Horizons in Asymmetric Organocatalysis: En Route to the Sustainability and New Applications
Abstract
:1. Introduction
2. Discussion
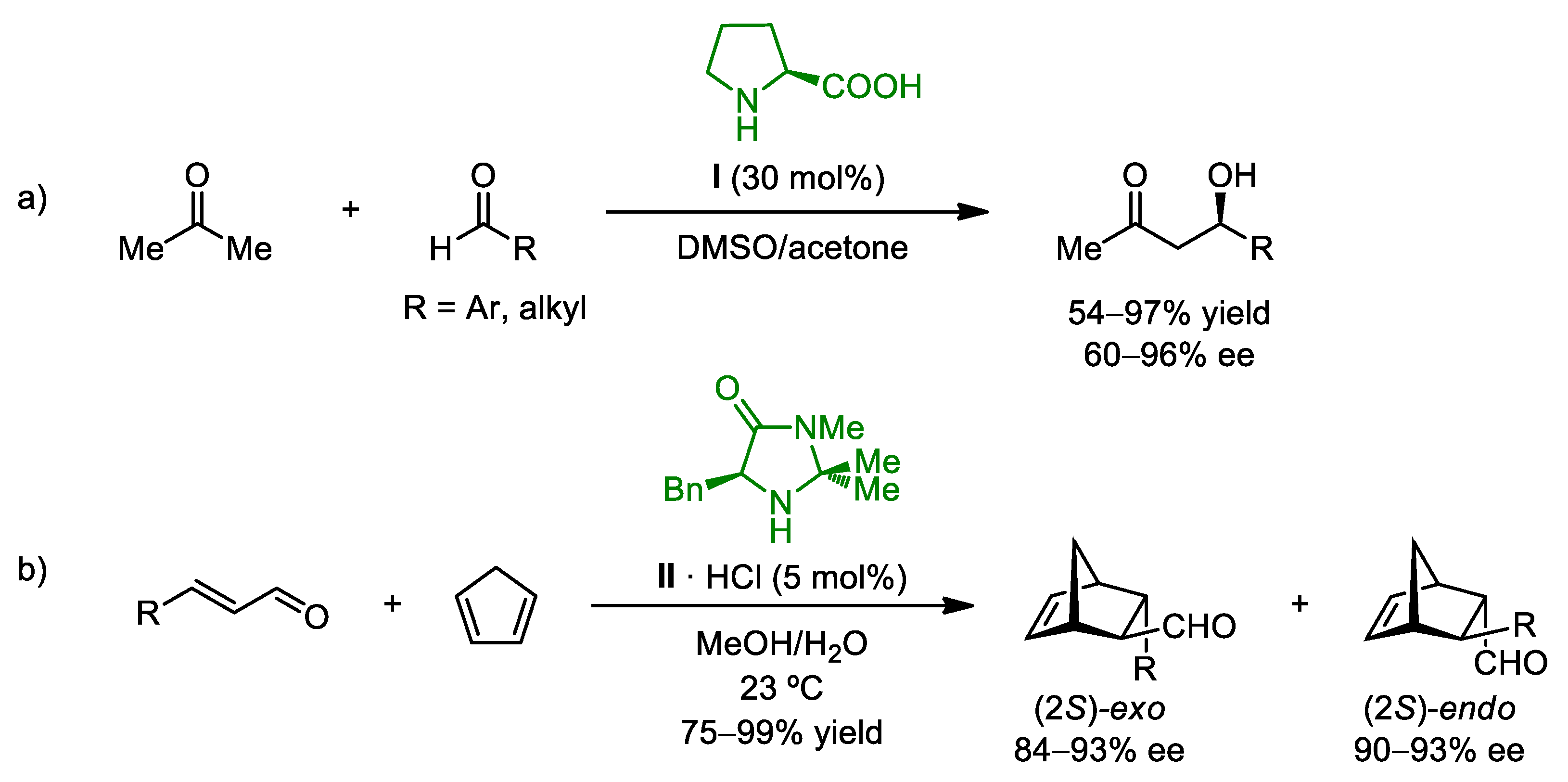
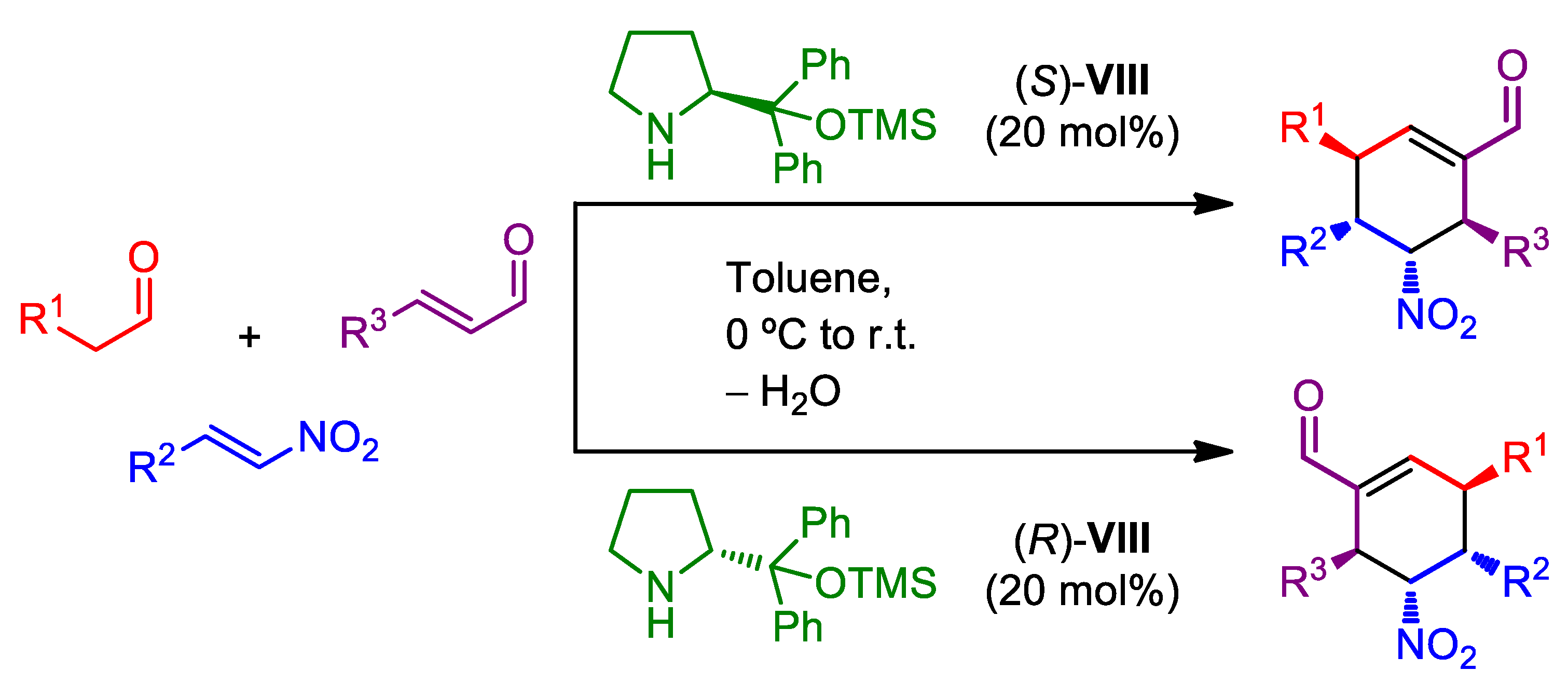
2.1. Organocatalytic Asymmetric Multicomponent Reactions
2.2. New Organocatalytic Applications
2.3. Increasingly Complex Catalytic Structures
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carlone, A.; Bernardi, L. Enantioselective Organocatalytic Approaches to Active Pharmaceutical Ingredients—Selected Industrial Examples. In Organocatalysis: Stereoselective Reactions and Applications in Organic Synthesis; Benaglia, M., Ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2021; pp. 401–434. [Google Scholar] [CrossRef]
- Marckwald, W. Ueber Asymmetrische Synthese. Ber. Dtsch. Chem. Ges. 1904, 37, 349–354. [Google Scholar] [CrossRef] [Green Version]
- List, B.; Lerner, R.A.; Barbas, C.F., III. Proline-Catalyzed Direct Asymmetric Aldol Reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels–Alder Reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Kelly, T.R.; Meghani, P.; Ekkundi, V.S. Diels–Alder reactions: Rate acceleration promoted by a biphenylenediol. Tetrahedron Lett. 1990, 31, 3381–3384. [Google Scholar] [CrossRef]
- Blake, J.F.; Jorgensen, W.L. Solvent Effects on a Diels–Alder Reaction from Computer Simulations. J. Am. Chem. Soc. 1991, 113, 7430–7432. [Google Scholar] [CrossRef]
- Severance, D.L.; Jorgensen, W.L. Effects of Hydration on the Claisen Rearrangement of Allyl Vinyl Ether from Computer Simulations. J. Am. Chem. Soc. 1992, 114, 10966–10968. [Google Scholar] [CrossRef]
- Curran, D.P.; Kuo, L.H. Altering the Stereochemistry of Allylation Reactions of Cyclic α-Sulfinyl Radicals with Diarylureas. J. Org. Chem. 1994, 59, 3259–3261. [Google Scholar] [CrossRef]
