Functionalized and Platinum-Decorated Multi-Layer Oxidized Graphene as a Proton, and Electron Conducting Separator in Solid Acid Fuel Cells
Abstract
:1. Introduction
2. Results and Discussion
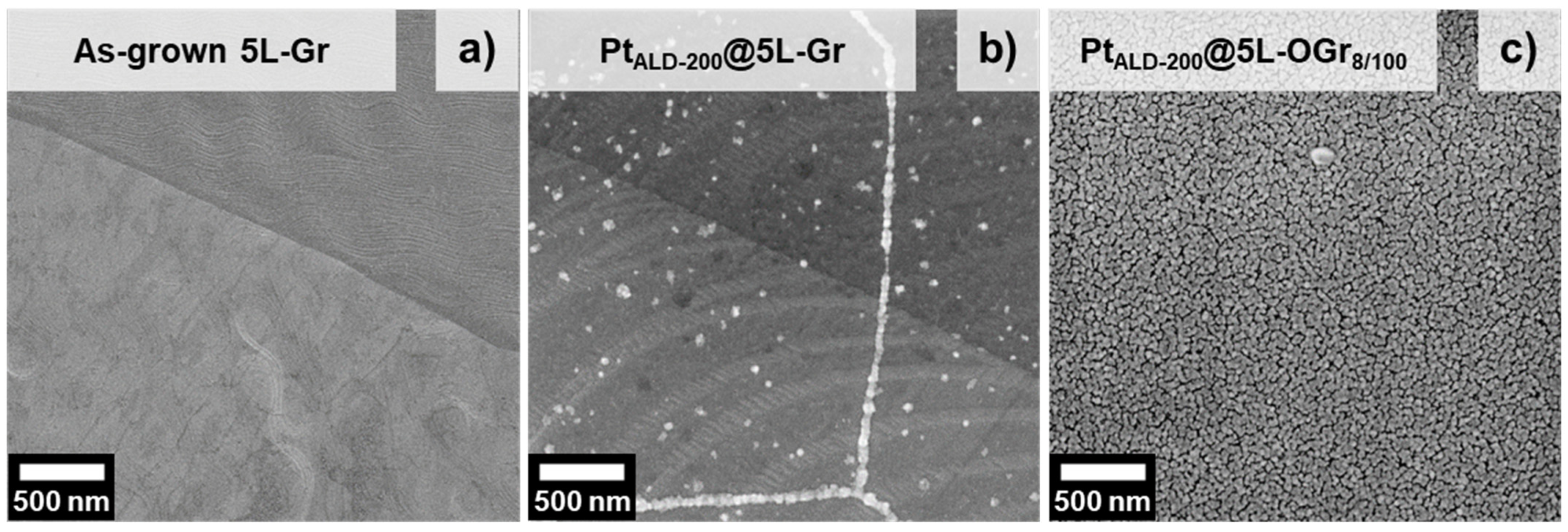
2.1. Platinum-Decorated Oxidized Graphene
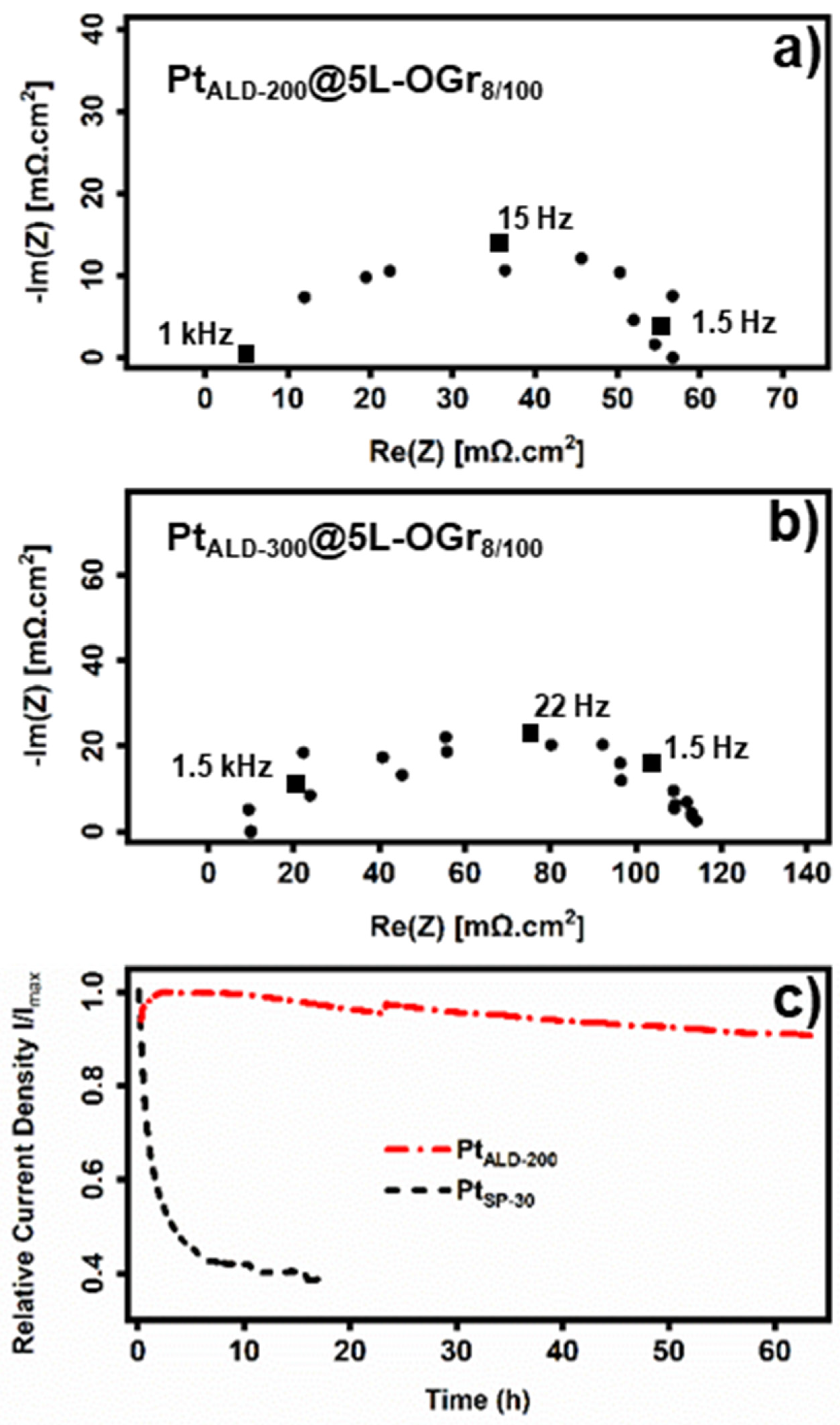
2.2. Electrochemical Measurements and Performance
3. Experimental, Materials and Methods
3.1. Graphene Preparation via Chemical Vapor Deposition (CVD) on Copper Substrate
3.2. Graphene Transfer
3.3. Oxygen Plasma Treatment
3.4. Catalyst Deposition
3.4.1. Atomic Layer Deposition (ALD)
3.4.2. Sputtering
3.5. Electrochemical Cell Characterization
3.5.1. Platinum-Decorated Oxidized Graphene Cell Fabrication
3.5.2. Impedance Spectroscopy
3.6. Characterization Methods
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boysen, D.A.; Uda, T.; Chisholm, C.R.I.; Haile, S.M. High-Performance Solid Acid Fuel Cells through Humidity Stabilization. Science 2004, 303, 68–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haile, S.M. Fuel cell materials and components. Acta Mater. 2003, 51, 5981–6000. [Google Scholar] [CrossRef]
- Uda, T.; Haile, S.M. Thin-Membrane Solid-Acid Fuel Cell. Electrochem. Solid-State Lett. 2005, 8, A245. [Google Scholar] [CrossRef] [Green Version]
- Haile, S.M.; Boysen, D.A.; Chisholm, C.R.I.; Merle, R.B. Solid acids as fuel cell electrolytes. Nature 2001, 410, 910–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, A.; Kitchaev, D.A.; Haile, S.M. Phase behavior and superprotonic conductivity in the Cs1−xRbxH2PO4 and Cs1−xKxH2PO4 systems. J. Mater. Chem. A 2014, 2, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Sanghvi, S.; Haile, S.M. Experimental Crystal Structure Determination. Solid State Ion. 2020, 349, 15291. [Google Scholar]
- Bagryantseva, I.N.; Ponomareva, V.G.; Khusnutdinov, V.R. Intermediate temperature proton electrolytes based on cesium dihydrogen phosphate and poly(vinylidene fluoride-co-hexafluoropropylene). J. Mater. Sci. 2021, 56, 14196–14206. [Google Scholar] [CrossRef]
- Navarrete, L.; Andrio, A.; Escolástico, S.; Moya, S.; Compañ, V.; Serra, J.M. Protonic Conduction of Partially-Substituted CsH2PO4 and the Applicability in Electrochemical Devices. Membranes 2019, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.; Daud, W.R.W.; Badiei, M.; Mohammad, A.B. Effect of surfactants in synthesis of CsH2PO4 as protonic conductive membrane. Bull. Mater. Sci. 2011, 34, 759–765. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, P.; Singh, J.; Veer, D.; Kumar, A.; Katiyar, R.S. Structural, thermal and electrical properties of composites electrolytes (1−x) CsH2PO4/x ZrO2 (0 ≤ x ≤ 0.4) for fuel cell with advanced electrode. SN Appl. Sci. 2021, 3, 46. [Google Scholar] [CrossRef]
