Ultrasound Assisted Synthesis of Gadolinium Oxide-Zeolitic Imidazolate Framework-8 Nanocomposites and Their Optimization for Photocatalytic Degradation of Methyl Orange Using Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Gd2O3-ZIF-8 Nanocomposites and Color Test of MO Dye Solution
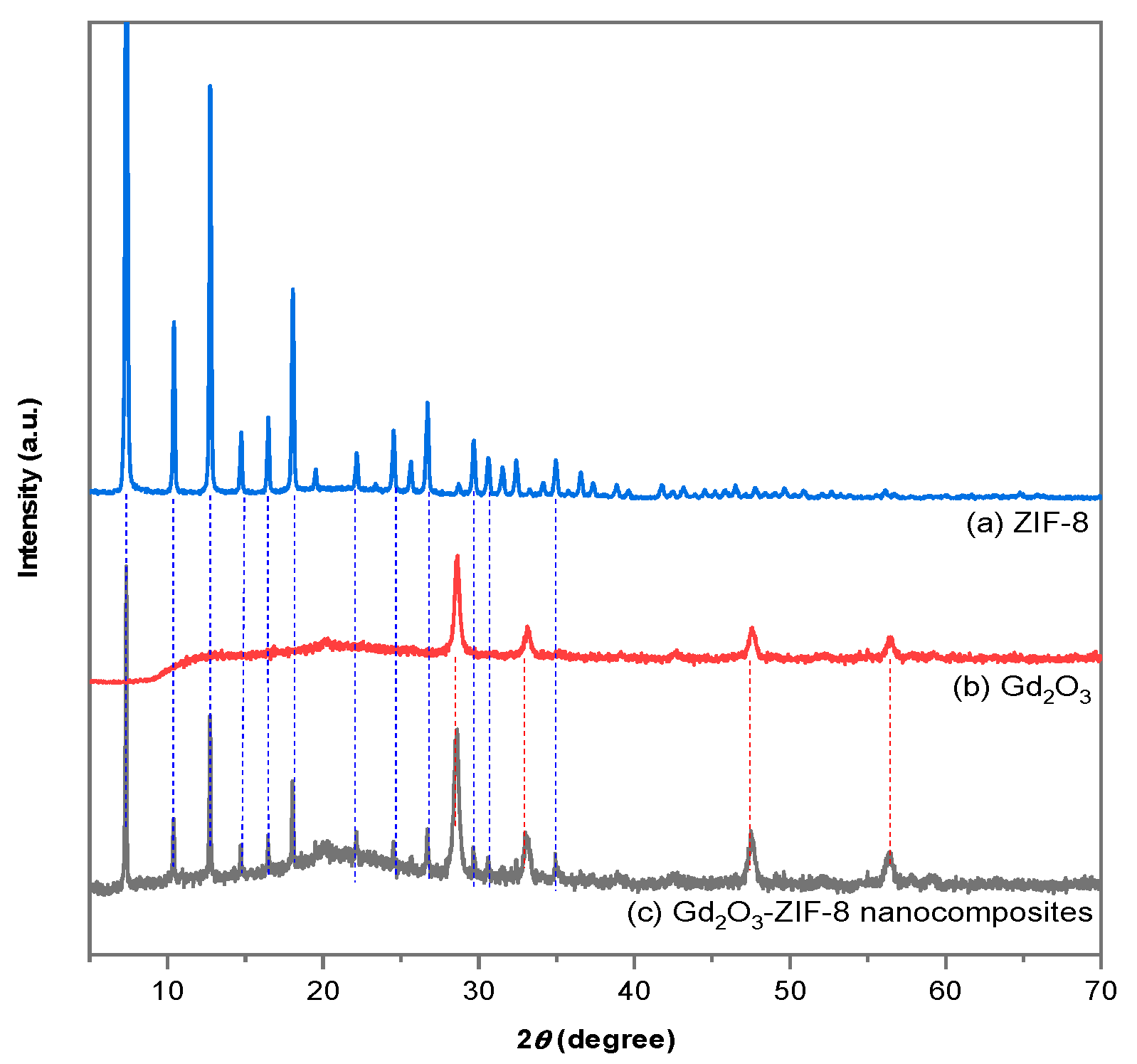
2.1.1. XRD Diffraction
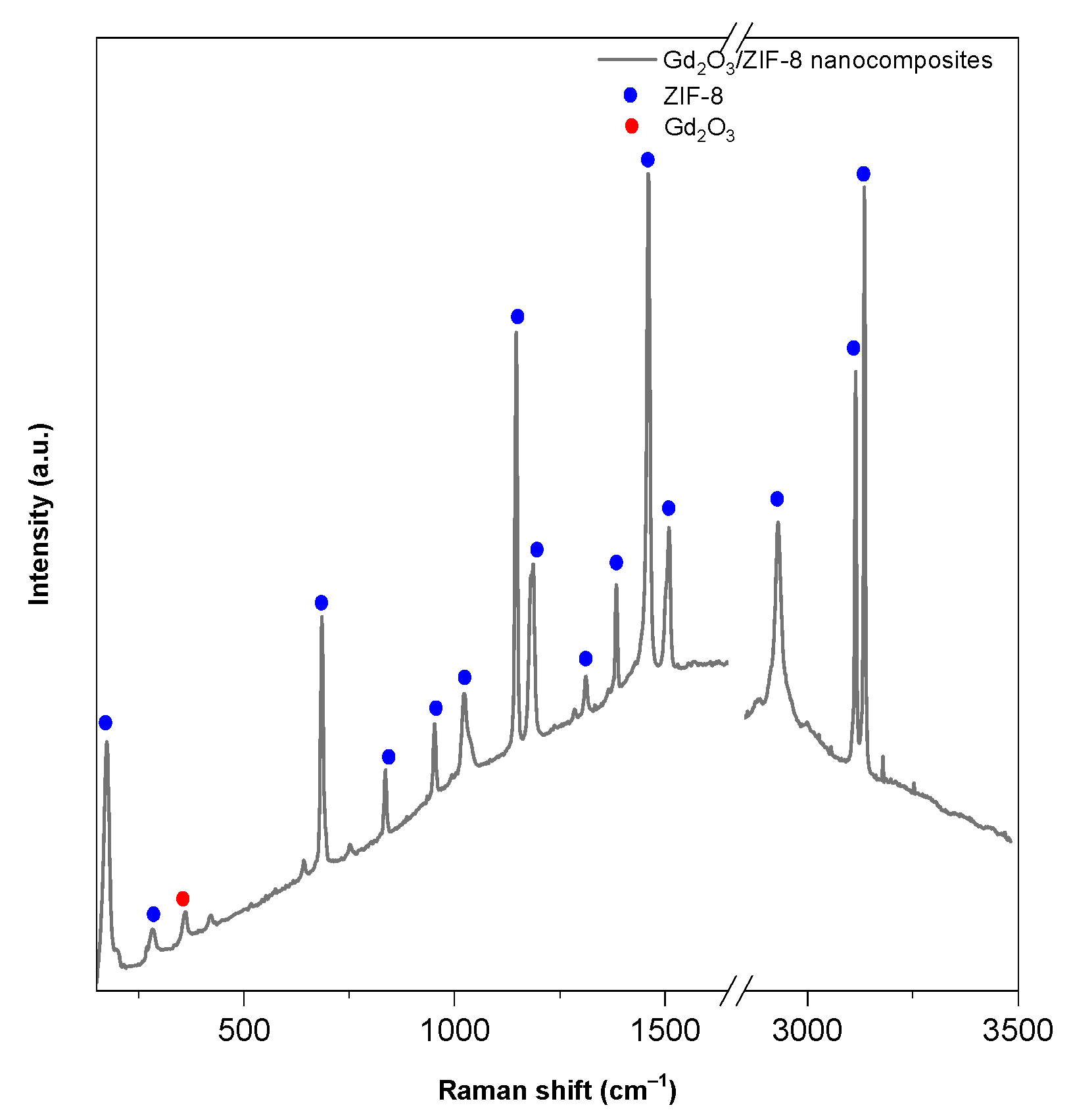
2.1.2. Raman Spectroscopy
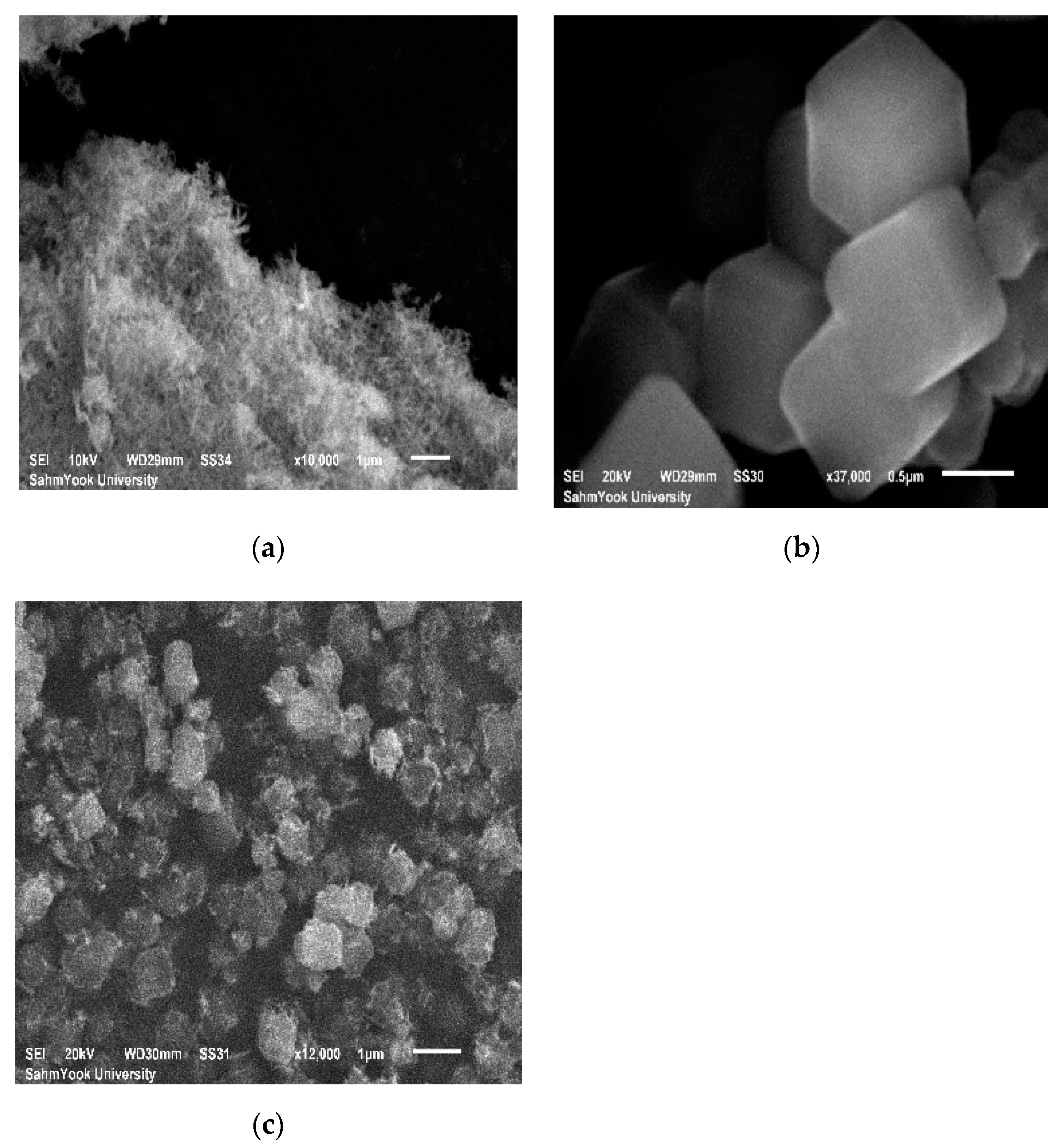
2.1.3. Scanning Electron Microscopy
2.2. Investigation of Photocatalytic Activity for Degradation of MO using Gd2O3-ZIF-8 Nanocomposites
2.3. Kinetics Study for Photocatalytic Degradation of MO
2.4. Color Test of Photocatalytic Degradation of MO Dye Solution
2.5. Box-Behnken Design with Response Surface Methodology (RSM)
2.5.1. Effect of pH
2.5.2. Effect of Photocatalyst Concentration
2.5.3. Effect of MO Concentration
2.5.4. Effect of H2O2 Concentration
2.6. Optimization for Photocatalytic Degradation of MO Using Response Surface Methodology (RSM)
2.7. Optimum Condition and Model Verification
3. Materials and Methods
3.1. Materials
3.2. Measurement Methods
3.2.1. Preparation of Gd2O3 Nanowires
3.2.2. Ultrasound Assisted Synthesis of Gd2O3-ZIF-8 Nanocomposites
3.2.3. Photocatalytic Activity of MO Degradation using Gd2O3-ZIF-8 Nanocomposites
3.2.4. Evaluation of Color Change during Photocatalytic Degradation of MO
3.2.5. Experimental Design with RSM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghiasi, E.; Azim, M. Removal of various textile dyes using LaMn(Fe)O3 and LaFeMn0.5O3 nanoperovskites; RSM optimization, isotherms and kinetics studies. J. Inorg. Organomet. Ploym. Mater. 2020, 30, 2789–2804. [Google Scholar] [CrossRef]
- Zhang, M.; Shang, Q.; Wan, Y.; Cheng, Q.; Liao, G.; Pan, Z. Self-template synthesis of double-shell TiO2@ ZIF-8 hollow nanospheres via sonocrystallization with enhanced photocatalytic activities in hydrogen generation. Appl. Catal. B Environ. 2019, 241, 149–158. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Zhao, G.; Li, C.; Liu, L.; Jiao, F. Fabrication of SnWO4/ZnFe-layered double hydroxide composites with enhanced photocatalytic degradation of methyl orange. J. Mater. Sci. Mater. Electron. 2020, 31, 12269–12281. [Google Scholar] [CrossRef]
