Highly Active Mo2C@WS2 Hybrid Electrode for Enhanced Hydrogen Evolution Reaction
Abstract
:1. Introduction
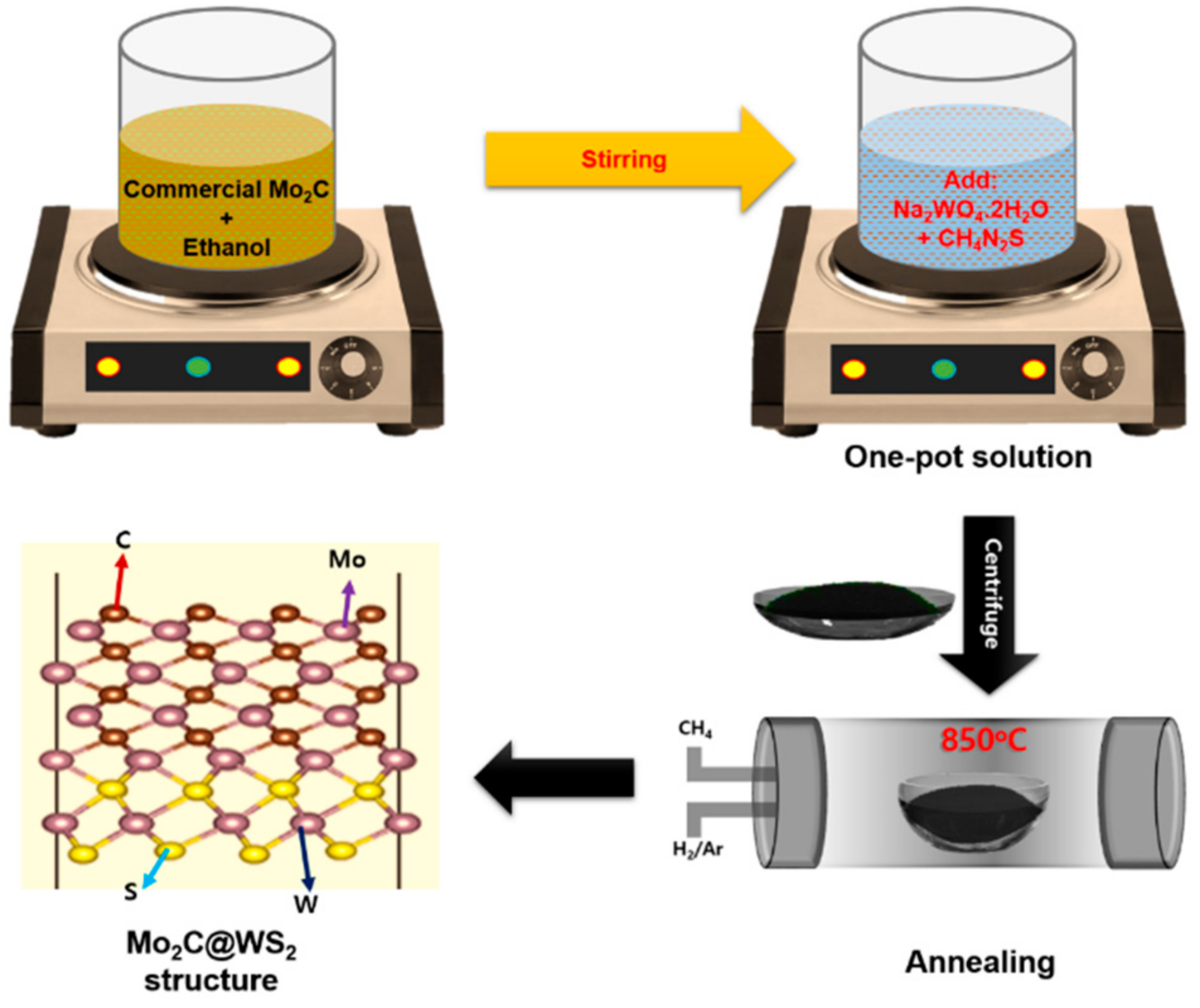
2. Experimental Section
Synthesis of Mo2C@WS2 Hybrid
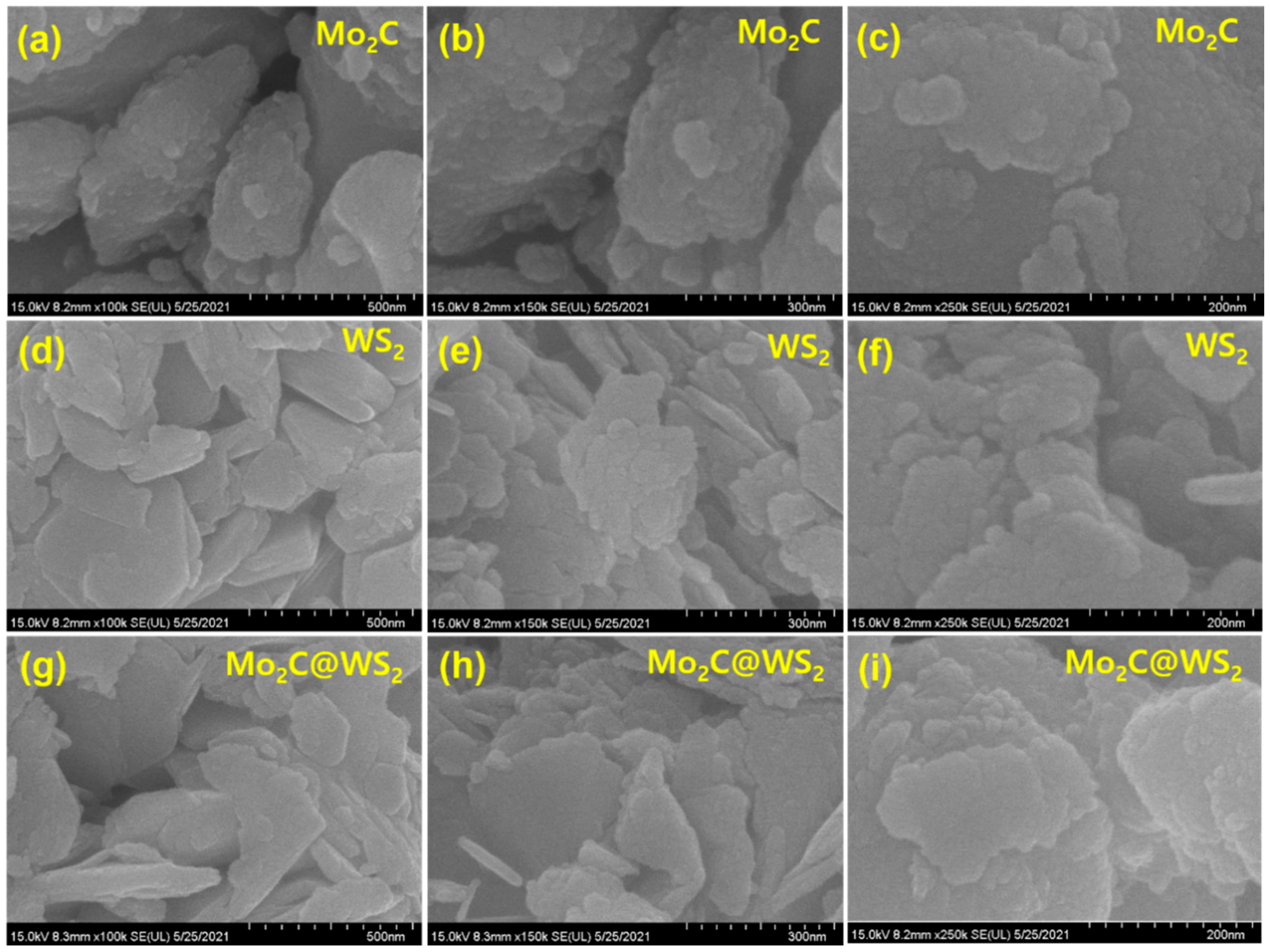
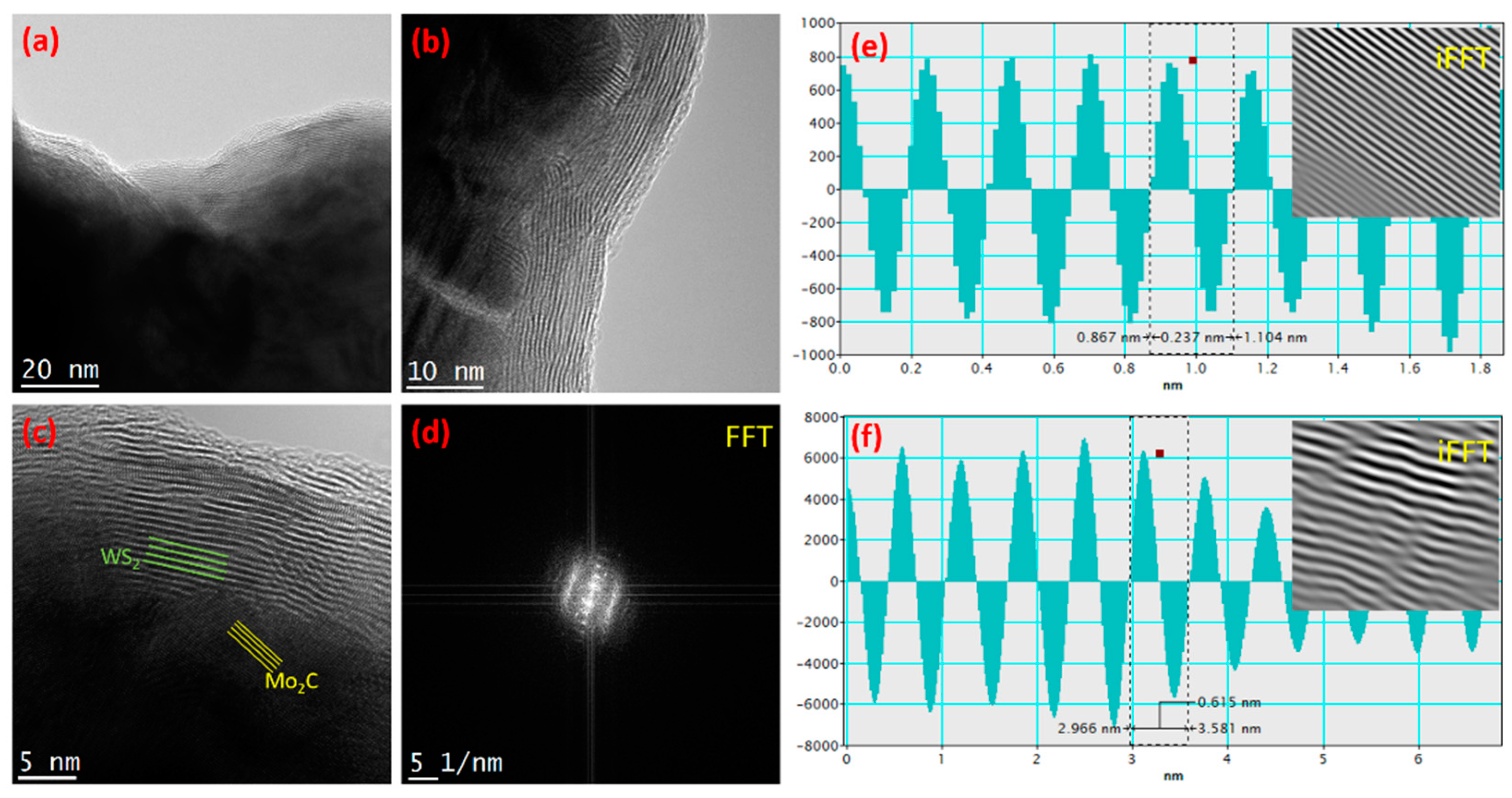
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dresselhaus, M.; Thomas, I. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Hussain, S.; Rabani, I.; Feroze, A.; Ali, M.; Seo, Y.-S.; Chun, S.-H.; Jung, J.; Kim, H.-S. Engineering MoTe2 and janus semote nanosheet structures: First-principles roadmap and practical uses in hydrogen evolution reactions and symmetric supercapacitors. Nano Energy 2021, 87, 106161. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Chen, W.; Wang, X. A review of phosphide-based materials for electrocatalytic hydrogen evolution. Adv. Energy Mater. 2015, 5, 1500985. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Murthy, A.P.; Madhavan, J.; Choi, M.Y. Fundamental aspects and recent advances in transition metal nitrides as electrocatalysts for hydrogen evolution reaction: A review. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100805. [Google Scholar] [CrossRef]
- Hussain, S.; Vikraman, D.; Feroze, A.; Song, W.; An, K.-S.; Kim, H.-S.; Chun, S.-H.; Jung, J. Synthesis of Mo2C and W2C nanoparticle electrocatalysts for the efficient hydrogen evolution reaction in alkali and acid electrolytes. Front. Chem. 2019, 7, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, M.; Pan, J.; He, T.; Yan, Y.; Xia, B.Y.; Wang, X. Molybdenum carbide-based electrocatalysts for hydrogen evolution reaction. Chem. Eur. J. 2017, 23, 10947–10961. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; English, C.R.; Meng, F.; Forticaux, A.; Hamers, R.J.; Jin, S. Highly active hydrogen evolution catalysis from metallic WS2 nanosheets. Energy Environ. Sci. 2014, 7, 2608–2613. