Evaluation of 3,3′-Triazolyl Biisoquinoline N,N′-Dioxide Catalysts for Asymmetric Hydrosilylation of Hydrazones with Trichlorosilane
Abstract
:1. Introduction
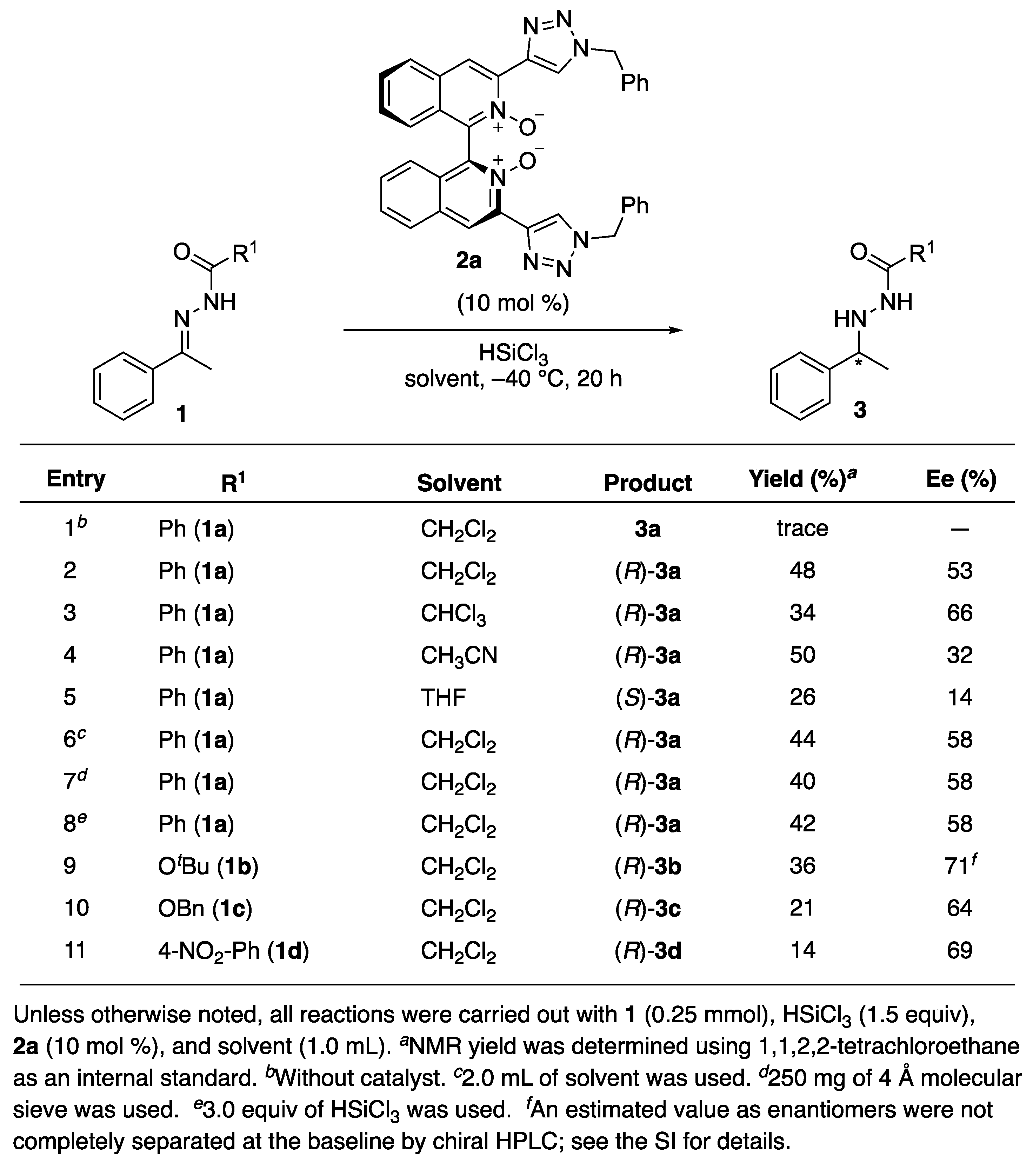
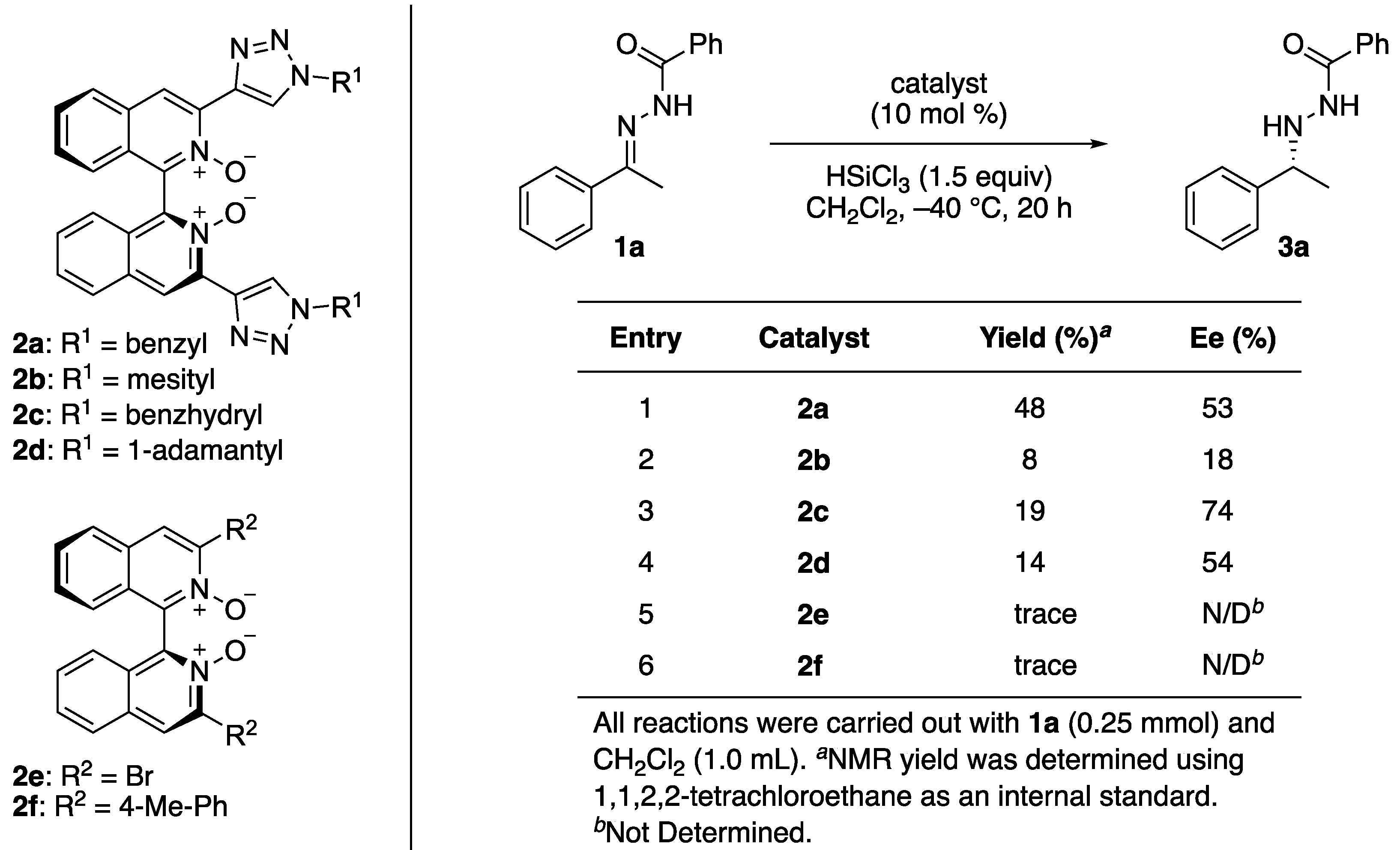
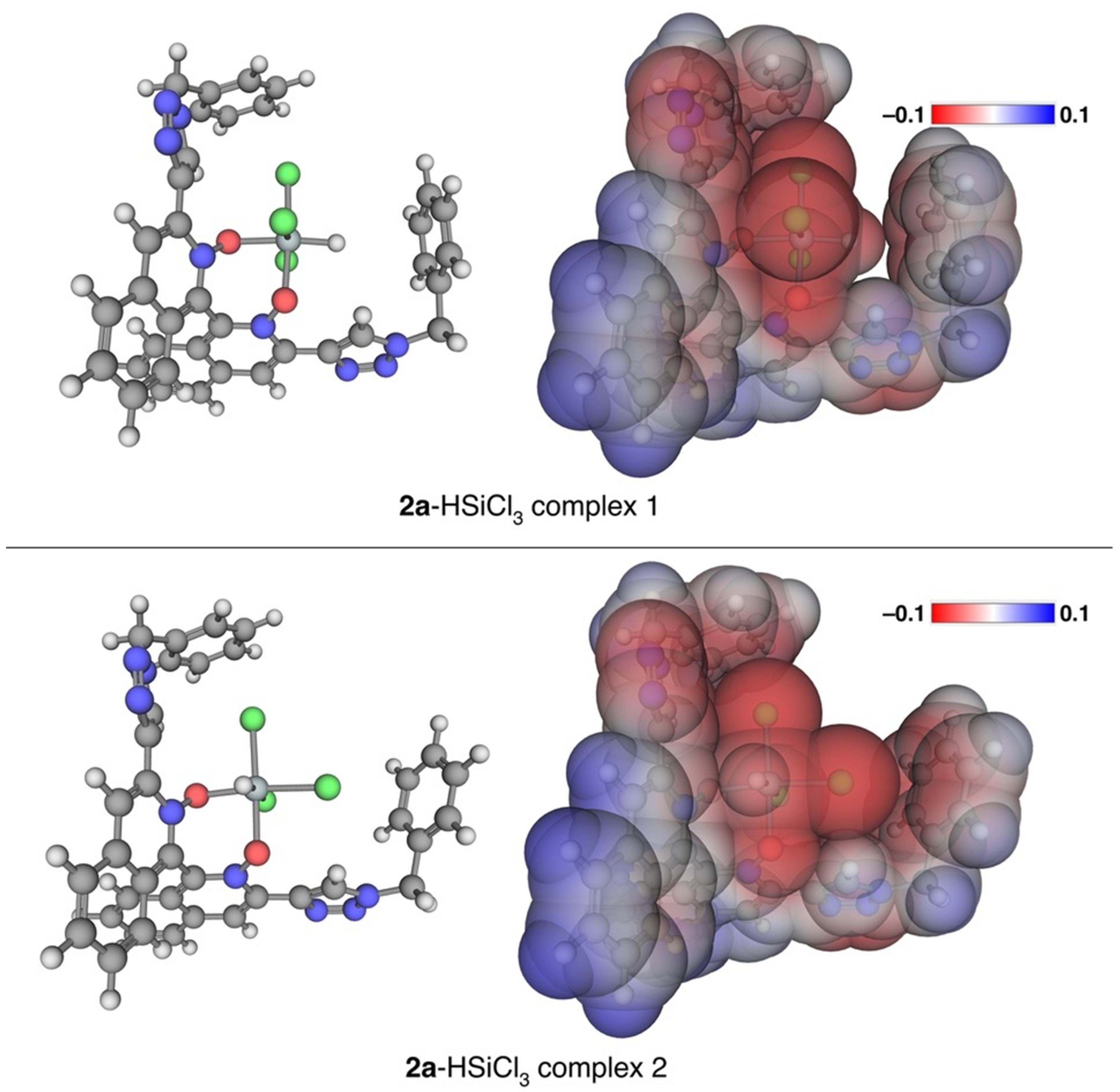
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdine, R.A.A.; Hedouin, G.; Colobert, F.; Wencel-Delord, J. Metal-Catalyzed Asymmetric Hydrogenation of C=N Bonds. ACS Catal. 2021, 11, 215–247. [Google Scholar] [CrossRef]
- Xie, J.-H.; Zhu, S.-F.; Zhou, Q.-L. Transition Metal-Catalyzed Enantioselective Hydrogenation of Enamines and Imines. Chem. Rev. 2011, 111, 1713–1760. [Google Scholar] [CrossRef]
- Fan, D.; Hu, Y.; Jiang, F.; Zhang, Z.; Zhang, W. Rhodium-Catalyzed Chemo- and Enantioselective Hydrogenation of Alkynyl-Aryl Hydrazones. Adv. Synth. Catal. 2018, 360, 2228–2232. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, Y.; Liu, Y.; Zhang, Z.; Liu, Y.; Zhang, W. Rh-Catalyzed Chemo- and Enantioselective Hydrogenation of Allylic Hydrazones. Chem. A Eur. J. 2017, 23, 1040–1043. [Google Scholar] [CrossRef]
- Haddad, N.; Qu, B.; Rodriguez, S.; van der Veen, L.; Reeves, D.C.; Gonnella, N.C.; Lee, H.; Grinberg, N.; Ma, S.; Krishnamurthy, D.; et al. Catalytic asymmetric hydrogenation of heterocyclic ketone-derived hydrazones, pronounced solvent effect on the inversion of configuration. Tetrahedron Lett. 2011, 52, 3718–3722. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Tan, L.; McWilliams, J.C.; Ramasamy, D.; Sheppard, R. Catalytic Enantioselective Hydrogenation of N-Alkoxycarbonyl Hydrazones: A Practical Synthesis of Chiral Hydrazines. Org. Lett. 2010, 12, 276–279. [Google Scholar] [CrossRef]
- Gavryushin, A.; Polborn, K.; Knochel, P. Novel chiral diphosphine ligands with a pinene core obtained via an allylphosphinite–allylphosphine oxide rearrangement. Tetrahedron Asymmetry 2004, 15, 2279–2288. [Google Scholar] [CrossRef]
