Photoelectrochemical Oxidation in Ambient Conditions Using Earth-Abundant Hematite Anode: A Green Route for the Synthesis of Biobased Polymer Building Blocks
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photolectrochemical Water Oxidation Using Hematite Nanorods
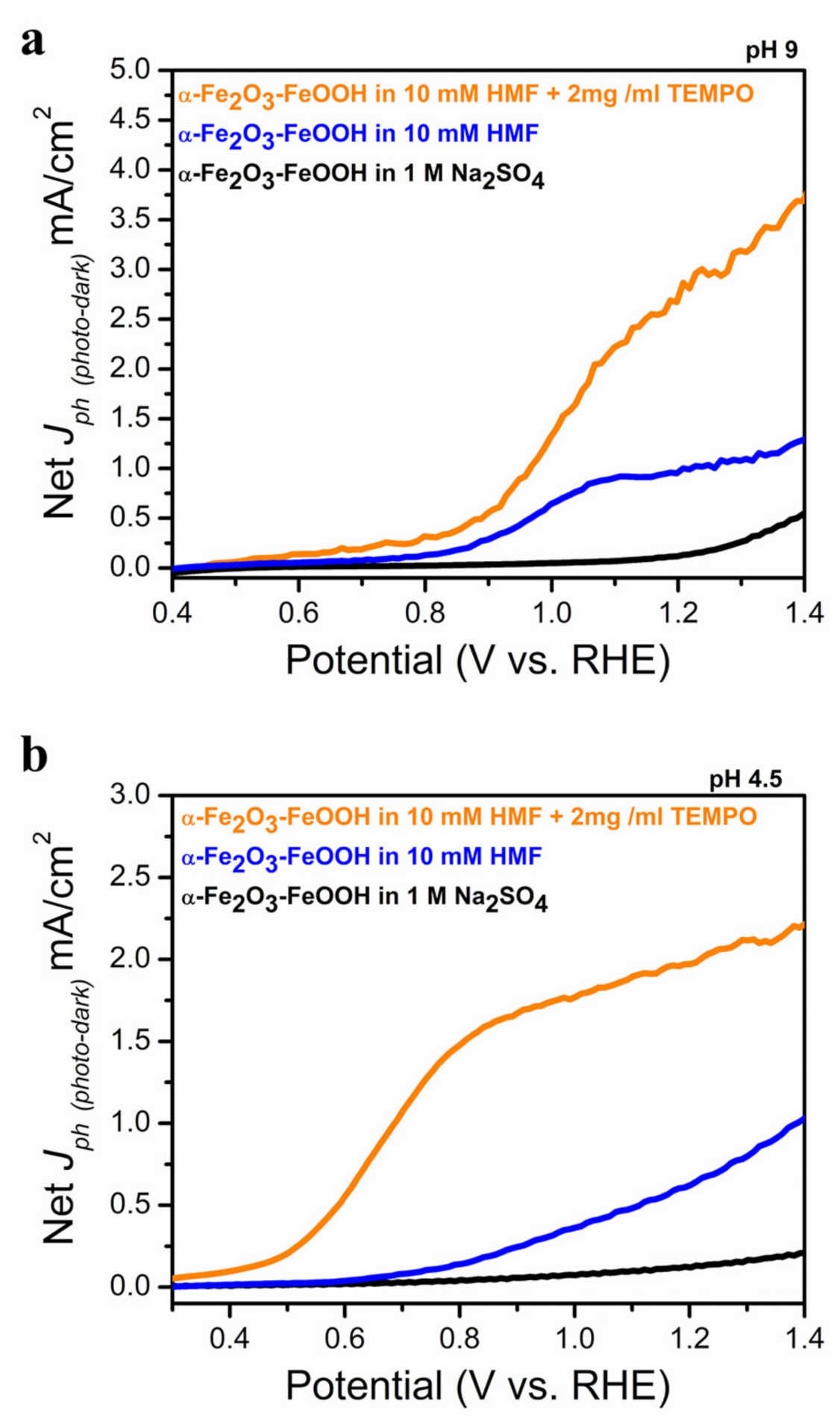
2.2. Photolectrochemical 5-HMF Oxidation
3. Experimental
3.1. Materials
3.2. Hematite Photoanode Synthesis
3.3. Photoelectrochemical Oxidation of 5-HMF, HMFCA, FFCA, and DFF
3.4. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chemicals. Available online: https://www.iea.org/fuels-and-technologies/chemicals (accessed on 1 June 2021).
