Enhanced Activity for CO Preferential Oxidation over CuO Catalysts Supported on Nanosized CeO2 with High Surface Area and Defects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of n-CeO2 and p-CeO2
2.2. Characterization of CuO/p-CeO2 and CuCeOX Catalysts
2.3. Catalytic Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Activity Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brian, B.C.H.; Heinzel, A. Materials for Fuel-Cell Technologies. Nature 2001, 414, 345–352. [Google Scholar]
- Devanathan, R. Recent developments in proton exchange membranes for fuel cells. Energy Environ. Sci. 2008, 1, 101–119. [Google Scholar] [CrossRef]
- Park, E.D.; Lee, D.; Lee, H.C. Recent progress in selective CO removal in a H2 rich stream. Catal. Today 2009, 139, 280–290. [Google Scholar] [CrossRef]
- Liu, K.; Wang, A.; Zhang, T. Rencent advance in preferential oxidation of CO reaction over platinum group metal catalysts. ACS Catal. 2012, 2, 1165–1178. [Google Scholar] [CrossRef]
- Wang, M.Q.; Yang, W.H.; Wang, H.H.; Chen, C.; Zhou, Z.Y.; Sun, S.G. Pyrolyzed Fe-N-C Composite as an Efficient Non-precious Metal Catalyst for Oxygen Reduction Reaction in Acidic Medium. ACS Catal. 2014, 4, 3928–3936. [Google Scholar] [CrossRef]
- Barroso-Martín, I.; Alberoni, C.; Rodríguze-Castellón, E.; Infantes-Molina, A.; Moretti, E. Recent advances in photo-assisted preferential CO oxidation in H2-rich stream. Curr. Opin. Green Sustain. Chem. 2020, 21, 9–15. [Google Scholar] [CrossRef]
- Gamarra, D.; Munuera, G.; Hungría, A.B.; Fernández-García, M.; Conesa, J.C.; Midgley, P.A.; Wang, X.Q.; Hanson, J.C.; Rodríguez, J.A.; Martínez-Arias, A. Structure-Activity relationship in nanostructured copper-ceria-based preferential CO oxidation catalysts. J. Phys. Chem. C 2007, 111, 11026–11038. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Gamarra, D.; Belver, C.; Fernández-García, M.; Martínez-Arias, A. Selective CO oxidation in excess H2 over copper-ceria catalysts: Identification of active entities/species. J. Am. Chem. Soc. 2007, 129, 12064–12065. [Google Scholar] [CrossRef]
