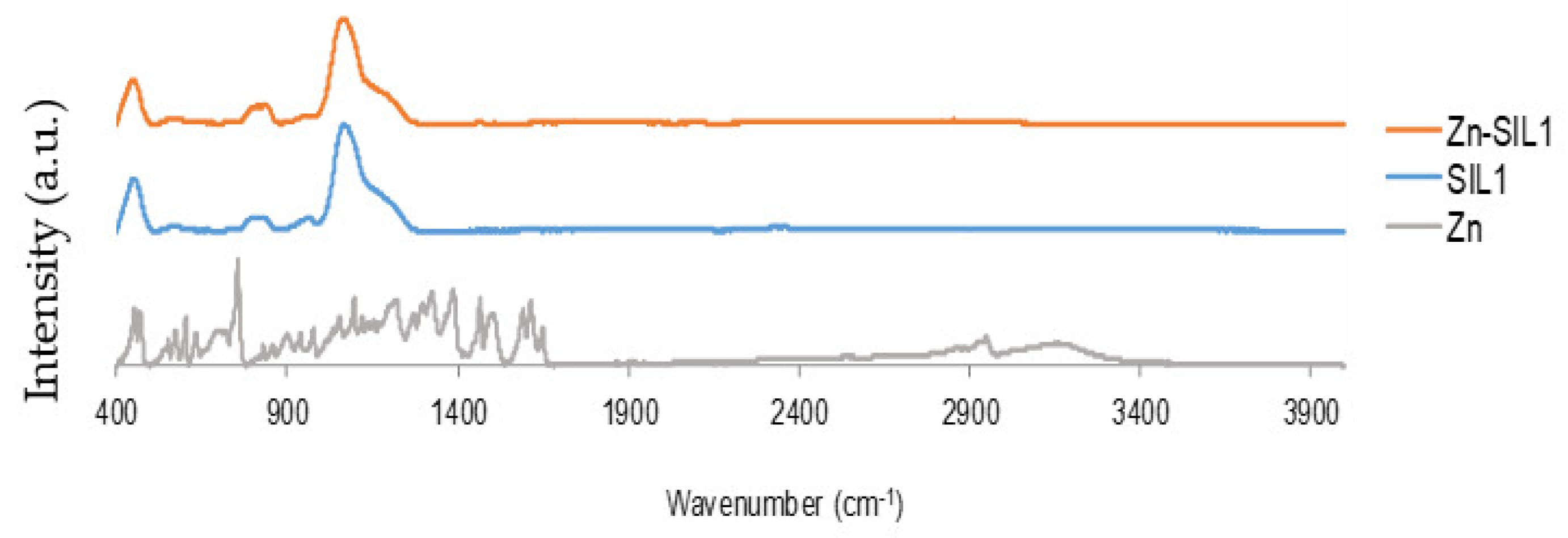
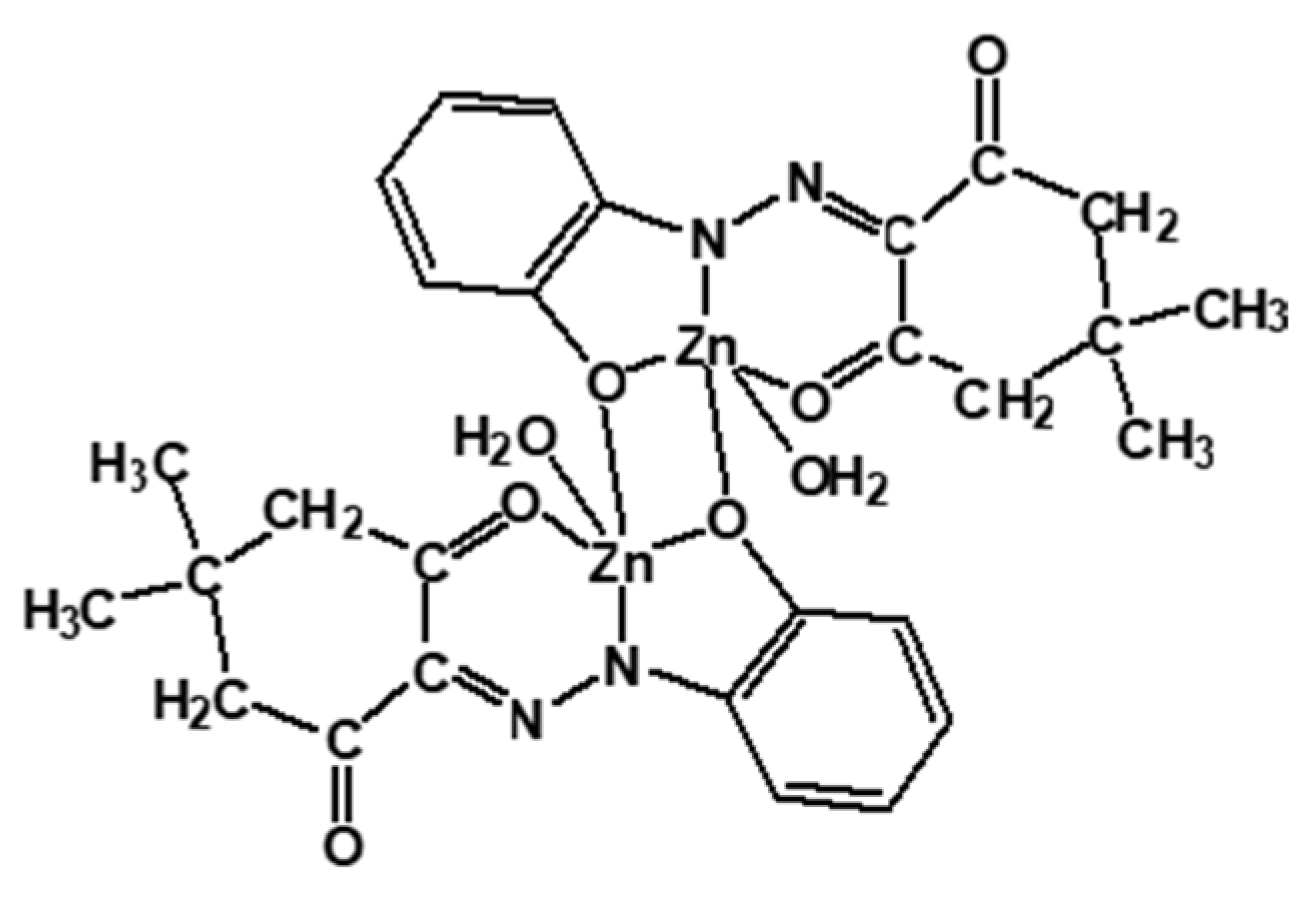




| Matrix | Material Reference | Substance Impregnated |
|---|---|---|
| Silica | Zn-SIL1 | Zn(II)-AHBD |
| Zn-SIL2 | Zn(II)-AHBD | |
| Zn-IL-SIL2 | Zn(II)-AHBD + IL | |
| Alginate | Zn-ALG | Zn(II)-AHBD |
| Zn-IL-ALG | Zn(II)-AHBD + IL | |
| IL-ALG | IL |
| Matrix | Aerogel Reference | Impregnation a | SBET (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|---|---|
| Silica | SIL1 | - | 882 | 2.85 | 12.93 |
| Zn-SIL1 | Zn(II)-AHBD | 772 | 2.96 | 15.35 | |
| SIL2 | - | 1099 | 2.44 | 8.91 | |
| Zn-SIL2 | Zn(II)-AHBD | 258 | 0.475 | 7.36 | |
| Zn-IL-SIL2 | Zn(II)-AHBD + IL | 245 | 0.445 | 7.25 | |
| Alginate | ALG | - | 271 | 2.27 | 33.56 |
| Zn-ALG | Zn(II)-AHBD | 253 | 2.18 | 34.46 | |
| Zn-IL-ALG | Zn(II)-AHBD + IL | <1 | - | - | |
| IL-ALG | IL | <1 | - | - |
| Matrix | Material Reference | Impregnation | Carbonate Formation a |
|---|---|---|---|
| Silica | Zn-SIL1 | Zn(II)-AHBD | 0% |
| Zn-SIL2 | Zn(II)-AHBD | 0% | |
| Zn-IL-SIL2 | Zn(II)-AHBD + IL | <5% | |
| Alginate | Zn-ALG | Zn(II)-AHBD | 0% |
| Zn-IL-ALG | Zn(II)-AHBD + IL | 35% | |