- Sigman, M.S.; Jacobsen, E.N. Schiff Base Catalysts for the Asymmetric Strecker Reaction Identified and Optimized from Parallel Synthetic Libraries. J. Am. Chem. Soc. 1998, 120, 4901–4902. [Google Scholar] [CrossRef]
- Bredig, G.; Fiske, P.S. Asymmetric Syntheses Caused by Catalyzers. Biochem. Z. 1913, 46, 7–23. [Google Scholar]
- Bredig, G.; Minaeff, M. Asymmetric synthesis by means of catalysts. II. Biochem. Z. 1932, 249, 241–244. [Google Scholar]
- Pracejus, H. Organische Katalysatoren, LXI. Asymmetrische Synthesen mit Ketenen, I. Alkaloid-katalysierte asymmetrische Synthesen von α-Phenyl-propionsäureestern. Justus Liebigs Ann. Chem. 1960, 634, 9–22. [Google Scholar] [CrossRef]
- Pracejus, H.; Mätje, H. Organische Katalysatoren. LXXI Asymmetrische Synthesen mit Ketenen. IV. Zusammenhänge zwischen dem räumlichen Bau einiger alkaloidartiger Katalysatoren und ihren stereospezifischen Wirkungen bei asymmetrischen Estersynthesen. J. Prakt. Chem. 1964, 24, 195–205. [Google Scholar] [CrossRef]
- Hiemstra, H.; Wynberg, H. Addition of Aromatic Thiols to Conjugated Cycloalkenones, Catalyzed by Chiral β-Hydroxy Amines. A Mechanistic Study of Homogeneous Catalytic Asymmetric Synthesis. J. Am. Chem. Soc. 1981, 103, 417–430. [Google Scholar] [CrossRef]
- Ukai, T.; Tanaka, R.; Dokawa, T. アチロイン縮合に於ける新觸媒群に就て (第1報) チアツオリウム鹽基或は共核開裂成績體の作用. J. Pharm. Soc. Jpn. 1943, 63, 296–300. [Google Scholar] [CrossRef]
- Makosza, M. Reactions of organic anions. XI. Catalytic alkylation of indene. Tetrahedron Lett. 1966, 7, 4621–4624. [Google Scholar] [CrossRef]
- Chinchilla, R.; Najera, C.; Sanchez-Agullo, P. Enantiomerically pure guanidine-catalysed asymmetric nitroaldol reaction. Tetrahedron Asymmetry 1994, 5, 1393–1402. [Google Scholar] [CrossRef]