- Davidson, M.G.; Elgie, S.; Parsons, S.; Young, T.J. Production of HMF, FDCA and their derived products: A review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies. Green Chem. 2021, 23, 3154–3171. [Google Scholar] [CrossRef]
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–345. [Google Scholar] [CrossRef]
- Vennestrøm, P.; Osmundsen, C.M.; Christensen, C.; Taarning, E. Beyond petrochemicals: The renewable chemicals industry. Angew. Chem. Int. Ed. 2011, 50, 10502–10509. [Google Scholar] [CrossRef]
- Cha, H.G.; Choi, K.-S. Combined biomass valorization and hydrogen production in a photoelectrochemical cell. Nat. Chem. 2015, 7, 328. [Google Scholar] [CrossRef] [PubMed]
- Lianos, P. Production of electricity and hydrogen by photocatalytic degradation of organic wastes in a photoelectrochemical cell: The concept of the photofuelcell: A review of a re-emerging research field. J. Hazard. Mater. 2011, 185, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 3. [Google Scholar] [CrossRef]
- Lhermitte, C.R.; Sivula, K. Alternative oxidation reactions for solar-driven fuel production. ACS Catal. 2019, 9, 2007–2017. [Google Scholar] [CrossRef]
- Kawde, A.; Annamalai, A.; Amidani, L.; Boniolo, M.; Kwong, W.L.; Sellstedt, A.; Glatzel, P.; Wågberg, T.; Messinger, J. Photo-electrochemical hydrogen production from neutral phosphate buffer and seawater using micro-structured p-Si photo-electrodes functionalized by solution-based methods. Sustain. Energy Fuels 2018, 2, 2215–2223. [Google Scholar] [CrossRef] [Green Version]
- Kawde, A.; Annamalai, A.; Sellstedt, A.; Uhlig, J.; Wågberg, T.; Glatzel, P.; Messinger, J. More than protection: The function of TiO2 interlayers in hematite functionalized Si photoanodes. Phys. Chem. Chem. Phys. 2020, 22, 28459–28467. [Google Scholar] [CrossRef] [PubMed]
- Kawde, A.; Annamalai, A.; Sellstedt, A.; Glatzel, P.; Wågberg, T.; Messinger, J. A microstructured p-Si photocathode outcompetes Pt as a counter electrode to hematite in photoelectrochemical water splitting. Dalton Trans. 2019, 48, 1166–1170. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Steier, L.; Son, M.-K.; Schreier, M.; Mayer, M.T.; Graätzel, M. Cu2O nanowire photocathodes for efficient and durable solar water splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef]
- Liang, Y.; Messinger, J. Improving BiVO4 photoanodes for solar water splitting through surface passivation. Phys. Chem. Chem. Phys. 2014, 16, 12014–12020. [Google Scholar] [CrossRef] [Green Version]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I--Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab.: Golden, CO, USA, 2004. [Google Scholar]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [Green Version]
- Jacquel, N.; Saint-Loup, R.; Pascault, J.-P.; Rousseau, A.; Fenouillot, F. Bio-based alternatives in the synthesis of aliphatic–aromatic polyesters dedicated to biodegradable film applications. Polymer 2015, 59, 234–242. [Google Scholar] [CrossRef]
- Davis, S.E.; Houk, L.R.; Tamargo, E.C.; Datye, A.K.; Davis, R.J. Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts. Catal. Today 2011, 160, 55–60. [Google Scholar] [CrossRef]
- Liguori, F.; Barbaro, P.; Calisi, N. Continuous-Flow Oxidation of HMF to FDCA by Resin-Supported Platinum Catalysts in Neat Water. ChemSusChem 2019, 12, 2558–2563. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, P.; Zhang, Z.; Yang, C.; Zhang, B.; Deng, K.; Bottle, S.; Zhu, H. Selective oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid using O2 and a photocatalyst of Co-thioporphyrazine bonded to g-C3N4. J. Am. Chem. Soc. 2017, 139, 14775–14782. [Google Scholar] [CrossRef]