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, F.; Kasiraju, S.; Xie, L.; Alam, M.K.; Chen, S.; Wang, D.; Ren, Z.; Wang, Z.; Grabow, L.C. Vertically aligned MoS2/Mo2C hybrid nanosheets grown on carbon paper for efficient electrocatalytic hydrogen evolution. ACS Catal. 2017, 7, 7312–7318. [Google Scholar] [CrossRef]
- Wang, F.; He, P.; Li, Y.; Shifa, T.A.; Deng, Y.; Liu, K.; Wang, Q.; Wang, F.; Wen, Y.; Wang, Z. Interface engineered WxC@WS2 nanostructure for enhanced hydrogen evolution catalysis. Adv. Funct. Mater. 2017, 27, 1605802. [Google Scholar]
- Li, Y.; Wu, X.; Zhang, H.; Zhang, J. Interface designing over WS2/W2C for enhanced hydrogen evolution catalysis. ACS Appl. Energy Mater. 2018, 1, 3377–3384. [Google Scholar] [CrossRef]
- Hussain, S.; Rabani, I.; Vikraman, D.; Feroze, A.; Ali, M.; Seo, Y.-S.; Kim, H.-S.; Chun, S.-H.; Jung, J. One-pot synthesis of w2c/ws2 hybrid nanostructures for improved hydrogen evolution reactions and supercapacitors. Nanomaterials 2020, 10, 1597. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Mu, X.; Li, Q.; Ma, L.; Xiong, Y.; Li, R. Rational design of ni-induced NC@Mo2C@MoS2 sphere electrocatalyst for efficient hydrogen evolution reaction in acidic and alkaline media. Int. J. Hydrogen Energy 2021, 46, 5250–5258. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Karuppasamy, K.; Feroze, A.; Kathalingam, A.; Sanmugam, A.; Chun, S.-H.; Jung, J.; Kim, H.-S. Engineering the novel MoSe2-Mo2C hybrid nanoarray electrodes for energy storage and water splitting applications. Appl. Catal. B 2020, 264, 118531. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Zhang, H.; Zhang, Y.; Liu, L.; Fang, L.; Yang, X.; Gu, X.; Wang, Y. Unique three-dimensional Mo2C@MoS2 heterojunction nanostructure with s vacancies as outstanding all-ph range electrocatalyst for hydrogen evolution. J. Catal. 2019, 371, 20–26. [Google Scholar] [CrossRef]