- Qi, L.; Yu, Q.; Dai, Y.; Tang, C.; Liu, L.; Zhang, H.; Gao, F.; Dong, L.; Chen, Y. Influence of cerium precursors on the structure and reducibility of mesoporous CuO-CeO2 catalysts for CO oxidation. Appl. Catal. B Environ. 2012, 119, 308–320. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, L.; Lu, J.; Chen, R.; Lei, Y.; Chen, K.; Han, C.; He, S.; Wan, G.; Luo, Y. A solvent-free method to rapidly synthesize CuO-CeO2 catalysts to enhance their CO preferential oxidation: Effects of Cu loading and calcination temperature. Mol. Catal. 2017, 443, 241–252. [Google Scholar] [CrossRef]
- Jampa, S.; Wangkawee, K.; Tantisriyanurak, S.; Changpradit, J.; Jamieson, A.M.; Chaisuwan, T.; Luengnaruemitchai, A.; Wongkasemjit, S. High performance and stability of copper loading on mesoporous ceria catalyst for preferential oxidation of CO in presence of excess of hydrogen. Int. J. Hydrogen Energy 2017, 42, 5537–5548. [Google Scholar] [CrossRef]
- Reis, C.G.M.; Almeida, K.A.; Silva, T.F.; Assaf, J.M. CO preferential oxidation reaction aspects in a nanocrystalline CuO/CeO2 catalyst. Catal. Today 2020, 344, 124–128. [Google Scholar] [CrossRef]
- Zou, Q.; Zhao, Y.; Jin, X.; Fang, J.; Li, D.; Li, K.; Lu, J.; Luo, Y. Ceria-nano supported copper oxide catalysts for CO preferential oxidation: Importance of oxygen species and metal-support interaction. Appl. Surf. Sci. 2019, 494, 1166–1176. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, Q.P.; Wang, X.L.; Ma, K.; Bai, X.Q.; Tan, S.R.; Tian, Y.; Ding, T.; Zheng, L.R.; Zhang, J.; et al. Enhanced catalytic performance for CO preferential oxidation over CuO catalysts supported on highly defective CeO2 nanocrystal. Appl. Surf. Sci. 2017, 422, 932–943. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, J.; Jing, G.; Zhang, H.; Zeng, S.; Tian, X.; Zou, X.; Wen, J.; Su, H.; Zhong, C.; et al. Structural origin of high catalytic activity for preferential CO oxidation over CuO/CeO2 nanocatalysts with different shapes. Appl. Catal. B Environ. 2018, 239, 665–676. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, R. Identification of the nano/micro structure of CeO2 (rod) and the essential role of interfacial copper-ceria interaction in CuCe (rod) for selective oxidation of CO in H2-rich streams. J. Power Sources 2017, 361, 39–53. [Google Scholar] [CrossRef]