- Lu, X.; Yang, X.; Tariq, M.; Li, F.; Steimecke, M.; Li, J.; Varga, A.; Bron, M.; Abel, B. Plasma-etched functionalized graphene as a metal-free electrode catalyst in solid acid fuel cells. J. Mater. Chem. A 2020, 8, 2445–2452. [Google Scholar] [CrossRef]
- Wagner, M.; Dreßler, C.; Lohmann-Richters, F.P.; Hanus, K.; Sebastiani, D.; Varga, A.; Abel, B. Mechanism of ion conductivity through polymer-stabilized CsH2PO4 nanoparticular layers from experiment and theory. J. Mater. Chem. A 2019, 7, 27367–27376. [Google Scholar] [CrossRef]
- Lohmann-Richters, F.P.; Odenwald, C.; Kickelbick, G.; Abel, B.; Varga, A. Facile and scalable synthesis of sub-micrometer electrolyte particles for solid acid fuel cells. RSC Adv. 2018, 8, 21806–21815. [Google Scholar] [CrossRef] [Green Version]
- Naumov, O.; Naumov, S.; Abel, B.; Varga, A. The stability limits of highly active nitrogen doped carbon ORR nano-catalysts: A mechanistic study of degradation reactions. Nanoscale 2018, 14, 6724–6733. [Google Scholar] [CrossRef]
- Lohmann-Richters, F.P.; Abel, B.; Varga, A. In situ determination of the electrochemically active platinum surface area: Key to improvement of solid acid fuel cells. J. Mater. Chem. A 2018, 6, 2700–2707. [Google Scholar] [CrossRef]
- Papandrew, A.B.; Chisholm, C.R.I.; Elgammal, R.A.; Ozer, M.M.; Zecevic, S.K. Advanced Electrodes for Solid Acid Fuel Cells by Platinum Deposition on CsH2PO4. Chem. Mater. 2011, 23, 1659–1667. [Google Scholar] [CrossRef] [Green Version]
- Papandrew, A.B.; Chisholm, C.R.I.; Zecevic, S.K.; Veith, G.M.; Zawodzinski, T.A. Activity and Evolution of Vapor Deposited Pt-Pd Oxygen Reduction Catalysts for Solid Acid Fuel Cells. J. Electrochem. Soc. 2013, 160, F175. [Google Scholar] [CrossRef]
- Lim, D.K.; Liu, J.; Pandey, S.A.; Paik, H.; Chisholm, C.R.I.; Hupp, J.T.; Haile, S.M. Atomic layer deposition of Pt@CsH2PO4 for the cathodes of solid acid fuel cells. Electrochim. Acta 2018, 288, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Daems, N.; Sheng, X.; Vankelecom, I.F.J.; Pescarmona, P.P. Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 4085–4110. [Google Scholar] [CrossRef]
- Samad, S.; Loh, K.S.; Wong, W.Y.; Lee, T.K.; Sunarso, J.; Chong, S.T.; Daud, W.R.W. Carbon and non-carbon support materials for platinum-based catalysts in fuel cells. Int. J. Hydrog. Energy 2018, 43, 7823–7854. [Google Scholar] [CrossRef]
- Yin, S.; Mu, S.; Lv, H.; Cheng, N.; Pan, M.; Fu, Z. A highly stable catalyst for PEM fuel cell based on durable titanium diboride support and polymer stabilization. Appl. Catal. B 2010, 93, 233–240. [Google Scholar] [CrossRef]
- He, P.; Liu, M.; Luo, J.L.; Sanger, A.R.; Chuang, K.T. Stabilization of Platinum Anode Catalyst in a H2S-O2 Solid Oxide Fuel Cell with an Intermediate TiO2 Layer. J. Electrochem. Soc. 2002, 149, A808. [Google Scholar] [CrossRef]
