Uniform Distribution of Pd on GO-C Catalysts for Enhancing the Performance of Air Cathode Microbial Fuel Cell
Abstract
:1. Introduction
2. Results and Discussion
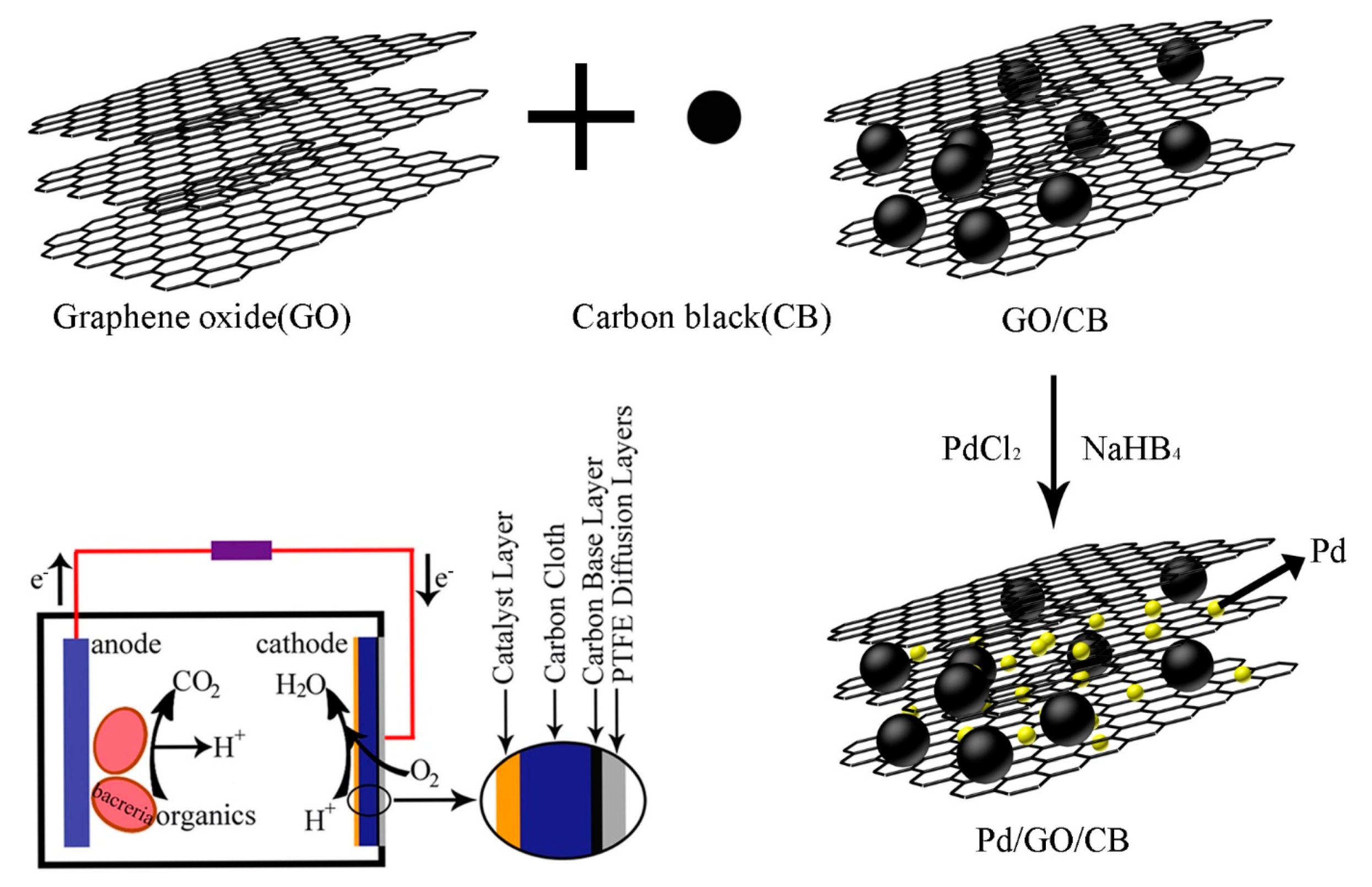
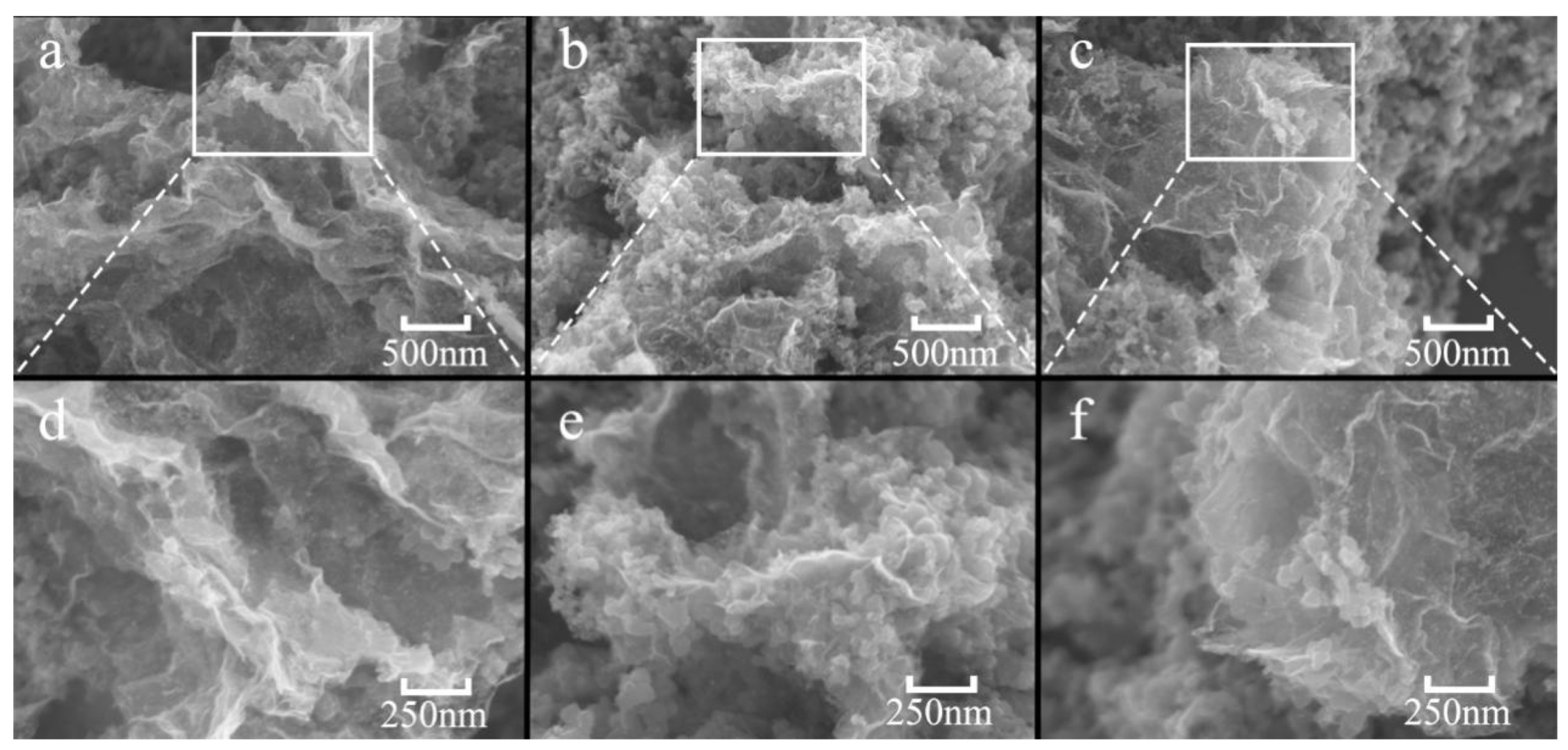
2.1. Characterization of Pd/GO-C Catalyst
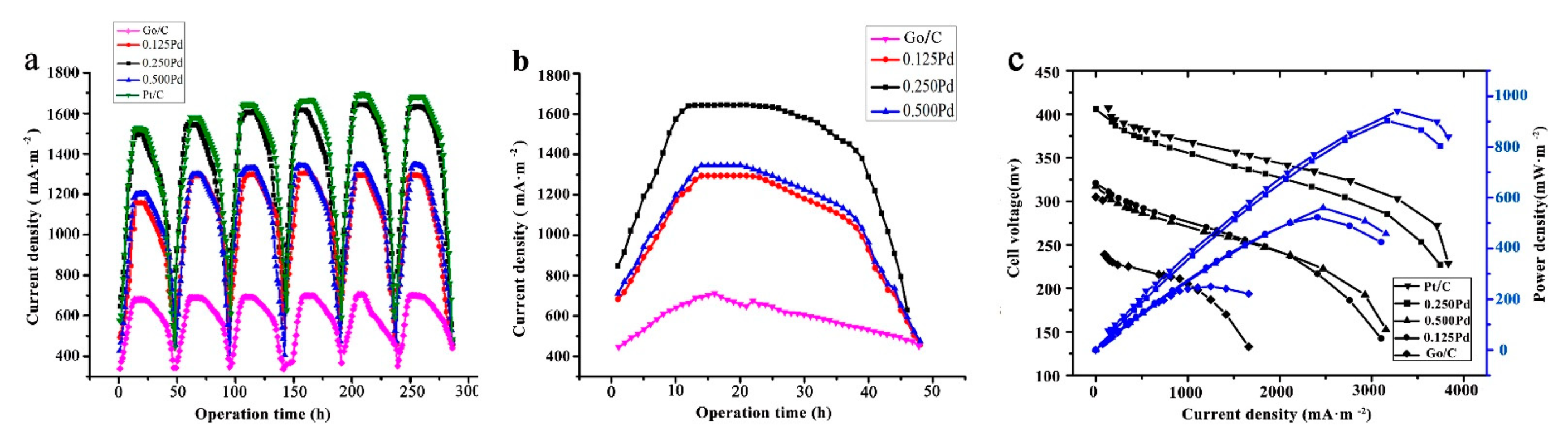
2.2. Performance of MFC with Catalysts in the Cathode
2.3. The Electrochemical Properties of MFC
3. Methods and Materials
3.1. Synthesis and Characterization of the Air Cathode
3.2. MFC Construction and Operation
3.3. Characterization and Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schroder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [Green Version]
- Allen, R.M.; Bennetto, H.P. Microbial Fuel-Cells Electricity Production from Carbohydrates. Appl. Biochem. Biotechnol. 2009, 39, 27–40. [Google Scholar]
- Potter, M.C. Electrical Effects Accompanying the Decomposition of Organic Compounds. Proc. R. Soc. B Biol. Sci. 1911, 84, 260–276. [Google Scholar]
- Li, F.; Sharma, Y.; Lei, Y.; Li, B.; Zhou, Q. Microbial fuel cells: The effects of configurations, electrolyte solutions, and electrode materials on power generation. Appl. Biochem. Biotechnol. 2010, 160, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Zhang, L.; Zhu, X.; Liao, Q. A monolithic air cathode derived from bamboo for microbial fuel cells. RSC Adv. 2017, 7, 28469–28475. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, Q.; Xia, X.; He, W.; Huang, X.; Logan, B.E. Addition of conductive particles to improve the performance of activated carbon air-cathodes in microbial fuel cells. Environ. Sci. Water Res. Technol. 2017, 3, 806–810. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Gokhale, R.; Rojas-Carbonell, S.; Stariha, L.; Gordon, J.; Artyushkova, K.; Atanassov, P. A family of Fe-N-C oxygen reduction electrocatalysts for microbial fuel cell (MFC) application: Relationships between surface chemistry and performances. Appl. Catal. B Environ. 