- Hussain, S.; Rabani, I.; Vikraman, D.; Feroze, A.; Ali, M.; Seo, Y.-S.; Song, W.; An, K.-S.; Kim, H.-S.; Chun, S.-H.; et al. MoS2@X2C (x = Mo or W) hybrids for enhanced supercapacitor and hydrogen evolution performances. Chem. Eng. J. 2021, 421, 127843. [Google Scholar] [CrossRef]
- Faizan, M.; Hussain, S.; Vikraman, D.; Ali, B.; Kim, H.-S.; Jung, J.; Nam, K.W. MoS2@Mo2C hybrid nanostructures formation as an efficient anode material for lithium-ion batteries. J. Mater. Res. Technol. 2021, 14, 2382–2393. [Google Scholar] [CrossRef]
- Hussain, S.; Rabani, I.; Vikraman, D.; Feroze, A.; Karuppasamy, K.; Haq, Z.U.; Seo, Y.-S.; Chun, S.-H.; Kim, H.-S.; Jung, J. Hybrid design using carbon nanotubes decorated with Mo2C and W2C nanoparticles for supercapacitors and hydrogen evolution reactions. ACS Sustain. Chem. Eng. 2020, 8, 12248–12259. [Google Scholar] [CrossRef]
- Liu, Z.; Li, N.; Su, C.; Zhao, H.; Xu, L.; Yin, Z.; Li, J.; Du, Y. Colloidal synthesis of 1t’phase dominated WS2 towards endurable electrocatalysis. Nano Energy 2018, 50, 176–181. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Xiao, Z.; Zhou, Y.; Chen, H.; Khalil, A.; Xiang, T.; Xu, J.; Chu, W.; Wu, X. Stable metallic 1T-WS2 nanoribbons intercalated with ammonia ions: The correlation between structure and electrical/optical properties. Adv. Mater. 2015, 27, 4837–4844. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Akbar, K.; Truong, L.; Kathalingam, A.; Chun, S.-H.; Jung, J.; Park, H.J.; Kim, H.-S. Improved hydrogen evolution reaction performance using MoS2–WS2 heterostructures by physicochemical process. ACS Sustain. Chem. Eng. 2018, 6, 8400–8409. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Hu, B.-C.; Wu, P.; Liang, H.-W.; Yu, Z.-L.; Lin, Y.; Zheng, Y.-R.; Li, Z.; Yu, S.-H. Mo2c nanoparticles embedded within bacterial cellulose-derived 3d n-doped carbon nanofiber networks for efficient hydrogen evolution. NPG Asia Mater. 2016, 8, e288. [Google Scholar] [CrossRef]