- Akiyama, T.; Itoh, J.; Yokota, K.; Fuchibe, K. Enantioselective Mannich-type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. 2004, 43, 1566–1568. [Google Scholar] [CrossRef]
- Uraguchi, D.; Terada, M. Chiral Brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 2004, 126, 5356–5357. [Google Scholar] [CrossRef]
- Herrera, R.P.; Sgarzani, V.; Bernardi, L.; Ricci, A. Catalytic Enantioselective Friedel–Crafts Alkylation of Indoles with Nitroalkenes by a Simple Thiourea Organocatalyst. Angew. Chem. Int. Ed. 2005, 44, 6576–6579. [Google Scholar] [CrossRef]
- Bertelsen, S.; Marigo, M.; Brandes, S.; Dinér, P.; Jørgensen, K.A. Dienamine Catalysis: Organocatalytic Asymmetric γ-Amination of α,β-Unsaturated Aldehydes. J. Am. Chem. Soc. 2006, 128, 12973–12980. [Google Scholar] [CrossRef]
- Jia, Z.-J.; Jiang, H.; Li, J.-L.; Gschwend, B.; Li, Q.-Z.; Yin, X.; Grouleff, J.; Chen, Y.-C.; Jørgensen, K.A. Trienamines in Asymmetric Organocatalysis: Diels–Alder and Tandem Reactions. J. Am. Chem. Soc. 2011, 133, 5053–5061. [Google Scholar] [CrossRef]
- Dessole, G.; Herrera, R.P.; Ricci, A. H-Bonding Organocatalysed Friedel–Crafts Alkylation of Aromatic and Heteroaromatic Systems with Nitroolefins. Synlett 2004, 2374–2378. [Google Scholar] [CrossRef]
- Alegre-Requena, J.V.; Marqués-López, E.; Herrera, R.P. Optimizing Accuracy and Computational Cost in Theoretical Squaramide Catalysis: The Henry Reaction. Chem. Eur. J. 2017, 23, 15336–15347. [Google Scholar] [CrossRef] [Green Version]
- Alegre-Requena, J.V.; Marqués-López, E.; Herrera, R.P. “Push-Pull π+/π-” (PPππ) Systems in Catalysis. ACS Catal. 2017, 7, 6430–6439. [Google Scholar] [CrossRef] [Green Version]
- Alegre-Requena, J.V.; Marqués-López, E.; Herrera, R.P. Trifunctional squaramide catalyst for efficient enantioselective Henry reaction activation. Adv. Synth. Catal. 2016, 358, 1801–1809. [Google Scholar] [CrossRef] [Green Version]