| IL-ALG | IL | 32% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paninho, A.B.; Mustapa, A.N.; Mahmudov, K.T.; Pombeiro, A.J.L.; Guedes da Silva, M.F.C.; Bermejo, M.D.; Martín, Á.; Cocero, M.J.; Nunes, A.V.M. A Bio-Based Alginate Aerogel as an Ionic Liquid Support for the Efficient Synthesis of Cyclic Carbonates from CO2 and Epoxides. Catalysts 2021, 11, 872. https://doi.org/10.3390/catal11080872
Paninho AB, Mustapa AN, Mahmudov KT, Pombeiro AJL, Guedes da Silva MFC, Bermejo MD, Martín Á, Cocero MJ, Nunes AVM. A Bio-Based Alginate Aerogel as an Ionic Liquid Support for the Efficient Synthesis of Cyclic Carbonates from CO2 and Epoxides. Catalysts. 2021; 11(8):872. https://doi.org/10.3390/catal11080872
Chicago/Turabian StylePaninho, Ana B., Ana N. Mustapa, Kamran T. Mahmudov, Armando J. L. Pombeiro, M. Fátima C. Guedes da Silva, María D. Bermejo, Ángel Martín, María J. Cocero, and Ana V. M. Nunes. 2021. "A Bio-Based Alginate Aerogel as an Ionic Liquid Support for the Efficient Synthesis of Cyclic Carbonates from CO2 and Epoxides" Catalysts 11, no. 8: 872. https://doi.org/10.3390/catal11080872
APA StylePaninho, A. B., Mustapa, A. N., Mahmudov, K. T., Pombeiro, A. J. L., Guedes da Silva, M. F. C., Bermejo, M. D., Martín, Á., Cocero, M. J., & Nunes, A. V. M. (2021). A Bio-Based Alginate Aerogel as an Ionic Liquid Support for the Efficient Synthesis of Cyclic Carbonates from CO2 and Epoxides. Catalysts, 11(8), 872. https://doi.org/10.3390/catal11080872