- Men, Y.-L.; You, Y.; Pan, Y.-X.; Gao, H.; Xia, Y.; Cheng, D.-G.; Song, J.; Cui, D.-X.; Wu, N.; Li, Y.; et al. Selective co evolution from photoreduction of CO2 on a metal-carbide-based composite catalyst. J. Am. Chem. Soc. 2018, 140, 13071–13077. [Google Scholar] [CrossRef]
- Gao, W.; Shi, Y.; Zhang, Y.; Zuo, L.; Lu, H.; Huang, Y.; Fan, W.; Liu, T. Molybdenum carbide anchored on graphene nanoribbons as highly efficient all-ph hydrogen evolution reaction electrocatalyst. ACS Sustain. Chem. Eng. 2016, 4, 6313–6321. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, Q.; Song, X.; Feng, K.; Nie, K.; Zhao, F.; Wang, Y.; Zeng, M.; Zhong, J.; Li, Y. Mo2C nanoparticles dispersed on hierarchical carbon microflowers for efficient electrocatalytic hydrogen evolution. ACS Nano 2016, 10, 11337–11343. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zheng, Y.; Li, A.; Ma, Y.; Huo, Q.; Qiao, Z.A.; Dai, S. Sprout-like growth of mesoporous mo2c/nc nanonetworks as efficient electrocatalysts for hydrogen evolution. ChemCatChem 2018, 10, 625–631. [Google Scholar] [CrossRef]
- Cui, W.; Cheng, N.; Liu, Q.; Ge, C.; Asiri, A.M.; Sun, X. Mo2C nanoparticles decorated graphitic carbon sheets: Biopolymer-derived solid-state synthesis and application as an efficient electrocatalyst for hydrogen generation. ACS Catal. 2014, 4, 2658–2661. [Google Scholar] [CrossRef]
- Tang, C.; Sun, A.; Xu, Y.; Wu, Z.; Wang, D. High specific surface area Mo2C nanoparticles as an efficient electrocatalyst for hydrogen evolution. J. Power Sources 2015, 296, 18–22. [Google Scholar] [CrossRef]