- Wagner, M.; Lorenz, O.; Lohmann-Richters, F.P.; Varga, A.; Abel, B. Study on solid electrolyte catalyst poisoning in solid acid fuel cells. J. Mater. Chem. A 2021, 9, 11347–11358. [Google Scholar] [CrossRef]
- Prokop, M.; Drakselova, M.; Bouzek, K. Review of the experimental study and prediction of Pt-based catalyst degradation during PEM fuel cell operation. Electrochim. Acta. 2020, 20, 20–27. [Google Scholar] [CrossRef]
- Beilstein, Z. Materials and characterization techniques for high-temperature polymer electrolyte membrane fuel cells. J. Nanotechnol. 2015, 6, 68–83. [Google Scholar]
- Cho, E.; Yitamben, E.N.; Iski, E.V.; Guisinger, N.P.; Kuech, T.F. Atomic-Scale Investigation of Highly Stable Pt Clusters Synthesized on a Graphene Support for Catalytic Applications. J. Phys. Chem. C 2012, 116, 26066–26071. [Google Scholar] [CrossRef]
- Tennyson, W.D.; Tian, M.; Papandrew, A.B.; Rouleau, C.M.; Puretzky, A.A.; Sneed, B.T.; More, K.L.; Veith, G.M.; Duscher, G.; Zawodzinski, T.A.; et al. Bottom up synthesis of boron-doped graphene for stable intermediate temperature fuel cell electrodes. Carbon 2017, 123, 605–615. [Google Scholar] [CrossRef]
- Thoi, V.S.; Usiskin, R.E.; Haile, S.M. Platinum-decorated carbon nanotubes for hydrogen oxidation and proton reduction in solid acid electrochemical cells. Chem. Sci. 2015, 6, 1570–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.B.R.; Bent, S.F. Formation of Continuous Pt Films on the Graphite Surface by Atomic Layer Deposition with Reactive O3. Chem. Mater. 2015, 27, 6802–6809. [Google Scholar] [CrossRef]
- Haile, S.M.; Chisholm, C.R.I.; Sasaki, K.; Boysen, D.A.; Uda, T. Solid acid proton conductors: From laboratory curiosities to fuel cell electrolytes. Faraday Discuss. 2007, 134, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Litster, S.; McLean, G. PEM fuel cell electrodes. J. Power Sources 2004, 130, 61–76. [Google Scholar] [CrossRef]
- Sasaki, K.; Wang, J.X.; Balasubramanian, M.; McBreen, J.; Uribe, F.; Adzic, R.R. Ultra-low platinum content fuel cell anode electrocatalyst with a long-term performance stability. Electrochim. Acta 2004, 49, 3873–3877. [Google Scholar] [CrossRef]
- Haug, A.T.; White, R.E.; Weidner, J.W.; Huang, W.; Shi, S.; Stoner, T.; Rana, N. Increasing Proton Exchange Membrane Fuel Cell Catalyst Effectiveness Through Sputter Deposition. J. Electrochem. Soc. 2002, 149, A280. [Google Scholar] [CrossRef]
- Cha, S.Y.; Lee, W.M. Performance of Proton Exchange Membrane Fuel Cell Electrodes Prepared by Direct Deposition of Ultrathin Platinum on the Membrane Surface. J. Electrochem. Soc. 1999, 146, 4055. [Google Scholar] [CrossRef]