- Gong, X.; Liu, B.; Kang, B.; Xu, G.; Wang, Q.; Jia, C.J.; Zhang, J. Boosting Cu-Ce interaction in CuxO/CeO2 nanocube catalysts for enhanced catalytic performance of preferential oxidation of CO in H2-rich gases. Mol. Catal. 2017, 436, 90–99. [Google Scholar] [CrossRef]
- Castañeda, R.; Pascual, L.; Martínez-Arias, A. Influence of sodium impurities on the properties of CeO2/CuO for carbon monoxide oxidation in a hydrogen-rich stream. Catal. Commun. 2018, 108, 88–92. [Google Scholar] [CrossRef]
- Ramachandran, M.; Subadevi, R.; Sivakumar, M. Role of pH on synthesis and characterization of cerium oxide (CeO2) nano particles by modified co-precipitation method. Vacuum 2019, 161, 220–224. [Google Scholar] [CrossRef]
- Li, W.; Hu, Y.; Jiang, H.; Yang, S.; Li, C. Facile synthesis of multi-shelled hollow Cu/CeO2 microspheres with promoted catalytic performance for preferential oxidation of CO. Mater. Chem. Phys. 2019, 226, 158–168. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, R. A new insight into the morphology effect of ceria on CuO/CeO2 catalysts for selective oxidation of CO in hydrogen-rich gas. Catal. Sci. Technol. 2016, 6, 3862–3871. [Google Scholar] [CrossRef]
- Gong, L.; Liu, C.; Liu, Q.; Dai, R.; Nie, X.; Lu, L.; Liu, G.; Hu, X. CuO/CeO2-MnO2 catalyst prepared by redox method for preferential oxidation of CO H2-rich gases. Catal. Surv. Asia 2019, 23, 1–9. [Google Scholar] [CrossRef]
- Arango-Díaz, A.; Cecilia, J.A.; Santos-Gómez, L.D. Characterization and performance in preferential oxidation of CO of CuO-CeO2 catalysts synthesized using polymethyl metacrylate (PMMA) as template. Int. J. Hydrogen Energy 2015, 40, 11254–11260. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Hu, W.; Huang, X.; Li, Q.; Xu, Y.; Zuo, Y.; Li, G. Anchoring high-concentration oxygen vacancies at interfaces of CeO2-x/Cu toward enhanced activity for preferential CO oxidation. ACS Appl. Mater. Interfaces 2015, 7, 22999–23007. [Google Scholar] [CrossRef]
