Optimized Synthesis Routes of MnOx-ZrO2 Hybrid Catalysts for Improved Toluene Combustion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Characterization
2.1.1. XRD Analysis
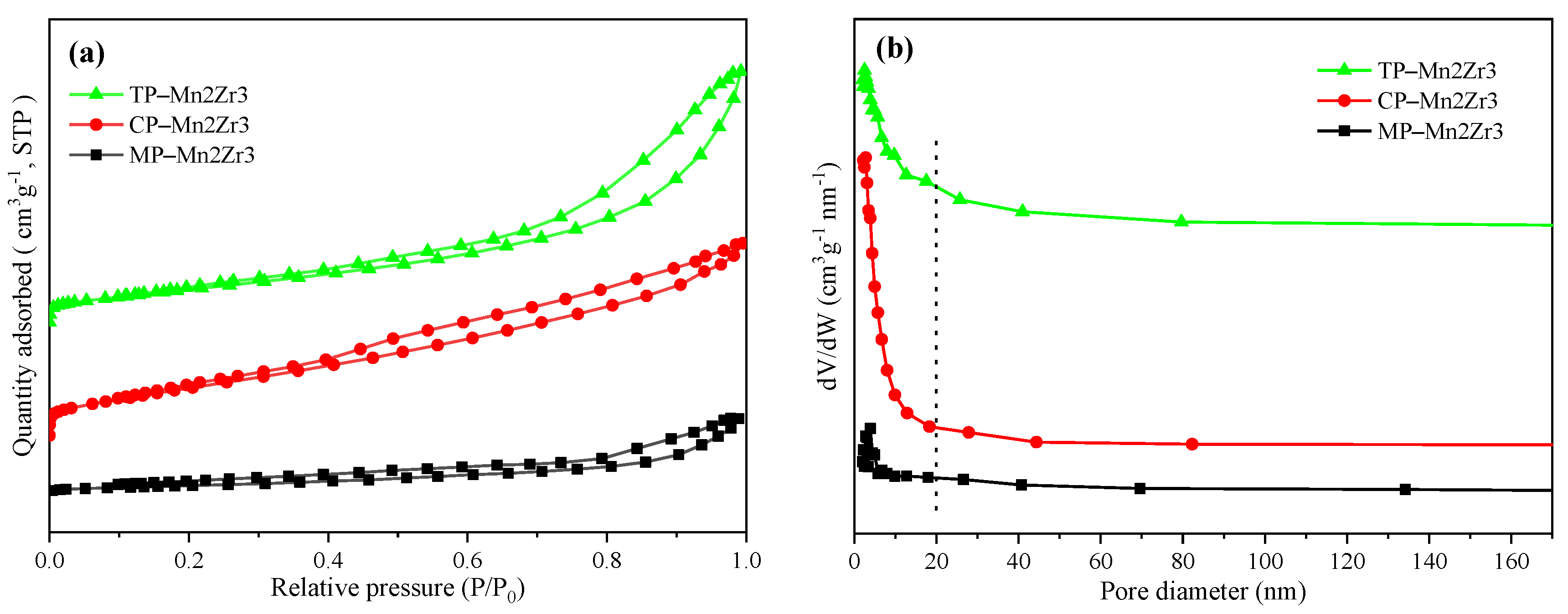
2.1.2. BET Analysis
2.1.3. HRTEM Analysis
2.1.4. XPS Analysis
2.1.5. H2-TPR Results Analysis
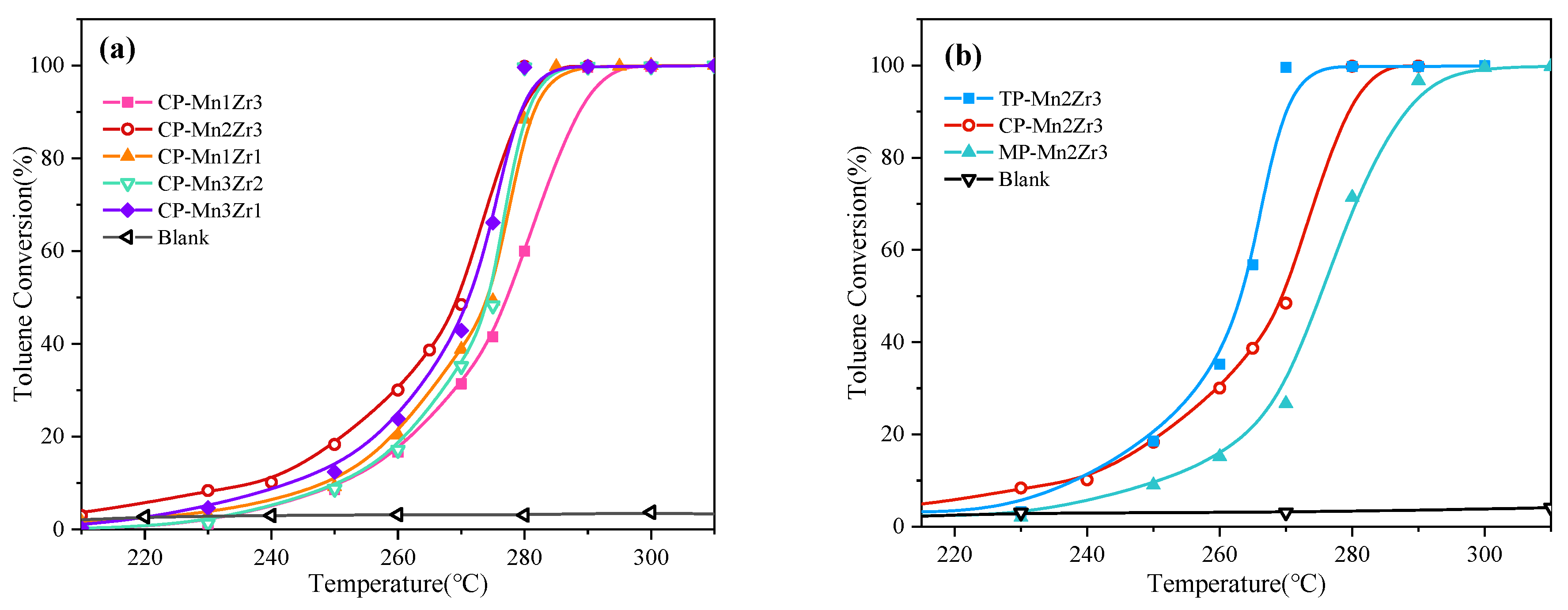
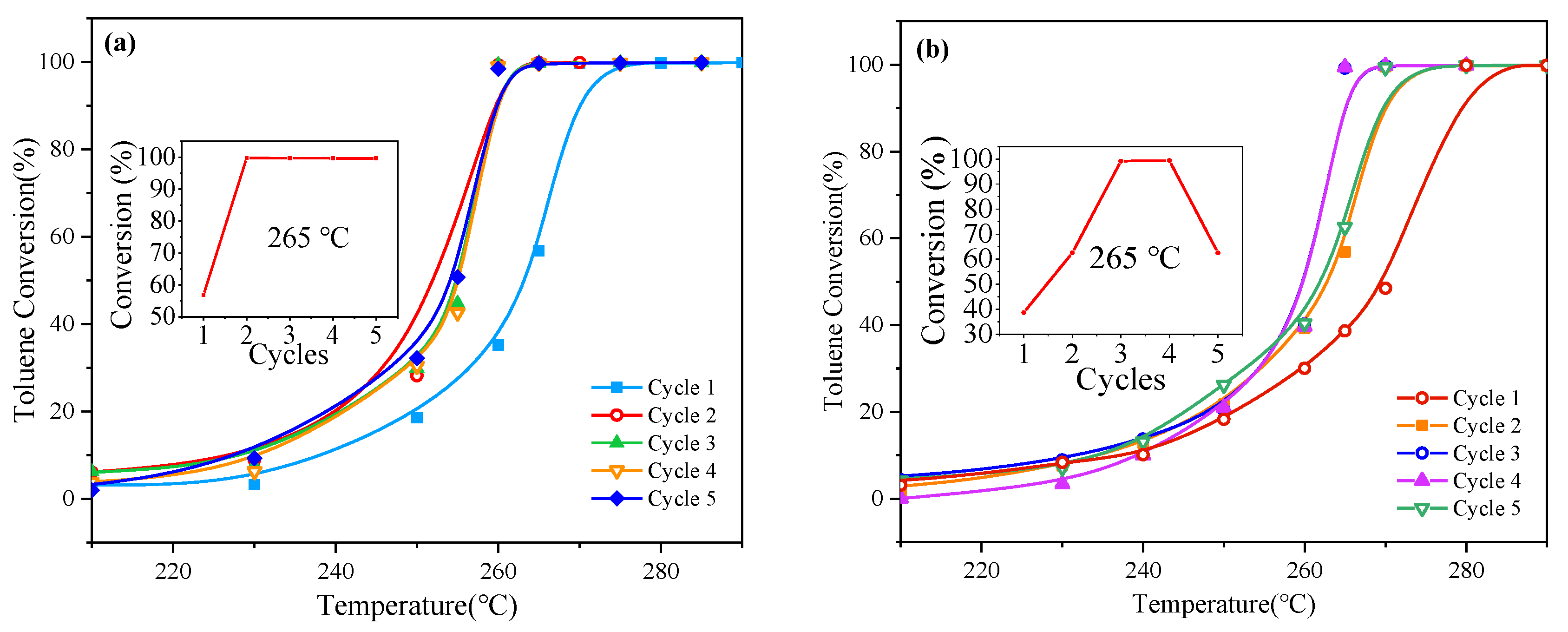
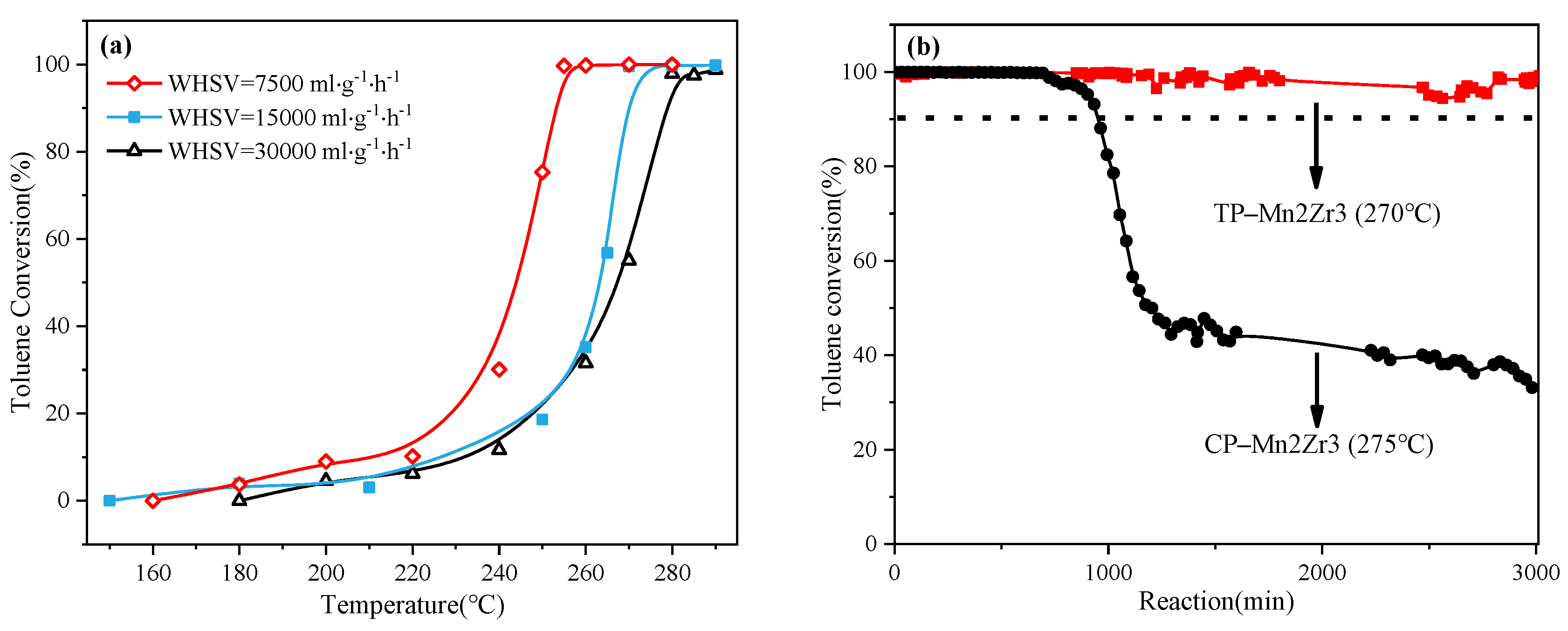
2.2. Evaluation of Catalytic Activity
3. Discussion
4. Materials and Methods
4.1. Materials
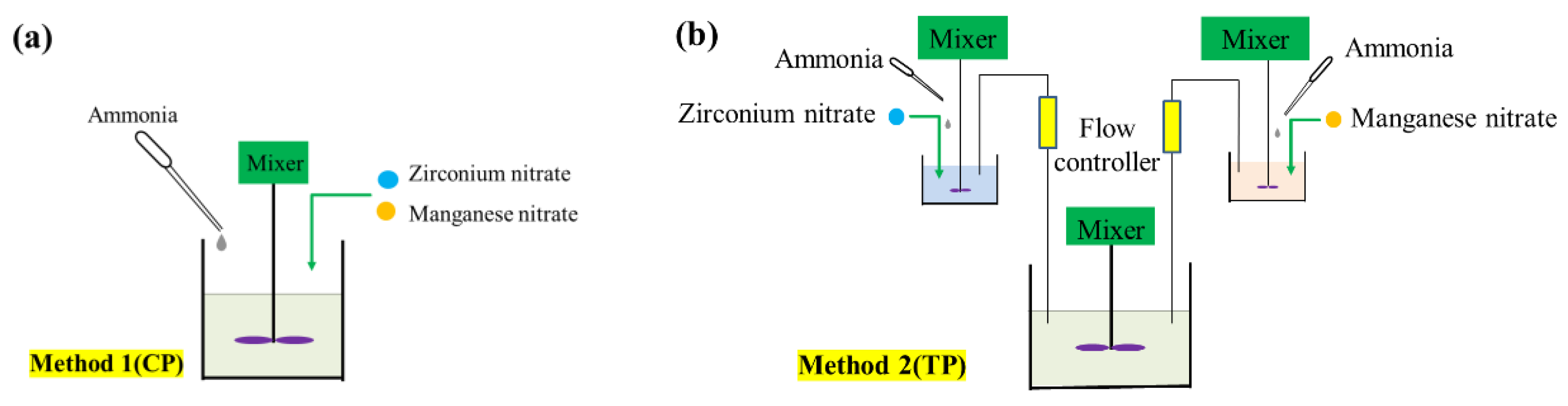
4.2. Catalyst Preparation
4.3. Catalyst Activity Evaluation
4.4. Catalyst Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Dai, H.; Du, Y.; Deng, J.; Zhang, L.; Zhao, Z.; Au, C.T. Controlled preparation and high catalytic performance of three-dimensionally ordered macroporous LaMnO3 with nanovoid skeletons for the combustion of toluene. J. Catal. 2012, 287, 149–160. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Saqer, S.M.; Kondarides, D.I.; Verykios, X.E. Catalytic Activity of Supported Platinum and Metal Oxide Catalysts for Toluene Oxidation. Top. Catal. 2009, 52, 517–527. [Google Scholar] [CrossRef]
- Mustafa, M.F.; Fu, X.; Liu, Y.; Abbas, Y.; Wang, H.; Lu, W. Volatile organic compounds (VOCs) removal in non-thermal plasma double dielectric barrier discharge reactor. J. Hazard. Mater. 2018, 347, 317–324. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Ma, X.Y.; Yu, X.L.; Yang, X.Q.; Lin, M.Y.; Ge, M.F. Hydrothermal Synthesis of a Novel Double-Sided Nanobrush Co3O4 Catalyst and Its Catalytic Performance for Benzene Oxidation. ChemCatChem 2019, 11, 1214–1221. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Lin, M.; Ge, M.; Zhao, Y.; Wang, F. Interface effect of mixed phase Pt/ZrO2 catalysts for HCHO oxidation at ambient temperature. J. Mater. Chem. A 2017, 5, 13799–13806. [Google Scholar] [CrossRef]