- Wee, J.H.; Lee, K.Y.; Kim, S.H. Fabrication methods for low-Pt-loading electrocatalysts in proton exchange membrane fuel cell systems. J. Power Sources 2007, 165, 667–677. [Google Scholar] [CrossRef]
- Singh, S.; Datta, J. Size control of Pt nanoparticles with stabilizing agent for better utilization of the catalyst in fuel cell reaction. J. Mater. Sci. 2010, 45, 3030–3040. [Google Scholar] [CrossRef]
- Hsieh, C.; Liu, Y.; Tzou, D.; Chen, Y. Platinum electrocatalysts attached to carbon nanotubes by atomic layer deposition with different cycle numbers. J. Taiwan Inst. Chem. Eng. 2014, 45, 186–191. [Google Scholar] [CrossRef]
- Liang, X.; Jiang, C. Atomic layer deposited highly dispersed platinum nanoparticles supported on non-functionalized multiwalled carbon nanotubes for the hydrogenation of xylose to xylitol. J. Nanopart. Res. 2013, 15, 1890. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Jin, B.; Liang, X.; Wang, Q.; Zhaoa, Z.; Li, Q. Pt–Carbon interaction-determined reaction pathway and selectivity for hydrogenation of 5-hydroxymethylfurfural over carbon supported Pt catalysts. Catal. Sci. Technol. 2021, 11, 1298–1310. [Google Scholar]
- Hsieh, C.; Chen, W.; Tzou, D.; Roy, A.K.; Hsiao, H. Atomic layer deposition of Pt nanocatalysts on graphene oxide nanosheets for electro-oxidation of formic acid. Int. J. Hydrog. Energy 2012, 37, 17837–17843. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom Catalysis Using Pt/Graphene Achieved through Atomic Layer Deposition. Sci. Rep. 2013, 3, 1775. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Lu, J.; Feng, H. Surface modification and functionalization of powder materials by atomic layer deposition: A review. RSC Adv. 2021, 11, 11918–11942. [Google Scholar] [CrossRef]
- Hsueh, Y.; Wang, C.; Kei, C.; Lin, Y.; Liu, C.; Perng, T. Fabrication of catalyst by atomic layer deposition for high specific power density proton exchange membrane fuel cells. J. Catal. 2012, 294, 63–68. [Google Scholar] [CrossRef]
- Shu, T.; Liao, S.; Hsieh, C.; Roy, A.K.; Liu, Y.; Tzou, D.; Chen, W. Fabrication of platinum electrocatalysts on carbon nanotubes using atomic layer deposition for proton exchange membrane fuel cells. Electrochim. Acta 2012, 75, 101–107. [Google Scholar] [CrossRef]
- Ritaka, M.; Leskelä, M. Handbook of Thin Film Material; Nalwa, H.S., Ed.; Academic Press: San Diego, CA, USA, 2001; Volume 1, p. 103. [Google Scholar]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Lee, W.; Bera, S.; Kim, C.M.; Koh, E.; Hong, W.; Oh, S.; Cho, E.; Kwon, S. Synthesis of highly dispersed Pt nanoparticles into carbon supports by fluidized bed reactor atomic layer deposition to boost PEMFC performance. NPG Asia Mater. 2020, 12, 40. [Google Scholar] [CrossRef]
- Biró, L.P.; Lambin, P. Grain boundaries in graphene grown by chemical vapor deposition. New J. Phys. 2013, 15, 035024. [Google Scholar] [CrossRef]