- Mann, J. (Ed.) Chemical Aspects of Biosynthesis. In Oxford Chemistry Primers; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Enders, D.; Hüttl, M.R.M.; Grondal, C.; Raabe, G. Control of four stereocentres in a triple cascade organocatalytic reaction. Nature 2006, 441, 861–863. [Google Scholar] [CrossRef]
- Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. [Google Scholar] [CrossRef]
- Chen, W.; Du, W.; Duan, Y.-Z.; Wu, Y.; Yang, S.-Y.; Chen, Y.-C. Enantioselective 1,3-Dipolar Cycloaddition of Cyclic Enones Catalyzed by Multifunctional Primary Amines: Beneficial Effects of Hydrogen Bonding. Angew. Chem. Int. Ed. 2007, 46, 7667–7670. [Google Scholar] [CrossRef]
- Nakoji, M.; Kanayama, T.; Okino, T.; Takemoto, Y. Chiral Phosphine-Free Pd-Mediated Asymmetric Allylation of Prochiral Enolate with a Chiral Phase-Transfer Catalyst. Org. Lett. 2001, 3, 3329–3331. [Google Scholar] [CrossRef]
- Nakoji, M.; Kanayama, T.; Okino, T.; Takemoto, Y. Pd-Catalyzed Asymmetric Allylic Alkylation of Glycine Imino Ester Using a Chiral Phase-Transfer Catalyst. J. Org. Chem. 2002, 67, 7418–7423. [Google Scholar] [CrossRef]
- Ibrahem, I.; Breistein, P.; Córdova, A. One-Pot Three-Component Catalytic Enantioselective Synthesis of Homoallylboronates. Angew. Chem. Int. Ed. 2011, 50, 12036–12041. [Google Scholar] [CrossRef]
- Jiang, G.; List, B. Direct Asymmetric α-Allylation of Aldehydes with Simple Allylic Alcohols Enabled by the Concerted Action of Three Different Catalysts. Angew. Chem. Int. Ed. 2011, 50, 9471–9474. [Google Scholar] [CrossRef]
- Beeson, T.D.; Mastracchio, A.; Hong, J.-B.; Ashton, K.; MacMillan, D.W.C. Enantioselective Organocatalysis Using SOMO Activation. Science 2007, 316, 582–585. [Google Scholar] [CrossRef]