- Li, L.; Chu, W.; Liu, Y. Insights into key parameters of MnO2 catalyst toward high catalytic combustion performance. J. Mater. Sci. 2021, 56, 6361–6373. [Google Scholar] [CrossRef]
- Xie, S.; Deng, J.; Liu, Y.; Zhang, Z.; Yang, H.; Jiang, Y.; Arandiyan, H.; Dai, H.; Au, C.T. Excellent catalytic performance, thermal stability, and water resistance of 3DOM Mn2O3-supported Au–Pd alloy nanoparticles for the complete oxidation of toluene. Appl. Catal. A Gen. 2015, 507, 82–90. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Ji, Z.; Lv, Y.; Cao, Y.; Tang, C.; Gao, F.; Dong, L.; Chen, Y. A comparative study of different doped metal cations on the reduction, adsorption and activity of CuO/Ce0.67M0.33O2 (M = Zr4+, Sn4+, Ti4+) catalysts for NO+CO reaction. Appl. Catal. B Environ. 2013, 130–131, 293–304. [Google Scholar] [CrossRef]
- Chung, W.-C.; Mei, D.-H.; Tu, X.; Chang, M.-B. Removal of VOCs from gas streams via plasma and catalysis. Catal. Rev. 2018, 61, 270–331. [Google Scholar] [CrossRef]
- Sihaib, Z.; Puleo, F.; Garcia-Vargas, J.M.; Retailleau, L.; Descorme, C.; Liotta, L.F.; Valverde, J.L.; Gil, S.; Giroir-Fendler, A. Manganese oxide-based catalysts for toluene oxidation. Appl. Catal. B Environ. 2017, 209, 689–700. [Google Scholar] [CrossRef]
- Craciun, R.; Nentwick, B.; Hadjiivanov, K.; Knözinger, H. Structure and redox properties of MnOx/Yttrium-stabilized zirconia (YSZ) catalyst and its used in CO and CH4 oxidation. Appl. Catal. A Gen. 2003, 243, 67–79. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B Environ. 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; He, H.; Wu, Z.; Wu, J.; Chen, L.; Ye, D.; Fu, M. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Appl. Catal. B Environ. 2018, 223, 91–102. [Google Scholar] [CrossRef]