- Gu, D.; Jia, C.J.; Bongard, H.; Spliethoff, B.; Weidenthaler, C.; Schmidt, W.; Schüth, F. Ordered mesoporous Cu-Ce-O catalysts for CO preferential oxidation in H2-rich gases: Influence of copper content and pretreatment conditions. Appl. Catal. B Environ. 2014, 152, 11–18. [Google Scholar] [CrossRef]
- Hou, H.; Liu, Y.; Liu, B.; Jing, P.; Gao, Y.; Zhang, L.; Niu, P.; Wang, Q.; Zhang, J. Modulating the operation temperature window of CO preferential oxidation in H2-rich gases on three dimensionally ordered macroporous CeO2-CuO catalysts by tuning their composition and incooperating Fe2O3 and Co3O4. Int. J. Hydrogen Energy 2015, 40, 878–890. [Google Scholar] [CrossRef]
- Monte, M.; Gamarra, D.; Cámara, A.L.; Rasmussen, S.B.; Gyorffy, N.; Schay, Z.; Martínez-Arias, A.; Conesa, J.C. Preferential oxidation of CO in excess H2 over CuO/CeO2 catalysts: Performance as a function of the copper coverage and exposed face present in the CeO2 support. Catal. Today 2014, 229, 104–113. [Google Scholar] [CrossRef]
- Hossain, S.T.; Azeeva, E.; Zhang, K.; Zell, E.T.; Bernard, D.T.; Balaz, S.; Wang, R. A comparative study of CO oxidation over Cu-O-Ce solid solutions and CuO/CeO2 nanorods catalysts. Appl. Surf. Sci. 2018, 455, 132–143. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Arango-Díaz, A.; Marrero-Jerez, J.; Núñez, P.; Moretti, E.; Storaro, L.; Rodríguez-Castellón, E. Catalytic behaviour of CuO-CeO2 system prepared by different synthetic methodologies in the CO-PROX reaction under CO2-H2O feed srteam. Catalysts 2017, 7, 160. [Google Scholar] [CrossRef]
- Wen, B.; He, M.Y. Study of the Cu-Ce synergism for NO reduction with CO in the presence of O2, H2O, and SO2 in FCC operation. Appl. Catal. B Environ. 2002, 37, 75–82. [Google Scholar] [CrossRef]
- Peng, C.T.; Lia, H.K.; Liaw, B.J.; Chen, Y.Z. Removal of CO in excess hydrogen over CuO/Ce1-xMnO2 catalysts. Chem. Eng. J. 2011, 172, 452–458. [Google Scholar] [CrossRef]
- Skårman, B.; Grandjean, D.; Benfield, R.E.; Hinz, A.; Andersson, A.; Wallenberg, L.R. Carbon monoxide on nanostructured CuOx/CeO2 composite particles characterized by HREM, XPS, XAS and high-energy diffraction. J. Catal. 2002, 211, 119–133. [Google Scholar] [CrossRef]
