Titania-Clay Mineral Composites for Environmental Catalysis and Photocatalysis
Abstract
:1. Introduction
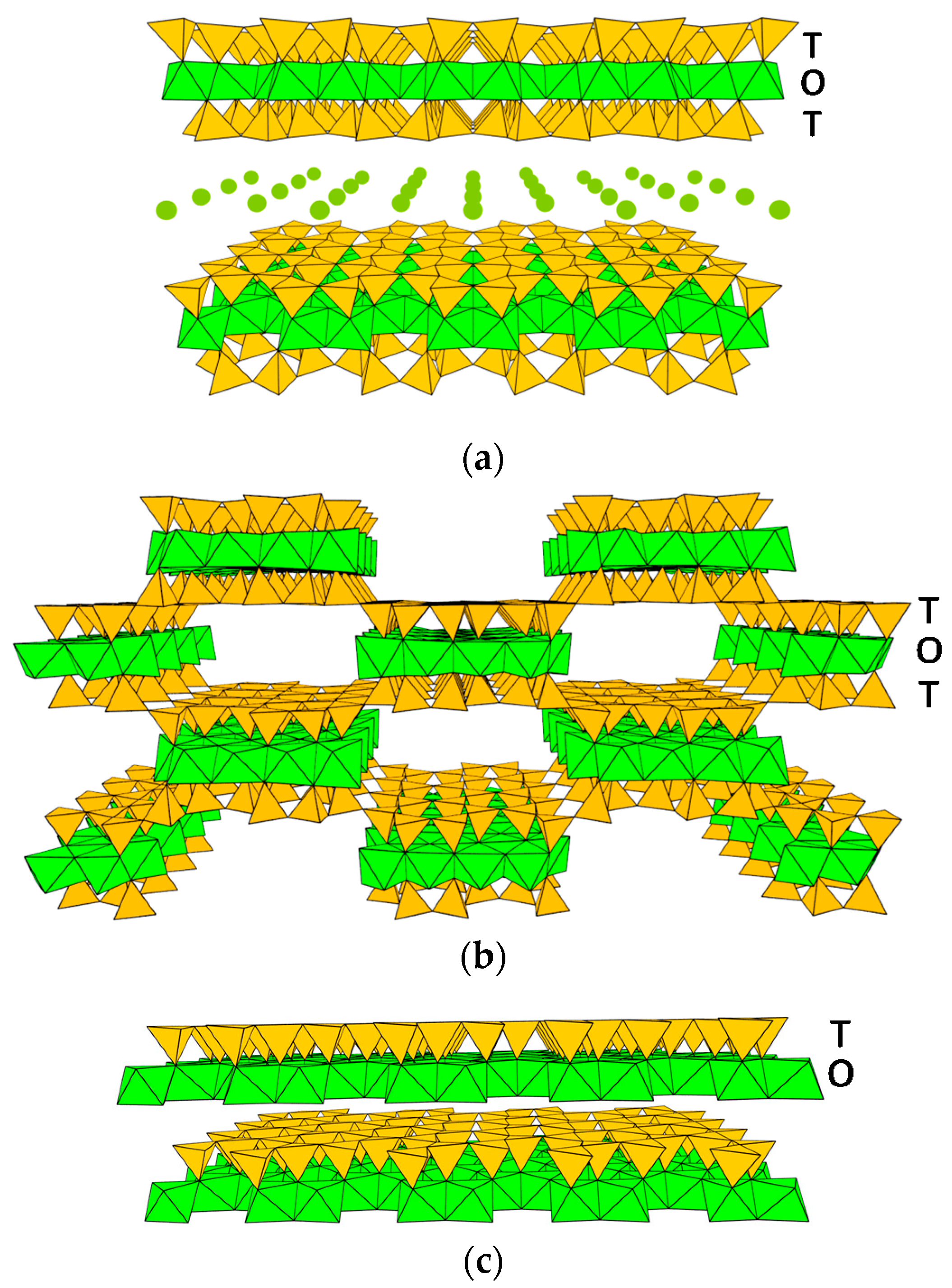
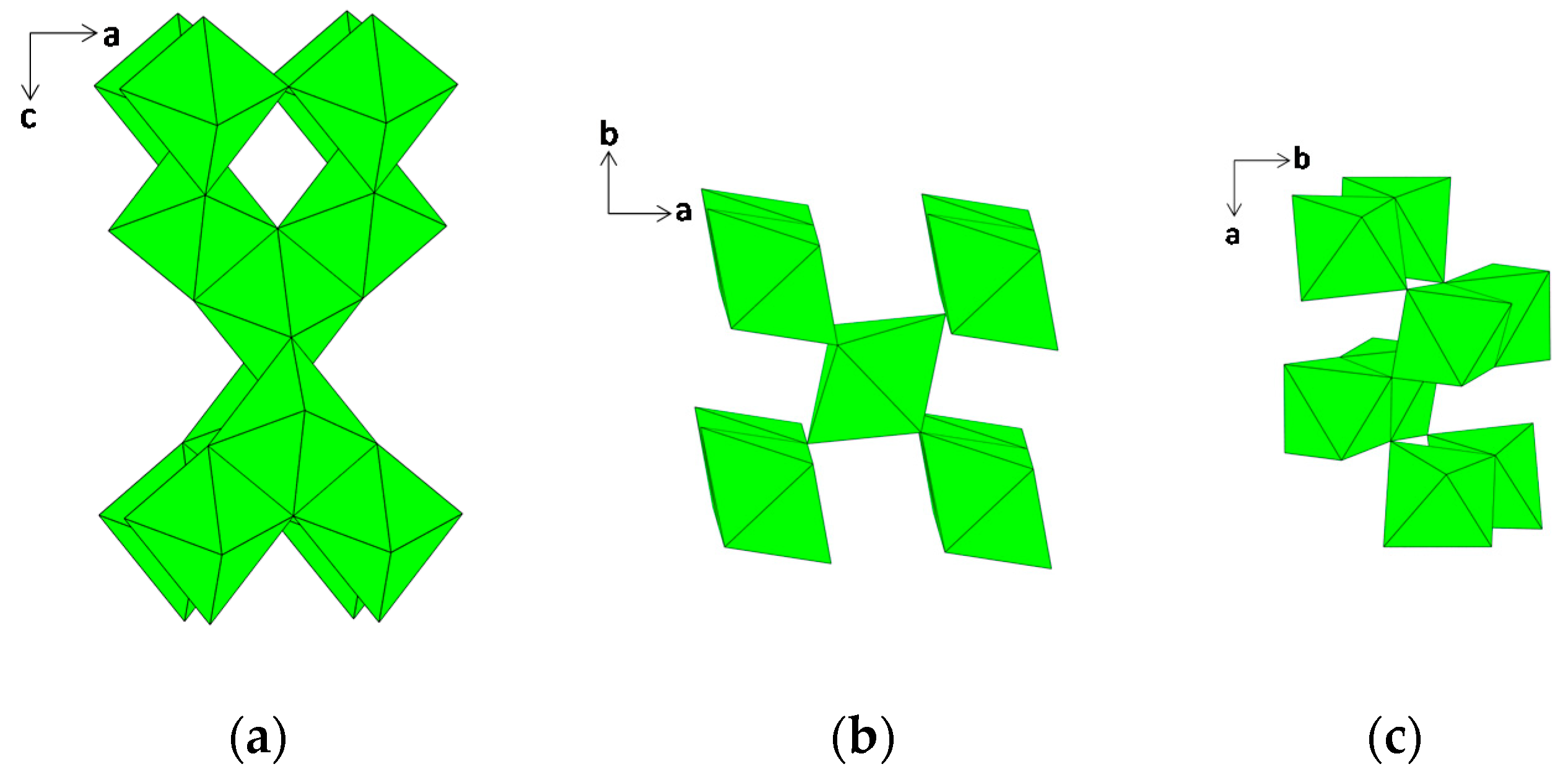
2. Clay Mineral Components
3. Titania Nanoparticles
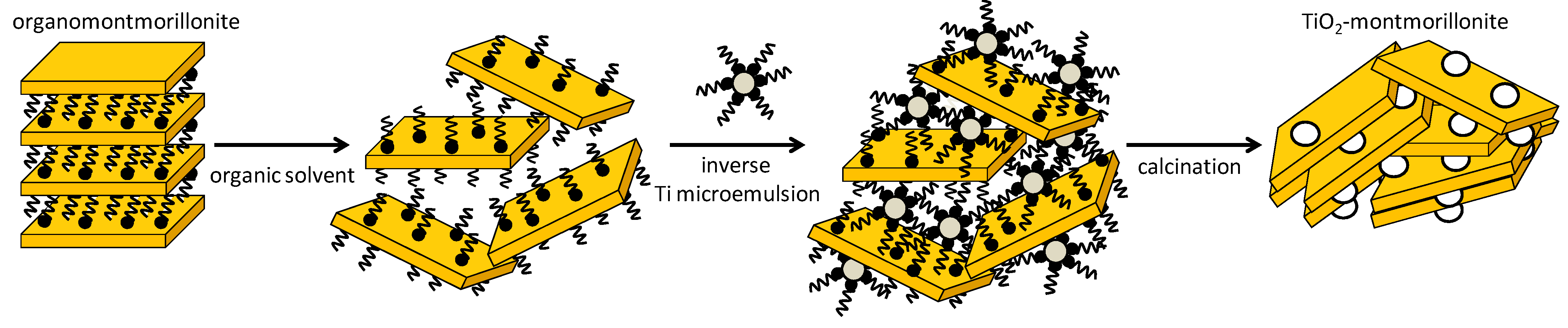
4. TiO2–Clay Mineral Composites
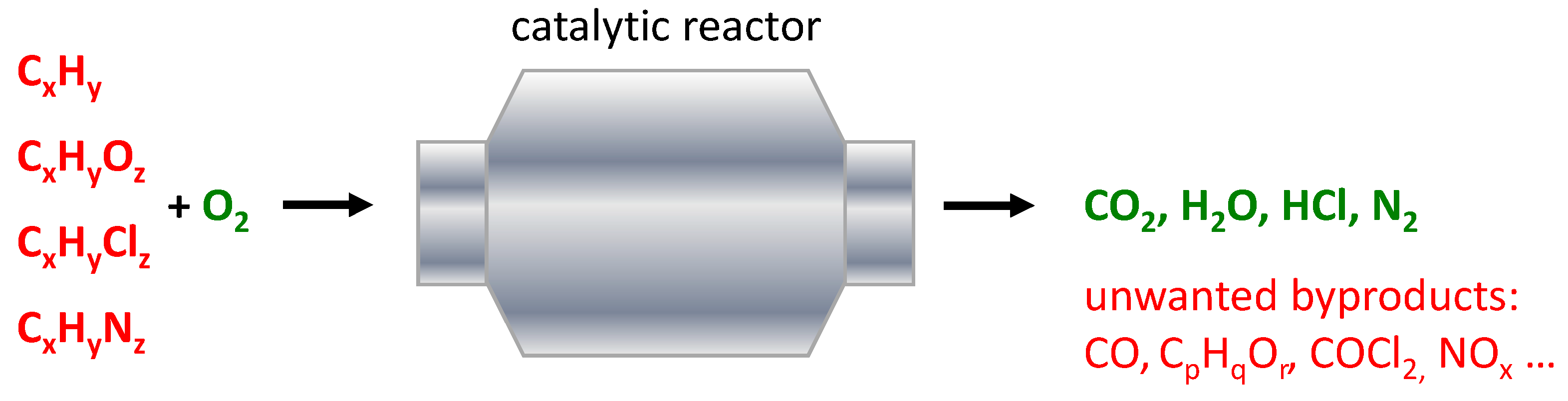
5. TiO2–Clay Mineral Composites for Catalytic Applications
5.1. Selective Catalytic Reduction of Nitrogen Oxides
5.1.1. V–TiO2–Clay Mineral Composites
5.1.2. TiO2-Clay Mineral Composites Doped with Other Transition Metal Elements
5.2. Total Oxidation of Volatile Organic Compounds
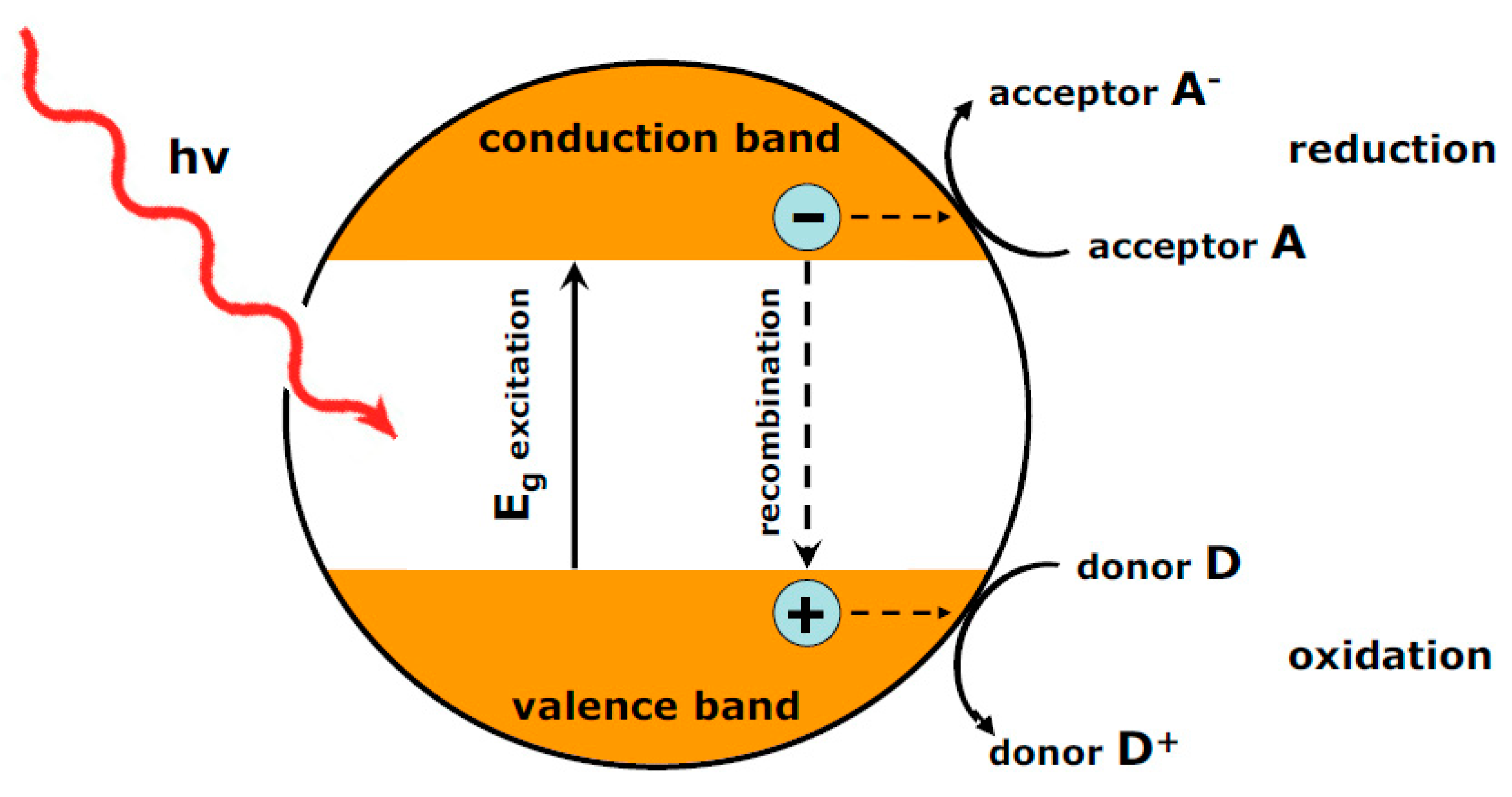
6. TiO2–Clay Mineral Composites for Photocatalytic Applications
6.1. Photodegradation of Organic Pollutants in Water
6.1.1. Photodegradation of Dyes
6.1.2. Photodegradation of Phenolic Compounds
6.1.3. Photodegradation of Emerging Pollutants
6.2. Photodegradation of Air Pollutants
6.2.1. Photodegradation of Volatile Organic Compounds
6.2.2. Photodegradation of Nitrogen Oxides
7. Concluding Remarks
Funding
Conflicts of Interest
References
- Friedrichsen, W.; Goehre, O. Oxidation Catalyst Containing Vanadium and Titanium. GB Patent 1140264, 2 September 1969. [Google Scholar]
- Grzybowska-Swierkosz, B. Vanadia-titania catalysts for oxidation of o-xylene and other hydrocarbons. Appl. Catal. A Gen. 1997, 157, 263–310. [Google Scholar] [CrossRef]
- Matsuda, S.; Takeuchi, M.; Hishinuma, T.; Nakajima, F.; Narita, T.; Watanabe, Y.; Imanari, M. Selective reduction of nitrogen oxides in combustion flue gases. J. Air Pollut. Control Assoc. 1978, 28, 350–353. [Google Scholar] [CrossRef]
- Nakajima, F.; Takeuchi, M.; Matsuda, S.; Uno, S.; Mori, T.; Watanabe, Y.; Imanari, M. Catalytic Process for Reducing Nitrogen oxides to Nitrogen. U.S. Patent 4085193, 18 April 1978. [Google Scholar]
- Matsuda, S.; Kato, A. Titanium oxide based catalysts—A review. Appl. Catal. 1983, 8, 149–165. [Google Scholar] [CrossRef]
- Coudurier, G.; Vedrine, J.C. EUROCAT oxide: An European’ V2O5−WO3/TiO2 SCR standard catalyst study. Characterisation by electron microscopies (SEM, HRTEM, EDX) and by atomic force microscopy. Catal. Today 2000, 56, 415–430. [Google Scholar] [CrossRef]
- Jung, H.; Park, E.; Kim, M.; Jurng, J. Pilot-scale evaluation of a novel TiO2-supported V2O5 catalyst for DeNOx at low temperatures at a waste incinerator. Waste Manag. 2017, 61, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A Review of low temperature NH3-SCR for removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Genet, M.J.; Delmon, B. Low-temperature oxidation of CO over gold supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, B.; Su, D.; Huang, W. Titania Morphology-Dependent Gold-Titania Interaction, Structure, and Catalytic Performance of Gold/Titania Catalysts. ChemCatChem 2015, 7, 3290–3298. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Shehzada, N.; Tahirc, M.; Joharia, K.; Murugesana, T.; Hussain, M.; Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Kavitha, B.; Reddy, P.A.K.; Kim, K.H. TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Klissurski, D.G. Surface chemistry of titania (anatase) and titania-supported catalysts. Chem. Soc. Rev. 1996, 25, 61–69. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef]
- Fernández-García, M.; Rodriguez, J.A. Metal Oxide Nanoparticles. In Encyclopedia of Inorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Myakonkaya, O.; Hu, Z.; Nazar, M.F.; Eastoe, J. Recycling Functional Colloids and Nanoparticles. Chem. Eur. J. 2010, 16, 11784–11790. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Gil, A.; Gandía, L.M.; Vicente, M.A. Recent Advances in the Synthesis and Catalytic Applications of Pillared Clays. Catal. Rev. 2000, 42, 145–212. [Google Scholar] [CrossRef]
- Serwicka, E.M.; Bahranowski, K. Environmental catalysis by tailored materials derived from layered minerals. Catal. Today 2004, 90, 85–92. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Catalysis by layered materials: A review. Microporous Mesoporous Mater. 2008, 107, 3–15. [Google Scholar] [CrossRef]
- Zhou, C.H. An overview on strategies towards clay-based designer catalysts for green and sustainable catalysis. Appl. Clay Sci. 2011, 53, 87–96. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G. Recent advances in synthesis and applications of clay-based photocatalysts: A review. Phys. Chem. Chem. Phys. 2014, 16, 8178–8192. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A Review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Deepracha, S.; Vibulyaseak, K.; Ogawa, M. Complexation of TiO2 with clays and clay minerals for hierarchically designed functional hybrids. In Advanced Supramolecular Nanoarchitectonics, 1st ed.; Ariga, K., Aono, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 125–150. [Google Scholar]
- Ruiz-Hitzky, E.; Aranda, P.; Akkari, M.; Khaorapapong, N.; Ogawa, M. Photoactive nanoarchitectures based on clays incorporating TiO2 and ZnO nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1140–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, A.; Vicente, M.A. Progress and perspectives on pillared clays applied in energetic and environmental remediation processes. Curr. Opin. Green Sustain. Chem. 2020, 21, 56–63. [Google Scholar] [CrossRef]
- Schoonheydt, R.A.; Johnston, C.T.; Bergaya, F. Clay minerals and their surfaces. In Developments in Clay Science; Schoonheydt, R., Johnston, C.T., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 9, pp. 1–21. [Google Scholar]
- Ariga, K.; Aono, M. Introduction: Nanoarchitechtonics for Materials Innovation. In Manipulation of Nanoscale Materials: An Introduction to Nanoarchitectonics; RSC: Cambridge, UK, 2012; pp. 1–3. [Google Scholar]
- Vaughan, D.E.W. Pillared clays—A historical perspective. Catal. Today 1988, 2, 187–198. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. General Introduction: Clays, Clay Minerals, and Clay Science. In Developments in Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5A, pp. 1–19. [Google Scholar]
- Brigatti, M.F.; Galan, E.; Theng, B.K.G. Structure and mineralogy of clay minerals. In Developments in Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5A, pp. 21–81. [Google Scholar]
- Sparks, D.L. Inorganic soil components. In Environmental Soil Chemistry, 2nd ed.; Sparks, D.L., Ed.; Academic Press: Burlington, MA, USA, 2003; pp. 43–73. [Google Scholar]
- Galan, E. Properties and applications of palygorskite-sepiolite clays. Clay Miner. 1996, 31, 443–453. [Google Scholar] [CrossRef]
- Steudel, A.; Emmerich, K. Strategies for the successful preparation of homoionic smectites. Appl. Clay Sci. 2018, 75–76, 13–21. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Sterte, J. Synthesis and properties of titanium oxide cross-linked montmorillonite. Clays Clay Miner. 1986, 34, 658–664. [Google Scholar] [CrossRef]
- Yamanaka, S.; Nishihara, T.; Hattori, M.; Suzuki, Y. Preparation and properties of titania pillared clay. Mater. Chem. Phys. 1987, 17, 87–101. [Google Scholar] [CrossRef]
- Bernier, A.; Admaiai, L.F.; Grange, P. Synthesis and characterization of titanium pillared clay. Influence of the temperature of preparation. Appl. Catal. 1991, 77, 269–281. [Google Scholar] [CrossRef]
- Lin, J.T.; Jong, S.J.; Cheng, S. A new method for preparing microporous titanium pillared clays. Microporous Mater. 1993, 1, 287–290. [Google Scholar] [CrossRef]
- Bahranowski, K.; Dula, R.; Komorek, J.; Romotowski, T.; Serwicka, E.M. Preparation, physicochemical and catalytic properties of vanadium doped alumina and titania-pillared montmorillonites. Stud. Surf. Sci. Catal. 1995, 91, 747–754. [Google Scholar]
- Kooli, F.; Bovey, J.; Jones, W. Dependence of the properties of titanium-pillared clays on the host matrix: A comparison of montmorillonite, saponite and rectorite pillared materials. J. Mater. Chem. 1997, 7, 153–158. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Selective catalytic reduction of NO with ammonia over V2O5 doped TiO2 pillared clay catalysts. Appl.Catal. B-Environ. 2000, 24, 13–21. [Google Scholar] [CrossRef]
- Yuan, P.; Yin, X.; He, H.; Yang, D.; Wang, L.; Zhu, J. Investigation on the delaminated-pillared structure of TiO2-PILC synthesized by TiCl4 hydrolysis method. Microporous Mesoporous Mater. 2006, 93, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Damardji, B.; Khalaf, H.; Duclaux, L.; David, B. Preparation of TiO2-pillared montmorillonite as photocatalyst Part I. Microwave calcination, characterisation, and adsorption of a textile azo dye. Appl. Clay Sci. 2009, 44, 201–205. [Google Scholar] [CrossRef]
- Chmielarz, L.; Piwowarska, Z.; Kuśtrowski, P.; Węgrzyn, A.; Gil, B.; Kowalczyk, A.; Dudek, B.; Dziembaj, R.; Michalik, M. Comparison study of titania pillared interlayered clays and porous clay heterostructures modified with copper and iron as catalysts of the DeNOx process. Appl. Clay Sci. 2011, 53, 164–173. [Google Scholar] [CrossRef]
- Bahranowski, K.; Włodarczyk, W.; Wisła-Walsh, E.; Gaweł, A.; Matusik, J.; Klimek, A.; Gil, B.; Michalik-Zym, A.; Dula, R.; Socha, R.P.; et al. [Ti,Zr]-pillared montmorillonite—A new quality with respect to Ti- and Zr-pillared clays. Microporous Mesoporous Mater. 2015, 202, 155–164. [Google Scholar] [CrossRef]
- Butman, M.F.; Ovchinnikov, N.L.; Karasev, N.S.; Kochkina, N.E.; Agafonov, A.V.; Vinogradov, A.V. Photocatalytic and adsorption properties of TiO2-pillared montmorillonite obtained by hydrothermally activated intercalation of titanium polyhydroxo complexes. Beilstein J. Nanotechnol. 2018, 9, 364–378. [Google Scholar] [CrossRef] [Green Version]
- González, B.; Trujillano, R.; Vicente, M.A.; Rives, V.; Korili, S.A.; Gil, A. Photocatalytic degradation of trimethoprim on doped Ti-pillared montmorillonite. Appl. Clay Sci. 2019, 167, 43–49. [Google Scholar] [CrossRef]
- Chauhan, M.; Saini, V.K.; Suthar, S. Ti-pillared montmorillonite clay for adsorptive removal of amoxicillin, imipramine, diclofenac-sodium, and paracetamol from water. J. Hazard. Mater. 2020, 399, 122832. [Google Scholar] [CrossRef]
- Yoneyama, H.; Haga, S.; Yamanaka, S. Photocatalytic activities of microcrystalline titania incorporated in sheet silicates of clay. J. Phys. Chem. 1989, 93, 4833–4837. [Google Scholar] [CrossRef]
- Del Castillo, H.L.; Grange, P. Preparation and catalytic activity of titanium pillared montmorillonite. Appl. Catal. A-Gen. 1993, 103, 23–34. [Google Scholar] [CrossRef]
- Del Castillo, H.L.; Gil, A.; Grange, P. Influence of the nature of titanium alkoxide and of the acid of hydrolysis in the preparation of titanium-pillared montmorillonites. J. Phys. Chem. Sol. 1997, 58, 1053–1062. [Google Scholar] [CrossRef]
- Ooka, C.; Akita, S.; Ohashi, Y.; Horiuchi, T.; Suzuki, K.; Komai, S.; Yoshida, H.; Hattori, T. Crystallization of hydrothermally treated TiO2 pillars in pillared montmorillonite for improvement of the photocatalytic activity. J. Mater. Chem. 1999, 100, 2943–2952. [Google Scholar] [CrossRef]
- Valverde, J.L.; Sánchez, P.; Dorado, F.; Asencio, I.; Romero, A. Preparation and characterization of Ti-pillared clays using Ti alkoxides. Influence of the synthesis parameters. Clays Clay Miner. 2003, 51, 41–51. [Google Scholar] [CrossRef]
- Ooka, C.; Yoshida, H.; Suzuki, K.; Hattori, T. Highly hydrophobic TiO2 pillared clay for photocatalytic degradation of organic compounds in water. Microporous Mesoporous Mater. 2004, 67, 143–150. [Google Scholar] [CrossRef]
- Sun, S.; Jiang, Y.; Yu, L.; Li, F.; Yang, Z.; Hou, T.; Hu, D.; Xia, M. Enhanced photocatalytic activity of microwave treated TiO2 pillared montmorillonite. Mater. Chem. Phys. 2006, 98, 377–381. [Google Scholar] [CrossRef]
- Rezala, H.; Khalaf, H.; Valverde, J.L.; Romero, A.; Molinari, A.; Maldotti, A. Photocatalysis with Ti-pillared clays for the oxofunctionalization of alkylaromatics by O2. Appl. Catal. A-Gen. 2009, 352, 234–242. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, Q.; Zhou, F.; Deng, X.; Li, F. Synthesis and photocatalytic performances of the TiO2 pillared montmorillonite. J. Hazard. Mater. 2012, 235–236, 186–193. [Google Scholar] [CrossRef]