- Hussain, S.; Zaidi, S.A.; Vikraman, D.; Kim, H.-S.; Jung, J. Facile preparation of molybdenum carbide (Mo2C) nanoparticles and its effective utilization in electrochemical sensing of folic acid via imprinting. Biosens. Bioelectron. 2019, 140, 111330. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Patil, S.A.; Truong, L.; Arbab, A.A.; Jeong, S.H.; Chun, S.-H.; Jung, J.; Kim, H.-S. Engineering MoSe2/WS2 hybrids to replace the scarce platinum electrode for hydrogen evolution reactions and dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2021, 13, 5061–5072. [Google Scholar] [CrossRef]
- Lin, H.; Shi, Z.; He, S.; Yu, X.; Wang, S.; Gao, Q.; Tang, Y. Heteronanowires of MoC–Mo2C as efficient electrocatalysts for hydrogen evolution reaction. Chem. Sci. 2016, 7, 3399–3405. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.-Y.; Lou, X.W.D. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Hussain, S.; Truong, L.; Karuppasamy, K.; Kim, H.-J.; Maiyalagan, T.; Chun, S.-H.; Jung, J.; Kim, H.-S. Fabrication of MoS2/WSe2 heterostructures as electrocatalyst for enhanced hydrogen evolution reaction. Appl. Surf. Sci. 2019, 480, 611–620. [Google Scholar] [CrossRef]
- Wang, X.; Sun, P.; Lu, H.; Tang, K.; Li, Q.; Wang, C.; Mao, Z.; Ali, T.; Yan, C. Aluminum-tailored energy level and morphology ofCo3−xAlxO4 porous nanosheets toward highly efficient electrocatalysts for water oxidation. Small 2019, 15, 1804886. [Google Scholar]
- Wang, X.; Zheng, B.; Yu, B.; Wang, B.; Hou, W.; Zhang, W.; Chen, Y. In situ synthesis of hierarchical MoSe2–CoSe2 nanotubes as an efficient electrocatalyst for the hydrogen evolution reaction in both acidic and alkaline media. J. Mater. Chem. A 2018, 6, 7842–7850. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, B.; Ramadoss, M.; Li, W.; Yang, D.; Wang, B.; Chen, Y. Scalable synthesis of heterogeneous W–W2C nanoparticle-embedded CNT networks for boosted hydrogen evolution reaction in both acidic and alkaline media. ACS Sustain. Chem. Eng. 2019, 7, 10016–10024. [Google Scholar] [CrossRef]