- Dhanalakshmi, S.; Kumar, P.S.; Karuthapandian, S.; Muthuraj, V.; Prithivikumaran, N. Design of Gd2O3 nanorods: A challenging photocatalyst for the degradation of neurotoxicity chloramphenicol drug. J. Mater. Sci. Mater. Electron. 2019, 30, 3744–3752. [Google Scholar] [CrossRef]
- Surendra, T.V.; Mohana, R.S.; Khan, M.R. Biogenic approach to synthesize rod shaped Gd2O3 nanoparticles and its optimization using response surface methodology-Box–Behnken design model. Biotechnol. Prog. 2019, 3, e2823–e2834. [Google Scholar]
- Liu, Y.; Liu, Z.; Huang, D.; Cheng, M.; Zeng, G.; Lai, C.; Zhang, C.; Zhou, C.; Wang, W.; Jiang, D.; et al. Metal or metal-containing nanoparticle@ MOF nanocomposites as a promising type of photocatalyst. Coord. Chem. Rev. 2019, 388, 63–78. [Google Scholar] [CrossRef]
- Jing, H.P.; Wang, C.C.; Zhang, Y.W.; Wang, P.; Li, R. Photocatalytic degradation of methylene blue in ZIF-8. RSC Adv. 2014, 4, 54454–54462. [Google Scholar] [CrossRef]
- Qi, X.; Shang, F.; Wang, T.; Ma, Y.; Yan, Y. In situ coupling of TiO2(B) and ZIF-8 with enhanced photocatalytic activity via effective defect. CrystEngComm 2020, 22, 4250–4259. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Yue, G.; Luo, X. Novel Ag@nitrogen-doped porous carbon composite with high electrochemical performance as anode materials for lithium-ion batteries. Nano-Micro Lett. 2017, 9, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraji, A.; Mehrdadi, N.; Mahmoodi, N.M.; Pardakhti, A. Enhanced photocatalytic activity by synergic action of ZIF-8 and NiFe2O4 under visible light irradiation. J. Mol. Struct. 2021, 1223, 129028. [Google Scholar] [CrossRef]
- Li, R.; Li, W.; Jin, C.; He, Q.; Wang, Y. Fabrication of ZIF-8@ TiO2 micron composite via hydrothermal method with enhanced absorption and photocatalytic activities in tetracycline degradation. J. Alloys Compd. 2020, 825, 154008–154017. [Google Scholar] [CrossRef]