- Sahel, K.; Bouhent, M.; Belkhadem, F.; Ferchichi, M.; Dappozze, F.; Guillard, C.; Figueras, F. Photocatalytic degradation of anionic and cationic dyes over TiO2 P25, and Ti-pillared clays and Ag-doped Ti-pillared clays. Appl. Clay Sci. 2014, 95, 205–210. [Google Scholar] [CrossRef]
- Kang, L.; Liu, H.; He, H.; Yang, C. Oxidative desulfurization of dibenzothiophene using molybdenum catalyst supported on Ti-pillared montmorillonite and separation of sulfones by filtration. Fuel 2018, 234, 1229–1237. [Google Scholar] [CrossRef]
- Fatimah, I.; Nurillahi, R.; Sahroni, I.; Muraza, O. TiO2-pillared saponite and photosensitization using a ruthenium complex for photocatalytic enhancement of the photodegradation of bromophenol blue. Appl. Clay Sci. 2019, 183, 105302. [Google Scholar] [CrossRef]
- Yoda, S.; Sakurai, Y.; Endo, A.; Miyata, T.; Yanagishita, H.; Otake, K.; Tsuchiya, T. Synthesis of titania pillared montmorillonite via intercalation of titanium alkoxide dissolved in supercritical carbon dioxide. J. Mater. Chem. 2004, 14, 2763–2767. [Google Scholar] [CrossRef]
- Einaga, H. Hydrolysis of titanium(IV) in aqueous (Na,H)Cl solution. J. Chem. Soc. Dalton Trans. 1979, 12, 1917–1919. [Google Scholar] [CrossRef]
- Comba, P.; Merbach, A. The titanyl question revisited. Inorg. Chem. 1987, 26, 1315–1323. [Google Scholar] [CrossRef]
- Vicente, M.A.; Bañares-Muñoz, M.A.; Toranzo, R.; Gandía, L.M.; Gil, A. Influence of the Ti precursor on the properties of Ti-pillared smectites. Clay Miner. 2001, 36, 125–138. [Google Scholar] [CrossRef]
- Binitha, N.N.; Sugunan, S. Preparation, characterization and catalytic activity of titania pillared montmorillonite clays. Microporous Mesoporous Mater. 2006, 93, 82–89. [Google Scholar] [CrossRef]
- Ming-Yuan, H.; Zhonghui, L.; Enze, M. Acidic and hydrocarbon catalytic properties of pillared clay. Catal. Today 1988, 2, 321–338. [Google Scholar] [CrossRef]
- Yang, G.; Lan, X.; Zhuang, J.; Ma, D.; Zhou, L.; Liu, X.; Han, X.; Bao, X. Acidity and defect sites in titanium silicalite catalyst. Appl. Catal. A-Gen. 2008, 337, 58–65. [Google Scholar] [CrossRef]
- Lambert, J.-F.; Poncelet, G. Acidity in pillared clays: Origin and catalytic manifestations. Top. Catal. 1997, 4, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Gil, A.; Korili, S.A.; Vicente, M.A. Recent advances in the control and characterization of the porous structure of pillared clay catalysts. Catal. Rev. 2008, 50, 153–221. [Google Scholar] [CrossRef]
- Gil, A.; Del Castillo, H.L.; Masson, J.; Court, J.; Grange, P. Selective dehydration of 1-phenylethanol to 3-oxa-2,4-diphenylpentane on titanium pillared montmorillonite. J. Mol. Catal. A-Chem. 1996, 107, 185–190. [Google Scholar] [CrossRef]
- Swarnakar, R.; Brandt, K.B.; Kydd, R.A. Catalytic activity of Ti- and Al-pillared montmorillonite and beidellite for cumene cracking and hydrocracking. Appl. Catal. A-Gen. 1996, 142, 61–71. [Google Scholar] [CrossRef]
- Belkhadem, F.; Clacens, J.-M.; Bengueddach, A.; Figueras, F. Acidity and Catalytic Properties for the alkylation of aromatics of PILCs with mixed oxide pillars prepared from two different bentonites. Appl. Catal. A-Gen. 2006, 298, 188–193. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef] [PubMed]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total Environ. 2020, 714, 136712. [Google Scholar] [CrossRef]
- Serwicka, E.M. Clays as catalysts for the removal of nitrogen oxides. Pol. J. Chem. 2001, 75, 307–328. [Google Scholar]
- Grzybek, T. Layered clays as SCR deNOx catalysts. Catal. Today 2007, 119, 125–132. [Google Scholar] [CrossRef]
- Yang, R.; Chen, J.; Kikkinides, E.; Cheng, L.; Cichanowicz, J. Pillared Clays as Superior Catalysts for Selective Catalytic Reduction of Nitric Oxide with Ammonia. Ind. Eng. Chem. Res. 1992, 31, 1440–1445. [Google Scholar] [CrossRef]
- Bahranowski, K.; Janas, J.; Machej, T.; Serwicka, E.M.; Vartikian, L. Vanadium-Doped Titania-Pillared Montmorillonite Clay as a Catalyst for Selective Catalytic Reduction of NO by Ammonia. Clay Miner. 1997, 32, 665–672. [Google Scholar] [CrossRef]
- Bahranowski, K.; Serwicka, E.M. ESR study of vanadium-doped alumina- and titania-pillared montmorillonites. Coll. Surf. 1993, 72, 153–160. [Google Scholar] [CrossRef]
- Bahranowski, K.; Labanowska, M.; Serwicka, E.M. ESR characterization of catalytically active V centres supported on alumina-, titania- and zirconia pillared montmorillonite clay. Appl. Magn. Reson. 1996, 10, 477–490. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Catalytic Performance and Characterization of VO2+-Exchanged Titania-Pillared Clays for Selective Catalytic Reduction of Nitric Oxide with Ammonia. J. Catal. 2000, 196, 73–85. [Google Scholar] [CrossRef]
- Chae, H.J.; Nam, I.S.; Ham, S.W.; Hong, S.B. Characteristics of vanadia on the surface of V2O5/Ti-PILC catalyst for the reduction of NOx by NH3. Appl. Catal. B-Environ. 2004, 5, 117–126. [Google Scholar] [CrossRef]
- Lai, J.-K.; Wachs, I.E. A Perspective on the Selective Catalytic Reduction (SCR) of NO with NH3 by Supported V2O5–WO3/TiO2 Catalysts. ACS Catal. 2018, 8, 6537–6551. [Google Scholar] [CrossRef]
- Zang, S.; Zhang, G.; Qiu, W.; Song, L.; Zhang, R.; He, H. Resistance to SO2 poisoning of V2O5/TiO2-PILC catalyst for the selective catalytic reduction of NO by NH3. Chin. J. Catal. 2016, 37, 888–897. [Google Scholar] [CrossRef]
- Del Castillo, H.L.; Gil, A.; Grange, P. Selective catalytic reduction of NO by NH3 on titanium pillared montmorillonite. Catal. Lett. 1996, 36, 237–239. [Google Scholar] [CrossRef]
- Del Castillo, H.L.; Gil, A.; Grange, P. Preparation and characterization of sulfated titanium-modified pillared montmorillonite. Catal. Lett. 1997, 43, 133–137. [Google Scholar]
- Khalfallah Boudali, L.; Ghorbel, A.; Grange, P. Selective catalytic reduction of NO by NH3 on sulfated titanium-pillared clay. Catal. Lett. 2003, 86, 251–256. [Google Scholar]
- Khalfallah Boudali, L.; Ghorbel, A.; Grange, P.; Figueras, F. Selective catalytic reduction of NO with ammonia over V2O5 supported sulfated titanium-pillared clay catalysts: Influence of V2O5 content. Appl. Catal. B-Environ. 2005, 59, 105–111. [Google Scholar] [CrossRef]
- Khalfallah Boudali, L.; Ghorbel, A.; Grange, P. SCR of NO by NH3 over V2O5 supported sulfated Ti-pillared clay: Reactivity and reducibility of catalysts. Appl. Catal. A-Gen. 2006, 305, 7–14. [Google Scholar] [CrossRef]
- Khalfallah Boudali, L.; Ghorbel, A.; Grange, P. Characterization and reactivity of WO3–V2O5 supported on sulfated titanium pillared clay catalysts for the SCR-NO reaction. C. R. Chim. 2009, 12, 779–786. [Google Scholar] [CrossRef]
- Arfaoui, J.; Boudali, L.K.; Ghorbel, A.; Delahay, G. Effect of vanadium on the behaviour of unsulfated and sulfated Ti-pillared clay catalysts in the SCR of NO by NH3. Catal. Today 2009, 142, 234–238. [Google Scholar] [CrossRef]
- Arfaoui, J.; Boudali, L.K.; Ghorbel, A.; Delahay, G. Influence of the nature of titanium source and of vanadia content on the properties of titanium-pillared montmorillonite. J. Phys. Chem. Sol. 2008, 69, 1121–1124. [Google Scholar] [CrossRef]
- Cheng, J.; Song, Y.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. A mechanistic investigation on the selective catalytic reduction of NO with ammonia over the V-Ce/Ti-PILC catalysts. Mol. Catal. 2018, 445, 111–123. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Li, J.; Zhu, J.; Ma, L. Novel V2O5-CeO2/TiO2 catalyst with low vanadium loading for the selective catalytic reduction of NOx by NH3. Appl. Catal. B-Environ. 2014, 158–159, 11–19. [Google Scholar] [CrossRef]
- Cai, M.; Bian, X.; Xie, F.; Wu, W.; Cen, P. Preparation and Performance of Cerium-Based Catalysts for Selective Catalytic Reduction of Nitrogen Oxides: A Critical Review. Catalysts 2021, 11, 361. [Google Scholar] [CrossRef]
- Cheng, J.; Ye, Q.; Zheng, C.; Cheng, S.; Kang, T.; Dai, H. Effect of ceria loading on Zr-pillared clay catalysts for selective catalytic reduction of NO with NH3. New J. Chem. 2019, 43, 10850–10858. [Google Scholar] [CrossRef]
- Cheng, L.S.; Yang, R.T.; Chen, N. Iron Oxide and Chromia Supported on Titania-Pillared Clay for Selective Catalytic Reduction of Nitric Oxide with Ammonia. J. Catal. 1996, 164, 70–81. [Google Scholar] [CrossRef]
- Yang, R.T.; Li, W.B. Ion-Exchanged Pillared Clays: A New Class of Catalysts for Selective Catalytic Reduction of NO by Hydrocarbons and by Ammonia. J. Catal. 1995, 155, 414–417. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Selective catalytic reduction of nitrogen oxides by ammonia over Fe3+-exchanged TiO2-pillared clay catalysts. J. Catal. 1999, 186, 254–268. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Acid- and base-treated Fe3+-TiO2-pillared clays for selective catalytic reduction of NO by NH3. Catal. Lett. 1999, 59, 39–44. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. FTIR and kinetic studies of the mechanism of Fe3+-exchanged TiO2-pillared clay catalyst for selective catalytic reduction of NO with ammonia. J. Catal. 2000, 190, 22–31. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T.; Zammit, K.D. Superior Pillared Clay Catalysts for Selective Catalytic Reduction of Nitrogen Oxides for Power Plant Emission Control. J. Air Waste Manag. Assoc. 2000, 50, 436–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, R.Q.; Yang, R.T. The promoting role of rare earth oxides on Fe-exchanged TiO2-pillared clay for selective catalytic reduction of nitric oxide by ammonia. Appl. Catal. B-Environ. 2000, 27, 87–95. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Zbroja, M.; Łasocha, W.; Dziembaj, R. Selective reduction of NO with NH3 over pillared clays modified with transition metals. Catal.Today 2004, 90, 43–49. [Google Scholar] [CrossRef]
- Chmielarz, L.; Piwowarska, Z.; Kuśtrowski, P.; Gil, B.; Adamski, A.; Dudek, B.; Michalik, M. Porous clay heterostructures (PCHs) intercalated with silica-titania pillars and modified with transition metals as catalysts for the DeNOx process. Appl. Catal. B-Environ. 2009, 91, 449–459. [Google Scholar] [CrossRef]
- Galarneau, A.; Barodawalla, A.; Pinnavaia, T.J. Porous clay heterostructures formed by gallery-templated synthesis. Nature 1995, 374, 529–531. [Google Scholar] [CrossRef]
- Xu, D.; Wu, W.; Wang, P.; Deng, J.; Yan, T.; Zhang, D. Boosting the Alkali/Heavy Metal Poisoning Resistance for NO Removal by Using Iron-Titanium Pillared Montmorillonite Catalysts. J. Hazard. Mater. 2020, 399, 122947. [Google Scholar] [CrossRef] [PubMed]
- Mrad, R.; Aissat, A.; Cousin, R.; Courcot, D.; Siffert, S. Catalysts for NOx selective catalytic reduction by hydrocarbons (HC-SCR). Appl. Catal. A-Gen. 2015, 504, 542–548. [Google Scholar]
- Yahiro, H.; Iwamoto, M. Copper ion-exchanged zeolite catalysts in deNOx reaction. Appl. Catal. A-Gen. 2001, 222, 163–181. [Google Scholar] [CrossRef]
- Yang, R.T.; Tharappiwattananon, N.; Long, R.Q. Ion-exchanged pillared clays for selective catalytic reduction of NO by ethylene in the presence of oxygen. Appl. Catal. B-Environ. 1998, 19, 289–304. [Google Scholar] [CrossRef]
- Valverde, J.L.; Lucas, A.D.; Sánchez, P.; Dorado, F.; Romero, A. Cation exchanged and impregnated Ti-pillared clays for selective catalytic reduction of NOx by propylene. Appl. Catal. B-Environ. 2003, 43. [Google Scholar] [CrossRef]
- Bahranowski, K.; Dula, R.; Łabanowska, M.; Serwicka, E.M. ESR Study of Cu Centers Supported on Al-, Ti-, and Zr-Pillared Montmorillonite Clays. Appl. Spectrosc. 1996, 50, 1439–1445. [Google Scholar] [CrossRef]
- Bahranowski, K.; Kielski, A.; Serwicka, E.M.; Wisła-Walsh, E.; Wodnicka, K. Influence of doping with copper on the texture of pillared montmorillonite catalysts. Microporous Mesoporous Mater. 2000, 41, 201–215. [Google Scholar] [CrossRef]
- Li, X.; Lu, G.; Qu, Z.; Zhang, D.; Liu, S. The role of titania pillar in copper-ion exchanged titania pillared clays for the selective catalytic reduction of NO by propylene. Appl. Catal. A-Gen. 2011, 398, 82–87. [Google Scholar] [CrossRef]
- Lu, G.; Li, X.; Qu, Z.; Zhao, Q.; Zhao, L.; Chen, G. Copper-ion exchanged Ti-pillared clays for selective catalytic reduction of NO by propylene. Chem. Eng. J. 2011, 168, 1128–1133. [Google Scholar] [CrossRef]