- Barwe, S.; Weidner, J.; Cychy, S.; Morales, D.M.; Dieckhöfer, S.; Hiltrop, D.; Masa, J.; Muhler, M.; Schuhmann, W. Electrocatalytic Oxidation of 5-(Hydroxymethyl) furfural Using High-Surface-Area Nickel Boride. Angew. Chem. Int. Ed. 2018, 57, 11460–11464. [Google Scholar] [CrossRef]
- Mi, Q.; Zhanaidarova, A.; Brunschwig, B.S.; Gray, H.B.; Lewis, N.S. A quantitative assessment of the competition between water and anion oxidation at WO 3 photoanodes in acidic aqueous electrolytes. Energy Environ. Sci. 2012, 5, 5694–5700. [Google Scholar] [CrossRef] [Green Version]
- Fuku, K.; Wang, N.; Miseki, Y.; Funaki, T.; Sayama, K. Photoelectrochemical Reaction for the Efficient Production of Hydrogen and High-Value-Added Oxidation Reagents. ChemSusChem 2015, 8, 1593–1600. [Google Scholar] [CrossRef]
- Han, G.; Jin, Y.-H.; Burgess, R.A.; Dickenson, N.E.; Cao, X.-M.; Sun, Y. Visible-light-driven valorization of biomass intermediates integrated with H2 production catalyzed by ultrathin Ni/CdS nanosheets. J. Am. Chem. Soc. 2017, 139, 15584–15587. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Dinh, H.N.; Miller, E. Photoelectrochemical Water Splitting; Springer: New York, NY, USA, 2013; Volume 344, ISBN 978-1-4614-8297-0. [Google Scholar]
- Vuyyuru, K.R.; Strasser, P. Oxidation of biomass derived 5-hydroxymethylfurfural using heterogeneous and electrochemical catalysis. Catal. Today 2012, 195, 144–154. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jang, J.W.; Youn, D.H.; Magesh, G.; Lee, J.S. A stable and efficient hematite photoanode in a neutral electrolyte for solar water splitting: Towards stability engineering. Adv. Energy Mater. 2014, 4, 1400476. [Google Scholar] [CrossRef]
- Vayssieres, L.; Beermann, N.; Lindquist, S.-E.; Hagfeldt, A. Controlled aqueous chemical growth of oriented three-dimensional crystalline nanorod arrays: Application to iron (III) oxides. Chem. Mater. 2001, 13, 233–235. [Google Scholar] [CrossRef]
- Kormányos, A.; Kecsenovity, E.; Honarfar, A.; Pullerits, T.; Janáky, C. Hybrid FeNiOOH/α-Fe2O3/Graphene Photoelectrodes with Advanced Water Oxidation Performance. Adv. Funct. Mater. 2020, 30, 2002124. [Google Scholar] [CrossRef]
- Wang, T.; Long, X.; Wei, S.; Wang, P.; Wang, C.; Jin, J.; Hu, G. Boosting Hole Transfer in the Fluorine-Doped Hematite Photoanode by Depositing Ultrathin Amorphous FeOOH/CoOOH Cocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 49705–49712. [Google Scholar] [CrossRef]
- Sayed, M.; Warlin, N.; Hulteberg, C.; Munslow, I.; Lundmark, S.; Pajalic, O.; Tunå, P.; Zhang, B.; Pyo, S.-H.; Hatti-Kaul, R. 5-Hydroxymethylfurfural from fructose: An efficient continuous process in a water-dimethyl carbonate biphasic system with high yield product recovery. Green Chem. 2020, 22, 5402–5413. [Google Scholar] [CrossRef]
- Bragd, P.; Van Bekkum, H.; Besemer, A. TEMPO-mediated oxidation of polysaccharides: Survey of methods and applications. Top. Catal. 2004, 27, 49–66. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef]
- Annamalai, A.; Shinde, P.S.; Subramanian, A.; Kim, J.Y.; Kim, J.H.; Choi, S.H.; Lee, J.S.; Jang, J.S. Bifunctional TiO2 underlayer for α-Fe2O3 nanorod based photoelectrochemical cells: Enhanced interface and Ti4+ doping. J. Mater. Chem. A 2015, 3, 5007–5013. [Google Scholar] [CrossRef]
- Gerken, J.B.; Pang, Y.Q.; Lauber, M.B.; Stahl, S.S. Structural Effects on the pH-Dependent Redox Properties of Organic Nitroxyls: Pourbaix Diagrams for TEMPO, ABNO, and Three TEMPO Analogs. J. Org. Chem. 2017, 83, 7323–7330. [Google Scholar] [CrossRef] [PubMed]
- Riva, S. Laccases: Blue enzymes for green chemistry. TRENDS Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef]