- Zeng, X.; Huang, L.; Wang, C.; Wang, J.; Li, J.; Luo, X. Sonocrystallization of ZIF-8 on electrostatic spinning TiO2 nanofibers surface with enhanced photocatalysis property through synergistic effect. ACS Appl. Mater. Inter. 2016, 8, 20274–20282. [Google Scholar] [CrossRef]
- Zheng, H.B.; Wu, D.; Wang, Y.L.; Liu, X.P.; Gao, P.Z.; Liu, W.; Rebrov, E.V. One-step synthesis of ZIF-8/ZnO composites based on coordination defect strategy and its derivatives for photocatalysis. J. Alloys Compd. 2020, 838, 155219–155229. [Google Scholar] [CrossRef]
- Chandra, R.; Singh, V.; Tomar, S.; Nath, M. Multi-core-shell composite SnO2 NPs@ZIF-8: Potential antiviral agent and effective photocatalyst for waste-water treatment. Environ. Sci. Pollut. Res. 2019, 26, 23346–23358. [Google Scholar] [CrossRef]
- Garcia, B.B.; Lourinho, G.; Romano, P.; Brito, P.S.D. Photocatalytic degradation of swine wastewater on aqueous TiO2 suspensions: Optimization and modeling via Box-Behnken design. Heliyon 2020, 6, e03293–e03300. [Google Scholar] [CrossRef] [PubMed]
- Gholamnia, R.; Abtahi, M.; Saeedi, R.; Khaloo, S.S. Synthesis and characterization of a new magnetic adsorbent for removal of 4-nitrophenol: Application of response surface methodology. Water Sci. Technol. 2019, 80, 1430–1442. [Google Scholar] [CrossRef]
- Azimi, F.; Nabizadeh, R.; Hassanvand, M.S.; Rastkari, N.; Nazmara, S.; Naddafi, K. Photochemical degradation of toluene in gas-phase under UV/visible light graphene oxide-TiO2 nanocomposite: Influential operating factors, optimization, and modeling. J. Environ. Health Sci. 2019, 17, 671–683. [Google Scholar] [CrossRef]
- Chawla, P.; Sharma, S.K.; Toor, A.P. Optimization and modeling of UV-TiO2 mediated photocatalytic degradation of golden yellow dye through response surface methodology. Chem. Eng. Commun. 2019, 206, 1123–1138. [Google Scholar] [CrossRef]
- Mortazavian, S.; Saber, A.; James, D.E. Optimization of photocatalytic degradation of Acid Blue 113 and Acid Red 88 textile dyes in a UV-C/TiO2 suspension system: Application of response surface methodology (RSM). Catalysts 2019, 9, 360. [Google Scholar] [CrossRef] [Green Version]