- Tappe, K.; Knochel, P. New efficient synthesis of Taniaphos ligands: Application in ruthenium- and rhodium-catalyzed enantioselective hydrogenations. Tetrahedron Asymmetry 2004, 15, 91–102. [Google Scholar] [CrossRef]
- Ireland, T.; Tappe, K.; Grossheimann, G.; Knochel, P. Synthesis of a New Class of Chiral 1,5-Diphosphanylferrocene Ligands and Their Use in Enantioselective Hydrogenation. Chem. A Eur. J. 2002, 8, 843–852. [Google Scholar] [CrossRef]
- Yamazaki, A.; Achiwa, I.; Horikawa, K.; Tsurubo, M.; Achiwa, K. Preparation and Application of a Novel Type of Chiral Biphosphine Ligand (NORPHOS-7X) Containing a Hetero-Functional Group. Synlett 1997, 1997, 455–456. [Google Scholar] [CrossRef]
- Burk, M.J.; Martinez, J.P.; Feaster, J.E.; Cosford, N. Catalytic Asymmetric Reductive Amination of Ketones via Highly Enantioselective Hydrogenation of the C=N Double Bond. Tetrahedron 1994, 50, 4399–4428. [Google Scholar] [CrossRef]
- Burk, M.J.; Gross, M.F. New Chiral 1,1′-Bis(phospholano)ferrocene Ligands for Asymmetric Catalysis. Tetrahedron Lett. 1994, 35, 9363–9366. [Google Scholar] [CrossRef]
- Burk, M.J.; Feaster, J.E. Enantioselective Hydrogenation of the C=N Group: A Catalytic Asymmetric Reductive Animation Procedure. J. Am. Chem. Soc. 1992, 114, 6266–6267. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Lu, S.-M.; Zhou, Y.-G. Highly Enantioselective Pd-Catalyzed Asymmetric Hydrogenation of Activated Imines. J. Org. Chem. 2007, 72, 3729–3734. [Google Scholar] [CrossRef]
- Chen, Z.-P.; Hu, S.-B.; Chen, M.-W.; Zhou, Y.-G. Synthesis of Chiral Fluorinated Hydrazines via Pd-Catalyzed Asymmetric Hydrogenation. Org. Lett. 2016, 18, 2676–2679. [Google Scholar] [CrossRef]
- Chen, Z.-P.; Hu, S.-B.; Zhou, J.; Zhou, Y.-G. Synthesis of Chiral Trifluoromethyl-Substituted Hydrazines via Pd-Catalyzed Asymmetric Hydrogenation and Reductive Amination. ACS Catal. 2015, 5, 6086–6089. [Google Scholar] [CrossRef]