- Dong, S.; Su, Y.; Liu, X.; Li, Q.; Yuan, M.; Zhou, H.; Deng, W. Experimental study on selective catalytic reduction of NO by C3H6 over Fe/Ti-PILC catalysts. J. Fuel Chem. Technol. 2018, 46, 1231–1239. [Google Scholar] [CrossRef]
- Qian, W.; Su, Y.; Yang, X.; Yuan, M.; Deng, W.; Zhao, B. Experimental study on selective catalytic reduction of NO with propene over iron based catalysts supported on aluminum pillared clays. J. Fuel Chem. Technol. 2017, 45, 1499–1507. [Google Scholar] [CrossRef]
- Wen, N.; Dong, S.; Su, Y.; Deng, W.; Zhao, B. Effect of synthesis parameters on catalytic performance of Fe/Ti-PILC catalysts for SCR-C3H6 and in situ DRIFTS study. J. Environ. Chem. Eng. 2020, 8, 104555. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Zbroja, M.; Rafalska-Łasocha, A.; Dudek, B.; Dziembaj, R. SCR of NO by NH3 on alumina or titania-pillared montmorillonite various modified with Cu or Co Part I. General characterization and catalysts screening. Appl. Catal. B-Environ. 2003, 45, 103–116. [Google Scholar] [CrossRef]
- Shen, B.; Yao, Y.; Ma, H.; Liu, T. Ceria Modified MnOx/TiO2-Pillared Clays Catalysts for the Selective Catalytic Reduction of NO with NH3 at Low Temperature. Chin. J. Catal. 2011, 32, 1803–1811. [Google Scholar] [CrossRef]
- Shen, B.X.; Ma, H.; Yao, Y. Mn-CeOx/Ti-PILCs for selective catalytic reduction of NO with NH3 at low temperature. J. Environ. Sci. 2012, 24, 499–506. [Google Scholar] [CrossRef]
- Wang, Z.; Jiao, M.; Chen, Z.; He, H.; Liu, L. Effects of montmorillonite and anatase TiO2 support on CeO2 catalysts during NH3-SCR reaction. Microporous Mesoporous Mater. 2021, 320, 111072. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, W.; Xie, A.; Wu, F.; Yao, C.; Li, X.; Zuo, S.; Liu, T. Effect of MnO2 polymorphs structure on the selective catalytic reduction of NOx with NH3 over TiO2–Palygorskite. Chem. Eng. J. 2016, 286, 291–299. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, G.; Mua, B.; Tang, Z.; Zhang, J. The promoting effect of palygorskite on CeO2-WO3-TiO2 catalyst for the selective catalytic reduction of NOx with NH3. Appl. Clay Sci. 2020, 192, 105641. [Google Scholar] [CrossRef]
- Soni, V.; Singh, P.; Shree, V.; Goel, V. Effects of VOCs on Human Health. In Air Pollution and Control. Energy, Environment, and Sustainability; Sharma, N., Agarwal, A., Eastwood, P., Gupta, T., Singh, A., Eds.; Springer: Singapore, 2018; pp. 119–142. [Google Scholar]
- Gelles, T.; Krishnamurthy, A.; Adebayo, B.; Rownaghi, A.; Rezaei, F. Abatement of Gaseous Volatile Organic Compounds: A Materials Perspective. Catal. Today 2019, 350, 3–18. [Google Scholar] [CrossRef]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B-Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Li, J.; Hu, M.; Zuo, S.; Wang, X. Catalytic combustion of volatile organic compounds on pillared interlayered clay (PILC)-based catalysts. Curr. Opin. Chem. Eng. 2018, 20, 93–98. [Google Scholar] [CrossRef]
- Liang, X.; Qi, F.; Liu, P.; Wei, G.; Su, X.; Ma, L.; He, H.; Lin, X.; Xi, Y.; Zhu, J.; et al. Performance of Ti-pillared montmorillonite supported Fe catalysts for toluene oxidation: The effect of Fe on catalytic activity. Appl. Clay Sci. 2016, 132–133, 96–104. [Google Scholar] [CrossRef]
- Napruszewska, B.; Michalik-Zym, A.; Rogowska, M.; Bielańska, E.; Rojek, W.; Gaweł, A.; Wójcik-Bania, M.; Bahranowski, K.; Serwicka, E.M. Novel Montmorillonite/TiO2/MnAl-Mixed Oxide Composites Prepared from Inverse Microemulsions as Combustion Catalysts. Materials 2017, 10, 1326. [Google Scholar] [CrossRef] [Green Version]
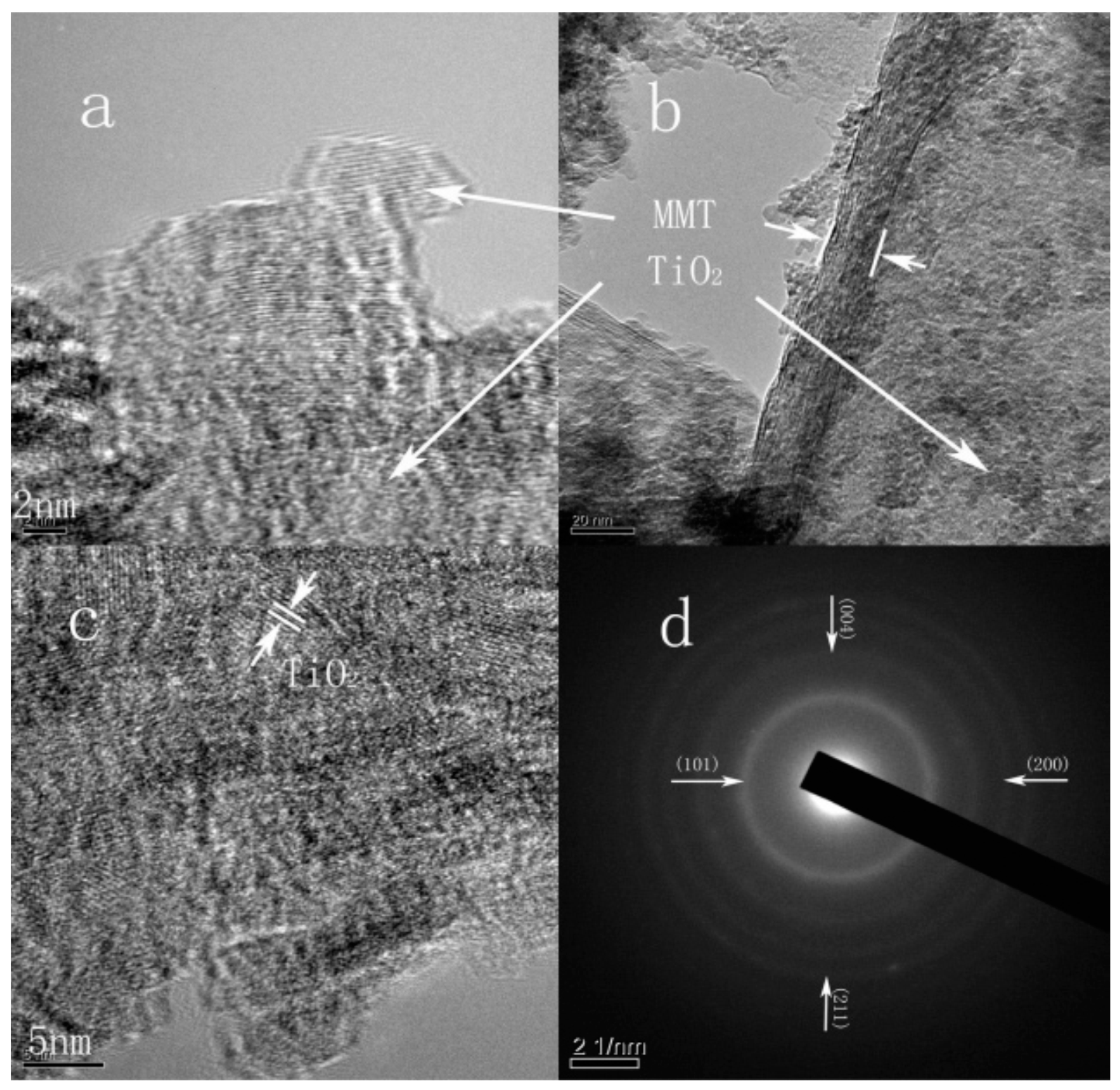
- Bahranowski, K.; Gaweł, A.; Klimek, A.; Michalik-Zym, A.; Napruszewska, B.D.; Nattich-Rak, M.; Rogowska, M.; Serwicka, E.M. Influence of purification method of Na-montmorillonite on textural properties of clay mineral composites with TiO2 nanoparticles. Appl. Clay Sci. 2017, 140, 75–80. [Google Scholar] [CrossRef]
- Michalik-Zym, A.; Dula, R.; Duraczyńska, D.; Kryściak-Czerwenka, J.; Machej, T.; Socha, R.P.; Włodarczyk, W.; Gaweł, A.; Matusik, J.; Bahranowski, K.; et al. Active, selective and robust Pd and/or Cr catalysts supported on Ti-, Zr- or [Ti,Zr]-pillared montmorillonites for destruction of chlorinated volatile organic compounds. Appl. Catal. B-Environ. 2015, 174–175, 293–307. [Google Scholar] [CrossRef]
- Bahranowski, K.; Gaweł, A.; Janik, R.; Komorek, J.; Machej, T.; Michalik, A.; Serwicka, E.M.; Włodarczyk, W. Catalytic Combustion of Trichloroethylene over Pd-Doped Ti-Pillared Montmorillonites. Pol. J. Chem. 2003, 77, 675–682. [Google Scholar]
- Zuo, S.; Ding, M.; Tong, J.; Feng, L.; Qi, C. Study on the preparation and characterization of a titanium-pillared clay-supported CrCe catalyst and its application to the degradation of a low concentration of chlorobenzene. Appl. Clay Sci. 2015, 105–106, 118–123. [Google Scholar] [CrossRef]
- He, X.; Tang, A.; Yang, H.; Ouyang, J. Synthesis and catalytic activity of doped TiO2-palygorskite composites. Appl. Clay Sci. 2011, 53, 80–84. [Google Scholar] [CrossRef]
- Huang, Q.; Zuo, S.; Zhou, R. Catalytic performance of pillared interlayered clays (PILCs) supported CrCe catalysts for deep oxidation of nitrogen-containing VOCs. Appl. Catal. B-Environ. 2010, 95, 327–334. [Google Scholar] [CrossRef]
- Shi, Z.; Huang, Q.; Yang, P.; Zhou, R. The catalytic performance of Ti-PILC supported CrOx–CeO2 catalysts for n-butylamine oxidation. J. Porous Mater. 2015, 22, 739–747. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [Green Version]
- Praneeth, N.V.S.; Paria, S. Clay-semiconductor nanocomposites for photocatalytic applications. In Clay Minerals: Properties, Occurrence and Uses; Sen, T.K., Ed.; Nova Science Publishers: Rourkela, India, 2017; Chapter 5; pp. 144–184. [Google Scholar]
- Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: A review. RSC Adv. 2018, 8, 5197–5211. [Google Scholar] [CrossRef] [Green Version]
- Tanguay, J.F.; Suib, S.L.; Coughlin, R.W. Dichloromethane photodegradation using titanium catalysts. J. Catal. 1989, 117, 335–347. [Google Scholar] [CrossRef]
- Ding, Z.; Zhu, H.Y.; Lu, G.Q.; Greenfield, P.F. Photocatalytic Properties of Titania Pillared Clays by Different Drying Methods. J. Colloid Interface Sci. 1999, 209, 193–199. [Google Scholar] [CrossRef]
- Yoshida, H.; Kawase, T.; Miyashita, Y.; Murata, C.; Ooka, C.; Hattori, T. Effect of Hydrothermal Treatment of Titania-pillared Montmorillonite for Photocatalytic Degradation of Dibutyl Phthalate in Water. Chem. Lett. 1999, 28, 715–716. [Google Scholar] [CrossRef]
- Cheng, S. From layer compounds to catalytic materials. Catal. Today 1999, 49, 303–312. [Google Scholar] [CrossRef]
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Perathoner, S.; Centi, G. Catalytic Wastewater Treatment Using Pillared Clays. In Pillared Clays and Related Catalysts; Gil, A., Korilli, S.A., Trujillano, R., Vicente, M.A., Eds.; Springer: New York, NY, USA, 2010; pp. 167–200. [Google Scholar]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Awate, S.; Suzuki, K. Enhanced Adsorption Capacity and Photo-Catalytic Oxidative Activity of Dyes in Aqueous Medium by Hydrothermally Treated Titania Pillared Clay. Adsorption 2001, 7, 319–326. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Y.; Ke, Q.; Yang, Y.; Yuan, J. Photocatalytic degradation of a cationic azo dye by TiO2/bentonite nanocomposite. J. Photochem. Photobiol. A Chem. 2002, 149, 169–174. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Orthman, J.A.; Li, J.Y.; Zhao, J.C.; Churchman, G.J.; Vansant, E.F. Novel Composites of TiO2(Anatase) and Silicate Nanoparticles. Chem. Mater. 2002, 14, 5037–5044. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhao, J.; Zhu, H.; Orthman, J. Photodegradation of dye pollutants on TiO2 nanoparticles dispersed in silicate under UV–VIS irradiation. Appl. Catal. B-Environ. 2002, 37, 331–338. [Google Scholar] [CrossRef]
- Djellabi, R.; Ghorab, M.F.; Cerrato, G.; Morandi, S.; Gatto, S.; Oldani, V.; Di Michele, A.; Bianchi, C.L. Photoactive TiO2–montmorillonite composite for degradation of organic dyes in water. J. Photochem. Photobiol. A Chem. 2014, 295, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Damardji, B.; Khalaf, H.; Duclaux, L.; David, B. Preparation of TiO2-pillared montmorillonite as photocatalyst Part II. Photocatalytic degradation of a textile azo dye. Appl. Clay Sci. 2009, 45, 98–104. [Google Scholar] [CrossRef]
- Miao, S.; Liu, Z.; Han, B.; Zhang, J.; Yu, X.; Du, J.; Sun, Z. Synthesis and characterization of TiO2–montmorillonite nanocomposites and their application for removal of methylene blue. J. Mater. Chem. 2006, 16, 579–584. [Google Scholar] [CrossRef]
- Liu, S.; Yang, J.H.; Choy, J.H. Microporous SiO2–TiO2 nanosols pillared montmorillonite for photocatalytic decomposition of methyl orange. J. Photochem. Photobiol. A Chem. 2006, 179, 75–80. [Google Scholar] [CrossRef]
- Zhang, G.K.; Ding, X.M.; He, F.S.; Yu, X.Y.; Zhou, J.; Hu, Y.J.; Xie, J.W. Low-temperature synthesis and photocatalytic activity of TiO2 pillared montmorillonite. Langmuir 2008, 24, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, M.; Zuo, S.; Yu, Y. Solvothermal preparation of TiO2/montmorillonite and photocatalytic activity. Appl. Clay Sci. 2009, 43, 156–159. [Google Scholar] [CrossRef]
- Ding, X.; An, T.; Li, G.; Zhang, S.; Chen, J.; Yuan, J.; Zhao, H.; Chen, H.; Sheng, G.; Fu, J. Preparation and characterization of hydrophobic TiO2 pillared clay: The effect of acid hydrolysis catalyst and doped Pt amount on photocatalytic activity. J. Colloid Interface Sci. 2008, 320, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Qian, Z.; Wang, H.; Gao, X.; Zhang, S.; Yang, M. Sol–gel immobilization of SiO2/TiO2 on hydrophobic clay and its removal of methyl orange from water. J. Sol-Gel Sci. Technol. 2008, 46, 195–200. [Google Scholar] [CrossRef]
- Dvininov, E.; Popovici, E.; Pode, R.; Cocheci, L.; Barvinschi, P.; Nica, V. Synthesis and characterization of TiO2-pillared Romanian clay and their application for azoic dyes photodegradation. J. Hazard. Mater. 2009, 167, 1050–1056. [Google Scholar] [CrossRef]
- Lin, J.J.; Cheng, I.J.; Wang, R.; Lee, R.J. Tailoring Basal Spacings of Montmorillonite by Poly(oxyalkylene)diamine Intercalation. Macromolecules 2001, 34, 8832–8834. [Google Scholar] [CrossRef]
- Chen, D.; Du, G.; Zhu, Q.; Zhou, F. Synthesis and characterization of TiO2 pillared montmorillonites: Application for methylene blue degradation. J. Colloid Interface Sci. 2013, 409, 151–157. [Google Scholar] [CrossRef]
- Yang, S.; Liang, G.; Gu, A.; Mao, H. Synthesis of TiO2 pillared montmorillonite with ordered interlayer mesoporous structure and high photocatalytic activity by an intra-gallery templating method. Mater. Res. Bull. 2013, 48, 3948–3954. [Google Scholar] [CrossRef]
- Letaief, S.; Ruiz-Hitzky, E. Silica–clay nanocomposites. Chem. Commun. 2003, 2996–2997. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Rodriguez, J.J. Titania–clay heterostructures with solar photocatalytic applications. Appl. Catal. B-Environ. 2015, 176–177, 278–287. [Google Scholar] [CrossRef]
- Huang, S.; Lu, X.; Li, Z.; Ravishankar, H.; Wang, J.; Wang, X. A biomimetic approach towards the synthesis of TiO2 /carbon-clay as a highly recoverable photocatalyst. J. Photochem. Photobiol. A Chem. 2018, 351, 131–138. [Google Scholar] [CrossRef]
- Huo, M.; Guo, H.; Jiang, Y.; Ju, H.; Xue, B.; Li, F. A facile method of preparing sandwich layered TiO2 in between montmorillonite sheets and its enhanced UV-light photocatalytic activity. J. Photochem. Photobiol. A Chem. 2018, 358, 121–129. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zuo, S.; Yu, Y. Preparation and photocatalytic activity of silver and TiO2 nanoparticles/montmorillonite composites. Appl. Clay Sci. 2007, 37, 275–280. [Google Scholar] [CrossRef]
- Li, J.; Wang, W. A study of photodegradation of sulforhodamine B on Au–TiO2/bentonite under UV and visible light irradiation. Solid State Sci. 2009, 11, 2037–2043. [Google Scholar] [CrossRef]