- Ghadiri, S.K.; Alidadi, H.; Tavakkoli, N.N.; Javid, A.; Roudbari, A.; Talebi, S.S.; Rezania, S. Valorization of biomass into amine-functionalized bio graphene for efficient ciprofloxacin adsorption in water-modeling and optimization study. PLoS ONE 2020, 15, e0231045–e0231063. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.N.; Cho, H.; Han, J.; Her, N.; Yoon, J. Photocatalytic degradation of acesulfame K: Optimization using the Box–Behnken design (BBD). Process Saf. Environ. Prot. 2018, 113, 10–21. [Google Scholar] [CrossRef]
- Kang, J.G.; Min, B.K.; Sohn, Y. Synthesis and characterization of Gd(OH)3 and Gd2O3 nanorods. Ceram. Int. 2015, 41, 1243–1248. [Google Scholar] [CrossRef]
- Paul, A.; Vyas, G.; Paul, P.; Srivastava, D.N. Gold-nanoparticle-encapsulated ZIF-8 for a mediator-free enzymatic glucose sensor by amperometry. ACS Appl. Nano Mater. 2018, 1, 3600–3607. [Google Scholar] [CrossRef]
- Zhou, K.; Mousavi, B.; Luo, Z.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. A 2017, 5, 952–957. [Google Scholar] [CrossRef]
- Pinheiro, D.; Devi, K.S.; Jose, A.; Karthik, K.; Sugunan, S.; Mohan, M.K. Experimental design for optimization of 4-nitrophenol reduction by green synthesized CeO2/g-C3N4/Ag catalyst using response surface methodology. J. Rare Earths 2020, 38, 1171–1177. [Google Scholar] [CrossRef]
- Jeon, S.; Ko, J.W.; Ko, W.B. Synthesis of Gd2O3 Nanoparticles and Their Photocatalytic Activity for Degradation of Azo Dyes. Catalysts 2021, 11, 742. [Google Scholar] [CrossRef]
- Tanaka, S.; Fujita, K.; Miyake, Y.; Miyamoto, M.; Hasegawa, Y.; Makino, T.; Denayer, J.F. Adsorption and diffusion phenomena in crystal size engineered ZIF-8 MOF. J. Phys. Chem. C 2015, 119, 28430–28439. [Google Scholar] [CrossRef] [Green Version]