- Yazyev, O.V.; Chen, Y.P. Polycrystalline graphene and other two-dimensional materials. Nat. Nanotechnol. 2014, 9, 755–767. [Google Scholar] [CrossRef]
- Shaina, P.R.; George, L.; Yadav, V.; Jaiswal, M. Estimating the thermal expansion coefficient of graphene: The role of graphene–substrate interactions. J. Phys.: Condens. Matter 2016, 28, 085301. [Google Scholar] [CrossRef]
- Li, P.; Jing, G.; Zhang, B.; Sando, S.; Cui, T. Single-crystalline monolayer and multilayer graphene nano switches. Appl. Phys. Lett. 2014, 104, 113110. [Google Scholar] [CrossRef]
- Sun, Z.; Raji, A.R.O.; Zhu, Y.; Xiang, C.; Yan, Z.; Kittrell, C.; Samuel, E.L.G.; Tour, J.M. Large-Area Bernal-Stacked Bi-, Tri-, and Tetralayer Graphene. ACS Nano 2012, 6, 9790–9796. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.S.; Riedl, C.; Krauss, B.; von Klitzing, K.; Starke, U.; Smet, J.H. Raman spectra of epitaxial graphene on SiC and of epitaxial graphene transferred to SiO2. Nano Lett. 2008, 8, 4320–4325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marichy, C.; Pinna, N. Carbon-nanostructures coated/decorated by atomic layer deposition: Growth and applications. Coord. Chem. Rev. 2013, 257, 3232–3253. [Google Scholar] [CrossRef]
- Bui, H.V.; Grillo, F.; Helmer, R.; Goulas, A.; van Ommen, J.R. Controlled Growth of Palladium Nanoparticles on Graphene Nanoplatelets via Scalable Atmospheric Pressure Atomic Layer Deposition. J. Phys. Chem. C 2016, 120, 8832–8840. [Google Scholar]
- Xin, Y.; Liu, J.; Jie, X.; Liu, W.; Liu, F.; Yin, Y.; Gu, J.; Zoua, Z. Preparation and electrochemical characterization of nitrogen doped graphene by microwave as supporting materials for fuel cell catalysts. Electrochim. Acta 2012, 60, 354–358. [Google Scholar] [CrossRef]
- Qi, Z.; Zhu, X.; Jin, H.; Zhang, T.; Kong, X.; Ruoff, R.S.; Qiao, Z.; Ji, H. Rapid Identification of the Layer Number of Large-Area Graphene on Copper. Chem. Mater. 2018, 30, 2067–2073. [Google Scholar] [CrossRef]
- Yen, W.C.; Chen, Y.Z.; Yeh, C.H.; He, J.H.; Chiu, P.W.; Chueh, Y.L. Direct growth of self-crystallized graphene and graphite nanoballs with Ni vapor-assisted growth: From controllable growth to material characterization. Sci. Rep. 2014, 4, 4739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaltonen, T.; Ritala, M.; Sajavaara, T.; Keinonen, J.; Leskelä, M. Atomic Layer Deposition of Platinum Thin Films. Chem. Mater. 2003, 15, 1924–1928. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, L.; Duan, X. Graphene–dielectric integration for graphene transistors. Mater. Sci. Eng. R 2010, 70, 354–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohmann, F.P.; Schulze, P.S.C.; Wagner, M.; Naumov, O.; Lotnyk, A.; Abel, B.; Varga, A. The next generation solid acid fuel cell electrodes: Stable, high performance with minimized catalyst loading. J. Mater. Chem. A 2017, 5, 15021–15025. [Google Scholar] [CrossRef]