- Xie, S.; Liu, Y.; Deng, J.; Zhao, X.; Yang, J.; Zhang, K.; Han, Z.; Dai, H. Three-dimensionally ordered macroporous CeO2-supported Pd@Co nanoparticles: Highly active catalysts for methane oxidation. J. Catal. 2016, 342, 17–26. [Google Scholar] [CrossRef]
- Afzal, S.; Quan, X.; Zhang, J. High surface area mesoporous nanocast LaMO3 (M = Mn, Fe) perovskites for efficient catalytic ozonation and an insight into probable catalytic mechanism. Appl. Catal. B Environ. 2017, 206, 692–703. [Google Scholar] [CrossRef]
- Zhao, Q.; Fu, L.; Jiang, D.; Ouyang, J.; Hu, Y.; Yang, H.; Xi, Y. Nanoclay-modulated oxygen vacancies of metal oxide. Commun. Chem. 2019, 2, 11. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. B Environ. 2010, 99, 353–363. [Google Scholar] [CrossRef]
- Tang, X.; Li, Y.; Huang, X.; Xu, Y.; Zhu, H.; Wang, J.; Shen, W. MnOx–CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature. Appl. Catal. B Environ. 2006, 62, 265–273. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, S.; Li, W.; Chen, Y. Porous Mn–Co mixed oxide nanorod as a novel catalyst with enhanced catalytic activity for removal of VOCs. Catal. Commun. 2014, 56, 134–138. [Google Scholar] [CrossRef]
- Morales, M.; Barbero, B.; Cadus, L. Total oxidation of ethanol and propane over Mn-Cu mixed oxide catalysts. Appl. Catal. B Environ. 2006, 67, 229–236. [Google Scholar] [CrossRef]
- Li, H.; Qi, G.; Tana; Zhang, X.; Huang, X.; Li, W.; Shen, W. Low-temperature oxidation of ethanol over a Mn0.6Ce0.4O2 mixed oxide. Appl. Catal. B Environ. 2011, 103, 54–61. [Google Scholar] [CrossRef]