- Kumari, G.; Jayaramulu, K.; Maji, T.K.; Narayana, C. Temperature induced structural transformations and gas adsorption in the zeolitic imidazolate framework ZIF-8: A Raman study. J. Phys. Chem. A 2013, 117, 11006–11012. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Y.; Li, Z. Ag loaded flower-like BaTiO3 nanotube arrays: Fabrication and enhanced photocatalytic property. CrystEngComm 2012, 14, 1473–1478. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Wojnárovits, L.; Pálfi, T.; Takács, E. Kinetics and mechanism of azo dye destruction in advanced oxidation processes. Radiat. Phys. Chem. 2007, 76, 1497–1501. [Google Scholar] [CrossRef]
- Hsiao, P.H.; Timjan, S.; Kuo, K.Y.; Juan, J.C.; Chen, C.Y. Optical management of CQD/AgNP@ SiNW arrays with highly efficient capability of dye degradation. Catalysts 2021, 11, 399. [Google Scholar] [CrossRef]
- Hsiao, P.H.; Li, T.C.; Chen, C.Y. ZnO/Cu2O/Si nanowire arrays as ternary heterostructure-based photocatalysts with enhanced photodegradation performances. Nanoscale Res. Lett. 2019, 14, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polleto, M. Comparative study of wood flour photodegradation of two wood species submitted to artificial weathering. Maderas Cienc. Tecnol. 2017, 19, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Baysal, E.; Degirmentepe, S.; Simsek, H. Some surface properties of thermally modified scots pine after artificial weathering. Maderas Cienc. Tecnol. 2014, 16, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.P.; Mehrvar, M. Photocatalytic treatment of an actual confectionery wastewater using Ag/TiO2/Fe2O3: Optimization of photocatalytic reactions using surface response methodology. Catalysts 2018, 8, 409. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Saeedi, R. Photocatalytic Degradation of Methyl Orange on Bi2O3 and Ag2O-Bi2O3 Nano Photocatalysts. Bull. Chem. React. Eng. Catal. 2017, 12, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Ghavami, M.; Mohammadi, R.; Koohi, M.; Kassaee, M.Z. Visible light photocatalytic activity of reduced graphene oxide synergistically enhanced by successive inclusion of γ-Fe2O3, TiO2, and Ag nanoparticles. Mater. Sci. Semicond. Process 2014, 26, 69–78. [Google Scholar] [CrossRef]