- Sibi, M.P.; Hasegawa, M. Organocatalysis in Radical Chemistry. Enantioselective α-Oxyamination of Aldehydes. J. Am. Chem. Soc. 2007, 129, 4124–4125. [Google Scholar] [CrossRef] [Green Version]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Silvi, M.; Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature 2018, 554, 41–49. [Google Scholar] [CrossRef]
- Westermann, B.; Neuhaus, C. Dihydroxyacetone in Amino Acid Catalyzed Mannich-Type Reactions. Angew. Chem. Int. Ed. Engl. 2005, 44, 4077–4079. [Google Scholar] [CrossRef]
- Chatel, G.; Varma, R.S. Ultrasound and microwave irradiation: Contributions of alternative physicochemical activation methods to Green Chemistry. Green Chem. 2019, 21, 6043–6050. [Google Scholar] [CrossRef]
- Kantam, M.L.; Rajasekhar, C.V.; Gopikrishna, G.; Reddy, K.R.; Choudary, B.M. Proline catalyzed two-component, three-component and self-asymmetric Mannich reactions promoted by ultrasonic conditions. Tetrahedron Lett. 2006, 47, 5965–5967. [Google Scholar] [CrossRef]
- Rodríguez, B.; Rantanen, T.; Bolm, C. Solvent-Free Asymmetric Organocatalysis in a Ball Mill. Angew. Chem. Int. Ed. 2006, 45, 6924–6926. [Google Scholar] [CrossRef]
- Chauhan, P.; Chimni, S.S. Mechanochemistry assisted asymmetric organocatalysis: A sustainable approach. Beilstein J. Org. Chem. 2012, 8, 2132–2141. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.L.; Franke, P.T.; Nielsen, L.T.; Daasbjerg, K.; Jørgensen, K.A. Anodic Oxidation and Organocatalysis: Direct Regio- and Stereoselective Access to meta-Substituted Anilines by α-Arylation of Aldehydes. Angew. Chem. Int. Ed. 2010, 49, 129–133. [Google Scholar] [CrossRef]
- Yan, M.; Kawamata, Y.; Baran, P.S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. [Google Scholar] [CrossRef]
- Kotrusz, P.; Kmentová, I.; Gotov, B.; Toma, Š.; Solčániová, E. Proline-catalysed asymmetric aldol reaction in the room temperature ionic liquid [bmim]PF6. Chem. Commun. 2002, 21, 2510–2511. [Google Scholar] [CrossRef]