- Chen, Z.-P.; Chen, M.-W.; Shi, L.; Yu, C.-B.; Zhou, Y.-G. Pd-catalyzed asymmetric hydrogenation of fluorinated aromatic pyrazol-5-ols via capture of active tautomers. Chem. Sci. 2015, 6, 3415–3419. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.; Liu, S.; Huang, K.; Zhang, X. Direct Catalytic Asymmetric Reductive Amination of Simple Aromatic Ketones. Org. Lett. 2013, 15, 4354–4357. [Google Scholar] [CrossRef] [PubMed]
- Schuster, C.H.; Dropinski, J.F.; Shevlin, M.; Li, H.; Chen, S. Ruthenium-Catalyzed Enantioselective Hydrogenation of Hydrazones. Org. Lett. 2020, 22, 7562–7566. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, D.; Hu, Y.; Chen, J.; Zhang, Z.; Zhang, W. Nickel-Catalyzed Asymmetric Hydrogenation of Hydrazones. Eur. J. Org. Chem. 2021, 2021, 3421–3425. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, C.; Ma, Y.; Zhang, C.; Li, A.; Tang, B.; Zhou, J.S. Nickel-Catalyzed N-Alkylation of Acylhydrazines and Arylamines Using Alcohols and Enantioselective Examples. Angew. Chem. Int. Ed. 2017, 56, 14702–14706. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Lim, L.H.; Chuanprasit, P.; Hirao, H.; Zhou, J.S. Nickel-Catalyzed Enantioselective Reductive Amination of Ketones with Both Arylamines and Benzhydrazide. Angew. Chem. Int. Ed. 2016, 55, 12083–12087. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, P.; Chuanprasit, P.; Hirao, H.; Zhou, J.S. Nickel-Catalyzed Asymmetric Transfer Hydrogenation of Hydrazones and Other Ketimines. Angew. Chem. Int. Ed. 2015, 54, 5112–5116. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Zhang, J.; Liu, Y.; Gridnev, I.D.; Zhang, W. Cobalt-Catalyzed Asymmetric Hydrogenation of C=N Bonds Enabled by Assisted Coordination and Nonbonding Interactions. Angew. Chem. Int. Ed. 2019, 58, 15767–15771. [Google Scholar] [CrossRef]
- Iwasaki, F.; Matsumura, K.; Onomura, O. Jpn. Kokai Tokkyo Koho (2001). Patent JP 2001270851A, 2 October 2001. [Google Scholar]
- Wang, T.; Di, X.; Wang, C.; Zhou, L.; Sun, J. Reductive Hydrazination with Trichlorosilane: A Method for the Preparation of 1,1-Disubstituted Hydrazines. Org. Lett. 2016, 18, 1900–1903. [Google Scholar] [CrossRef]