- Louie, M.W.; Haile, S.M. Platinum thin film anodes for solid acid fuel cells. Energy Env. Sci. 2011, 4, 4230–4238. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Mortazavi, B. Ultrahigh thermal conductivity and strength in direct-gap semiconducting graphene-like BC6N: A first-principles and classical investigation. Carbon 2021, 182, 373–383. [Google Scholar] [CrossRef]
- Lee, W.; Lim, G.; Ko, S.H. Significant thermoelectric conversion efficiency enhancement of single layer graphene with substitutional silicon dopants. Nano Energy 2021, 87, 106188. [Google Scholar] [CrossRef]
- Gong, S.; Jiang, L.; Cheng, Q. Robust bioinspired graphene-based nanocomposites via synergistic toughening of zinc ions and covalent bonding. J. Mater. Chem. A 2016, 4, 17073–17079. [Google Scholar] [CrossRef]
- Li, W.; Chen, B.; Meng, C.; Fang, W.; Xiao, Y.; Li, X.Y.; Hu, Z.F.; Xu, Y.X.; Tong, L.; Wang, H.Q.; et al. Ultrafast All-Optical Graphene Modulator. Nano Lett. 2014, 14, 955–959. [Google Scholar] [CrossRef]
- El Abbassi, M.; Sangtarash, S.; Liu, X.; Perrin, M.L.; Braun, O.; Lambert, C.; Jan van der Zant, H.S.; Yitzchaik, S.; Decurtins, S.; Liu, S.; et al. Robust graphene-based molecular devices. Nat. Nanotechnol. 2019, 14, 957–961. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Chaudhary, V.; Kumar, A.; Sinhaa, S.R.P. Investigation of electronic properties of chemical vapor deposition grown single layer graphene via doping of thin transparent conductive films. RSC Adv. 2021, 11, 3096. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Wang, S.; Minami, D.; Kaneko, K. Robust graphene-based monoliths of homogeneous ultramicroporosity. Carbon 2015, 87, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Nirmalraj, P.N.; Lutz, T.; Kumar, S.; Duesberg, G.S.; Boland, J.J. Nanoscale Mapping of Electrical Resistivity and Connectivity in Graphene Strips and Networks. Nano Lett. 2011, 11, 16–22. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.M.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Hwang, E.H.; Sarma, S.D. Acoustic phonon scattering limited carrier mobility in two-dimensional extrinsic graphene. Phys. Rev. B. 2008, 77, 115449. [Google Scholar] [CrossRef] [Green Version]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Haas, J.; Marchenkov, A.N.; et al. Electronic Confinement and Coherence in Patterned Epitaxial Graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Zhang, Y.; Stormer, H.L.; Kim, P. Temperature dependent electron transport in graphene. Eur. Phys. J. Spec. Top. 2007, 148, 15–18. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Bolotin, K.I.; Sikes, K.J.; Hone, J.; Stormer, H.L.; Kim, P. Temperature-Dependent Transport in Suspended Graphene. Phys. Rev. Lett. 2008, 101, 096802. [Google Scholar] [CrossRef] [Green Version]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, A.K. Giant Intrinsic Carrier Mobilities in Graphene and Its Bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Yang, D.; Lau, A.; Ma, T.; Lin, H.; Jia, B. Hybridized Graphene for Supercapacitors: Beyond the Limitation of Pure Graphene. Small 2021, 17, 2007311. [Google Scholar] [CrossRef]
- Fang, X.; Yu, X.; Zheng, H.; Jin, H.; Wang, L.; Cao, M.S. Temperature- and thickness-dependent electrical conductivity of few-layer graphene and graphene nanosheets. Phys. Lett. A 2015, 379, 2245–2251. [Google Scholar] [CrossRef]
- Griffin, E.; Mogg, L.; Hao, G.; Kalon, G.; Bacaksiz, C.; Lopez-Polin, G.; Zhou, T.Y.; Guarochico, V.; Cai, J.; Neumann, C.; et al. Proton and Li-Ion Permeation through Graphene with Eight-Atom-Ring Defects. Acs Nano 2020, 14, 7280–7286. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, Y.; Jiang, S.; Li, Z.; He, G.; Wu, H. Enhanced proton conductivity of Nafion nanohybrid membrane incorporated with phosphonic acid functionalized graphene oxide at elevated temperature and low humidity. J. Membr. Sci. 2016, 518, 243–253. [Google Scholar] [CrossRef]
- Hatakeyama, K.; Karim, M.R.; Ogata, C.; Tateishi, H.; Taniguchi, T.; Koinuma, M.; Hayami, S.; Matsumoto, Y. Optimization of proton conductivity in graphene oxide by filling sulfate ions. Chem. Commun. 2014, 50, 14527–14530. [Google Scholar] [CrossRef]
- Hamidah, N.L.; Shintani, M.; Fauzi, A.S.A.; Putri, G.K.; Kitamura, S.; Hatakeyama, K.; Sasaki, M.; Quitain, A.T.; Kida, T. Graphene Oxide Membranes with Cerium-Enhanced Proton Conductivity for Water Vapor Electrolysis. ACS Appl. Nano Mater. 2020, 3, 4292–4304. [Google Scholar] [CrossRef]
- Hu, S.; Lozada-Hidalgo, M.; Wang, F.C.; Mishchenko, A.; Schedin, F.; Nair, R.R.; Hill, E.W.; Boukhvalov, D.W.; Katsnelson, M.I.; Dryfe, R.A.W.; et al. Proton transport through one-atom-thick crystals. Nature 2014, 516, 227–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozada-Hidalgo, M.; Hu, S.; Marshall, O.; Mishchenko, A.; Grigorenko, A.N.; Dryfe, R.A.W.; Radha, B.; Grigorieva, I.V.; Geim, A.K. Sieving hydrogen isotopes through two-dimensional crystals. Science 2016, 351, 68–70. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.Z.; Yang, Q.; Kuang, W.J.; Stebunov, Y.V.; Xiong, W.Q.; Yu, J.; Nair, R.R.; Katsnelson, M.I.; Yuan, S.J.; Grigorieva, I.V.; et al. Limits on gas impermeability of graphene. Nature 2020, 579, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lee, G.; Gong, C.; Colombo, L.; Cho, K. Grain Boundary Effect on Electrical Transport Properties of Graphene. J. Phys. Chem. C 2014, 118, 2338–2343. [Google Scholar] [CrossRef]