- Haji, S.; Benstaali, B.; Al-Bastaki, N. Degradation of methyl orange by UV/H2O2 advanced oxidation process. Chem. Eng. Sci. 2011, 168, 134–139. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Pang, Y.L.; Law, Z.X.; Lim, S.; Chan, Y.Y.; Shuit, S.H.; Chong, W.C.; Lai, C.W. Enhanced photocatalytic degradation of methyl orange by coconut shell–derived biochar composites under visible LED light irradiation. Environ. Sci. Pollut. Res. 2021, 28, 27457–27473. [Google Scholar] [CrossRef]
- Sabour, M.R.; Amiri, A. Comparative study of ANN and RSM for simultaneous optimization of multiple targets in Fenton treatment of landfill leachate. Waste Manag. 2017, 65, 54–62. [Google Scholar] [CrossRef]
- Doğdu, O.G.; Baldan, P.N.; Ökten, H.E.; Yalçuk, A. A box–behnken design (Bbd) optimization of the photocatalytic degradation of 2, 4-dichlorophenoxyacetic acid (2, 4-d) using TiO2/H2O2. Desalin. Water Treat. 2018, 123, 188–195. [Google Scholar]
- Abd, R.N.F.; Shamsuddin, M. Catalytic reduction of 4-nitrophenol over biostabilized gold nanoparticles supported onto thioctic acid functionalized silica-coated magnetite nanoparticles and optimization using response surface methodology. Inorg. Nano-Metal Chem. 2020, 50, 489–500. [Google Scholar]
- Fu, F.; Zheng, B.; Xie, L.H.; Du, H.; Du, S.; Dong, Z. Size-controllable synthesis of zeolitic imidazolate framework/carbon nanotube composites. Crystals 2018, 8, 367. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef]
- Kurz, W.; Yetisen, A.K.; Kaito, M.V.; Fuchter, M.J.; Jakobi, M.; Elsner, M.; Koch, A.W. UV-Sensitive Wearable Devices for Colorimetric Monitoring of UV Exposure. Adv. Opt. Mater. 2020, 8, 1901969–1901977. [Google Scholar] [CrossRef] [Green Version]





| Times | ΔL | Δa | Δb | ΔE |
|---|---|---|---|---|
| 0 min | 0 | 0 | 0 | 0 |
| 10 min | 8.73 | −7.8 | −22.68 | 25.52 |
| 20 min | 12.78 | −12.94 | −50.06 | 54.11 |
| 30 min | 15.7 | −13.06 | −67.06 | 70.10 |
| 40 min | 16.3 | −12.98 | −70.15 | 73.17 |
| Range and Levels | |||||
|---|---|---|---|---|---|
| Independent Variables | Unit | Symbol | Low (−1) | Medium (0) | High (+1) |