- Zhang, G.; Ding, X.; Hu, Y.; Huang, B.; Zhang, X.; Qin, X.; Zhou, J.; Xie, J. Photocatalytic degradation of 4BS dye by N, S-codoped TiO2 pillared montmorillonite photocatalysts under visible-light irradiation. J. Phys. Chem. C 2008, 112, 17994–17997. [Google Scholar] [CrossRef]
- Chen, K.; Li, J.; Li, J.; Zhang, Y.; Wang, W. Synthesis and characterization of TiO2–montmorillonites doped with vanadium and/or carbon and their application for the photodegradation of sulphorhodamine B under UV–vis irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2010, 360, 47–56. [Google Scholar] [CrossRef]
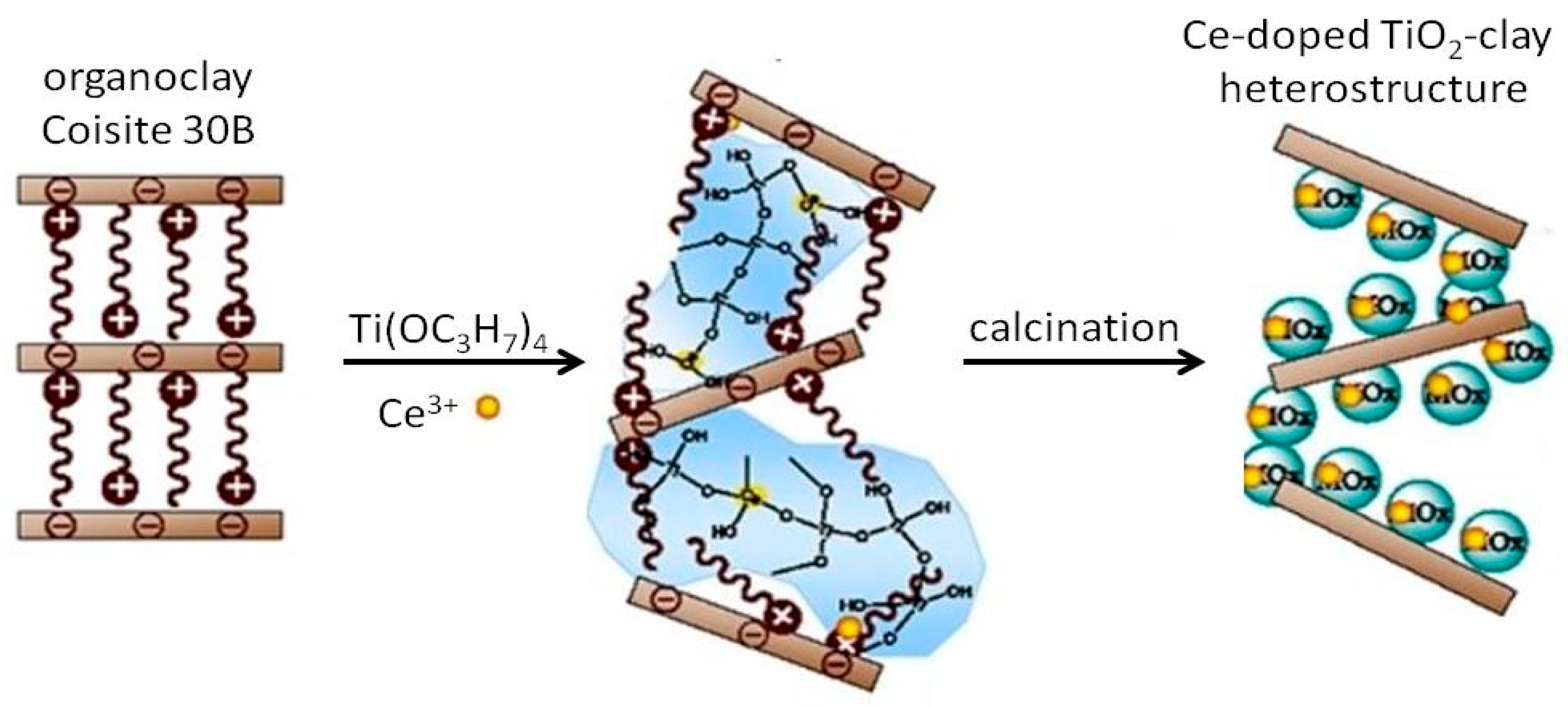
- Belver, C.; Bedia, J.; Álvarez-Montero, M.A.; Rodriguez, J.J. Solar photocatalytic purification of water with Ce-doped TiO2/clay heterostructures. Catal.Today 2016, 266, 36–45. [Google Scholar] [CrossRef]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Santamaria Amato, L.; Maddalena, P. Charge Carrier Processes and Optical Properties in TiO2 and TiO2-Based Heterojunction Photocatalysts: A Review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Kainth, S.; Basu, S. Effect of g-C3N4 loading on TiO2/Bentonite nanocomposites for efficient heterogeneous photocatalytic degradation of industrial dye under visible light. J. Alloys Compd. 2018, 764, 406–415. [Google Scholar] [CrossRef]
- Daniel, L.M.; Frost, R.L.; Zhu, H.Y. Synthesis and characterisation of clay-supported titania photocatalysts. J. Colloid Interface Sci. 2007, 316, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Jia, Y.; Jing, Y.; Sun, J.; Yao, Y. Synthesis and photocatalytic activity of TiO2-hectorite composites. Appl. Clay Sci. 2009, 46, 114–116. [Google Scholar] [CrossRef]
- Deepracha, S.; Bureekaew, S.; Ogawa, M. Synergy effects of the complexation of a titania and a smectite on the film formation and its photocatalyst’ performance. Appl. Clay Sci. 2019, 169, 129–134. [Google Scholar] [CrossRef]
- You, R.; Chen, J.; Hong, M.; Li, J.; Hong, X. Facile Synthesis of g-C3N4/TiO2/Hectorite Z-Scheme Composite and Its Visible Photocatalytic Degradation of Rhodamine B. Materials 2020, 13, 5304. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.C.R.; Torchia, C.B.; Lago, R.M. Floating photocatalysts based on TiO2 supported on high surface area exfoliated vermiculite for water decontamination. Catal. Commun. 2006, 7, 538–541. [Google Scholar] [CrossRef]
- Chong, M.N.; Vimonses, V.; Lei, S.; Jin, B.; Chow, C.; Saint, C. Synthesis and characterisation of novel titania impregnated kaolinite nano-photocatalyst. Microporous Mesoporous Mater. 2009, 117, 233–242. [Google Scholar] [CrossRef]
- Vimonses, V.; Chong, M.N.; Jin, B. Evaluation of the physical properties and photodegradation ability of titania nanocrystalline impregnated onto modified kaolin. Microporous Mesoporous Mater. 2010, 132, 201–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Gan, H.; Zhang, G. A novel mixed-phase TiO2/kaolinite composites and their photocatalytic activity for degradation of organic contaminants. Chem. Eng. J. 2011, 172, 936–943. [Google Scholar] [CrossRef]
- Kutláková, K.M.; Tokarský, J.; Kovář, P.; Vojtěšková, S.; Kovářová, A.; Smetana, B.; Kukutschová, J.; Čapková, P.; Matějka, V. Preparation and characterization of photoactive composite kaolinite/TiO2. J. Hazard. Mater. 2011, 188, 212–220. [Google Scholar] [CrossRef]
- Barbosa, L.V.; Marçal, L.; Nassar, E.J.; Calefi, P.S.; Vicente, M.A.; Trujillano, R.; Rives, V.; Gil, A.; Korili, S.A.; Ciuffi, K.J. Kaolinite-titanium oxide nanocomposites prepared via sol-gel as heterogeneous photocatalysts for dyes degradation. Catal. Today 2015, 246, 133–142. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Sharma, M.; Basu, S. Enhanced heterogeneous photodegradation of VOC and dye using microwave synthesized TiO2/Clay nanocomposites: A comparison study of different type of clays. J. Alloys Compd. 2017, 694, 574–580. [Google Scholar] [CrossRef]
- Wu, A.; Wang, D.; Wei, C.; Zhang, X.; Liu, Z.; Feng, P.; Oua, X.; Qianga, Y.; Garcia, C.; Niu, J. A comparative photocatalytic study of TiO2 loaded on three natural clays with different morphologies. Appl. Clay Sci. 2019, 183, 105352. [Google Scholar] [CrossRef]
- Bel Hadjltaief, H.; Ben Zina, M.; Galvez, M.E.; Da Costa, P. Photocatalytic degradation of methyl green dye in aqueous solution over natural clay-supported ZnO–TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2016, 315, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo-Fierro, X.; González, S.; Jaramillo, H.A.; Medina, F. Synthesis of the ZnTiO3/TiO2 Nanocomposite Supported in Ecuadorian Clays for the Adsorption and Photocatalytic Removal of Methylene Blue Dye. Nanomaterials 2020, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Feng, S.; Yang, Z.; Ding, S. Low-temperature synthesis of heterogeneous crystalline TiO2–halloysite nanotubes and their visible light photocatalytic activity. J. Mater. Chem. A 2013, 1, 8045–8054. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Guo, H.; Ding, S. Low temperature synthesis of polyaniline–crystalline TiO2–halloysite composite nanotubes with enhanced visible light photocatalytic activity. J. Colloid Interface Sci. 2015, 458, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zheng, P. Adsorption and photodegradation of methylene blue on TiO2-halloysite adsorbents. Korean J. Chem. Eng. 2014, 31, 2051–2056. [Google Scholar] [CrossRef]
- Zheng, P.; Du, Y.; Chang, P.R.; Ma, X. Amylose–halloysite–TiO2 composites: Preparation, characterization and photodegradation. Appl. Surf. Sci. 2015, 329, 256–261. [Google Scholar] [CrossRef]
- Aranda, P.; Kun, R.; Martin-Luengo, M.A.; Letaïef, S.; Dékány, I.; Ruiz-Hitzky, E. Titania-sepiolite nanocomposites prepared by a surfactant templating colloidal route. Chem. Mater. 2008, 20, 84–91. [Google Scholar] [CrossRef]
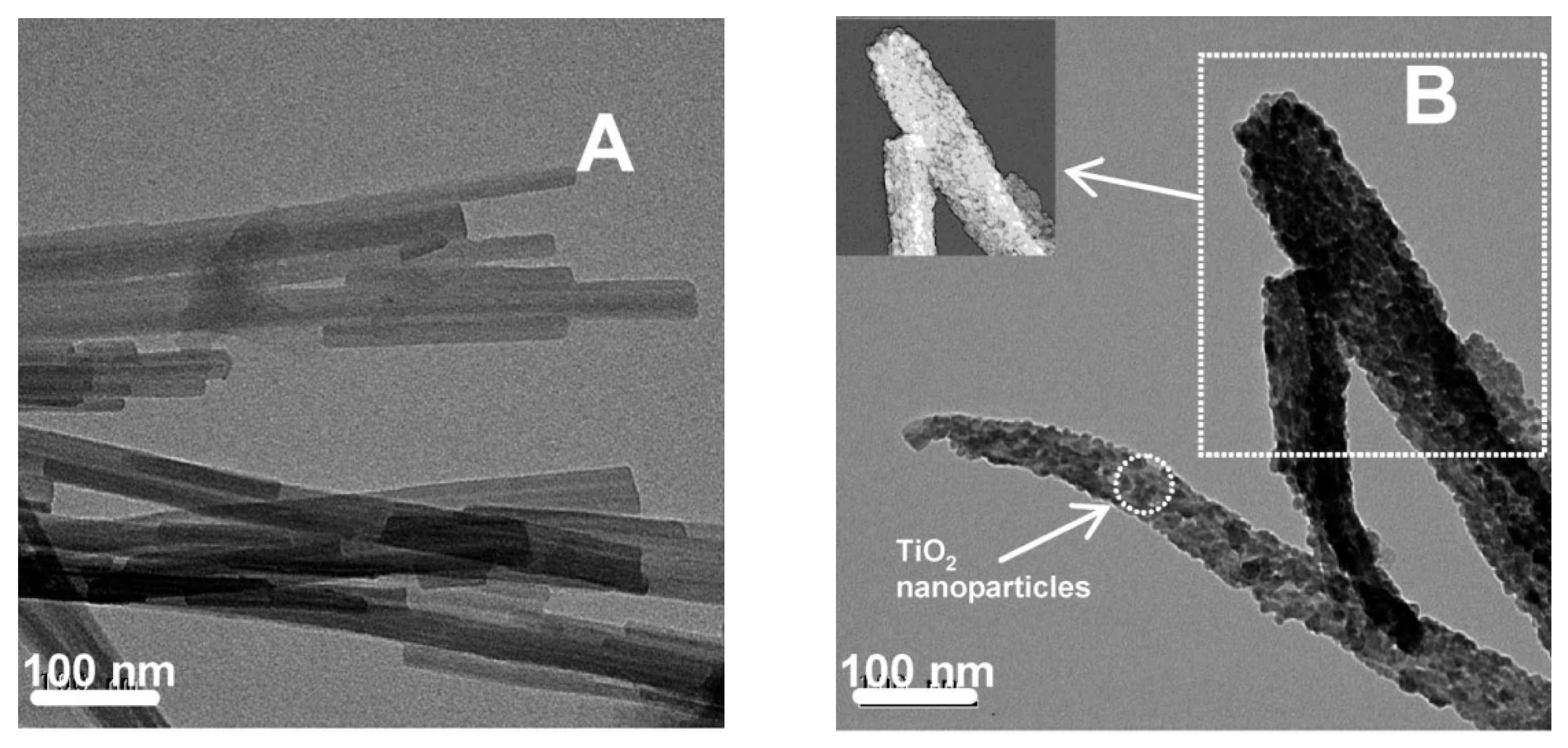
- Bouna, L.; Rhouta, B.; Amjoud, M.; Maury, F.; Lafont, M.C.; Jada, A.; Senocq, F.; Daoudi, L. Synthesis, characterization and photocatalytic activity of TiO2 supported natural palygorskite microfibers. Appl. Clay Sci. 2011, 52, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Stathatos, E.; Papoulis, D.; Aggelopoulos, C.A.; Panagiotaras, D.; Nikolopoulou, A. TiO2/palygorskite composite nanocrystalline films prepared by surfactant templating route: Synergistic effect to the photocatalytic degradation of an azo-dye in water. J. Hazard. Mater. 2012, 211–212, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Zhang, G. Photocatalytic degradation of organic contaminants by TiO2/sepiolite composites prepared at low temperature. Chem. Eng. J. 2011, 173, 1–10. [Google Scholar] [CrossRef]
- Zhou, F.; Yan, C.; Wang, H.; Zhou, S.; Komarneni, S. Sepiolite-TiO2 nanocomposites for photocatalysis: Synthesis by microwave hydrothermal treatment versus calcination. Appl. Clay Sci. 2017, 146, 246–253. [Google Scholar] [CrossRef]
- Zhou, F.; Yan, C.; Liang, T.; Sun, Q.; Wang, H. Photocatalytic degradation of Orange G using sepiolite-TiO2 nanocomposites: Optimization of physicochemical parameters and kinetics studies. Chem. Eng. Sci. 2018, 183, 231–239. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, J.; Liu, N. Characterization of the structure and catalytic activity of copper modified palygorskite/TiO2 (Cu2+-PG/TiO2) catalysts. Mater. Sci. Eng. A 2006, 431, 256–262. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, J.; Liu, N. Surface characteristics and photoactivity of silver-modified palygorskite clays coated with nanosized titanium dioxide particles. Mater. Charact. 2017, 58, 249–255. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, F.; Zhang, W.; Li, R.; Zhong, H.; Zhao, Y.; Zhang, Y.; Wang, X. Photo degradation of methyl orange by attapulgite–SnO2–TiO2 nanocomposites. J. Hazard. Mater. 2009, 171, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Zhou, S.; Chen, H.; Zhao, Y.; Wang, X. Exceptional visible-light-induced photocatalytic activity of attapulgite–BiOBr–TiO2 nanocomposites. Appl. Clay Sci. 2014, 90, 135–140. [Google Scholar] [CrossRef]
- Chen, D.; Du, Y.; Zhu, H.; Deng, Y. Synthesis and characterization of a microfibrous TiO2–CdS/palygorskite nanostructured material with enhanced visible-light photocatalytic activity. Appl. Clay Sci. 2014, 87, 285–291. [Google Scholar] [CrossRef]
- Liu, R.; Ji, Z.; Wang, J.; Zhang, J. Solvothermal synthesized Ag-decorated TiO2/sepiolite composite with enhanced UV–vis and visible light photocatalytic activity. Microporous Mesoporous Mater. 2018, 266, 268–275. [Google Scholar] [CrossRef]
- Kuang, M.; Zhang, J.; Wang, W.; Chen, J.; Cao, Y.; Wang, J.; Ji, Z. Ternary Ag-deposited TiO2/palygorskite composites with synergistic effect for enhanced photocatalytic activity. Solid State Sci. 2019, 97, 106015. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Peng, T.; You, H.; Qin, Y.; Zeng, L. Effects of muscovite matrix on photocatalytic degradation in TiO2/muscovite nanocomposites. Appl. Clay Sci. 2019, 179, 105155. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Peng, T.; You, H.; Zeng, L.; Qin, Y. Preparation and Visible Photocatalytic Properties of N-Doped TiO2/Muscovite Nanocomposites. Clays Clay Miner. 2021, 69, 254–262. [Google Scholar] [CrossRef]
- Bruce, R.M.; Santodonato, J.; Neal, M.W. Summary Review of the Health Effects Associated With Phenol. Toxicol. Ind. Health 1987, 3, 535–568. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Dombi, A.; Mogyorósi, K.; Dékány, I. Photocatalytic water treatment with different TiO2 nanoparticles and hydrophilic/hydrophobic layer silicate adsorbents. Colloids Surf. A Physicochem. Eng. Asp. 2004, 230, 89–97. [Google Scholar] [CrossRef]
- Pichat, P.; Khalaf, H.; Tabet, D.; Houari, M.; Saidi, M. Ti-montmorillonite as photocatalyst to remove 4-chlorophenol in water and methanol in air. Environ. Chem. Lett. 2005, 2, 191–194. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Li, J.Y.; Zhao, J.C.; Churchman, G.J. Photocatalysts prepared from layered clays and titanium hydrate for degradation of organic pollutants in water. Appl. Clay Sci. 2005, 28, 79–88. [Google Scholar] [CrossRef]
- Kun, R.; Mogyorosi, K.; Dékány, I. Synthesis and structural and photocatalytic properties of TiO2/montmorillonite nanocomposites. Appl. Clay Sci. 2006, 32, 99–110. [Google Scholar] [CrossRef]
- Ménesi, J.; Körösi, L.; Bazsó, É.; Zöllmer, V.; Richardt, A.; Dékány, I. Photocatalytic oxidation of organic pollutants on titania–clay composites. Chemosphere 2008, 70, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Li, G.; Nie, X.; An, T.; Zhang, S.; Zhao, H. Synthesis and characterization of novel SiO2 and TiO2 co-pillared montmorillonite composite for adsorption and photocatalytic degradation of hydrophobic organic pollutants in water. Catal. Today 2011, 164, 364–369. [Google Scholar] [CrossRef]
- Manova, E.; Aranda, P.; Angeles Martín-Luengo, M.; Letaïef, S.; Ruiz-Hitzky, E. New titania-clay nanostructured porous materials. Microporous Mesoporous Mater. 2010, 131, 252–260. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, H.; Liu, J.; Gao, X.; Martens, W.N.; Frost, R.L.; Shen, Y.; Yuan, Z. A mesoporous structure for efficient photocatalysts: Anatase nanocrystals attached to leached clay layers. Microporous Mesoporous Mater. 2008, 112, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Xuzhuang, Y.; Yang, D.; Huaiyong, Z.; Jiangwen, L.; Martins, W.N.; Frost, R.; Daniel, L.; Yuenian, S. Mesoporous Structure with Size Controllable Anatase Attached on Silicate Layers for Efficient Photocatalysis. J. Phys. Chem. C 2009, 113, 8243–8248. [Google Scholar] [CrossRef]
- Décsiné Gombos, E.; Krakkó, D.; Záray, G.; Illés, Á.; Dóbé, S.; Szegedi, Á. Laponite immobilized TiO2 catalysts for photocatalytic degradation of phenols. J. Photochem. Photobiol. A: Chem. 2020, 387, 112045. [Google Scholar] [CrossRef]
- Carriazo, J.G.; Moreno-Forero, M.; Molina, R.A.; Moreno, S. Incorporation of titanium and titanium–iron species inside a smectite-type mineral for photocatalysis. Appl. Clay Sci. 2010, 50, 401–408. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Tang, C.; Lv, J.; Zhong, H.; Zhao, Y.; Wang, X. Palygorskite and SnO2–TiO2 for the photodegradation of phenol. Appl. Clay Sci. 2011, 51, 68–73. [Google Scholar]