- Simonis, P.; Goffaux, C.; Thiry, P.A.; Biro, L.P.; Lambin, P.; Meunier, V. STM study of a grain boundary in graphite. Surf. Sci. 2002, 511, 319–322. [Google Scholar] [CrossRef]
- Gao, W.; Wu, G.; Janicke, M.T.; Cullen, D.A.; Mukundan, R.; Baldwin, J.K.; Brosha, E.L.; Galande, C.; Ajayan, P.M.; More, K.L.; et al. Ozonated Graphene Oxide Film as a Proton-Exchange Membrane. Angew. Chem. Int. Ed. Engl. 2014, 53, 3588–3593. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Pumera, M. The CVD graphene transfer procedure introduces metallic impurities which alter the graphene electrochemical properties. Nanoscale 2014, 6, 472–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehnert, J.; Spemann, D.; Hatahet, M.H.; Mändl, S.; Mensing, M.; Finzel, A.; Varga, A.; Rauschenbach, B. Graphene on silicon dioxide via carbon ion implantation in copper with PMMA-free transfer. Appl. Phys. Lett. 2017, 110, 233114. [Google Scholar] [CrossRef]
- Olesik, J.W.; Gray, P.J. Considerations for measurement of individual nanoparticles or microparticles by ICP-MS: Determination of the number of particles and the analyte mass in each particle. J. Anal. At. Spectrom. 2012, 27, 1143–1155. [Google Scholar] [CrossRef]
- dos Anjos, S.L.; Alves, J.C.; Rocha Soares, S.A.; Araujo, R.G.O.; de Oliveira, O.M.C.; Queiroz, A.F.S.; Ferreira, S.L.C. Multivariate optimization of a procedure employing microwave-assisted digestion for the determination of nickel and vanadium in crude oil by ICP OES. Talanta 2018, 178, 842–846. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, K.; Yamaji, K.; Tsuda, T.; Matsumoto, H.; Sato, T.; Izumi, R.; Torimotoc, T.; Kuwabata, S. Highly durable Pt nanoparticle-supported carbon catalysts for the oxygen reduction reaction tailored by using an ionic liquid thin layer. J. Mater. Chem. A 2016, 4, 12152–12157. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatahet, M.H.; Wagner, M.; Prager, A.; Helmstedt, U.; Abel, B. Functionalized and Platinum-Decorated Multi-Layer Oxidized Graphene as a Proton, and Electron Conducting Separator in Solid Acid Fuel Cells. Catalysts 2021, 11, 947. https://doi.org/10.3390/catal11080947
Hatahet MH, Wagner M, Prager A, Helmstedt U, Abel B. Functionalized and Platinum-Decorated Multi-Layer Oxidized Graphene as a Proton, and Electron Conducting Separator in Solid Acid Fuel Cells. Catalysts. 2021; 11(8):947. https://doi.org/10.3390/catal11080947
Chicago/Turabian StyleHatahet, Mhamad Hamza, Maximilian Wagner, Andrea Prager, Ulrike Helmstedt, and Bernd Abel. 2021. "Functionalized and Platinum-Decorated Multi-Layer Oxidized Graphene as a Proton, and Electron Conducting Separator in Solid Acid Fuel Cells" Catalysts 11, no. 8: 947. https://doi.org/10.3390/catal11080947