2017, 205, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, J.; Zhou, Y.; Fu, Q.; Yang, W.; Zhu, X.; Liao, Q. A green, cheap, high-performance carbonaceous catalyst derived from Chlorella pyrenoidosa for oxygen reduction reaction in microbial fuel cells. Int. J. Hydrogen Energy 2017, 42, 27657–27665. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, N.; Yin, F.; Yang, T.; Wu, P.; Dang, Z.; Liu, M.; Wei, X. Biomass-derived heteroatoms-doped mesoporous carbon for efficient oxygen reduction in microbial fuel cells. Biosens. Bioelectron. 2017, 98, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, Y.; Zhuang, S.; Liang, Z.; Li, B. Enhancement of Electricity Generation in Single Chamber Microbial Fuel Cell Using Binuclear-Cobalt-Phthalocyanine and Cerium Oxide Co-Supported on Ordered Mesoporous Carbon as Cathode Catalyst. J. Electrochem. Soc. 2019, 166, F9–F17. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Obaid, M.; Poo, K.-M.; Ali Abdelkareem, M.; Talas, S.A.; Fadali, O.A.; Kim, H.Y.; Chae, K.-J. Fe/Fe2O3 nanoparticles as anode catalyst for exclusive power generation and degradation of organic compounds using microbial fuel cell. Chem. Eng. J. 2018, 349, 800–807. [Google Scholar] [CrossRef]
- Vicha, J.; Foroutan-Nejad, C.; Pawlak, T.; Munzarova, M.L.; Straka, M.; Marek, R. Understanding the Electronic Factors Responsible for Ligand Spin-Orbit NMR Shielding in Transition-Metal Complexes. J. Chem. Theory Comput. 2015, 11, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.A.; Liu, H.; Logan, B.E. Power Densities Using Different Cathode Catalysts (Pt and CoTMPP) and Polymer Binders (Nafion and PTFE) in Single Chamber Microbial Fuel Cells. Environ. Sci. Technol. 2006, 40, 364–369. [Google Scholar] [CrossRef]
- Sanchez, D.V.P.; Huynh, P.; Kozlov, M.E.; Baughman, R.H.; Vidic, R.D.; Yun, M. Carbon Nanotube/Platinum (Pt) Sheet as an Improved Cathode for Microbial Fuel Cells. Energy Fuels 2010, 24, 5897–5902. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Ahadzadeh, I. A dual-chambered microbial fuel cell with Ti/nano-TiO2/Pd nano-structure cathode. J. Power Sources 2012, 220, 292–297. [Google Scholar] [CrossRef]