- Chen, W.; Tan, C.; Wang, H.; Ye, X. The Development of Organocatalytic Asymmetric Reduction of Carbonyls and Imines Using Silicon Hydrides. Eur. J. Org. Chem. 2021, 2021, 3091–3112. [Google Scholar] [CrossRef]
- Faísca Phillips, A.M.; Pombeiro, A.J.L. Recent advances in organocatalytic enantioselective transfer hydrogenation. Org. Biomol. Chem. 2017, 15, 2307–2340. [Google Scholar] [CrossRef]
- Rossi, S.; Benaglia, M.; Massolo, E.; Raimondi, L. Organocatalytic strategies for enantioselective metal-free reductions. Catal. Sci. Technol. 2014, 4, 2708. [Google Scholar] [CrossRef] [Green Version]
- Kočovský, P.; Malkov, A.V. Lewis Bases as Catalysts in the Reduction of Imines and Ketones with Silanes (n→σ*). In Lewis Base Catalysis in Organic Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; Volume 3, pp. 1077–1112. ISBN 9783527675142. [Google Scholar]
- Jones, S.; Warner, C.J.A. Trichlorosilane mediated asymmetric reductions of the C=N bond. Org. Biomol. Chem. 2012, 10, 2189. [Google Scholar] [CrossRef]
- Guizzetti, S.; Benaglia, M. Trichlorosilane-Mediated Stereoselective Reduction of C=N Bonds. Eur. J. Org. Chem. 2010, 2010, 5529–5541. [Google Scholar] [CrossRef]
- Onomura, O.; Kouchi, Y.; Iwasaki, F.; Matsumura, Y. New organic activators for the enantioselective reduction of aromatic imines with trichlorosilane. Tetrahedron Lett. 2006, 47, 3751–3754. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, F.; Onomura, O.; Mishima, K.; Kanematsu, T.; Maki, T.; Matsumura, Y. First chemo- and stereoselective reduction of imines using trichlorosilane activated with N-formylpyrrolidine derivatives. Tetrahedron Lett. 2001, 42, 2525–2527. [Google Scholar] [CrossRef]
- Malkov, A.V.; Vranková, K.; Stončius, S.; Kočovský, P. Asymmetric Reduction of Imines with Trichlorosilane, Catalyzed by Sigamide, an Amino Acid-Derived Formamide: Scope and Limitations. J. Org. Chem. 2009, 74, 5839–5849. [Google Scholar] [CrossRef] [PubMed]