- Gutierrez-Ortiz, J.I.; de Rivas, B.; Lopez-Fonseca, R.; Martin, S.; Gonzalez-Velasco, J.R. Structure of Mn-Zr mixed oxides catalysts and their catalytic performance in the gas-phase oxidation of chlorocarbons. Chemosphere 2007, 68, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Fernández López, E.; Sánchez Escribano, V.; Resini, C.; Gallardo-Amores, J.M.; Busca, G. A study of coprecipitated Mn–Zr oxides and their behaviour as oxidation catalysts. Appl. Catal. B Environ. 2001, 29, 251–261. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Vinokurov, Z.S.; Afonasenko, T.N.; Tsyrul’nikov, P.G.; Tsybulya, S.V.; Saraev, A.A.; Kaichev, V.V. Reduction of mixed Mn-Zr oxides: In situ XPS and XRD studies. Dalton Trans. 2015, 44, 15499–15507. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Shih, W.Y.; Chang, H.L.; Shih, W.H. Redox Activity and NO Storage Capacity of MnOx−ZrO2 with Enhanced Thermal Stability at Elevated Temperatures. Ind. Eng. Chem. Res. 2010, 49, 1725–1731. [Google Scholar] [CrossRef]
- Zuo, J.; Chen, Z.; Wang, F.; Yu, Y.; Wang, L.; Li, X. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Novel Mn–Zr Mixed Oxide Catalysts. Ind. Eng. Chem. Res. 2014, 53, 2647–2655. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Uphade, B.S.; Pataskar, S.G. Low temperature complete combustion of dilute methane over Mn-doped ZrO2 catalysts: Factors influencing the reactivity of lattice oxygen and methane combustion activity of the catalyst. Appl. Catal. A Gen. 2002, 227, 29–41. [Google Scholar] [CrossRef]