- Pu, Z.Y.; Lu, J.Q.; Luo, M.F.; Xie, Y.L. Study of oxygen vacancies in Ce0.9Pr0.1O2-δ solid solution by in Situ X-ray diffraction and in Situ Raman spectroscopy. J. Phys. Chem. C 2007, 111, 18695–18702. [Google Scholar] [CrossRef]
- Barbato, P.S.; Colussi, S.; Benedetto, A.D.; Landi, G.; Lisi, L.; Llorca, J.; Trovarelli, A. Origin of high activity and selectivity of CuO/CeO2 catalysts prepared by solution combustion synthesis in CO-PROX reaction. J. Phys. Chem. C 2016, 120, 13039–13048. [Google Scholar] [CrossRef]
- Fan, J.; Wu, X.; Wu, X.; Liang, Q.; Ran, R.; Weng, D. Thermal ageing of Pt on low-surface-area CeO2-ZrO2-La2O3 mixed oxides: Effect on the OSC performance. Appl. Catal. B Environ. 2008, 81, 38–48. [Google Scholar] [CrossRef]
- Li, X.; Quek, X.Y.; Ligthart, D.A.J.M.; Guo, M.; Zhang, Y.; Li, C.; Yang, Q.; Hensen, E.J.M. CO-PROX reactions on copper cerium oxide catalysts prepared by melt infiltration. Appl. Catal. B Environ. 2012, 123, 424–432. [Google Scholar] [CrossRef]
- Sedmak, G.; Hočevar, S.; Levec, J. Kinetics of selective CO oxidation in excess of H2 over the nanostructured Cu0.1Ce0.9O2−y catalyst. J. Catal. 2003, 213, 135–150. [Google Scholar] [CrossRef]
- Davó-Quiňonero, A.; Navlani-García, M.; Lozano-Castelló, D.; Bueno-López, A.; Anderson, J.A. Role of Hydrogen groups in the preferential oxidation of CO over copper oxide catalysts. ACS Catal. 2016, 6, 1723–1731. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.W.; Du, P.P.; Zou, S.H.; He, H.Y.; Wang, R.X.; Jin, Z.; Shi, S.; Huang, Y.Y.; Si, R.; Song, Q.S.; et al. Highly dispersed copper oxide cluster as active species in copper-ceria catalyst for preferential oxidation of carbon monoxide. ACS Catal. 2015, 5, 2088–2099. [Google Scholar] [CrossRef]
- Polster, C.S.; Nair, H.; Baertsch, C.D. Study of active sites and mechanism responsible for highly selective CO oxidation in H2 rich atmospheres on a mixed Cu and Ce oxide catalyst. J. Catal. 2009, 266, 308–319. [Google Scholar] [CrossRef]



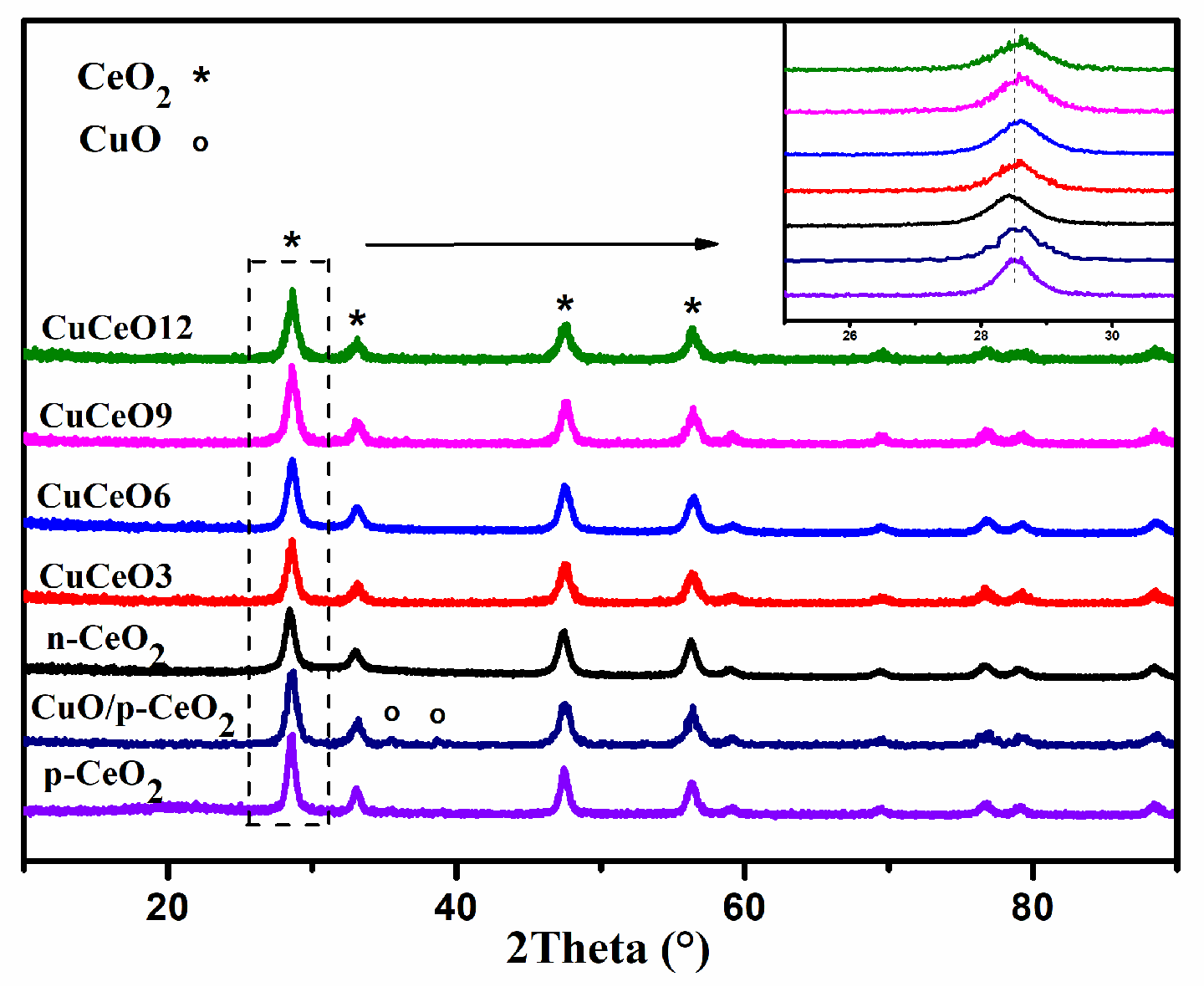

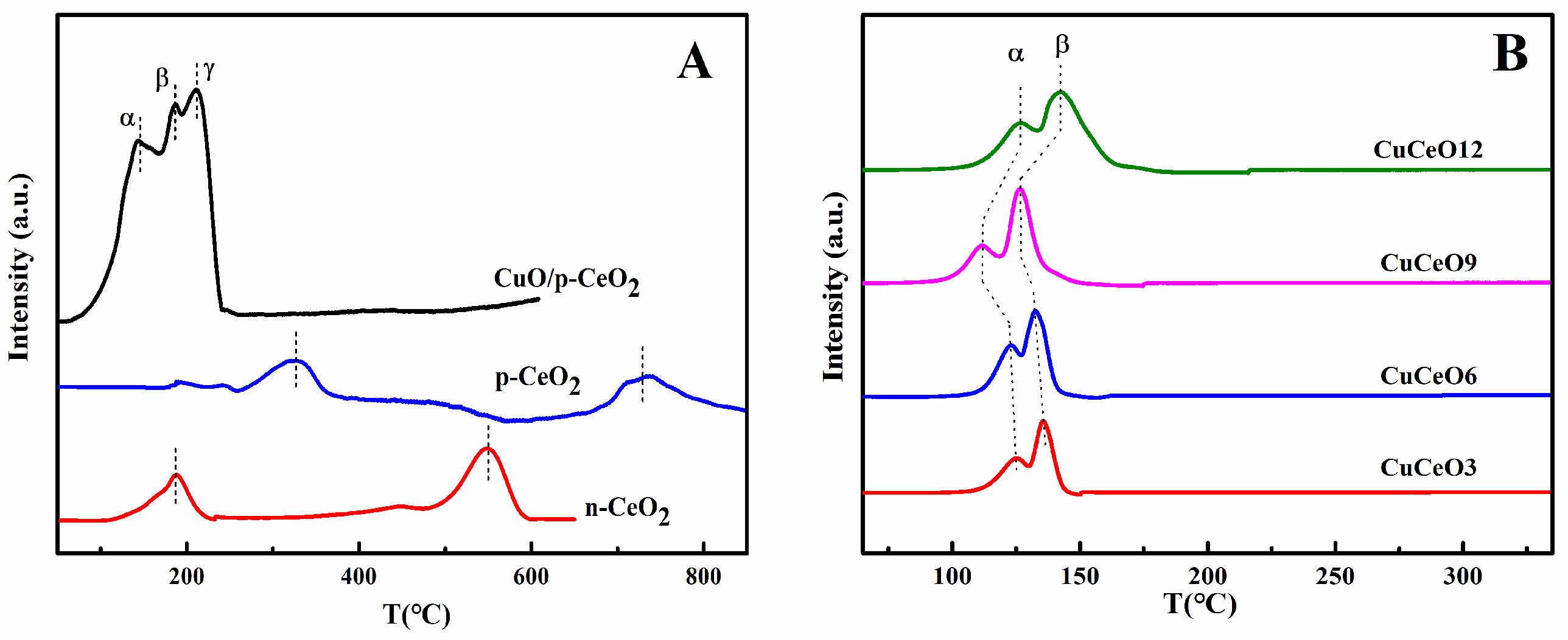
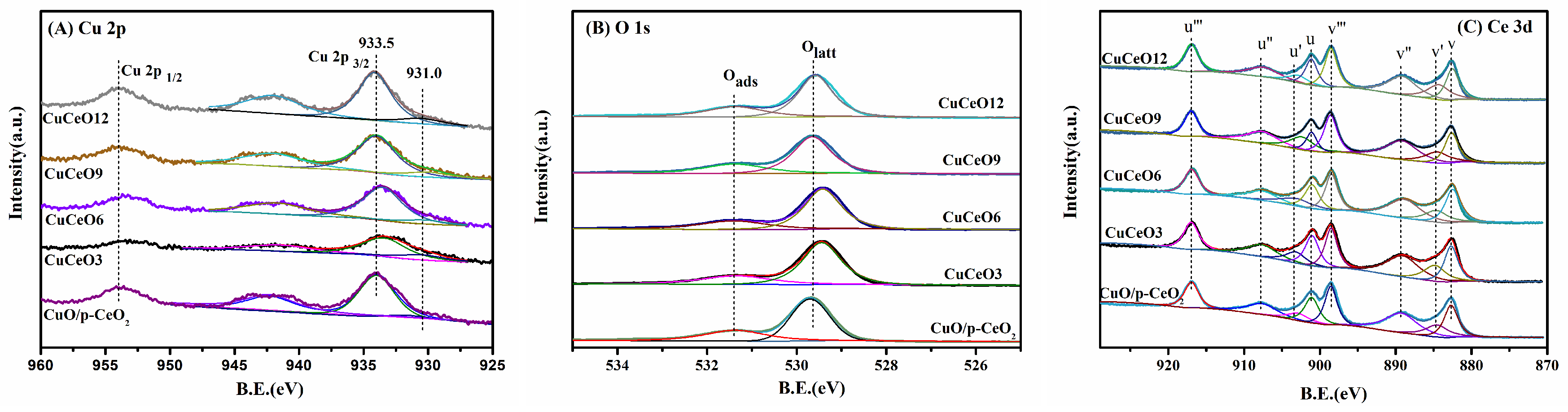