- Malkov, A.V.; Stončius, S.; MacDougall, K.N.; Mariani, A.; McGeoch, G.D.; Kočovský, P. Formamides derived from N-methyl amino acids serve as new chiral organocatalysts in the enantioselective reduction of aromatic ketimines with trichlorosilane. Tetrahedron 2006, 62, 264–284. [Google Scholar] [CrossRef]
- Malkov, A.V.; Mariani, A.; MacDougall, K.N.; Kočovský, P. Role of Noncovalent Interactions in the Enantioselective Reduction of Aromatic Ketimines with Trichlorosilane. Org. Lett. 2004, 6, 2253–2256. [Google Scholar] [CrossRef]
- Genoni, A.; Benaglia, M.; Mattiolo, E.; Rossi, S.; Raimondi, L.; Barrulas, P.C.; Burke, A.J. Synthesis of an advanced precursor of Rivastigmine: Cinchona -derived quaternary ammonium salts as organocatalysts for stereoselective imine reductions. Tetrahedron Lett. 2015, 56, 5752–5756. [Google Scholar] [CrossRef]
- Barrulas, P.C.; Genoni, A.; Benaglia, M.; Burke, A.J. Cinchona-Derived Picolinamides: Effective Organocatalysts for Stereoselective Imine Hydrosilylation. Eur. J. Org. Chem. 2014, 2014, 7339–7342. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.-Y.; Zhang, M.-M.; Shu, C.; Zhang, Y.-H.; Liao, L.-H.; Yuan, W.-C.; Zhang, X.-M. Enantioselective Lewis-Base-Catalyzed Asymmetric Hydrosilylation of Substituted Benzophenone N-Aryl Imines: Efficient Synthesis of Chiral (Diarylmethyl)amines. Adv. Synth. Catal. 2014, 356, 3539–3544. [Google Scholar] [CrossRef]
- Zhang, Z.; Rooshenas, P.; Hausmann, H.; Schreiner, P. Asymmetric Transfer Hydrogenation of Ketimines with Trichlorosilane: Structural Studies. Synthesis 2009, 2009, 1531–1544. [Google Scholar] [CrossRef] [Green Version]
- Guizzetti, S.; Benaglia, M.; Rossi, S. Highly Stereoselective Metal-Free Catalytic Reduction of Imines: An Easy Entry to Enantiomerically Pure Amines and Natural and Unnatural α-Amino Esters. Org. Lett. 2009, 11, 2928–2931. [Google Scholar] [CrossRef]
- Wang, C.; Wu, X.; Zhou, L.; Sun, J. A highly enantioselective organocatalytic method for reduction of aromatic N-alkyl ketimines. Chem. A Eur. J. 2008, 14, 8789–8792. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Deng, J.; Lin, W.; Zhang, X. Enantioselective hydrosilylation of ketimines with trichlorosilane promoted by chiral N-picolinoylaminoalcohols. Tetrahedron Lett. 2007, 48, 7934–7937. [Google Scholar] [CrossRef]