- Rathi, B.S.; Kumar, P.S.; Show, P.L. A Review on Effective Removal of Emerging Contaminants from Aquatic Systems: Current Trends and Scope for Further Research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef]
- Ooka, C.; Yoshida, H.; Horio, M.; Suzuki, K.; Hattori, T. Adsorptive and photocatalytic performance of TiO2 pillared montmorillonite in degradation of endocrine disruptors having different hydrophobicity. Appl. Catal. B-Environ. 2003, 41, 313–321. [Google Scholar] [CrossRef]
- Sasai, R.; Watanabe, R.; Yamada, T. Preparation and characterization of titania- and organo-pillared clay hybrid photocatalysts capable of oxidizing aqueous bisphenol A under visible light. Appl. Clay Sci. 2014, 93–94, 72–77. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Rodriguez, J.J. Zr-doped TiO2 supported on delaminated clay materials for solar photocatalytic treatment of emerging pollutants. J. Hazard. Mater. 2017, 322, 233–242. [Google Scholar] [CrossRef]
- Tobajas, M.; Belver, C.; Rodriguez, J.J. Degradation of emerging pollutants in water under solar irradiation using novel TiO2-ZnO/clay nanoarchitectures. Chem. Eng. J. 2017, 309, 596–606. [Google Scholar] [CrossRef]
- Belver, C.; Hinojosa, M.; Bedia, J.; Tobajas, M.; Alvarez, M.; Rodríguez-González, V.; Rodriguez, J. Ag-Coated Heterostructures of ZnO-TiO2/Delaminated Montmorillonite as Solar Photocatalysts. Materials 2017, 10, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaizoğullar, A.İ. TiO2/ZnO Supported on Sepiolite: Preparation, Structural Characterization, and Photocatalytic Degradation of Flumequine Antibiotic in Aqueous Solution. Chem. Eng. Commun. 2017, 204, 689–697. [Google Scholar] [CrossRef]
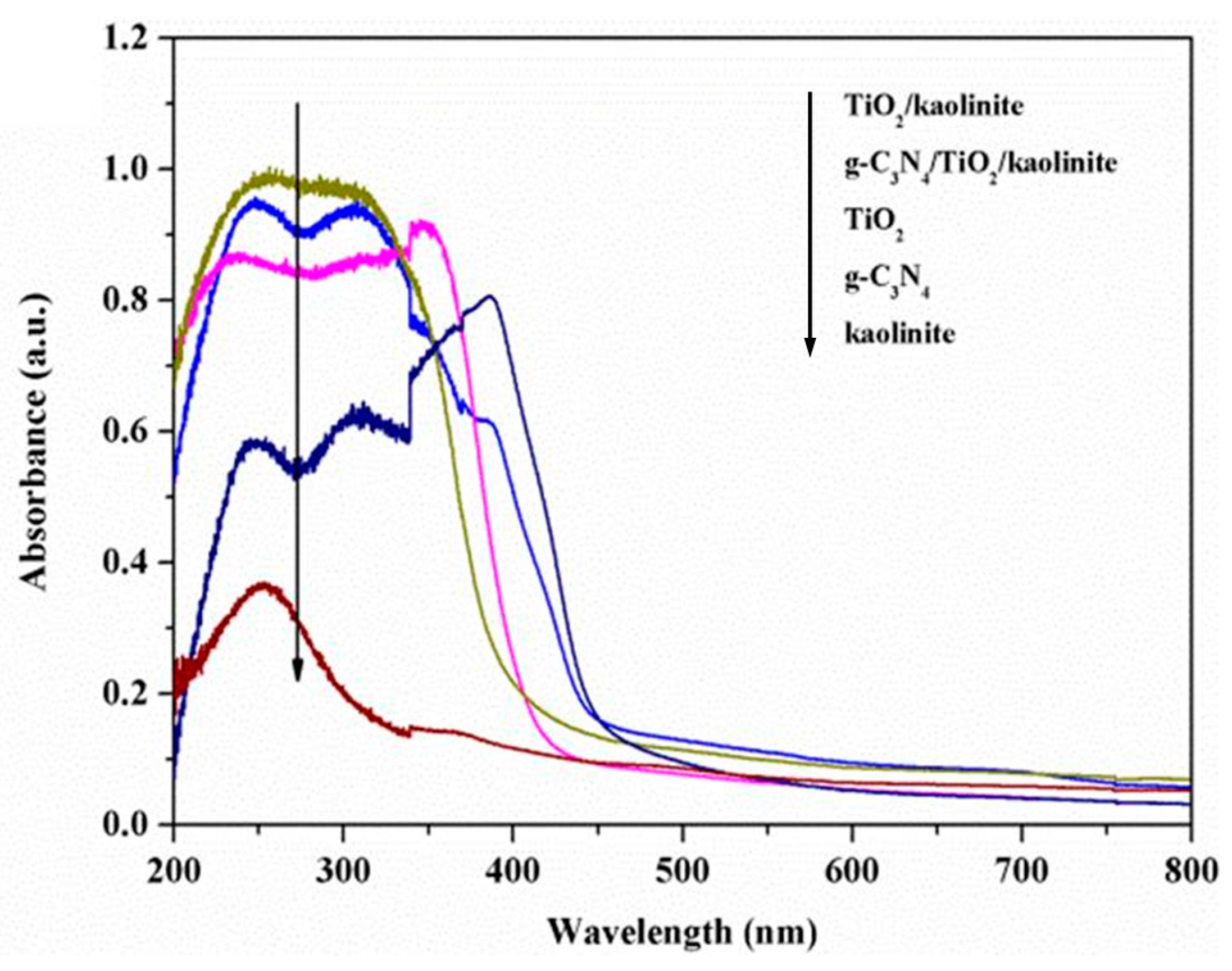
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B-Environ. 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Hu, X.; Sun, Z.; Song, J.; Zhang, G.; Li, C.; Zheng, S. Synthesis of novel ternary heterogeneous BiOCl/TiO2/sepiolite composite with enhanced visible-light-induced photocatalytic activity towards tetracycline. J. Colloid Interface Sci. 2019, 533, 238–250. [Google Scholar] [CrossRef]
- Belessi, V.; Lambropoulou, D.; Konstantinou, I.; Katsoulidis, A.; Pomonis, P.; Petridis, D.; Albanis, T. Structure and photocatalytic performance of TiO2/clay nanocomposites for the degradation of dimethachlor. Appl. Catal. B-Environ. 2007, 73, 292–299. [Google Scholar] [CrossRef]
- Paul, B.; Martens, W.N.; Frost, R.L. Immobilised anatase on clay mineral particles as a photocatalyst for herbicides degradation. Appl. Clay Sci. 2012, 57, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Belver, C.; Han, C.; Rodriguez, J.J.; Dionysiou, D.D. Innovative W-doped titanium dioxide anchored on clay for photocatalytic removal of atrazine. Catal. Today 2017, 280, 21–28. [Google Scholar] [CrossRef]
- Fermoso, J.; Sánchez, B.; Suarez, S. Air purification applications using photocatalysis. In Nanostructured Photocatalysts; Boukherroub, R., Ogale, S.B., Robertson, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 99–128. [Google Scholar]
- Ooka, C. Effect of surface hydrophobicity of TiO2-pillared clay on adsorption and photocatalysis of gaseous molecules in air. Appl. Catal. A-Gen. 2004, 260, 47–53. [Google Scholar] [CrossRef]
- Kibanova, D.; Cervini-Silva, J.; Destaillats, H. Efficiency of Clay−TiO2 Nanocomposites on the Photocatalytic Elimination of a Model Hydrophobic Air Pollutant. Environ. Sci. Technol. 2009, 43, 1500–1506. [Google Scholar] [CrossRef] [Green Version]
- Kibanova, D.; Trejo, M.; Destaillats, H.; Cervini-Silva, J. Synthesis of hectorite-TiO2 and kaolinite-TiO2 nanocomposites with photocatalytic activity for the degradation of model air pollutants. Appl. Clay Sci. 2009, 42, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Kibanova, D.; Sleiman, M.; Cervini-Silva, J.; Destaillats, H. Adsorption and photocatalytic oxidation of formaldehyde on a clay-TiO2 composite. J. Hazard. Mater. 2012, 211, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, M.; Zhou, Y.; Wood, B.; Wang, L.Z.; Rudolph, V.; Lu, G.Q. (Max). Highly Thermostable Anatase Titania-Pillared Clay for the Photocatalytic Degradation of Airborne Styrene. Environ. Sci. Technol. 2009, 43, 538–543. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Stathatos, E.; Toli, D.; Christoforidis, K.C.; Fernández-García, M.; Li, H.; Yin, S.; Sato, T. Halloysite–TiO2 nanocomposites: Synthesis, characterization and photocatalytic activity. Appl. Catal. B-Environ. 2013, 132, 416–422. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Nikolopoulou, A.; Christoforidis, K.; Fernández-Garcia, M.; Li, H.; Shu, Y.; Sato, T. Palygorskite–TiO2 nanocomposites: Part 2. photocatalytic activities in decomposing air and organic pollutants. Appl. Clay Sci. 2013, 83, 198–202. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Stathatos, E.; Christoforidis, K.C.; Fernández-García, M.; Li, H.; Shu, Y.; Sato, T.; Katsuki, H. Three-phase nanocomposites of two nanoclays and TiO2: Synthesis, characterization and photacatalytic activities. Appl. Catal. B Environ. 2014, 147, 526–533. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Zhang, J.; Xie, S.; Wang, X.; Ji, Z. Honeycomb-like micro-mesoporous structure TiO2/sepiolite composite for combined chemisorption and photocatalytic elimination of formaldehyde. Microporous Mesoporous Mater. 2017, 248, 234–245. [Google Scholar] [CrossRef]
- Portela, R.; Jansson, I.; Suárez, S.; Villarroel, M.; Sánchez, B.; Avila, P. Natural silicate-TiO2 hybrids for photocatalytic oxidation of formaldehyde in gas phase. Chem. Eng. J. 2017, 310, 560–570. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, H.; Guo, S.; Wang, J.; Liu, J. Synthesis of Cu/TiO2/organo-attapulgite fiber nanocomposite and its photocatalytic activity for degradation of acetone in air. Appl. Surf. Sci. 2016, 362, 257–264. [Google Scholar] [CrossRef]
- Suárez, S.; Coronado, J.M.; Portela, R.; Martín, J.C.; Yates, M.; Avila, P.; Sánchez, B. On the Preparation of TiO2−Sepiolite Hybrid Materials for the Photocatalytic Degradation of TCE: Influence of TiO2 Distribution in the Mineralization. Environ. Sci. Technol. 2008, 42, 5892–5896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewer, T.L.R.; Suárez, S.; Coronado, J.M.; Portela, R.; Avila, P.; Sanchez, B. Hybrid photocatalysts for the degradation of trichloroethylene in air. Catal. Today 2009, 143, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Suárez, S.; Hewer, T.L.R.; Portela, R.; Hernández-Alonso, M.D.; Freire, R.S.; Sánchez, B. Behaviour of TiO2–SiMgOx hybrid composites on the solar photocatalytic degradation of polluted air. Appl. Catal. B-Environ. 2011, 101, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, G.; He, Z.; An, T. Adsorption and degradation of model volatile organic compounds by a combined titania–montmorillonite–silica photocatalyst. J. Hazard. Mater. 2011, 190, 416–423. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, C.; Lu, J.; Liu, H.; Huang, L.; Chen, T.; Chen, D. Catalytic degradation of gaseous benzene by using TiO2/goethite immobilized on palygorskite: Preparation, characterization and mechanism. Solid State Sci. 2015, 49, 1–9. [Google Scholar] [CrossRef]
- Russell, H.S.; Frederickson, L.B.; Hertel, O.; Ellerman, T.; Jensen, S.S. A Review of Photocatalytic Materials for Urban NOx Remediation. Catalysts 2021, 11, 675. [Google Scholar] [CrossRef]
- Nikolopoulou, A.; Papoulis, D.; Komarneni, S.; Tsolis-Katagas, P.; Panagiotaras, D.; Kacandes, G.H.; Zhang, P.; Yin, S.; Sato, T. Solvothermal preparation of TiO2/saponite nanocomposites and photocatalytic activity. Appl. Clay Sci. 2009, 46, 363–368. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Nikolopoulou, A.; Tsolis-Katagas, P.; Panagiotaras, D.; Kacandes, H.G.; Zhang, P.; Yin, S.; Sato, T.; Katsuki, H. Palygorskite- and Halloysite-TiO2 nanocomposites: Synthesis and photocatalytic activity. Appl. Clay Sci. 2010, 50, 118–124. [Google Scholar] [CrossRef]
- Papoulis, D.; Somalakidi, K.; Todorova, N.; Trapalis, C.; Panagiotaras, D.; Sygkridou, D.; Stathos, E.; Gianni, E.; Mavrikos, A.; Komarneni, S. Sepiolite/TiO2 and metal ion modified sepiolite/TiO2 nanocomposites: Synthesis, characterization and photocatalytic activity in abatement of NOx gases. Appl. Clay Sci. 2019, 179, 105156. [Google Scholar] [CrossRef]
- Todorova, N.; Giannakopoulou, T.; Karapati, S.; Petridis, D.; Vaimakis, T.; Trapalis, C. Composite TiO2/clays materials for photocatalytic NOx oxidation. Appl. Surf. Sci. 2014, 319, 113–120. [Google Scholar]
- Bloh, J.Z.; Folli, A.; Macphee, D.E. Photocatalytic NOx abatement: Why the selectivity matters. RSC Adv. 2014, 4, 45726–45734. [Google Scholar] [CrossRef]






| TiO2–Clay Mineral Composite | Synthesis | Reaction Conditions | Maximum NO Conversion/Selectivity to N2 | Ref. |
|---|---|---|---|---|
| V/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 400 °C, Ti-PILC SSA = 346 m2g−1; V-doping by cation exchange or co-pillaring, calcination 400 °C; best catalyst 3.4 wt % V by cation exchange | 200–450 °C, NO = NH3 = l000 ppm, O2 = 2%; balance = He; GHSV = 10,000 h−1 | 100% conversion at 275 °C, 100% selectivity (for the best catalyst) | [87] |
| V/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 350 °C, Ti-PILC SSA = 310 m2g−1; V-doping by cation exchange, calcination 400 °C; best catalyst 3.5 wt % V | 200–400 °C, NO = NH3 = l000 ppm, O2 = 2%; 1000 ppm SO2 (when used), 8% H2O vapor (when used); balance = He; GHSV = 75,000 h−1 | 94.5% conversion at 375 °C, 98.8% selectivity, negligible inhibition by SO2 and H2O (for the best catalyst) | [90] |
| V/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 350 °C, Ti-PILC SSA = 310 m2g−1; V-doping by impregnation, calcination 500 °C; best catalyst 4.4 wt % V2O5 | 200–450 °C, NO = NH3 = l000 ppm, O2 = 2%; 1000 ppm SO2 (when used), 8% H2O vapor (when used); balance = He; GHSV = 75,000 h−1 | 93.0% conversion at 400 °C, 98.9% selectivity (for the best catalyst); slight improvement upon exposure to SO2 and H2O and co-doping with W | [91] |
| V/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 400 °C, Ti-PILC SSA = 223 m2g−1; V-doping by impregnation, calcination 500 °C; best catalyst 3.47 wt % V2O5 | 120–480 °C, NO = NH3 = l000 ppm, O2 = 8%; 500 ppm SO2 (when used), 10% H2O vapor (when used); balance = He; GHSV = 75,000 h−1 | ca. 99.0% conversion at 260 °C, ca.98% selectivity, (for the best catalyst); moderate loss of activity upon time-on-stream exposure to SO2 and H2O | [93] |
| V,Ce/Ti-pillared Mt | Ti(OC4H9)4 precursor + Mt, acid treated Mt, calcination 400 °C, V,Ce-doping by sequential impregnation, calcination 400 °C; best catalyst 1 wt % V + 4 wt % Ce | 100–450 °C, NO = 1000 ppm, NH3 = l100 ppm, O2 = 4%; 5% H2O; balance = N2; GHSV = 50,000 h−1 | 97% conversion at 370 °C | [102] |
| S,Ti-pillared Mt | TiCl4 precursor + Mt, H2SO4 as sulfating agent, S,Ti-PILC SSA = 152 m2g−1; | 100–400 °C, NO = NH3 = l000 ppm, O2 = 2.5%; balance = He; GHSV = 46,000 h−1 | 91.6% conversion at 400 °C, 71% selectivity | [97] |
| V/S,Ti-pillared Mt | TiCl4 precursor + Mt, H2SO4 as sulfating agent, S,Ti-PILC SSA = 152 m2g−1; V-doping by impregnation, calcination 400 °C; best catalyst 3.0 wt % V, SSA = 131 m2g−1 | 100–400 °C, NO = NH3 = l000 ppm; O2 = 2.5%; balance = He; GHSV = 46,000 h−1 | 99.9% conversion at 300 °C, 79% selectivity (for the best catalyst) | [97] |
| Fe/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 350 °C, Ti-PILC SSA = 310 m2g−1; Fe-doping by CE, calcination 400 °C; best catalyst 5.9% wt % Fe, SSA = 245 m2g−1 | 300–450 °C, NO = NH3 = l000 ppm, O2 = 2%; 1000 ppm SO2 (when used), 8% H2O vapor (when used); balance = N2; GHSV = 113,000 h−1 | 92.5% conversion at 400 °C, 99.5% selectivity (for the best catalyst), activity enhancement (98%) upon exposure to SO2 and H2O | [108] |
| Fe/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 400 °C, Ti-PILC SSA = 212 m2g−1; Fe-doping by CE, calcination 550 °C, 8.90 wt % Fe2O3, SSA = 195 m2g−1 | 100–550 °C, NO = NH3 = 2500 ppm, O2 = 2.5%; balance = He; 0.1 g catalyst, 40 mL/min−1 flow rate | 98% conversion at 450 °C, 94% selectivity | [53] |