- Shi, H.; Auerbach, S.M.; Ramasubramaniam, A. First-Principles Predictions of Structure–Function Relationships of Graphene-Supported Platinum Nanoclusters. J. Phys. Chem. C 2016, 120, 11899–11909. [Google Scholar] [CrossRef]
- Yang, H.N.; Lee, D.C.; Park, K.W.; Kim, W.J. Platinum–boron doped graphene intercalated by carbon black for cathode catalyst in proton exchange membrane fuel cell. Energy 2015, 89, 500–510. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Li, Y.; Tang, L.; Li, J. Preparation and electrochemical performance for methanol oxidation of pt/graphene nanocomposites. Electrochem. Commun. 2009, 11, 846–849. [Google Scholar] [CrossRef]
- Şanlı, L.I.; Bayram, V.; Yarar, B.; Ghobadi, S.; Gürsel, S.A. Development of graphene supported platinum nanoparticles for polymer electrolyte membrane fuel cells: Effect of support type and impregnation–reduction methods. Int. J. Hydrogen Energy 2016, 41, 3414–3427. [Google Scholar] [CrossRef]
- McKerracher, R.D.; Figueredo-Rodríguez, H.A.; Ponce de León, C.; Alegre, C.; Baglio, V.; Aricò, A.S.; Walsh, F.C. A high-performance, bifunctional oxygen electrode catalysed with palladium and nickel-iron hexacyanoferrate. Electrochim. Acta 2016, 206, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Alegre, C.; Modica, E.; Lo Vecchio, C.; Sebastian, D.; Lazaro, M.J.; Arico, A.S.; Baglio, V. Carbon Nanofibers as Advanced Pd Catalyst Supports for the Air Electrode of Alkaline Metal-Air Batteries. ChemPlusChem 2015, 80, 1384–1388. [Google Scholar] [CrossRef]
- Juárez-Marmolejo, L.; Pérez-Rodríguez, S.; Montes de Oca-Yemha, M.G.; Palomar-Pardavé, M.; Romero-Romo, M.; Ezeta-Mejía, A.; Morales-Gil, P.; Martínez-Huerta, M.V.; Lázaro, M.J. Carbon supported PdM (M = Fe, Co) electrocatalysts for formic acid oxidation. Influence of the Fe and Co precursors. Int. J. Hydrogen Energy 2019, 44, 1640–1649. [Google Scholar] [CrossRef] [Green Version]