- Malkov, A.V.; Liddon, A.J.P.S.; Ramírez-López, P.; Bendová, L.; Haigh, D.; Kočovský, P. Remote Chiral Induction in the Organocatalytic Hydrosilylation of Aromatic Ketones and Ketimines. Angew. Chem. Int. Ed. 2006, 45, 1432–1435. [Google Scholar] [CrossRef]
- Žeimytė, S.; Stončius, S. Chiral bipyridine-annulated bicyclo [3.3.1] nonane N-oxide organocatalysts for stereoselective allylation and hydrosilylation reactions. Tetrahedron 2021, 78, 131831. [Google Scholar] [CrossRef]
- Wrzeszcz, Z.; Siedlecka, R. Heteroaromatic N-Oxides in Asymmetric Catalysis: A Review. Molecules 2020, 25, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, M.; Wang, J.; Wu, S.; Zhao, Y.; Ma, Y.; Xing, Y.; Cao, F.; Li, L.; Li, Z.; Zhu, H. Catalytic Mechanism Study on the 1,2- and 1,4-Transfer Hydrogenation of Ketimines and β-Enamino Esters Catalyzed by Axially Chiral Biscarboline-Based Alcohols. Adv. Synth. Catal. 2019, 361, 4602–4610. [Google Scholar] [CrossRef]
- Xing, Y.; Wu, S.; Dong, M.; Wang, J.; Liu, L.; Zhu, H. Synthesis and application of axially chiral biscarbolines with functional N-O and sulfone for 1,2-transfer hydrogenations of ketimines. Tetrahedron 2019, 75, 130495. [Google Scholar] [CrossRef]
- Pei, Y.-N.; Deng, Y.; Li, J.-L.; Liu, L.; Zhu, H.-J. New chiral biscarboline N,N′-dioxide derivatives as catalyst in enantioselective reduction of ketoimines with trichlorosilane. Tetrahedron Lett. 2014, 55, 2948–2952. [Google Scholar] [CrossRef]
- Pan, W.; Deng, Y.; He, J.-B.; Bai, B.; Zhu, H.-J. Highly efficient asymmetric-axle-supported N–O amides in enantioselective hydrosilylation of ketimines with trichlorosilane. Tetrahedron 2013, 69, 7253–7257. [Google Scholar] [CrossRef] [Green Version]
- Warner, C.J.A.; Berry, S.S.; Jones, S. Evaluation of bifunctional chiral phosphine oxide catalysts for the asymmetric hydrosilylation of ketimines. Tetrahedron 2019, 75, 130733. [Google Scholar] [CrossRef]