- Zalewska, K.; Branco, L.C. Organocatalysis with Chiral Ionic Liquids. Mini-Rew. Org. Chem. 2014, 11, 141–153. [Google Scholar] [CrossRef]
- Alonso, D.A.; Burlingham, S.-J.; Chinchilla, R.; Guillena, G.; Ramón, D.J.; Tiecco, M. Asymmetric Organocatalysis in Deep Eutectic Solvents. Eur. J. Org. Chem. 2021, 2021, 4065–4071. [Google Scholar] [CrossRef]
- Fanjul-Mosteirín, N.; del Amo, V. Organocatalytic transformations in deep eutectic solvents: Green methodologies made greener. Tetrahedron 2021, 84, 131967. [Google Scholar] [CrossRef]
- Sakthivel, K.; Notz, W.; Bui, T.; Barbas, C.F., III. Amino Acid Catalyzed Direct Asymmetric Aldol Reactions: A Bioorganic Approach to Catalytic Asymmetric Carbon-Carbon Bond-Forming Reactions. J. Am. Chem. Soc. 2001, 123, 5260–5267. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Noto, R. Water in Stereoselective Organocatalytic Reactions. Adv. Synth. Catal. 2009, 351, 33–57. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Avila-Ortiz, C.G.; Juaristi, E. Novel Methodologies for Chemical Activation in Organic Synthesis under Solvent-Free Reaction Conditions. Molecules 2020, 25, 3579. [Google Scholar] [CrossRef]
- Cozzi, F. Immobilization of Organic Catalysts: When, Why, and How. Adv. Synth. Catal. 2006, 348, 1367–1390. [Google Scholar] [CrossRef]
- Ferré, M.; Pleixats, R.; Man, M.W.C.; Cattoën, X. Recyclable organocatalysts based on hybrid silicas. Green Chem. 2016, 18, 881–922. [Google Scholar] [CrossRef] [Green Version]
- Bruckmann, A.; Krebs, A.; Bolm, C. Organocatalytic reactions: Effects of ball milling, microwave and ultrasound irradiation. Green Chem. 2008, 10, 1131–1141. [Google Scholar] [CrossRef]