- Jesús Lázaro, M.; Ascaso, S.; Pérez-Rodríguez, S.; Calderón, J.C.; Gálvez, M.E.; Jesús Nieto, M.; Moliner, R.; Boyano, A.; Sebastián, D.; Alegre, C.; et al. Carbon-based catalysts: Synthesis and applications. C. R. Chim. 2015, 18, 1229–1241. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Sebastián, D.; Alegre, C.; Aricò, A.S.; Baglio, V. Carbon-supported Pd and Pd-Co cathode catalysts for direct methanol fuel cells (DMFCs) operating with high methanol concentration. J. Electroanal. Chem. 2018, 808, 464–473. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhu, E.; McLouth, T.; Chiu, C.Y.; Huang, X.; Huang, Y. Stabilization of high-performance oxygen reduction reaction Pt electrocatalyst supported on reduced graphene oxide/carbon black composite. J. Am. Chem. Soc. 2012, 134, 12326–12329. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Lee, J.; Park, S.-K.; Kim, H.; Chung, T.D.; Piao, Y. Enhanced electrocatalysis of PtRu onto graphene separated by Vulcan carbon spacer. J. Power Sources 2013, 222, 261–266. [Google Scholar] [CrossRef]
- Wu, Q.; Rao, Z.; Yuan, L.; Jiang, L.; Sun, G.; Ruan, J.; Zhou, Z.; Sang, S. Carbon supported PdO with improved activity and stability for oxygen reduction reaction in alkaline solution. Electrochim. Acta 2014, 150, 157–166. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Y.; Zhang, L.; Tang, T.; Xiao, H.; Chen, W.; Zhao, M.; Jia, J.; Zhu, H. Facile synthesis of PdO-doped Co3O4 nanoparticles as an efficient bifunctional oxygen electrocatalyst. Appl. Catal. B Environ. 2019, 243, 175–182. [Google Scholar] [CrossRef]
- Li, H.C.; Zhang, Y.J.; Hu, X.; Liu, W.J.; Chen, J.J.; Yu, H.Q. Metal-Organic Framework Templated Pd@PdO–Co3O4 Nanocubes as an Efficient Bifunctional Oxygen Electrocatalyst. Adv. Energy Mater. 2018, 8, 1702734. [Google Scholar] [CrossRef]
- Lv, M.; Li, W.; Liu, H.; Wen, W.; Dong, G.; Liu, J.; Peng, K. Enhancement of the formic acid electrooxidation activity of palladium using graphene/carbon black binary carbon supports. Chin. J. Catal. 2017, 38, 939–947. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Dai, Y.; Chan, Y.; Jiang, B.; Wang, L.; Zou, J.; Pan, K.; Fu, H. Bifunctional Ag/Fe/N/C Catalysts for Enhancing Oxygen Reduction via Cathodic Biofilm Inhibition in Microbial Fuel Cells. ACS Appl. Mater. Interfaces 2016, 8, 6992–7002. [Google Scholar] [CrossRef]