| pH | - | 3 | 5 | 7 | |
| Dose of catalyst | g/L | 0.1 | 0.3 | 0.5 | |
| Concentration of methyl orange | mM | 0.0630 | 0.0840 | 0.1050 | |
| Concentration of H2O2 | mg /L | 111 | 333 | 555 | |
| Degradation Percentage | Degradation Percentage | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | Experimental | Predicted | Run | Experimental | Predicted | ||||||||
| 1 | 1 | 0 | 1 | 0 | 46.93 | 46.25 | 15 | 0 | 1 | 1 | 0 | 57.62 | 58.39 |
| 2 | 0 | 0 | 0 | 0 | 64.76 | 64.46 | 16 | 0 | 0 | −1 | −1 | 52.22 | 51.45 |
| 3 | 0 | 0 | 1 | −1 | 32.12 | 32.11 | 17 | −1 | 0 | −1 | 0 | 94.79 | 95.74 |
| 4 | 0 | 0 | 1 | 1 | 48.63 | 49.06 | 18 | 0 | −1 | 0 | 1 | 44.80 | 44.43 |
| 5 | 1 | 1 | 0 | 0 | 50.35 | 50.30 | 19 | 0 | 1 | 0 | −1 | 35.54 | 36.18 |
| 6 | 0 | 1 | 0 | 1 | 59.59 | 58.78 | 20 | 0 | 0 | 0 | 0 | 63.72 | 64.46 |
| 7 | 0 | −1 | −1 | 0 | 69.74 | 69.04 | 21 | 1 | 0 | −1 | 0 | 67.85 | 68.68 |
| 8 | 0 | −1 | 1 | 0 | 43.85 | 43.89 | 22 | 0 | 1 | −1 | 0 | 76.03 | 76.07 |
| 9 | −1 | −1 | 0 | 0 | 67.89 | 67.60 | 23 | −1 | 0 | 1 | 0 | 75.89 | 75.34 |
| 10 | −1 | 0 | 0 | 1 | 78.78 | 79.54 | 24 | 1 | −1 | 0 | 0 | 45.98 | 46.24 |
| 11 | −1 | 0 | 0 | −1 | 52.29 | 52.04 | 25 | −1 | 1 | 0 | 0 | 85.69 | 85.09 |
| 12 | 0 | 0 | 0 | 0 | 64.90 | 64.46 | 26 | 0 | −1 | 0 | −1 | 27.91 | 28.99 |
| 13 | 1 | 0 | 0 | 1 | 42.66 | 42.98 | 27 | 0 | 0 | −1 | 1 | 72.88 | 72.55 |
| 14 | 1 | 0 | 0 | −1 | 33.12 | 32.44 | - | - | - | - | - | - | - |
| Source | Sum of Squares | Df | Mean Square | F Value | p Value | |
|---|---|---|---|---|---|---|
| Model | 7655.69 | 14 | 546.84 | 710.13 | <0.0001 | Significant |
| 2364.71 | 1 | 2364.71 | 3070.86 | <0.0001 | - | |
| 348.02 | 1 | 348.02 | 451.95 | <0.0001 | - | |
| 1375.69 | 1 | 1375.69 | 1786.50 | <0.0001 | - | |
| 1085.62 | 1 | 1085.62 | 1409.81 | <0.0001 | - | |
| 45.10 | 1 | 45.10 | 58.56 | <0.0001 | - | |
| 1.03 | 1 | 1.03 | 1.34 | 0.2702 | - | |
| 71.86 | 1 | 71.86 | 93.32 | <0.0001 | - | |
| 13.96 | 1 | 13.96 | 18.13 | 0.0011 | - | |
| 12.79 | 1 | 12.79 | 16.61 | 0.0015 | - | |
| 4.31 | 1 | 4.31 | 5.59 | 0.0357 | - | |