- Zeng, K.; Li, X.; Wang, C.; Wang, Z.; Guo, P.; Yu, J.; Zhang, C.; Zhao, X.S. Three-dimensionally macroporous MnZrOx catalysts for propane combustion: Synergistic structure and doping effects on physicochemical and catalytic properties. J. Colloid Interface Sci. 2020, 572, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, X.; Jing, M.; Song, W.; Liu, J.; Ge, M. Defective MnxZr1-xO2 Solid Solution for the Catalytic Oxidation of Toluene: Insights into the Oxygen Vacancy Contribution. ACS Appl. Mater. Interfaces 2019, 11, 730–739. [Google Scholar] [CrossRef]
- Fan, X.; Li, L.; Jing, F.; Li, J.; Chu, W. Effects of preparation methods on CoAlOx/CeO2 catalysts for methane catalytic combustion. Fuel 2018, 225, 588–595. [Google Scholar] [CrossRef]
- Li, L.; Luo, J.; Liu, Y.; Jing, F.; Su, D.; Chu, W. Self-Propagated Flaming Synthesis of Highly Active Layered CuO-delta-MnO2 Hybrid Composites for Catalytic Total Oxidation of Toluene Pollutant. ACS Appl. Mater. Interfaces 2017, 9, 21798–21808. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Li, H.; Chen, S.; Chen, Y. Preparation of CeO2–ZrO2–Al2O3 composite with layered structure for improved Pd-only three-way catalyst. J. Mater. Sci. 2017, 52, 9615–9629. [Google Scholar] [CrossRef]
- Hou, Z.; Feng, J.; Lin, T.; Zhang, H.; Zhou, X.; Chen, Y. The performance of manganese-based catalysts with Ce0.65Zr0.35O2 as support for catalytic oxidation of toluene. Appl. Surf. Sci. 2018, 434, 82–90. [Google Scholar] [CrossRef]
- Azalim, S.; Franco, M.; Brahmi, R.; Giraudon, J.M.; Lamonier, J.F. Removal of oxygenated volatile organic compounds by catalytic oxidation over Zr-Ce-Mn catalysts. J. Hazard. Mater. 2011, 188, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Ma, X.Y.; Yu, X.L.; Ge, M.F. Exploration of strong metal-support interaction in zirconia supported catalysts for toluene oxidation. Appl. Catal. B Environ. 2020, 263, 118355. [Google Scholar] [CrossRef]