- Krištofíková, D.; Modrocká, V.; Mečiarová, M.; Šebesta, R. Green Asymmetric Organocatalysis. ChemSusChem 2020, 13, 2828–2858. [Google Scholar] [CrossRef]
- Antenucci, A.; Dughera, S.; Renzi, P. Green Chemistry Meets Asymmetric Organocatalysis: A Critical Overview on Catalysts Synthesis. ChemSusChem 2021, 14, 2785–2853. [Google Scholar] [CrossRef]
- Zhu, J.; Bienaymé, H. (Eds.) Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Zhu, J.; Wang, Q.; Wang, M.-X. (Eds.) Multicomponent Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Herrera, R.P.; Marqués-López, E. (Eds.) Multicomponent Reactions. Concepts and Applications for Design and Synthesis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Walji, A.M.; MacMillan, D.W.C. Strategies to Bypass the Taxol Problem. Enantioselective Cascade Catalysis, a New Approach for the Efficient Construction of Molecular Complexity. Synlett 2007, 2007, 1477–1489. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Navas, J.; Sabater, M.J. Advances in One-Pot Synthesis through Borrowing Hydrogen Catalysis. Chem. Rev. 2018, 118, 1410–1459. [Google Scholar] [CrossRef]
- Yu, X.; Wang, W. Organocatalysis: Asymmetric cascade reactions catalysed by chiral secondary amines. Org. Biomol. Chem. 2008, 6, 2037–2046. [Google Scholar] [CrossRef]
- Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem. 2010, 2, 167–178. [Google Scholar] [CrossRef]
- Vetica, F.; de Figueiredo, R.M.; Orsini, M.; Tofani, D.; Gasperi, T. Recent Advances in Organocatalytic Cascade Reactions toward the Formation of Quaternary Stereocenters. Synthesis 2015, 47, 2139–2184. [Google Scholar] [CrossRef]
- Evans, C.S.; Davis, L.O. Recent Advances in Organocatalyzed Domino C–C Bond-Forming Reactions. Molecules 2018, 23, 33. [Google Scholar] [CrossRef] [Green Version]