- Warner, C.J.A.; Reeder, A.T.; Jones, S. P-Chiral phosphine oxide catalysed reduction of prochiral ketimines using trichlorosilane. Tetrahedron Asymmetry 2016, 27, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Cauteruccio, S.; Dova, D.; Benaglia, M.; Genoni, A.; Orlandi, M.; Licandro, E. Synthesis, Characterisation, and Organocatalytic Activity of Chiral Tetrathiahelicene Diphosphine Oxides. Eur. J. Org. Chem. 2014, 2014, 2694–2702. [Google Scholar] [CrossRef]
- Pei, D.; Wang, Z.; Wei, S.; Zhang, Y.; Sun, J. S-Chiral Sulfinamides as Highly Enantioselective Organocatalysts. Org. Lett. 2006, 8, 5913–5915. [Google Scholar] [CrossRef]
- Pei, D.; Zhang, Y.; Wei, S.; Wang, M.; Sun, J. Rationally-Designed S- Chiral Bissulfinamides as Highly Enantioselective Organocatalysts for Reduction of Ketimines. Adv. Synth. Catal. 2008, 350, 619–623. [Google Scholar] [CrossRef]
- Sun, S.; Reep, C.; Zhang, C.; Captain, B.; Peverati, R.; Takenaka, N. Design and synthesis of 3,3′-triazolyl biisoquinoline N,N′-dioxides via Hiyama cross-coupling of 4-trimethylsilyl-1,2,3-triazoles. Tetrahedron Lett. in press. [CrossRef]
- Morgante, P.; Captain, B.; Chouinard, C.D.; Peverati, R.; Takenaka, N. Synthesis of electrophilic N-heterocyclic carbenes based on azahelicene. Tetrahedron Lett. 2020, 61, 152143. [Google Scholar] [CrossRef]
- Reep, C.; Morgante, P.; Peverati, R.; Takenaka, N. Axial-Chiral Biisoquinoline N, N ′-Dioxides Bearing Polar Aromatic C-H Bonds as Catalysts in Sakurai-Hosomi-Denmark Allylation. Org. Lett. 2018, 20, 5757–5761. [Google Scholar] [CrossRef]
- Peng, Z.; Takenaka, N. Applications of Helical-Chiral Pyridines as Organocatalysts in Asymmetric Synthesis. Chem. Rec. 2013, 13, 28–42. [Google Scholar] [CrossRef]