- Han, Z.; Yu, Q.; Teng, Z.; Wu, B.; Xue, Z.; Qin, Q. Effects of manganese content and calcination temperature on Mn/Zr-PILM catalyst for low-temperature selective catalytic reduction of NOx by NH3 in metallurgical sintering flue gas. Environ. Sci. Pollut. Res. Int. 2019, 26, 12920–12927. [Google Scholar] [CrossRef]
- Huang, N.; Qu, Z.; Dong, C.; Qin, Y.; Duan, X. Superior performance of α@β-MnO2 for the toluene oxidation: Active interface and oxygen vacancy. Appl. Catal. A Gen. 2018, 560, 195–205. [Google Scholar] [CrossRef]
- Mitran, G.; Chen, S.; Seo, D.-K. Role of oxygen vacancies and Mn4+/Mn3+ ratio in oxidation and dry reforming over cobalt-manganese spinel oxides. Mol. Catal. 2020, 483, 110704. [Google Scholar] [CrossRef]
- Wang, W.L.; Meng, Q.; Xue, Y.; Weng, X.; Sun, P.; Wu, Z. Lanthanide perovskite catalysts for oxidation of chloroaromatics: Secondary pollution and modifications. J. Catal. 2018, 366, 213–222. [Google Scholar] [CrossRef]
- Tang, W.; Li, W.; Li, D.; Liu, G.; Wu, X.; Chen, Y. Synergistic Effects in Porous Mn–Co Mixed Oxide Nanorods Enhance Catalytic Deep Oxidation of Benzene. Catal. Lett. 2014, 144, 1900–1910. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.; Liu, Z.; Yao, L.; Yu, P.; Wei, P.; Xu, Y.; Jiang, X. High Catalytic Performance of Mn-Doped Ce-Zr Catalysts for Chlorobenzene Elimination. Nanomaterials 2019, 9, 675. [Google Scholar] [CrossRef] [Green Version]
- Dravid, V.P.; Ravikumar, V.; Notis, M.R.; Lyman, C.E.; Dhalenne, G.; Revcolevschi, A. Stabilization of Cubic Zirconia with Manganese Oxide. J. Am. Ceram. Soc. 1994, 77, 2758–2762. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, C.; Wang, H.; Zhang, M. Synthesis of highly efficient MnOx catalyst for low-temperature NH3-SCR prepared from Mn-MOF-74 template. Mater. Lett. 2016, 168, 17–19. [Google Scholar] [CrossRef]