- Wiensch, J.D.; John, J.; Velazquez, J.M.; Torelli, D.A.; Pieterick, A.P.; McDowell, M.T.; Sun, K.; Zhao, X.; Brunschwig, B.S.; Lewis, N.S. Comparative study in acidic and alkaline media of the effects of ph and crystallinity on the hydrogen-evolution reaction on MoS2 and MoSe2. ACS Energy Lett. 2017, 2, 2234–2238. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Han, J.; Zhang, Y.; Zhang, X.; Xu, P.; Yuan, Q.; Samad, L.; Wang, X.; Wang, Y.; Zhang, Z.; et al. Contributions of phase, sulfur vacancies, and edges to the hydrogen evolution reaction catalytic activity of porous molybdenum disulfide nanosheets. J. Am. Chem. Soc. 2016, 138, 7965–7972. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Vikraman, D.; Hussain, M.; Kim, H.-S.; Jung, J. Highly Active Mo2C@WS2 Hybrid Electrode for Enhanced Hydrogen Evolution Reaction. Catalysts 2021, 11, 1060. https://doi.org/10.3390/catal11091060
Hussain S, Vikraman D, Hussain M, Kim H-S, Jung J. Highly Active Mo2C@WS2 Hybrid Electrode for Enhanced Hydrogen Evolution Reaction. Catalysts. 2021; 11(9):1060. https://doi.org/10.3390/catal11091060
Chicago/Turabian StyleHussain, Sajjad, Dhanasekaran Vikraman, Manzoor Hussain, Hyun-Seok Kim, and Jongwan Jung. 2021. "Highly Active Mo2C@WS2 Hybrid Electrode for Enhanced Hydrogen Evolution Reaction" Catalysts 11, no. 9: 1060. https://doi.org/10.3390/catal11091060
APA StyleHussain, S., Vikraman, D., Hussain, M., Kim, H.-S., & Jung, J. (2021). Highly Active Mo2C@WS2 Hybrid Electrode for Enhanced Hydrogen Evolution Reaction. Catalysts, 11(9), 1060. https://doi.org/10.3390/catal11091060