- Honarfar, A.; Mourad, H.; Lin, W.; Polukeev, A.; Rahaman, A.; Abdellah, M.; Chábera, P.; Pankratova, G.; Gorton, L.; Zheng, K. Photoexcitation Dynamics in Electrochemically Charged CdSe Quantum Dots: From Hot Carrier Cooling to Auger Recombination of Negative Trions. ACS Appl. Energy Mater. 2020, 3, 12525–12531. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Industrial oxidations with organocatalyst TEMPO and its derivatives. Org. Process Res. Dev. 2010, 14, 245–251. [Google Scholar] [CrossRef]
- Dijksman, A.; Arends, I.W.; Sheldon, R.A. Polymer immobilised TEMPO (PIPO): An efficient catalyst for the chlorinated hydrocarbon solvent-free and bromide-free oxidation of alcohols with hypochlorite. Chem. Commun. 2000, 66, 271–272. [Google Scholar] [CrossRef]
- Liu, S.; Liang, H.; Sun, T.; Yang, D.; Cao, M. A recoverable dendritic polyamidoamine immobilized TEMPO for efficient catalytic oxidation of cellulose. Carbohydr. Polym. 2018, 202, 563–570. [Google Scholar] [CrossRef]
- Mani, P.; Fidal Kumar, V.T.; Keshavarz, T.; Chandra, T.S.; Kyazze, G. The role of natural laccase redox mediators in simultaneous dye decolorization and power production in microbial fuel cells. Energies 2018, 11, 3455. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Wu, Y.; Huang, J.; Liu, Y. Environmentally Friendly and Recyclable Natural-Mediator-Modified Magnetic Nanoparticles for Laccase-Catalyzed Decolorization. J. Chem. 2019, 2019, 4140565. [Google Scholar] [CrossRef]




| Experiment pH 12.5 | ||||||||
| Experiment at 1.1 VRHE | 5-HMF Conversion (%) | Time (h) | Product Profile (Selectivity %) | pH | ||||
| 5-HMFCA | DFF | FFCA | FDCA | Start | End | |||
| 10 mM 5-HMF | 11.7 | 20 | 83.9 | 7.8 | 6.4 | 1.8 | 12.5 | 10 |
| 10 mM 5-HMF + 1 mg/mL TEMPO | 42 | 20 | 71.1 | 1.4 | 4.1 | 23.4 | 12.5 | 11 |
| 10 mM 5-HMF + 2 mg/mL TEMPO | 96.6 | 2.5 | 0.4 | 0.3 | 18.4 | 80.9 | 12.5 | 11 |
| 10 mM 5-HMF + 2 mg/mL TEMPO | 99.2 | 5.5 | 0.5 | 2.2 | 6.4 | 90.7 | 12.5 | 11 |
| Experiment pH 9.0 | ||||||||
| Experiment at 1.1 VRHE | Conversion (%) | Time (h) | Product profile (Selectivity %) | pH | ||||
| 5-HMFCA | DFF | FFCA | FDCA | Start | End | |||
| 10 mM 5-HMF + 2 mg/mL TEMPO | 47.3 | 20 | 1.5 | 31.7 | 66.2 | 1.6 | 9.0 | 5 |
| 10 mM 5-HMFCA + 2 mg/mL TEMPO | 50.9 | 20 | - | - | 5.7 | 94.2 | 9.0 | 6 |
| 10 mM DFF + 2 mg/mL TEMPO | 90.1 | 20 | - | - | 96.1 | 2.6 | 9.0 | 5 |
| 10 mM FFCA + 2 mg/mL TEMPO | 40.6 | 20 | - | - | - | 97.4 | 9.0 | 5 |
| Experiment pH 4.5 | ||||||||
| Experiment at 1.1 VRHE | Conversion (%) | Time (h) | Product profile (Selectivity %) | pH | ||||
| 5-HMFCA | DFF | FFCA | FDCA | Start | End | |||
| 10 mM 5-HMF + 2 mg/mL TEMPO | 51.7 | 20 | 1.6 | 29.6 | 67.3 | 1.5 | 4.5 | 4 |
| 10 mM 5-HMF + 0.5 mg/mL Laccase | 14.9 | 20 | 21.4 | 53.5 | 24.1 | 1.0 | 4.5 | 4 |
| 10 mM 5-HMF + 2 mg/mL TEMPO + 0.5 mg/mL Laccase | 78.3 | 20 | 0.7 | 7.7 | 85.9 | 5.7 | 4.5 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawde, A.; Sayed, M.; Shi, Q.; Uhlig, J.; Pullerits, T.; Hatti-Kaul, R. Photoelectrochemical Oxidation in Ambient Conditions Using Earth-Abundant Hematite Anode: A Green Route for the Synthesis of Biobased Polymer Building Blocks. Catalysts 2021, 11, 969. https://doi.org/10.3390/catal11080969
Kawde A, Sayed M, Shi Q, Uhlig J, Pullerits T, Hatti-Kaul R. Photoelectrochemical Oxidation in Ambient Conditions Using Earth-Abundant Hematite Anode: A Green Route for the Synthesis of Biobased Polymer Building Blocks. Catalysts. 2021; 11(8):969. https://doi.org/10.3390/catal11080969
Chicago/Turabian StyleKawde, Anurag, Mahmoud Sayed, Qi Shi, Jens Uhlig, Tönu Pullerits, and Rajni Hatti-Kaul. 2021. "Photoelectrochemical Oxidation in Ambient Conditions Using Earth-Abundant Hematite Anode: A Green Route for the Synthesis of Biobased Polymer Building Blocks" Catalysts 11, no. 8: 969. https://doi.org/10.3390/catal11080969