- Mecheri, B.; Iannaci, A.; D’Epifanio, A.; Mauri, A.; Licoccia, S. Carbon-Supported Zirconium Oxide as a Cathode for Microbial Fuel Cell Applications. ChemPlusChem 2016, 81, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, S.A.; Logan, B.E. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, R. Electrochemical investigations on carbon supported palladium catalysts. Appl. Catal. A Gen. 1997, 153, 9–20. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Alegre, C.; Sebastian, D.; Stassi, A.; Arico, A.S.; Baglio, V. Investigation of Supported Pd-Based Electrocatalysts for the Oxygen Reduction Reaction: Performance, Durability and Methanol Tolerance. Materials 2015, 8, 7997–8008. [Google Scholar] [CrossRef] [Green Version]
- Santoro, C.; Kodali, M.; Kabir, S.; Soavi, F.; Serov, A.; Atanassov, P. Three-dimensional graphene nanosheets as cathode catalysts in standard and supercapacitive microbial fuel cell. J. Power Sources 2017, 356, 371–380. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Mu, K.; Zhao, X.; Luo, D.; Yu, X.; Li, W.; Chu, J.; Yang, J.; Yang, Q. Uniform Distribution of Pd on GO-C Catalysts for Enhancing the Performance of Air Cathode Microbial Fuel Cell. Catalysts 2021, 11, 888. https://doi.org/10.3390/catal11080888
Wang J, Mu K, Zhao X, Luo D, Yu X, Li W, Chu J, Yang J, Yang Q. Uniform Distribution of Pd on GO-C Catalysts for Enhancing the Performance of Air Cathode Microbial Fuel Cell. Catalysts. 2021; 11(8):888. https://doi.org/10.3390/catal11080888
Chicago/Turabian StyleWang, Jingzhen, Kaijie Mu, Xuedong Zhao, Dianliang Luo, Xiaodi Yu, Wenpeng Li, Jie Chu, Jing Yang, and Qinzheng Yang. 2021. "Uniform Distribution of Pd on GO-C Catalysts for Enhancing the Performance of Air Cathode Microbial Fuel Cell" Catalysts 11, no. 8: 888. https://doi.org/10.3390/catal11080888
APA StyleWang, J., Mu, K., Zhao, X., Luo, D., Yu, X., Li, W., Chu, J., Yang, J., & Yang, Q. (2021). Uniform Distribution of Pd on GO-C Catalysts for Enhancing the Performance of Air Cathode Microbial Fuel Cell. Catalysts, 11(8), 888. https://doi.org/10.3390/catal11080888