- Cuervo, M.R.; Diaz, E.; de Rivas, B.; Lopez-Fonseca, R.; Ordonez, S.; Gutierrez-Ortiz, J.I. Inverse gas chromatography as a technique for the characterization of the performance of Mn/Zr mixed oxides as combustion catalysts. J. Chromatogr. A 2009, 1216, 7873–7881. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, H.; Dong, C.; Qu, Z. Evolution and enhancement of the oxygen cycle in the catalytic performance of total toluene oxidation over manganese-based catalysts. J. Catal. 2019, 380, 21–31. [Google Scholar] [CrossRef]
- Li, L.; Wahab, M.A.; Li, H.; Zhang, H.; Deng, J.; Zhai, X.; Masud, M.K.; Hossain, M.S.A. Pt-Modulated CuMnOx Nanosheets as Catalysts for Toluene Oxidation. ACS Appl. Nano Mater. 2021, 4, 6637–6647. [Google Scholar] [CrossRef]
- Mo, S.P.; Zhang, Q.; Li, J.Q.; Sun, Y.H.; Ren, Q.M.; Zou, S.B.; Zhang, Q.; Lu, J.H.; Fu, M.L.; Mo, D.Q.; et al. Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: Oxygen-Vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl. Catal. B Environ. 2020, 264, 118464. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, H.; Zhang, Z.; Li, L. Improved reactivity for toluene oxidation on MnOx/CeO2-ZrO2 catalyst by the synthesis of cubic-tetragonal interfaces. Appl. Surf. Sci. 2021, 539, 148188. [Google Scholar] [CrossRef]
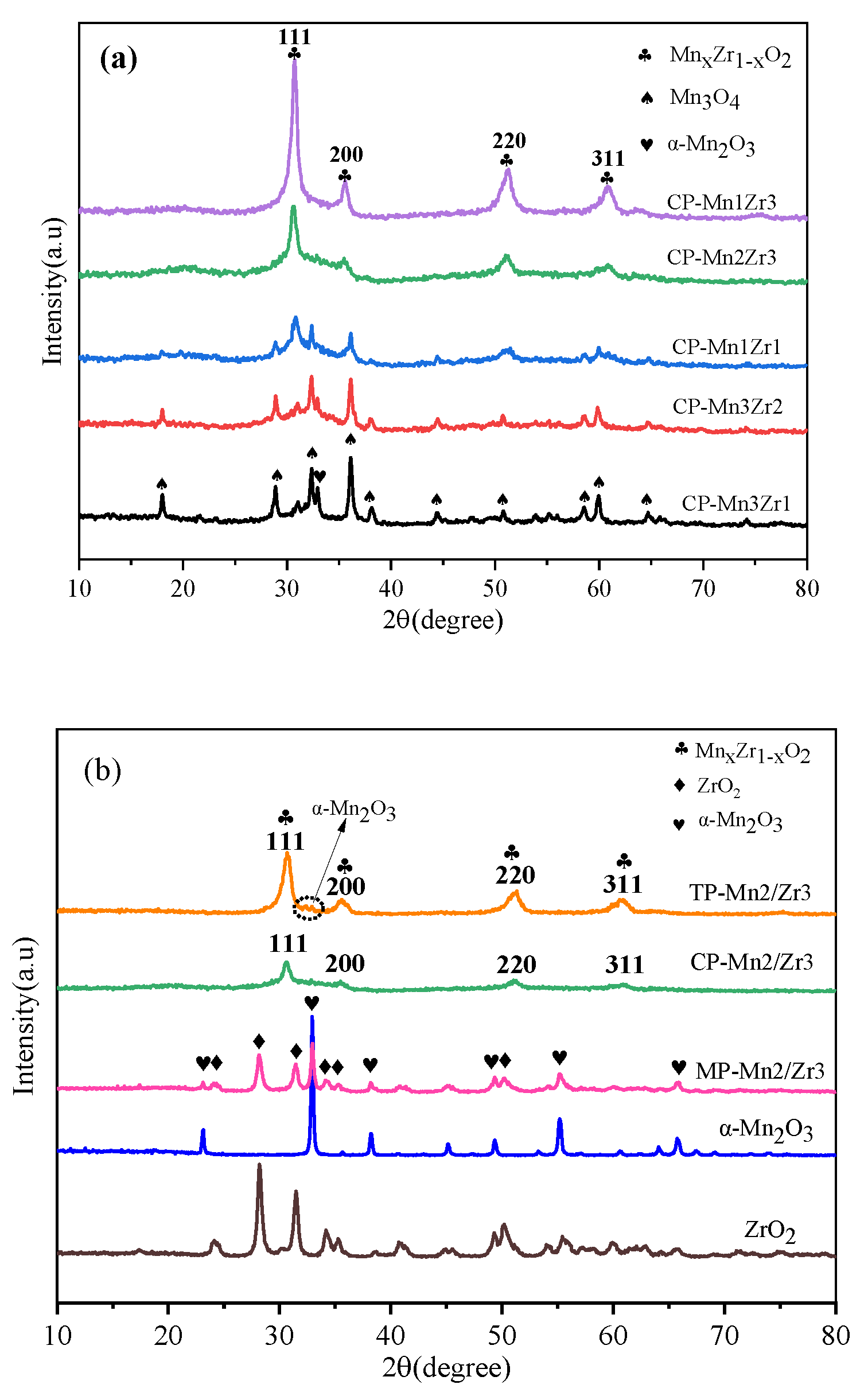
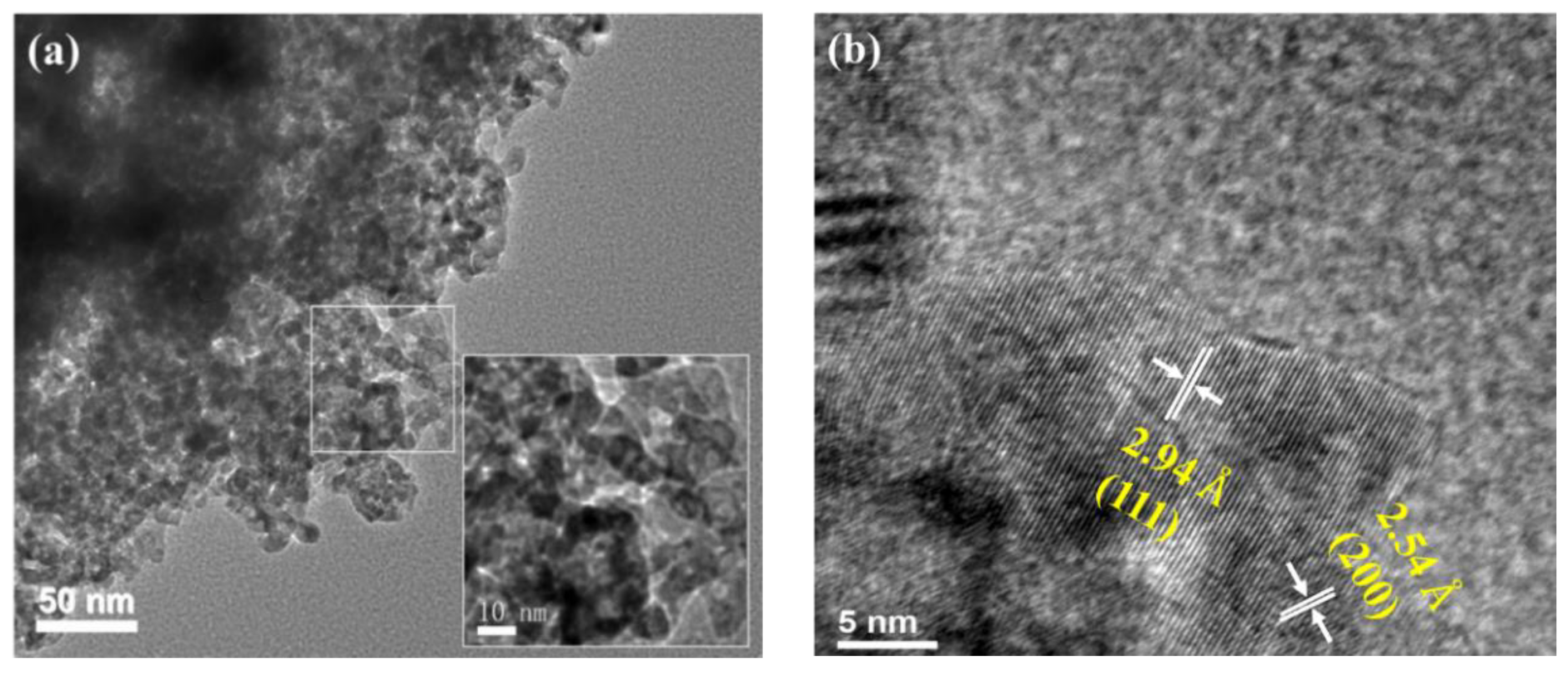
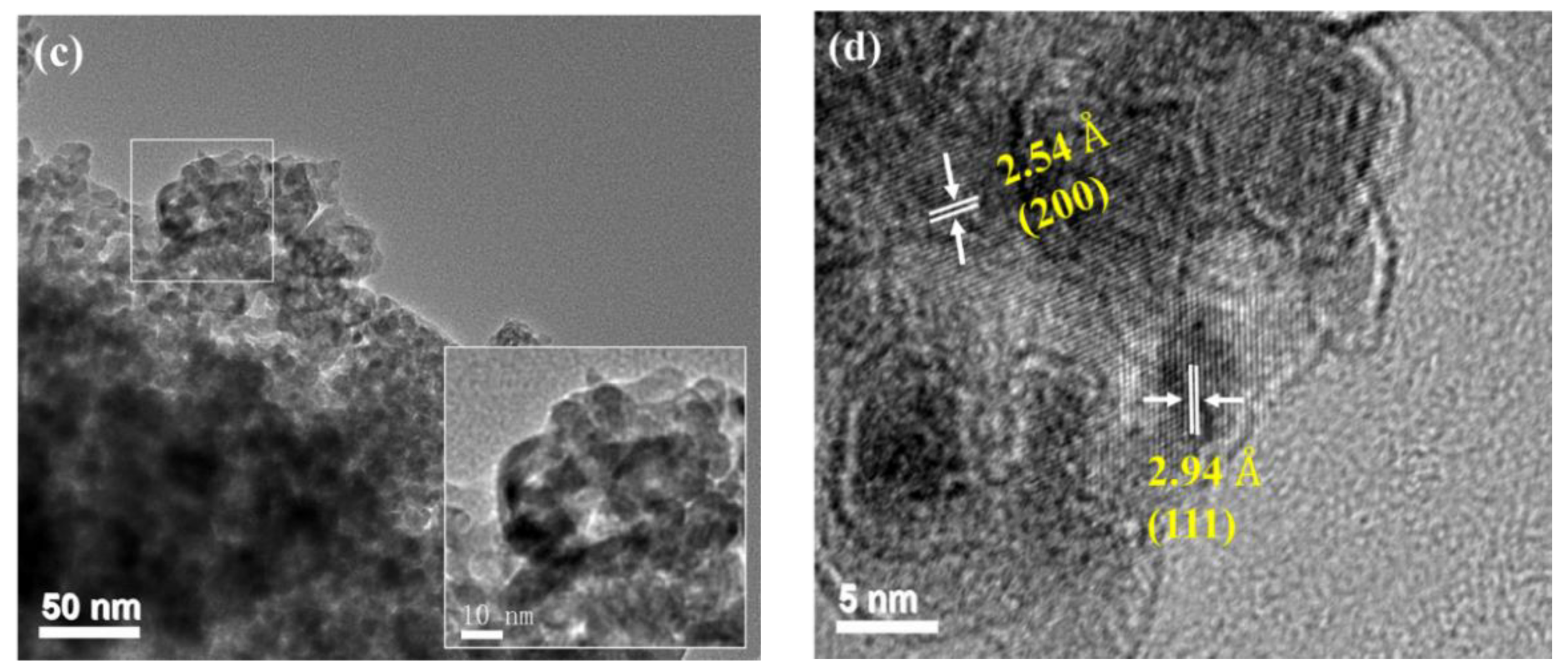
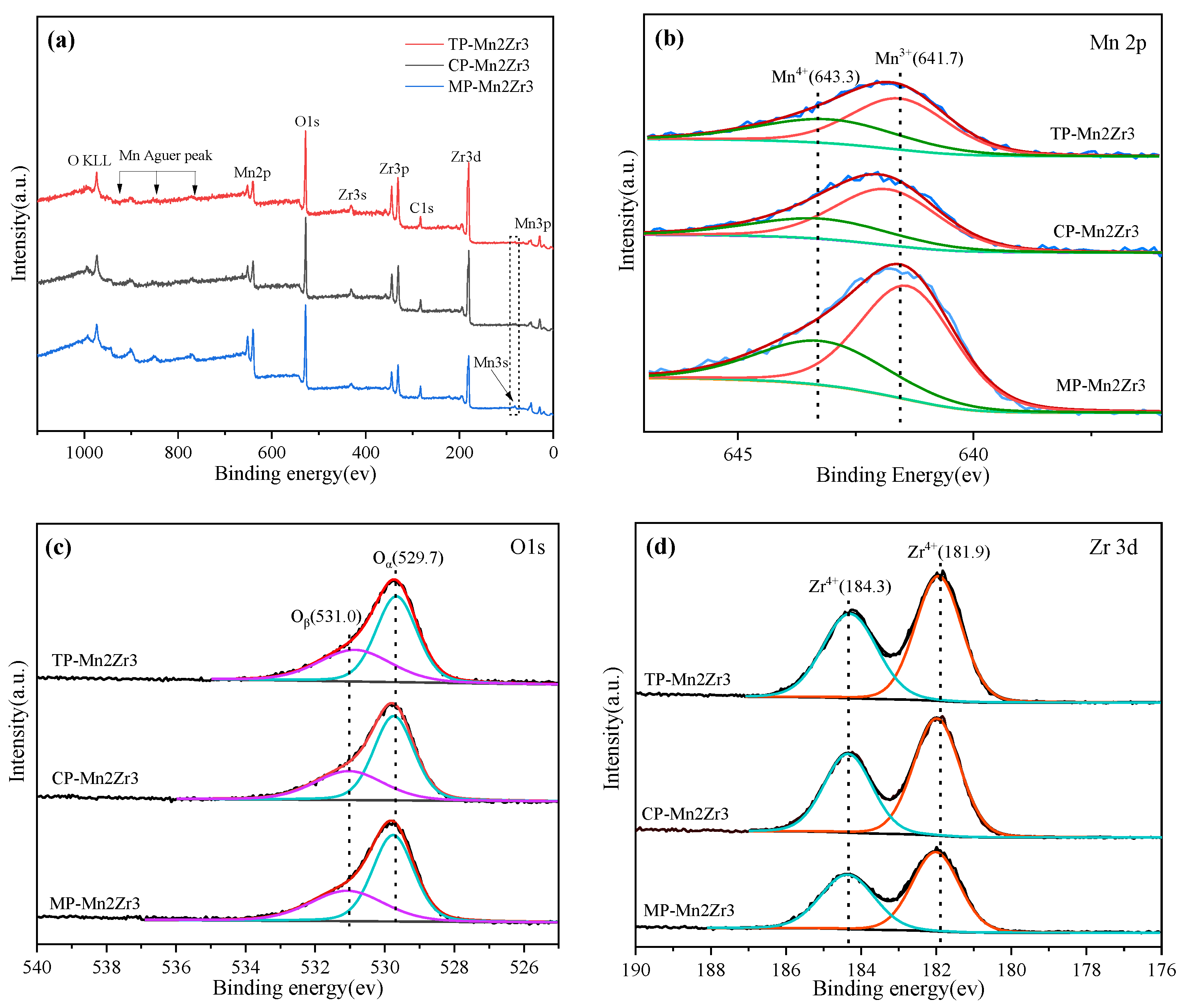

| Samples | a (Å) | b (Å) | c (Å) | v (Å3) | Grain Size (nm) a |
|---|---|---|---|---|---|
| TP-Mn2Zr3 | 5.04 | - | - | 128 | 8.4 |