| Fe,Cr/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 300 °C, Ti-PILC SSA = 308 m2g−1; Fe,Cr-doping by impregnation, calcination 400 °C; best catalyst 7.5% wt % Fe2O3 + 2.5% wt % Cr2O3 | 175–425 °C, NO = NH3 = l000 ppm, O2 = 2%; 1000 ppm SO2 (when used), 8% H2O vapor (when used); balance = N2; GHSV = 60,000 h−1 | 99% conversion at 375 °C, >99% selectivity (for the best catalyst), ca. 40% activity loss upon exposure to SO2 and H2O | [106] |
| Fe,Ce/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 350 °C, Ti-PILC SSA = 310 m2g−1; Ce and Fe-doping by sequential CE, calcination 400 °C; 5.45 wt % Fe, 0.51 wt % Ce | 300–450 °C, NO = NH3 = l000 ppm, O2 = 2%; 1000 ppm SO2 (when used), 8% H2O vapor (when used); balance = N2; GHSV = 226,000 h−1 | 91.5% conversion at 400 °C, 100% selectivity (for the best catalyst), activity enhancement (96%) upon exposure to SO2 and H2O | [108] |
| Cu/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 400 °C, Ti-PILC SSA = 212 m2g−1; Cu-doping by CE, calcination 550 °C, 1.51 wt % CuO, SSA = 198 m2g−1 | 100–550 °C, NO = NH3 = 2500 ppm, O2 = 2.5%; balance = He; 0.1 g catalyst, 40 mL/min−1 flow rate | 93% conversion at 500 °C, 94% selectivity | [53] |
| Co/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 350 °C, Ti-PILC SSA = 279 m2g−1; Co-doping by CE, calcination 350 °C, best catalyst SSA = 296 m2g−1 | 75–475 °C, NO = NH3 = 2500 ppm, O2 = 2.5%; balance = He; 0.1 g catalyst, 40 mL/min−1 flow rate | 100% conversion at 450 °C, 99% selectivity | [128] |
| Mn/Ti-pillared Mt | Ti(OC4H9)4 precursor + Mt, drying 80 °C, Mn-doping by impregnation, calcination 550 °C, best catalyst 8 wt % Mn, SSA = 134 m2g−1 | 80–240 °C, NO = NH3 = 600 ppm; O2 = 3%; balance = N2; GHSV = 50,000 h−1 | 100% conversion at 240 °C | [129] |
| Fe/Ti,Si-PCH | HDTMA-Mt + Si(OC2H5)4 + Ti(OC3H7)4, calcination 600 °C, Fe-doping by CE, calcination 600 °C; best catalyst 7.61 wt % Fe2O3, SSA = 525 m2g−1 | 100–550 °C, NO = NH3 = 2500 ppm, O2 = 2.5%; 2000 ppm SO2 (when used), 5% H2O vapor (when used); balance = He; 0.1 g catalyst, 40 mL/min−1 flow rate | 100% conversion at 400 °C, 98% selectivity (for the best catalyst), slight deactivation upon exposure to SO2 and H2O | [114] |
| Cu/Ti,Si-PCH | HDTMA-Mt + Si(OC2H5)4 + Ti(OC3H7)4, calcination 600 °C, Cu-doping by CE, calcination 600 °C; best catalyst 1.97 wt % CuO, SSA = 541 m2g−1 | 100–550 °C, NO = NH3 = 2500 ppm; O2 = 2.5%; 2000 ppm SO2 (when used), 5% H2O vapor (when used) balance = He; 0.1 g catalyst, 40 mL/min−1 flow rate | 97% conversion at 500 °C, 90% selectivity (for the best catalyst), slight deactivation upon exposure to SO2 and H2O | [114] |
| Ce,Ti/Mt | Ti(OC4H9)4 precursor + Ce precursor + Mt, sol–gel, calcination 400 °C; disordered structure, best catalyst 10.27 wt % CeO2, SSA = 109 m2g−1 | 100–400 °C, NO = NH3 = 500 ppm; O2 = 3%; balance = N2; GHSV = 100,000 h−1 | ca. 95% conversion in 260–475 °C range (for the best catalyst), selectivity not discussed | [131] |
| Mn/TiO2/Pal | Pal slurry + TiO2 from TiCl4 precursor (2:1 mass ratio) + in situ synthesized γ-MnO2; best catalyst 5 wt % MnO2, calcination 300 °C | 50–400 °C, NO = NH3 = 1000 ppm; O2 = 3%; balance = N2; GHSV = 25,000 h−1 | 98% conversion at 300 °C, 95% selectivity (for the best catalyst) | [132] |
| Ce,W/TiO2/Pal | TiO2 + Pal + Ce,V impregnating solution, drying 110 °C, calcination 500 °C; best catalyst 10 wt % CeO2, 5 wt % WO3, 20 wt % Pal, SSA = 102 m2g−1 | 50–400 °C, NO = NH3 = 500 ppm; O2 = 5%; balance = N2; GHSV = 30,000 h−1 | 98.4% conversion at 360 °C | [133] |
| Tio2–Clay MineralComposite | Synthesis | Reaction Conditions | Maximum NO Conversion/Selectivity to N2 | Ref. |
|---|---|---|---|---|
| Cu/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 300 °C, Ti-PILC SSA = 383 m2g−1; Cu-doping by CE or impregnation, calcination 400 °C; best catalyst CE 5.9 wt % Cu, SSA = 346 m2g−1 | 250–500 °C, NO = C2H4 = l000 ppm, O2 = 2%; 500 ppm SO2 (when used), 5% H2O vapor (when used), balance = He; 0.5 g catalyst, flow rate 250 mL/min−1 | 55% conversion at 300 °C (for the best catalyst), slight inhibition by SO2 and H2O | [119] |
| Ga/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 300 °C, Ti-PILC SSA = 383 m2g−1; Ga-doping by CE, calcination 400 °C, 5.6 wt % Ga | 250–500 °C, NO = C2H4 = l000 ppm, O2 = 2%; balance = He; 0.5 g catalyst, flow rate 250 mL/min−1 | 37% conversion at 450 °C | [119] |
| Fe/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 300 °C, Ti-PILC SSA = 383 m2g−1; Fe-doping by CE, calcination 400 °C, 6.8 wt % Ga | 250–500 °C, NO = C2H4 = l000 ppm, O2 = 2%; balance = He; 0.5 g catalyst, flow rate 250 mL/min−1 | 31% conversion at 450 °C | [119] |
| Cu/Ti-pillared Mt | Ti(OCH3)4 precursor + Mt, calcination 500 °C, Ti-PILC SSA = 295 m2g−1; Cu-doping by CE or impregnation, calcination 400 °C; best catalyst CE 5.9 wt % Cu, SSA = 234 m2g−1 | 220–450 °C, NO = C3H6 = l000 ppm; O2 = 5%; 5% H2O vapor (when used); balance = He; GHSV = 15,000 h−1 | 55% conversion at 260 °C (for the best catalyst) | [120] |
| Fe/Ti-pillared Mt | Ti(OCH3)4 precursor + Mt, calcination 500 °C, Ti-PILC SSA = 295 m2g−1; Fe-doping by CE, calcination 400 °C; best catalyst 8 wt % Fe, SSA = 218 m2g−1 | 220–450 °C, NO = C3H6 = l000 ppm; O2 = 5%; balance = He; GHSV = 15,000 h−1 | 31% conversion at 300 °C (for the best catalyst) | [120] |
| Ni/Ti-pillared Mt | Ti(OCH3)4 precursor + Mt, calcination 500 °C, Ti-PILC SSA = 295 m2g−1; Ni-doping by CE, calcination 400 °C; best catalyst 3.4 wt % Ni, SSA = 236 m2g−1 | 220–450 °C, NO = C3H6 = l000 ppm; O2 = 5%; balance = He; GHSV = 15,000 h−1 | 30% conversion at 400 °C (for the best catalyst) | [120] |
| Fe/Ti-pillared Mt | TiCl4 precursor + Mt calcination 500 °C, Ti-PILC SSA = 203 m2g−1; Fe-doping by CE, calcination 500 °C; best catalyst 19.2 wt % Fe, SSA = 190 m2g−1 | 150–600 °C, NO = C3H6 = l000 ppm; O2 = 1%; 200 ppm SO2 (when used), 10% H2O (when used); balance = He; GHSV = 12,000 h−1 | 100% conversion at 400 °C, (for the best catalyst), 95% selectivity; slight inhibiton by SO2 and H2O | [125] |
| TiO2–Clay Mineral Composite | Synthesis | Reaction Conditions | TX | VOC | Ref. |
|---|---|---|---|---|---|
| Fe/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 500 °C, SSA = 230 m2g−1; Fe-doping by CE, calcination 500 °C; best catalyst 3.8% Fe2O3 wt %, SSA = 185 m2g−1 | 250–450 °C, toluene = l000 ppm, air; 0.1 g catalyst, flow rate 100 mL/min−1 | T90 = 347 °C (for the best catalyst) | Toluene | [138] |
| Mn/TiO2/Mt | HDTMA-Mt + TiO2 IM + MnAl Ht IM, calcination 450 and 600 °C, best catalyst calcination 450 °C, 17.5 wt % MnO, SSA = 178 m2g−1 | 100–400 °C, toluene = 500 ppm, air; GHSV = 10,000 h−1 | T90 = 239 °C (for the best catalyst) | Toluene | [139] |
| Pd,Cr/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 400 °C, Ti-PILC SSA = 379 m2g−1; Pd,Cr-doping by impregnation, calcination 500 °C, best catalyst 1 wt % Pd, SSA = 392 m2g−1 | 200–550 °C, DCM = 530 ppm, air; 10% H2O vapor; GHSV = 10,000 h−1 | T90 = 339 °C (for the best catalyst) | Dichloromethane (DCM) | [141] |
| Pd,Cr/Ti,Zr-pillared Mt | TiCl4 +ZrOCl2 precursor + Mt, calcination 400 °C, Ti,Zr-PILC SSA = 379 m2g−1; Pd,Cr-doping by impregnation, calcination 500 °C, best catalyst 1 wt % Pd, SSA = 342 m2g−1 | 200–550 °C, DCM = 530 ppm, air; 10% H2O vapor; GHSV = 10,000 h−1 | T90 = 395 °C (for the best catalyst) | Dichloromethane (DCM) | [141] |
| Pd,Cr/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 400 °C, Ti-PILC SSA = 379 m2g−1; Pd,Cr-doping by impregnation, calcination 500 °C, best catalyst 0.5 wt % Pd + 0.5 wt % Cr, SSA = 378 m2g−1 | 200–550 °C, TCE = 340 ppm, air; 10% H2O vapor; GHSV = 10,000 h−1 | T90 = 338 °C (for the best catalyst) | Trichloro- ethylene (TCE) | [141] |
| Pd,Cr/Ti,Zr-pillared Mt | TiCl4 +ZrOCl2 precursor + Mt, calcination 40, 0 °C, Ti,Zr-PILC SSA = 379 m2g−1; Pd,Cr-doping by impregnation, calcination 500 °C, best catalyst 0.5 wt % Pd + 0.5 wt % Cr, SSA = 352 m2g−1 | 200–550 °C, TCE = 340 ppm, air; 10% H2O vapor; GHSV = 10,000 h−1. | T90 = 328 °C (for the best catalyst) | Trichloro- ethylene (TCE) | [141] |
| Cr,Ce/Ti-pillared Mt | Ti(OC4H9)4 precursor + Mt, calcination 550 °C, Ti-PILC SSA = 225 m2g−1; Cr,Ce-doping by impregnation, calcination 500 °C, best catalyst 8.6 wt % Cr + 1.4 wt % Ce, SSA = 179 m2g−1 | 200–550 °C, CB = 500 ppm, air; GHSV = 20,000 h−1 | T100 = 250 °C (for the best catalyst) | Chloro- benzene (CB) | [143] |
| V,W/TiO2-Pal | TiO2 and Pal mixture, V,W-doping by impregnation, calcination 500 °C, best catalyst mass ratio (VOx+WOy+TiO2):Pal = 2 | 200–350 °C, o-DCB = 3%, air; GHSV = 28,000 h−1 | T90 = 300 °C (for the best catalyst) | o-Dichloro- benzen (o-DCB) | [144] |
| Cr,Ce/Ti-pillared Mt | Ti(OC4H9)4 precursor + Mt, calcination 500 °C, Ti-PILC SSA = 207 m2g−1; Cr,Ce- doping by impregnation, calcination 500 °C, 6.9 wt % Cr + 1.1 wt % Ce, SSA = 183 m2g−1 | 120–340 °C, n-butylamine = 1000 ppm, air; GHSV = 20,000 h−1 | T90 = 231 °C | n-Butylamine | [145] |
| Cr,Ce/Ti-pillared Mt | Ti(OC4H9)4 precursor + Mt, calcination 500 °C, Ti-PILC SSA = 207 m2g−1; Cr,Ce-doping by impregnation, calcination 500 °C, 6.9 wt % Cr + 1.1 wt % Ce, SSA = 183 m2g−1 | 140–340 °C, ethylenediamine = 1000 ppm, air; GHSV = 20,000 h−1 | T90 = 254 °C | Ethylene-diamine | [145] |
| Cr,Ce/Ti-pillared Mt | Ti(OC4H9)4 precursor + Mt, calcination 500 °C, Ti-PILC SSA = 207 m2g−1; Cr,Ce-doping by impregnation, calcination 500 °C, 6.9 wt % Cr + 1.1 wt % Ce, SSA = 183 m2g−1 | 200–340 °C, acetonitrile = 1000 ppm, air; GHSV = 20,000 h−1 | T90 = 277 °C | Acetonitrile | [145] |
| TiO2–Clay Mineral Composite | Synthesis | Experimental Conditions | Dye | Degradation Rate | Ref. |
|---|---|---|---|---|---|
| Ti-pillared Mt | Ti(OC3H7)4 precursor + Mt, calcination 500 °C, SSA = 140 m2g−1 | UV irradiation CA: 1 mg mL−1 dye 10 ppm | MB | 85%—70 min | [157] |
| Ti-pillared Mt | Ti(OC3H7)4 precursor + Mt, post-intercalation hydrothermal treatment 250 °C, calcination 500 °C, SSA = 216 m2g−1 | UV irradiation CA: 1 mg mL−1 Dye: 10 ppm | MB | 99%—70 min | [157] |
| Ti-pillared Mt | Ti(OC4H9)4 precursor + Mt, calcination 400 °C, SSA = 368 m2g−1 | UV irradiation CA: 1 mg mL−1 Dye: 3 × 10−5 mmol mL−1 | MB | 58%—50 min | [64] |
| Ti-pillared Mt | Ti(OC4H9)4 precursor + Mt, clay/TiO2 sol microwave treated, calcination 400 °C, SSA = 405 m2g−1 | UV irradiation CA: 1 mg mL−1 Dye: 3 × 10−5 mmol mL−1 | MB | 93%—50 min | [64] |
| Ti-pillared Mt | Ti(OC3H7)4 precursor + POP surfactant + Mt, calcination 400 °C, SSA = 244 m2g−1 | UV irradiation CA: 0.2 mg mL−1 dye: 0.03 mg mL−1 | MB | 98%—90 min | [170] |
| Ti-pillared Mt | Ti(OC3H7)4 precursor + CTA-Mt, calcination 500 °C, SSA = 194 m2g−1 | UV irradiation CA: 0.2 mg mL−1 dye: 0.03 mg mL−1 | MB | 99%—60 min | [171] |
| Ti-pillared Mt | Ti(OC4H9)4 precursor + ODMBAC in ethanol + Mt, calcination 500 °C, SSA = 352 m2g−1 | UV irradiation CA: 0.5 mg mL−1 dye: 10−4 mmol/ mL−1 | MB | 98%—25 min | [172] |
| Ag/Ti-pillared Mt | TiCl4 precursor + Mt, calcination 500 °C, Ti-PILC SSA = 323 m2g−1, Ag doping: AgNO3 solution reduced with ascorbic acid, SSA = 323 m2g−1 | UV irradiation CA: 0.2 mg mL−1 dye: 10−4 mmol mL−1 | MB | 100%—15 min | [177] |
| TiO2/Mt | Mt impregnated with TiCl4 precursor, calcination at 350 °C, SSA = 52 m2g−1 | UV irradiation CA: 0.16 mg mL−1 dye: 10−4 mmol mL−1 | MB | 93%—360 min | [161] |
| TiO2/Mt | TiCl3 precursor + Mt, solvothermal treatment in H2O and C2H5OH, hexamethylene tetramine precipitant, hydrothermal treatment at 90/190 °C, no calcination, SSA = 102 m2g−1 | UV irradiation CA: 0.5 mg mL−1 dye: 1.2 × 10−4 mmol mL−1 | MB | 100%—40 min | [166] |
| Ti-pillared Mt | TiCl4 precursor + Mt, hydrothermal treatment 115 °C during intercalation, calcination 500 °C, SSA = 135 m2g−1 | UV irradiation CA: 1 mg mL−1 dye: 0.04 mg mL−1 | RhB | 98%—100 min | [55] |
| Ce,TiO2/Mt | Ti(OC3H7)4 precursor + Cloisite®30B + Ce(NO3)3 solution induced hydrolysis, calcination 500 °C; 0.25 wt % Ce, SSA = 211 m2g−1 | Solar irradiation CA: 0.5 mg mL−1 dye: 0.025 mg mL−1 (after saturation with adsorbed RhB in dark) | RhB | 100%—400 min | [181] |
| TiO2/Mt | Mt impregnated with TiCl4 precursor, calcination at 350 °C, SSA = 52 m2g−1 | UV irradiation CA: 0.16 mg mL−1 dye: 10−4 mmol mL−1 | RhB | 80%—360 min | [161] |