- Chen, J.; Captain, B.; Takenaka, N. Helical Chiral 2,2′-Bipyridine N- Monoxides as Catalysts in the Enantioselective Propargylation of Aldehydes with Allenyltrichlorosilane. Org. Lett. 2011, 13, 1654–1657. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, N.; Sarangthem, R.S.; Captain, B. Helical Chiral Pyridine N -Oxides: A New Family of Asymmetric Catalysts. Angew. Chem. Int. Ed. 2008, 47, 9708–9710. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Reep, C.; Jarvis, J.; Naumann, B.; Captain, B.; Takenaka, N. Asymmetric Catalytic Ketimine Mannich Reactions and Related Transformations. Catalysts 2021, 11, 712. [Google Scholar] [CrossRef]
- Sugiura, M.; Sato, N.; Kotani, S.; Nakajima, M. Lewis base-catalyzed conjugate reduction and reductive aldol reaction of α,β-unsaturated ketones using trichlorosilane. Chem. Commun. 2008, 2, 4309. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Porterfield, M.A.; Wheeler, S.E. Explaining the Disparate Stereoselectivities of N-Oxide Catalyzed Allylations and Propargylations of Aldehydes. Org. Lett. 2012, 14, 5310–5313. [Google Scholar] [CrossRef] [PubMed]
- Denmark, S.E.; Beutner, G.L. Lewis Base Catalysis in Organic Synthesis. Angew. Chem. Int. Ed. 2008, 47, 1560–1638. [Google Scholar] [CrossRef] [PubMed]
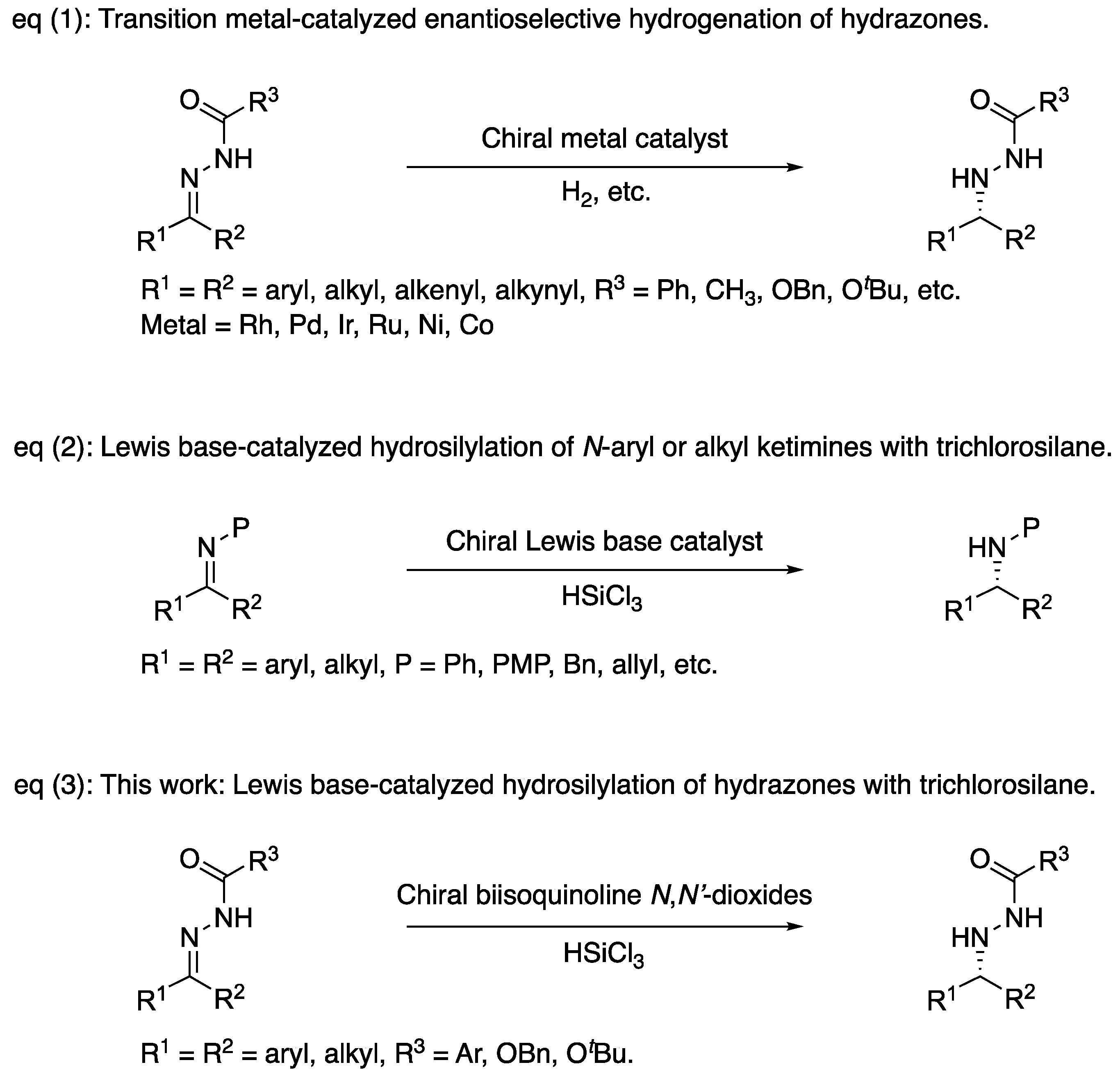


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Xu, C.; Jarvis, J.; Nader, P.; Naumann, B.; Soliven, A.; Peverati, R.; Takenaka, N. Evaluation of 3,3′-Triazolyl Biisoquinoline N,N′-Dioxide Catalysts for Asymmetric Hydrosilylation of Hydrazones with Trichlorosilane. Catalysts 2021, 11, 1103. https://doi.org/10.3390/catal11091103
Sun S, Xu C, Jarvis J, Nader P, Naumann B, Soliven A, Peverati R, Takenaka N. Evaluation of 3,3′-Triazolyl Biisoquinoline N,N′-Dioxide Catalysts for Asymmetric Hydrosilylation of Hydrazones with Trichlorosilane. Catalysts. 2021; 11(9):1103. https://doi.org/10.3390/catal11091103
Chicago/Turabian StyleSun, Shiyu, Changgong Xu, Jamielyn Jarvis, Phillip Nader, Brandon Naumann, Abigail Soliven, Roberto Peverati, and Norito Takenaka. 2021. "Evaluation of 3,3′-Triazolyl Biisoquinoline N,N′-Dioxide Catalysts for Asymmetric Hydrosilylation of Hydrazones with Trichlorosilane" Catalysts 11, no. 9: 1103. https://doi.org/10.3390/catal11091103