| Catalyst | SBET (m2·g−1) | Dpore (nm) | Vpore (cm3·g−1) | Lattice Parameters (nm) a |
|---|---|---|---|---|
| p-CeO2 | 35 | 18.7 | 0.083 | 0.5414 |
| n-CeO2 | 130 | 5.6 | 0.176 | 0.5417 |
| CuCeO3 | 95 | 8.9 | 0.187 | 0.5411 |
| CuCeO6 | 94 | 9.4 | 0.198 | 0.5409 |
| CuCeO9 | 92 | 9.3 | 0.186 | 0.5405 |
| CuCeO12 | 86 | 6.4 | 0.130 | 0.5417 |
| CuO/p-CeO2 | 24 | 20.3 | 0.051 | 0.5413 |
| Catalysts | α Peak | β Peak | γ Peak | Total | |||
|---|---|---|---|---|---|---|---|
| Peak Temp. (°C) | H2 Cons. (µmol·g−1) | Peak Temp. (°C) | H2 Cons. (µmol·g−1) | Peak Temp. (°C) | H2 Cons. (µmol·g−1) | H2 Cons. (µmol·g−1) | |
| CuO/p-CeO2 | 142 | 632 | 185 | 648 | 210 | 713 | 1993 |
| CuCeO3 | 124 | 468 | 136 | 513 | - | - | 981 |
| CuCeO6 | 121 | 616 | 133 | 851 | - | - | 1467 |
| CuCeO9 | 111 | 716 | 127 | 1291 | - | - | 2007 |
| CuCeO12 | 127 | 667 | 147 | 1775 | - | - | 2442 |
| Catalysts | Ce3+/(Ce3++Ce4+) (%) | Isat/Imp | Olatt/Ototal (%) | Cu Content (wt.%) a | Cu/Cu+Ce (at%) b | A584/A460 |
|---|---|---|---|---|---|---|
| CuO/p-CeO2 | 11.79 | 0.41 | 74.63 | 8.9 | 28.8 | 0.15 |
| CuCeO3 | 16.61 | 0.35 | 79.42 | 3.1 | 18.3 | 0.23 |
| CuCeO6 | 17.83 | 0.32 | 76.52 | 5.9 | 25.0 | 0.28 |
| CuCeO9 | 20.82 | 0.27 | 72.96 | 8.9 | 29.3 | 0.39 |
| CuCeO12 | 16.23 | 0.38 | 69.42 | 11.8 | 32.9 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, L.; Jie, W.; Liu, Y.; Lin, X.; Deng, W.; Qiu, M.; Hu, X.; Liu, Q. Enhanced Activity for CO Preferential Oxidation over CuO Catalysts Supported on Nanosized CeO2 with High Surface Area and Defects. Catalysts 2021, 11, 884. https://doi.org/10.3390/catal11080884
Gong L, Jie W, Liu Y, Lin X, Deng W, Qiu M, Hu X, Liu Q. Enhanced Activity for CO Preferential Oxidation over CuO Catalysts Supported on Nanosized CeO2 with High Surface Area and Defects. Catalysts. 2021; 11(8):884. https://doi.org/10.3390/catal11080884
Chicago/Turabian StyleGong, Lei, Weiwei Jie, Yumeng Liu, Xinchen Lin, Wenyong Deng, Mei Qiu, Xiuxia Hu, and Qian Liu. 2021. "Enhanced Activity for CO Preferential Oxidation over CuO Catalysts Supported on Nanosized CeO2 with High Surface Area and Defects" Catalysts 11, no. 8: 884. https://doi.org/10.3390/catal11080884