| CP-Mn2Zr3 | 5.05 | - | - | 128.8 | 11.3 |
| MP-Mn2Zr3 | - | - | - | - | 21.5 |
| ZrO2 | 5.87 | 4.86 | 5.20 | 148.3 | 16.6 |
| α-Mn2O3 | 9.42 | 9.42 | 9.42 | 835.9 | 32.2 |
| Samples | SBET (m2/g) | Average Pore Diameter (nm) a | Pore Volume (cm3/g) b |
|---|---|---|---|
| TP-Mn/Zr = 2/3 | 99.7 | 10.8 | 0.27 |
| CP-Mn/Zr = 2/3 | 139.5 | 5.9 | 0.20 |
| MP-Mn/Zr = 2/3 | 23.7 | 13.5 | 0.08 |
| Samples | Surface Element (at%) | Bind Energy | Surface Element Molar Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn 2p | Zr 3d | O 1s | Mn4+ | Mn3+ | Oα | Oβ | Mn4+/Mn3+ | Oβ/Oα | |
| TP-Mn2Zr3 | 15.92 | 18.63 | 65.46 | 643.3 | 641.7 | 529.7 | 531.0 | 0.68 | 0.65 |
| CP-Mn2Zr3 | 17.37 | 17.69 | 64.94 | 643.5 | 641.9 | 529.7 | 531.0 | 0.50 | 0.59 |
| MP-Mn2Zr3 | 29.70 | 11.04 | 59.25 | 643.4 | 641.5 | 529.7 | 531.1 | 0.53 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Li, L.; Liu, R.; Li, H.; Lan, L.; Zhou, W. Optimized Synthesis Routes of MnOx-ZrO2 Hybrid Catalysts for Improved Toluene Combustion. Catalysts 2021, 11, 1037. https://doi.org/10.3390/catal11091037
Huang X, Li L, Liu R, Li H, Lan L, Zhou W. Optimized Synthesis Routes of MnOx-ZrO2 Hybrid Catalysts for Improved Toluene Combustion. Catalysts. 2021; 11(9):1037. https://doi.org/10.3390/catal11091037
Chicago/Turabian StyleHuang, Xin, Luming Li, Rong Liu, Hongmei Li, Li Lan, and Weiqi Zhou. 2021. "Optimized Synthesis Routes of MnOx-ZrO2 Hybrid Catalysts for Improved Toluene Combustion" Catalysts 11, no. 9: 1037. https://doi.org/10.3390/catal11091037
APA StyleHuang, X., Li, L., Liu, R., Li, H., Lan, L., & Zhou, W. (2021). Optimized Synthesis Routes of MnOx-ZrO2 Hybrid Catalysts for Improved Toluene Combustion. Catalysts, 11(9), 1037. https://doi.org/10.3390/catal11091037