| ² | 75.00 | 1 | 75.00 | 97.40 | <0.0001 | - |
| ² | 185.88 | 1 | 185.88 | 241.38 | <0.0001 | - |
| ² | 57.79 | 1 | 57.79 | 75.05 | <0.0001 | - |
| ² | 1445.08 | 1 | 1445.08 | 1876.62 | <0.0001 | - |
| Residual | 9.24 | 12 | 0.7700 | - | - | - |
| Lack of Fit | 8.41 | 10 | 0.8408 | 2.02 | 0.3762 | Insignificant |
| Pure Error | 0.8323 | 2 | 0.4162 | - | - | - |
| Total | 7664.94 | 26 | - | - | - | - |
| NO. | X1 | X2 | X3 | X4 | Experimental | Predicted | Deviation (%) |
|---|---|---|---|---|---|---|---|
| 1. | 3.3 | 0.40 | 0.0635 | 431 | 98.05 | 97.24 | 0.82 |
| 2. | 3.0 | 0.10 | 0.0630 | 419 | 85.22 | 85.53 | 0.36 |
| 3. | 3.0 | 0.40 | 0.0630 | 203 | 83.10 | 83.63 | 0.64 |
| 4. | 7.0 | 0.39 | 0.0635 | 375 | 68.22 | 69.29 | 1.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.-G.; Ko, J.-W.; Ko, W.-B. Ultrasound Assisted Synthesis of Gadolinium Oxide-Zeolitic Imidazolate Framework-8 Nanocomposites and Their Optimization for Photocatalytic Degradation of Methyl Orange Using Response Surface Methodology. Catalysts 2021, 11, 1022. https://doi.org/10.3390/catal11091022
Jeon S-G, Ko J-W, Ko W-B. Ultrasound Assisted Synthesis of Gadolinium Oxide-Zeolitic Imidazolate Framework-8 Nanocomposites and Their Optimization for Photocatalytic Degradation of Methyl Orange Using Response Surface Methodology. Catalysts. 2021; 11(9):1022. https://doi.org/10.3390/catal11091022
Chicago/Turabian StyleJeon, Su-Gyeong, Jeong-Won Ko, and Weon-Bae Ko. 2021. "Ultrasound Assisted Synthesis of Gadolinium Oxide-Zeolitic Imidazolate Framework-8 Nanocomposites and Their Optimization for Photocatalytic Degradation of Methyl Orange Using Response Surface Methodology" Catalysts 11, no. 9: 1022. https://doi.org/10.3390/catal11091022
APA StyleJeon, S.-G., Ko, J.-W., & Ko, W.-B. (2021). Ultrasound Assisted Synthesis of Gadolinium Oxide-Zeolitic Imidazolate Framework-8 Nanocomposites and Their Optimization for Photocatalytic Degradation of Methyl Orange Using Response Surface Methodology. Catalysts, 11(9), 1022. https://doi.org/10.3390/catal11091022