| TiO2/Mt | Ti(OC3H7)4 precursor + Cloisite®30B, induced hydrolysis, calcination 550 °C, SSA = 143 m2g−1 | Solar irradiation CA: 0.5 mg mL−1 dye: 0.025 mg mL−1 (after saturation with adsorbed RhB in dark) | RhB | 100%—6 h | [174] |
| TiO2/C-Mt | Polydopamine modified exfoliated Mt coated with P25, carbonized at 600 °C | Solar irradiation CA: 0.5 mg mL−1 dye: 12 ppm | RhB | 100% -30 min | [175] |
| Ti-pillared Mt | Ti(OC3H7)4 precursor + Mt, calcination 500 °C, SSA = 140 m2g−1 | UV irradiation CA: 1 mg mL−1 dye 10 ppm | VPB | 58%—70 min | [161] |
| Ti-pillared Mt | Ti(OC3H7)4 precursor + Mt, post-intercalation hydrothermal treatment 250 °C, calcination 500 °C, SSA = 216 m2g−1 | UV irradiation CA: 1 mg mL−1 Dye: 10 ppm | VPB | 93%—70 min | [157] |
| Ti-pillared Mt | Ti(OC4H9)4 precursor + Mt, calcination 500 °C | UV irradiation CA: 3 mg mL−1 Dye: 0.14 mg mL−1 | CR GTL | 90%—240 min | [158] |
| Ti-pillared Mt | TiCl4 precursor + Mt, hydrothermal treatment 115 °C during intercalation, calcination 500 °C, SSA = 135 m2g−1 | UV irradiation CA: 1 mg mL−1 dye: 0.04 mg mL−1 | MO | 100%—100 min | [55] |
| TiO2/Mt | Mt impregnated with TiCl4 precursor, calcination at 350 °C, SSA = 52 m2g−1 | UV irradiation CA: 0.16 mg mL−1 dye: 10−4 mmol mL−1 | MO | 36%—360 min | [161] |
| TiO2/Mt | Mt (pH controlled delamination) + TiOSO4 precursor (pH controlled hydrolysis), pH controlled coagulation, hydrothermal treatment, no calcination | UV irradiation CA: 0.4 mg mL−1 dye: 0.02 mg mL−1 | MO | 100%—50 min | [176] |
| TiO2-SiO2/Mt | Sol from Ti(OC4H9)4 precursor mixed with sol from Si(OC2H5)4 precursor, added to CTA-Mt. Solid product not calcined. | UV irradiation CA: 0.2 mg mL−1 dye: 0.015 mg mL−1 | MO | 97%—18 h | [168] |
| Au/Ti-pillared Mt | Ti(OC4H9)4 precursor + Mt, calcination 400 °C, Ti-PILC SSA = 238 m2g−1, Au doping: treatment with HAuCl4 solution + NaOH precipitant, SSA = 178 m2g−1 | UV irradiation CA: 0.5 mg mL−1 dye: 2 × 10−5 mmol mL−1 | SRB | 100%—120 min | [178] |
| Au/Ti-pillared Mt | Ti(OC4H9)4 precursor + Mt, calcination 400 °C, Ti-PILC SSA = 238 m2g−1, Au doping: treatment with HAuCl4 solution + NaOH precipitant, SSA = 178 m2g−1 | Visible light irradiation CA: 0.5 mg mL−1 dye: 2 × 10−5 mmol mL−1 | SRB | 75%—21 h | [178] |
| V,C–TiO2/Mt | Ti(OC3H7)4 precursor + urea + vanadyl acetylacetonate + Mt, (120 mmol Ti/g Mt), calcination 500 °C, 0.09 wt % V, SSA = 86 m2g−1 | Visible light irradiation CA: 0.5 mg mL−1 dye: 2 × 10−5 mmol mL−1 | SRB | 67%—18 h | [180] |
| Ti-pillared Mt | Ti(OC3H7)4 precursor + CTA-Mt, calcination 500 °C | UV irradiation CA: 0.4 mg mL−1 dye: 0.02 mg mL−1 | CR | 100%—30 min | [169] |
| TiO2/Mt | Mt impregnated with TiCl4 precursor, calcination at 350 °C, SSA = 52 m2g−1 | UV irradiation CA: 0.16 mg mL−1 dye: 10−4 mmol mL−1 | CR | 23%—360 min | [161] |
| Ti-pillared Mt | Ti(OC3H7)4 precursor + Mt, post-synthesis microwave treatment, calcination 400 °C, SSA = 151 m2g−1 | UV irradiation CA: 2.5 mg mL−1 dye: 0.1 mg mL−1 | SR3BL | 95%—220 min | [162] |
| Ti-pillared Mt | TiCl4 precursor + Mt, hydrothermal treatment 70 °C during intercalation, no calcination, SSA = 277 m2g−1 | UV irradiation CA: 1 mg mL−1 dye: 0.03 mg mL−1 | ARG | 100%—60 min | [165] |
| TiO2/Mt | Mt impregnated with TiCl4 precursor, calcination at 350 °C, SSA = 52 m2g−1 | UV irradiation CA: 0.16 mg mL−1 dye: 10−4 mmol mL−1 | CV | 97%—360 min | [161] |
| Ag/Ti-pillared Mt | Ti(OC2H5)4 precursor + Mt, calcination 500 °C, Ti-PILC SSA = 208 m2g−1, Ag doping: treatment with AgNO3 solution reduced with ascorbic acid, SSA = 195 m2g−1 | UV irradiation CA: 0.5 mg mL−1 dye: 2.1 × 10−5 mmol mL−1 | RB | 15%—600 min | [67] |
| N,S,Ti-pillared Mt | TiCl4 precursor + CS(NH2)2 + Mt, Ti:S = 1:4, calcination 350 °C, SSA = 277 m2g−1 | Visible light irradiation CA: 2 mg mL−1 dye: 0.03 mg mL−1 | 4BS | 96%—60 min | [179] |
| g-C3N4/TiO2-Bent | Ti(OC4H9)4 precursor + Bent at pH = 3, calcination 550 °C, addition of g-C3N4 slurry under sonication, drying 60 °C; 40 wt % g-C3N4, SSA = 70 m2g−1 | Visible light irradiation CA: 1 mg mL−1 dye: 40 ppm | RBR-X3BS | 90%—100 min | [183] |
| TiO2–Clay Mineral Composite | Synthesis | Experimental Conditions | Dye | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|
| g-C3N4/TiO2-Hec | Ti(OC3H7)4 precursor + fluorinated Hect, calcination 500 °C, SSA = 229 m2g−1; TiO2-Hect mixed with g-C3N4 by grinding, SSA = 219 m2g−1 | visible light irradiation CA: 0.03 mg mL−1 dye: 10 ppm | RhB | 94%—120 min | [187] |
| TiO2/Hal | Ti(OC3H7)4 precursor + Hal, hydrothermal treatment at 65 °C, no calcination, SSA = 112 m2g−1 | solar irradiation CA: 0.5 mg mL−1 dye: 0.01 mg mL−1 | RhB | 89%—6 h | [198] |
| TiO2/Hal | Ti(OC3H7)4 precursor + Hal, hydrothermal treatment at 65 °C, no calcination, SSA = 112 m2g−1 | visible light irradiation CA: 0.5 mg mL−1 dye: 0.01 mg mL−1 | RhB | 69%—6 h | [198] |
| PANI–TiO2/Hal | Ti(OC3H7)4 precursor + aniline + FeCl3 + Hal, hydrothermal treatment at 65 °C | visible light irradiation CA: 0.5 mg mL−1 dye: 0.01 mg mL−1 | RhB | 77%—6 h | [199] |
| TiO2/Mus | Mus + TiOSO4 precursor, calcination 400 °C | UV irradiation CA: 0.5 mg mL−1 dye: 0.02 mg mL−1 | RhB | 98%—120 mn | [215] |
| N–TiO2/Mus | Mus + TiOSO4 precursor, calcination 400 °C, grinding with urea, calcination 400 °C | visible light irradiation CA: 0.5 mg mL−1 dye: 0.02 mg mL−1 | RhB | 96%—120 mn | [216] |
| TiO2/Lap | Ti(OC3H7)4 precursor + Lap + PEO, hydrothermal treatment 100 °C, calcination 500 °C, SSA = 379 m2g−1 | UV irradiation CA: 1 mg mL−1 dye: 0.01 mg mL−1 | RhG | 97%—60 min | [159] |
| TiO2/Kao | Ti(OC3H7)4 precursor + Kao, calcination 400 °C, SSA = 16 m2g−1 | UV irradiation CA: 10 mg mL−1 dye: 0.025 mg mL−1 | MB | 99%—5 min | [193] |
| TiO2/Kao | Ti(OC4H9)4 precursor + Kao microwave treated at 180 °C, mixture microwave treated at 180 °C; calcination 550 °C, SSA = 36 m2g−1 | UV irradiation CA: 0.2 mg mL−1 dye: 1.9 × 10−5 mmol mL−1 | MB | 54%—60 min | [194] |
| TiO2/Hal | Ti(OC3H7)4 precursor + Hal, calcination 300 °C | UV irradiation CA: 0.5 mg mL−1 dye: 10−4 mmol mL−1 | MB | 82%—4 h | [200] |
| TiO2/amylose/Hal | Hal + amylose ground together, dispersed in DMSO/H2O, amylose/Hal extracted from the supernatant; Ti(OC3H7)4 precursor + amylose/Hal, drying 110 °C | UV irradiation CA: 1 mg/mL dye: 0.032 mg/mL | MB | 91%—10 h | [201] |
| Ag–TiO2/Pal | Pal calcined at 500 °C + AgNO3, heated at 200 °C in N2 + Ti(OC4H9)4 precursor, calcination 400 °C | UV irradiation CA: 0.25 mg dye: 1.6 × 10−3 mmol mL−1 | MB | 87%—120 min | [206] |
| CdS–TiO2/Pal | CTA-Pal + Ti(OC4H9)4 precursor, calcination 500 °C + CdSO4, calcination 500 °C + Na2S | visible light irradiation dye: 0.02 mg mL−1 | MB | 37%—150 min | [212] |
| TiO2/Kao | Ti(OC4H9)4 precursor + K outgassed at 750 °C, calcination 600 °C, SSA = 35 m2g−1 | UV irradiation CA: 10 mg mL−1 dye: 0.04 mg mL−1 | CR | 100%—6 h | [189] |
| TiO2/Kao | Ti(OC4H9)4 precursor + acid and base treated Kao outgassed at 750 °C, calcination 600 °C, SSA = 58 m2g−1 | UV irradiation CA: 10 mg mL−1 dye: 5.7 × 10−5 mmol mL−1 | CR | 95%—3 h | [190] |
| TiO2/Hal | Ti(OC3H7)4 precursor + Hal, hydrothermal treatment at 65 °C, no calcination, SSA = 112 m2g−1 | solar irradiation CA: 0.5 mg mL−1 dye: 0.02 mg mL−1 | GV | 60%—6 h | [198] |
| TiO2/Hal | Ti(OC3H7)4 precursor + Hal, hydrothermal treatment at 65 °C, no calcination, SSA = 112 m2g−1 | visible light irradiationCA: 0.5 mg mL−1dye: 0.02 mg mL−1 | GV | 51%—6 h | [198] |
| ZnO–TiO2/clay | TiO2 deposited on clay dehydrated at 400 °C by MOCVD of Ti(OC3H7)4 at 600 °C; ZnO deposited by sol–gel from Zn acetate precursor, calcination 300 °C, SSA = 105 m2g−1 | UV irradiation CA: 4 mg mL−1 dye: 0.075 mg mL−1 | MG | 100%—30 min | [196] |
| TiO2/Kao | Ti(OC4H9)4 precursor + Kao, drying 80 °C, no calcination, SSA = 63 m2g−1 | solar irradiation CA: 1 mg mL−1 dye: 0.01 mg mL−1 | MO | 83%—150 min | [195] |
| TiO2/Hal | Ti(OC4H9)4 precursor + Kao, drying 80 °C, no calcination, SSA = 124 m2g−1 | solar irradiation CA: 1 mg mL−1 dye: 0.01 mg mL−1 | MO | 65%—150 min | [195] |
| TiO2/Pal | Ti(OC4H9)4 precursor + Kao, drying 80 °C, no calcination, SSA = 194 m2g−1 | solar irradiation CA: 1 mg mL−1 dye: 0.01 mg mL−1 | MO | 45%—150 min | [196] |
| BiOBr–TiO2/Att | Att + Bi(NO3)3 +NH4Br precursor, calcination 320 °C + Ti(OC4H9)4 precursor, calcination 320 °C | visible light irradiation CA: 1 mg mL−1 dye: 0.02 mg mL−1 | MO | 97%—120 min | [211] |
| Ag–TiO2/Sep | Ti(OC4H9)4 precursor + AgNO3, reduced with ascorbic acid + Sep, hydrothermal treatment at 150 °C; 5 wt % Ag | UV/visible light irradiation CA: 1 mg mL−1 dye: 0.02 mg mL−1 | MO | 100%—50 min | [213] |
| Ag–TiO2/Sep | Ti(OC4H9)4 precursor + AgNO3, reduced with ascorbic acid + Sep, hydrothermal treatment at 150 °C; 5 wt % Ag | visible light irradiation CA: 1 mg mL−1 dye: 0.02 mg mL−1 | MO | 86%—220 min | [213] |
| SnO2–TiO2/Att | Att + SnCl2 precursor, calcination 300 °C + Ti(OC4H9)4 precursor, calcination 300 °C, SSA = 147 m2g−1 | UV irradiation CA: 1 mg mL−1 dye: 0.02 mg mL−1 | MO | 99%—30 min | [210] |
| TiO2/Kao | Ti(OC3H7)4 precursor + Kao, calcination 400 °C, SSA = 16 m2g−1 | UV irradiation CA: 10 mg mL−1 dye: 0.025 mg mL−1 | MOII | 93%—5 min | [193] |
| TiO2/Kao | TiCl4 precursor + acid treated Kao, drying at 70 °C, calcination 400 °C, SSA = 114 m2g−1 | UV irradiation CA: 1.5 mg mL−1 dye: 0.03–0.05 mg mL−1 | ARG | 100%—60 min | [191] |
| TiO2/Sep | Sep + Ti(OC3H7)4 precursor, heated at 70 °C, SSA = 247 m2g−1 | UV irradiation CA: 1.5 mg/mL dye: 0.03 mg/mL | ARG | 100%—120 min | [205] |
| TiO2/Pal | CTA-Pal + Ti(OC3H7)4 precursor, calcination 600 °C | UV irradiation CA: 1 mg mL−1 dye: 10−5 mmol mL−1 | OG | 100%—90 min | [203] |
| TiO2/Sep | Sep + Ti(OC3H7)4 precursor, microwave hydrothermal treatment, SSA = 161 m2g−1 | UV irradiation CA: 0.3 mg mL−1 dye: 0.01 mg mL−1 | OG | 90%—120 min | [206] |
| Ru(Bpy)3/Ti-pillared Sap | Ti(OC3H7)4 precursor, calcination 400 °C, SSA = 154 m2g−1; + Ru(BPy)3, SSA = 150 m2g−1 | UV irradiation CA: 0.4 mg mL−1 dye: 0.02 mg mL−1 | BPB | 97%—120 min | [69] |
| Ru(Bpy)3/Ti-pillared Sap | Ti(OC3H7)4 precursor, calcination 400 °C, SSA = 154 m2g−1; + Ru(BPy)3, SSA = 150 m2g−1 | visible light irradiation CA: 0.4 mg mL−1 dye: 0.02 mg mL−1 | BPB | 90%—120 min | [69] |
| TiO2/Lap | TiO2 colloid from Ti(OC3H7)4 precursor-treated hydrothermally + Lap + PEO, 40 mmol Ti/g Lap, hydrothermal treatment 180 °C, calcination 500 °C, | UV irradiation CA: 1 mg mL−1 dye: 1.8 × 10−5 mmol mL−1 | SRB | 92%—90 min | [184] |
| TiO2/Kao | TiOSO4 precursor + raw Kao, calcination 600 °C, SSA = 32 m2g−1 | UV irradiation CA: 1.5 mg mL−1 dye: 0.03—0.05 mg mL−1 | AO7 | 70%—60 min | [192] |
| TiO2–Clay Mineral Composite | Synthesis | Experimental Conditions | Pollutant | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|
| TiO2/Mt | Ti(OC3H7)4 precursor + Mt, acidifiedto pH = 1, + Mt, drying at 50 °C, SSA = 251 m2g−1 | UV irradiation CA: 1 mg mL−1 P: 5 × 10−4 mmol mL−1 | Ph | 52%—120 min | [221] |
| TiO2/Mt | Ti(OC3H7)4 precursor + Cloisite®30B, induced hydrolysis, calcination 550 °C, SSA = 143 m2g−1 | solar irradiation CA: 0.5 mg mL−1 P: 0.025 mg mL−1 (after saturation with adsorbed Ph in dark) | Ph | ca. 80%—10 h | [174] |
| Fe,Ti-pillared Mt | TiCl4 precursor + Fe(NO3)3 + Mt, calcination 400 °C, SSA = 129 m2g−1 | UV irradiation CA: 5 mg mL−1 P: 5 × 10−4 mmol mL−1 | Ph | 90%—240 min | [228] |
| Ce,TiO2/Mt | Ti(OC3H7)4 precursor + Cloisite®30B + Ce(NO3)3 solution induced hydrolysis, calcination 500 °C; 0.25 wt % Ce, SSA = 211 m2g−1 | solar irradiation CA: 0.5 mg mL−1 dye: 0.025 mg mL−1 (after saturation with adsorbed RhB in dark) | Ph | 80%—600 min | [181] |
| TiO2/Lap | TiOSO4 precursor + Lap, hydrothermal treatment at 150 °C, calcination 500 °C, SSA = 323 m2g−1 | UV irradiation CA: 0.6 mg mL−1 P: 5 × 10−5 mmol mL−1 | Ph | 100%—80 min | [227] |
| TiO2/Lap | Ti(OC3H7)4 precursor + Lap, hydrothermal treatment 100 °C, calcination 500 °C, SSA = 435 m2g−1 | UV irradiation CA: 1 mg mL−1 P: 10 ppm | Ph | 94%—240 min | [220] |
| SnO2–TiO2/Pal | Pal + SnCl2 precursor, calcination 300 °C + Ti(OC4H9)4 precursor, calcination 300 °C, SSA = 147 m2g−1 | UV irradiation CA: 1 mg mL−1 dye: 0.08 mg mL−1 | Ph | 100%—100 min | [229] |
| Ti-pillared Mt | Ti(OC3H7)4 precursor + Mt, drying at 50 °C, no calcination | UV irradiation CA: 6 mg mL−1 P: 2.5 × 10−3 mmol mL−1 | CP | 75%—600 min | [218] |
| TiO2/HDPM-Mt | Sonicated HDPM-Mt + Ti(OC3H7)4 precursor, hydrothermal treatment at 150 °C | UV irradiation CA: 6 mg mL−1 P: 2.5 × 10−3 mmol mL−1 | CP | 45%—600 min | [218] |
| Ti-pillared Mt | Ti(OC4H9)4 precursor + Mt, calcination 450 °C, SSA = 133 m2g−1 | UV irradiation CA: 2 mg mL−1 P: 0.02 mg mL−1 | TCP | 100%—120 min | [223] |
| Ti,Si-pillared Mt | Ti(OC4H9)4 precursor + Si(OC2H5)4 precursor + Mt, calcination 450 °C, SSA = 259 m2g−1 | UV irradiation CA: 2 mg mL−1 P: 0.02 mg mL−1 | TCP | 40%—120 min | [223] |
| TiO2/Lap | TiOSO4 precursor + Lap, hydrothermal treatment at 150 °C, calcination 500 °C, SSA = 323 m2g−1 | UV irradiation CA: 0.6 mg mL−1 P: 5 × 10−5 mmol/ mL−1 | TCP | 100%—80 min | [227] |
| TiO2/Kao | TiCl4 precursor + acid treated Kao, drying at 70 °C, calcination 400 °C, SSA = 114 m2g−1 | UV irradiation CA: 1 mg mL−1 P: 0.01 mg mL−1 | NP | 85%—240 min | [191] |
| TiO2/Sep | Sep + Ti(OC3H7)4 precursor, heated at 70 °C, SSA = 247 m2g−1 | UV irradiation CA: 1.5 mg mL−1 P: 0.01 mg mL−1 | NP | 79%—240 min | [205] |
| TiO2–Clay Mineral Composite | Synthesis | Experimental Conditions | Pollutant | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|
| Ti-pillared Mt | Ti(OC3H7)4 precursor + Mt, hydrothermal treatment 200 °C, calcination 500 °C, SSA = 233 m2g−1 | UV irradiation CA: 1 mg mL−1 P: 17 ppm | DBP | 90%—180 min | [152] |
| Zr–TiO2/Mt | Ti(OC3H7)4 + Zr(OC4H9)4 precursor + Cloisite®30B-induced hydrolysis, calcination 500 °C, SSA = 168 m2g−1 | Solar irradiation CA: 0.25 mg mL−1 P: 0.01 mg mL−1 | ANT | ca. 90%—360 min | [233] |
| ZnO–TiO2/Mt | Ti(OC3H7)4 precursor + Cloisite®30B + Zn(CH3COO)2 precursor-induced hydrolysis, calcination 500 °C, SSA = 165 m2g−1 | Solar irradiation CA: 0.25 mg mL−1 P: 0.01 mg mL−1 | ANT | 100%—600 min | [234] |
| Ag–ZnO–TiO2/Mt | Ti(OC3H7)4 precursor + Cloisite®30B, + Zn(CH3COO)2 precursor-induced hydrolysis, calcination 500 °C, + AgNO3, photoreduction, 0.91 wt % Ag, SSA = 144 m2g−1 | Solar irradiation CA: 0.25 mg mL−1 P: 0.005 mg mL−1 | ANT | 95%—360 min | [235] |
| ZnO–TiO2/Mt | Ti(OC3H7)4 precursor + Cloisite®30B, + Zn(CH3COO)2 precursor-induced hydrolysis, calcination 500 °C, SSA = 165 m2g−1 | Solar irradiation CA: 0.25 mg mL−1 P: 0.01 mg mL−1 | ACE | 100%—600 min | [234] |
| Ag–ZnO–TiO2/Mt | Ti(OC3H7)4 precursor + Cloisite®30B + Zn(CH3COO)2 precursor-induced hydrolysis, calcination 500 °C, + AgNO3 under UV irradiation, 0.91 wt % Ag, SSA = 144 m2g−1 | Solar irradiation CA: 0.25 mg mL−1 P: 0.005 mg mL−1 | ACE | 100%—360 min | [235] |
| g-C3N4–TiO2/Kao | Ti(OC3H7)4 precursor + Kao, calcination 500 °C +H2SO4 + g-C3N4, SSA = 52 m2g−1 | Solar irradiation 2 mg mL−1 P: 10 ppm | CIP | 92%—240 min | [237] |
| Cr,Ti-pillared Mt | TiCl4 precursor + Cr3+ + Mt, calcination 500 °C, SSA = 272 m2g−1 | UV irradiation CA: 1 mg mL−1 P: 0.025 mg/mL | TRI | 76%—180 min | [56] |
| BiOCl–TiO2/Sep | Sequential precipitation of TiO2 and BiOCl onto Sep from TiSO4 and Bi(NO3)3 precursors, calcination 500 °C, SSA = 97 m2g−1 | Visible light irradiation CA: 0.6 mg mL−1 P: 0.05 mg mL−1 | TCY | >90%—180 min | [238] |
| Ag–ZnO–TiO2/Mt | Ti(OC3H7)4 precursor + Cloisite®30B + Zn(CH3COO)2 precursor-induced hydrolysis, calcination 500 °C + AgNO3, photoreduction, 0.91 wt % Ag, SSA = 144 m2g−1 | Solar irradiation CA: 0.25 mg mL−1 P: 0.005 mg mL−1 | ATR | 96%—360 min | [235] |
| W,TiO2/Mt | Ti(OC3H7)4 precursor + Cloisite®30B + (NH4)6H2W12O40 · xH2O precursor-induced hydrolysis, calcination 500 °C, 5 M% W, SSA = 148 m2g−1 | Solar irradiation CA: 0.25 mg mL−1 P: 0.0025 mg mL−1 | ATR | 100%—240 min | [241] |
| W,TiO2/Mt | Ti(OC3H7)4 precursor + Cloisite®30B + (NH4)6H2W12O40 · xH2O precursor-induced hydrolysis, calcination 500 °C, 5 M% W, SSA = 148 m2g−1 | Visible light irradiation CA: 0.25 mg mL−1 P: 0.0025 mg mL−1 | ATR | 100%—240 min | [241] |
| TiO2/Lap | Ti(OC3H7)4 precursor + Lap, hydrothermal treatment at 60 °C, calcination 500 °C, SSA = 320 m2g−1 | UV irradiation CA: 0.2 mg TiO2 mL−1 P: 0.002 mg mL−1 | DIM | 90%—180 min | [239] |
| TiO2/Mt | Ti(OC3H7)4 precursor + Mt, hydrothermal treatment at 60 °C, calcination 500 °C, SSA = 183 m2g−1 | UV irradiation CA: 0.2 mg TiO2 mL−1 P: 0.002 mg mL−1 | DIM | 86%—180 min | [239] |
| TiO2/Lap | TiOSO4 precursor + Lap, hydrothermal treatment at 200 °C, calcination 500 °C, SSA = 232 m2g−1 | UV irradiation CA: 1 mg mL−1 P: 10 ppm | ALA | 66%—60 min | [240] |
| TiO2/Lap | TiOSO4 precursor + Lap, hydrothermal treatment at 200 °C, calcination 500 °C, SSA = 232 m2g−1 | UV irradiation CA: 1 mg mL−1 P: 10 ppm | BRO | 84%—60 min | [240] |
| TiO2/Lap | TiOSO4 precursor + Lap, hydrothermal treatment at 200 °C, calcination 500 °C, SSA = 232 m2g−1 | UV irradiation CA: 1 mg mL−1 P: 5 ppm | CHT | 77%—60 min | [240] |
| TiO2/Lap | TiOSO4 precursor + Lap, hydrothermal treatment at 200 °C, calcination 500 °C, SSA = 232 m2g−1 | UV irradiation CA: 1 mg mL−1 P: 5 ppm | IMA | 100%—45 min | [240] |
| TiO2/Lap | TiOSO4 precursor + Lap, hydrothermal treatment at 200 °C, calcination 500 °C, SSA = 232 m2g−1 | UV irradiation CA: 1 mg mL−1 P: 5 ppm | SUS | 80%—60 min | [240] |
| TiO2–Clay Mineral Composite | Synthesis | Experimental Conditions | Pollutant | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|
| TiO2/Hec | Ti(OC3H7)4 precursor + Hec, hydrothermal treatment at 180 °C, SSA = 140 m2g−1 | UVA irradiation P: 185 ppbv RH = 10% Circulating flow reactor | Toluene | 26% removal | [244] |
| TiO2/Hec | Ti(OC3H7)4 precursor + Hec, hydrothermal treatment at 180 °C, SSA = 140 m2g−1 | UVC irradiation P: 165 ppbv RH = 10% Circulating flow reactor | Toluene | 58% removal | [244] |
| TiO2/Kao | Ti(OC3H7)4 precursor + Hec, hydrothermal treatment at 180 °C, SSA = 16 m2g−1 | UVA irradiation P: 170 ppbv RH = 10% Circulating flow reactor | Toluene | 4% removal | [244] |
| TiO2/Kao | Ti(OC3H7)4 precursor + Hec, hydrothermal treatment at 180 °C, SSA = 16 m2g−1 | UVC irradiation P: 162 ppbv RH = 10% Circulating flow reactor | Toluene | 50% removal | [244] |
| Ti-pillared Mt/SiO2 | Ti(OC4H9)4 precursor + Mt, + acid-treated silica gel, calcination 450 °C, SSA = 306 m2g−1 | UV irradiation CA: 5 g P: 500 ppb Fluidized bed ractor | Toluene | 100%—120 min | [257] |
| TiO2/Hal | Ti(OC3H7)4 precursor + Hal, hydrothermal treatment 180 °C, SSA = 183 m2g−1 | UV irradiation CA: 0.04 g P: 800 ppmv RH = 75% Flow reactor | Toluene | R = 4.2 × 10−8 mol s−1g−1 | [248] |
| TiO2/Hal | Ti(OC3H7)4 precursor + Hal, hydrothermal treatment 180 °C, SSA = 183 m2g−1 | Solar irradiation CA: 0.04 g P: 800 ppmv RH = 75% Flow reactor | Toluene | R = 2.6 × 10−8 mol s−1g−1 | [248] |
| TiO2/Pal | Ti(OC3H7)4 precursor + Pal, hydrothermal treatment 180 °C, SSA = 240 m2g−1 | UV irradiation CA: 0.04 g P: 800 ppmv RH = 75% Flow reactor | Toluene | R = 3.6 × 10−8 mol s−1g−1 | [249] |
| TiO2/Pal | Ti(OC3H7)4 precursor + Pal, hydrothermal treatment 180 °C, SSA = 240 m2g−1 | Solar irradiation CA: 0.04 g P: 800 ppmv RH = 75% Flow reactor | Toluene | R = 2.4 × 10−8 mol s−1g−1 | [249] |
| TiO2/Hal/Pal | Ti(OC3H7)4 precursor + Hal + Pal, hydrothermal treatment 180 °C, SSA = 207 m2g−1 | UV irradiation CA: 0.04 g P: 800 ppmv RH = 75% Flow reactor | Toluene | R = 4.5 × 10−8 mol s−1g−1 | [250] |
| TiO2/Hal/Pal | Ti(OC3H7)4 precursor + Hal + Pal, hydrothermal treatment 180 °C, SSA = 207 m2g−1 | Solar irradiation CA: 0.04 g P: 800 ppmv RH = 75% Continuous flow reactor | Toluene | R = 3.9 × 10−8 mol s−1g−1 | [250] |
| TiO2/Lap | Ti(OC3H7)4 precursor + Lap + PEO, hydrothermal treatment 100 °C, calcination 1000 °C, SSA = 1040 m2g−1 | UVC irradiation CA: 4 g P: 300 ppmv RH = 0% Fluidized bed reactor | Styrene | 87% | [247] |
| TiO2/Lap | Ti(OC3H7)4 precursor + Lap + PEO, hydrothermal treatment 100 °C, calcination 1000 °C, SSA = 1040 m2g−1 | UVC irradiation CA: 4 g P: 300 ppmv RH = 20% Fluidized bed reactor | Styrene | 5% | [247] |
| TiO2/Fe2O3/Pal | Pal + goethite + Ti(OC4H9)4 precursor, calcination 350 °C, SSA = 97 m2g−1 | UV irradiation P: 30 mg/m3 | Benzene | 70%—180 min | [258] |
| TiO2/Hec | Ti(OC3H7)4 precursor + Hec, hydrothermal treatment at 180 °C, SSA = 140 m2g−1 | UVA irradiation P: 110–130 ppbv RH = 10% Circulating flow reactor | Formaldehyde | 55% removal | [246] |
| TiO2/Hec | Ti(OC3H7)4 precursor + Hec, hydrothermal treatment at 180 °C, SSA = 140 m2g−1 | UVC irradiation P: 110–130 ppbv RH = 10% Circulating flow reactor | Formaldehyde | 62% removal | [246] |
| TiO2/Sep | Acid treated Sep + (NaPO3)6 dispersant, + TiCl4 precursor + (NH4)2SO4, + NH3(aq), hydrolysis/precipitation, calcination 550 °C, SSA = 135 m2g−1 | UV irradiation CA: 10 g P: 6.56 mg (m3)−1 Batch reator | Formaldehyde | 90%—12 h | [251] |
| Ti-pillared Mt | Ti(OC3H7)4 precursor + Mt, Microwave calcination, 500 °C, SSA = 165 m2g−1 | UV irradiation CA: 50 mg P: 1.34 × 10−5 mmol mL−1 Continuous flow reactor | Methanol | ca. 50% removal | [219] |
| Ti-pillared Mt/SiO2 | Ti(OC4H9)4 precursor + Mt, + acid-treated silica gel, calcination 450 °C, SSA = 306 m2g−1 | UV irradiation CA: 5 g P: 500 ppb Fluidized bed ractor | Ethyl acetate | 100%—120 min | [257] |
| Ti-pillared Mt/SiO2 | Ti(OC4H9)4 precursor + Mt, + acid treated silica gel, calcination 450 °C, SSA = 306 m2g−1 | UV irradiation CA: 5 g P: 500 ppb Fluidized bed ractor | Ethanethiol | 100%—120 min | [257] |
| TiO2/Sep | Ceramic dough made of Sep + commercial TiO2 + H2O, formation of extrudates, calcination 500 °C, SSA = 153 m2g−1 | Solar irradiation CA: 35 mL P: 150 ppm Flow reactor | Trichloro-ethylene | 100% removal | [256] |
| TiO2/Hal | Ti(OC3H7)4 precursor + Hal, hydrothermal treatment 180 °C, SSA = 167 m2g−1 | UV irradiation CA: 0.04 g P: 1 ppm Flow reactor | NO | 66% removal | [250] |
| TiO2/Pal | Ti(OC3H7)4 precursor + Pal, hydrothermal treatment 180 °C, SSA = 242 m2g−1 | UV irradiation CA: 0.04 g P: 1 ppm Flow reactor | NO | 60% removal | [250] |
| TiO2/Hal/Pal | Ti(OC3H7)4 precursor + Hal + Pal, hydrothermal treatment 180 °C, SSA = 206 m2g−1 | UV irradiation CA: 0.04 g P: 1 ppm Flow reactor | NO | 69% removal | [250] |
| TiO2/Hal | Ti(OC3H7)4 precursor + Hal, hydrothermal treatment 180 °C, SSA = 167 m2g−1 | Visible light irradiation CA: 0.04 g P: 1 ppm Flow reactor | NO | 21% removal | [250] |
| TiO2/Pal | Ti(OC3H7)4 precursor + Pal, hydrothermal treatment 180 °C, SSA = 242 m2g−1 | Visible light irradiation CA: 0.04 g P: 1 ppm flow reactor | NO | 20% removal | [250] |
| TiO2/Hal/Pal | Ti(OC3H7)4 precursor + Hal + Pal, hydrothermal treatment 180 °C, SSA = 228 m2g−1 | Visible light irradiation CA: 0.04 g P: 1 ppm Flow reactor | NO | 26% removal | [250] |
| TiO2/Talc | Sonicated Talc suspension + P25 | UV irradiation P: 1 ppm RH = 50% Flow reactor | NO | 35% removal | [263] |
| TiO2/Talc | Sonicated Talc suspension + P25 | Visible light irradiation P: 1 ppm RH = 50% Flow reactor | NO | 23% removal | [263] |
| TiO2/Sep | Ti(OC3H7)4 precursor + Sep, hydrothermal treatment 180 °C, SSA = 189 m2g−1 | UV irradiation CA: 1 g P: 1 ppm RH = 50% Flow reactor | NO | 35% removal | [262] |
| TiO2/Zn-Sep | Zn-exchanged Sep + Ti(OC3H7)4 precursor, hydrothermal treatment 180 °C, SSA = 179 m2g−1 | UV irradiation CA: 1 g P: 1 ppm RH = 50% Flow reactor | NO | 38% removal | [262] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serwicka, E.M. Titania-Clay Mineral Composites for Environmental Catalysis and Photocatalysis. Catalysts 2021, 11, 1087. https://doi.org/10.3390/catal11091087
Serwicka EM. Titania-Clay Mineral Composites for Environmental Catalysis and Photocatalysis. Catalysts. 2021; 11(9):1087. https://doi.org/10.3390/catal11091087
Chicago/Turabian StyleSerwicka, Ewa M. 2021. "Titania-Clay Mineral Composites for Environmental Catalysis and Photocatalysis" Catalysts 11, no. 9: 1087. https://doi.org/10.3390/catal11091087
APA StyleSerwicka, E. M. (2021). Titania-Clay Mineral Composites for Environmental Catalysis and Photocatalysis. Catalysts, 11(9), 1087. https://doi.org/10.3390/catal11091087