- Bortolami, M.; Leonelli, F.; Feroci, M.; Vetica, F. Step Economy in the Stereoselective Synthesis of Functionalized Oxindoles via Organocatalytic Domino/One-pot Reactions. Curr. Org. Chem. 2021, 25, 1321–1344. [Google Scholar] [CrossRef]
- Enders, D.; Grondal, C.; Hüttl, M.R.M. Asymmetric Organocatalytic Domino Reactions. Angew. Chem. Int. Ed. 2007, 46, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Guillena, G.; Ramón, D.J.; Yus, M. Organocatalytic enantioselective multicomponent reactions (OEMCRs). Tetrahedron Asymmetry 2007, 18, 693–700. [Google Scholar] [CrossRef]
- Parvin, T.; Yadava, R.; Choudhury, L.H. Recent applications of thiourea-based organocatalysts in asymmetric multicomponent reactions (AMCRs). Org. Biomol. Chem. 2020, 18, 5513–5532. [Google Scholar] [CrossRef]
- Volla, C.M.R.; Atodiresei, I.; Rueping, M. Catalytic C−C Bond-Forming Multi-Component Cascade or Domino Reactions: Pushing the Boundaries of Complexity in Asymmetric Organocatalysis. Chem. Rev. 2014, 114, 2390–2431. [Google Scholar] [CrossRef] [PubMed]
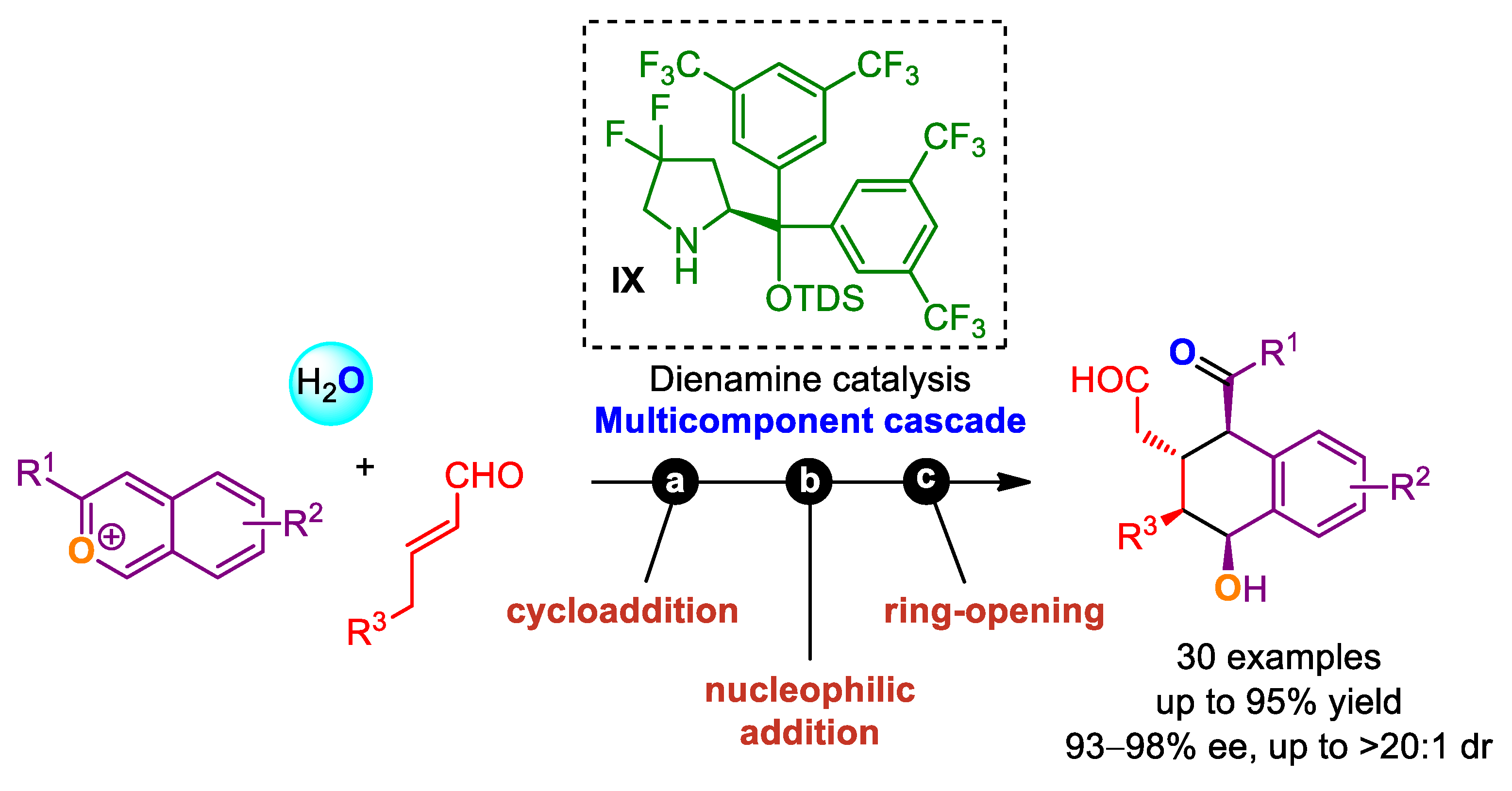
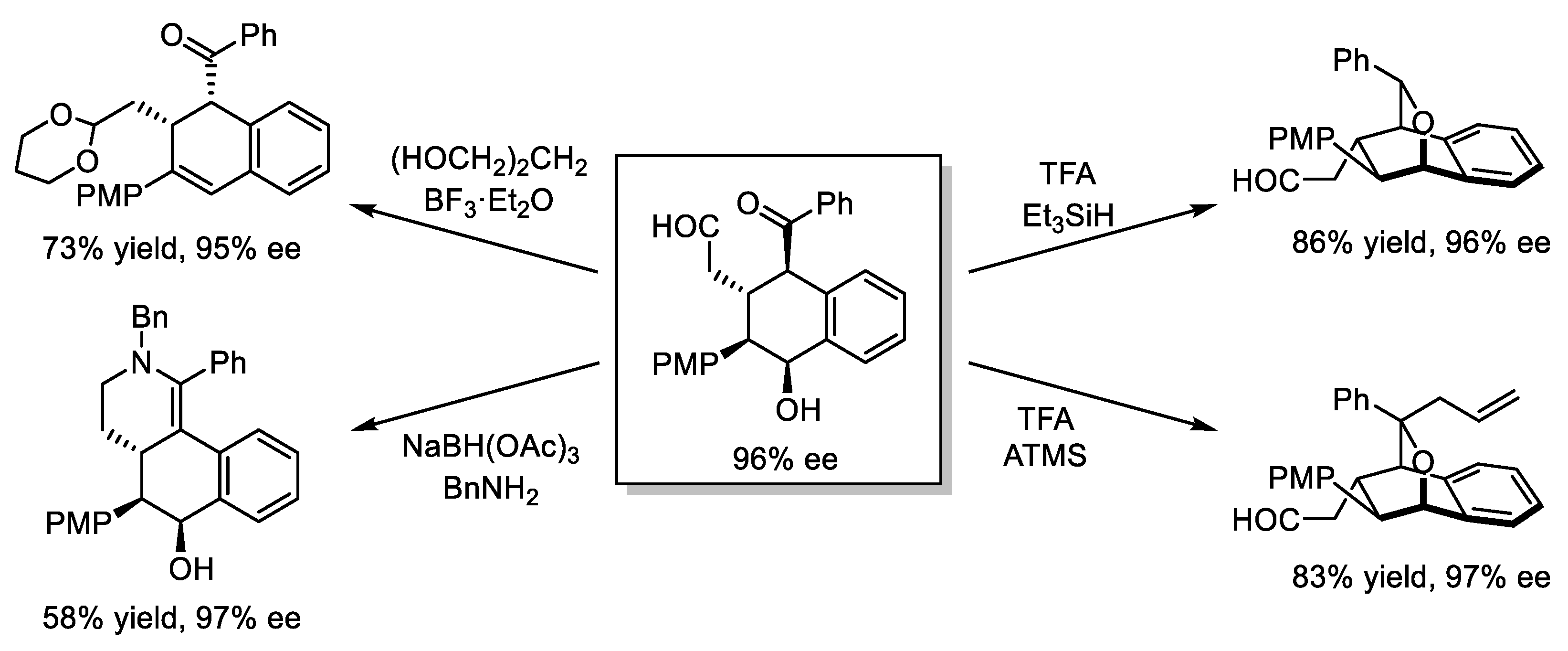
- Liu, Y.; Izzo, J.A.; McLeod, D.; Ričko, S.; Svenningsen, E.B.; Poulsen, T.B.; Jørgensen, K.A. Organocatalytic Asymmetric Multicomponent Cascade Reaction for the Synthesis of Contiguously Substituted Tetrahydronaphthols. J. Am. Chem. Soc. 2021, 143, 8208–8220. [Google Scholar] [CrossRef]
- Francotte, E.; Lindner, W. (Eds.) Chirality in Drug Research; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Nicolaou, K.C.; Montagnon, T.; Snyder, S.A. Tandem reactions, cascade sequences, and biomimetic strategies in total synthesis. Chem. Commun. 2003, 5, 551–564. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, R.M.; Christmann, M. Organocatalytic Synthesis of Drugs and Bioactive Natural Products. Eur. J. Org. Chem. 2007, 2007, 2575–2600. [Google Scholar] [CrossRef]
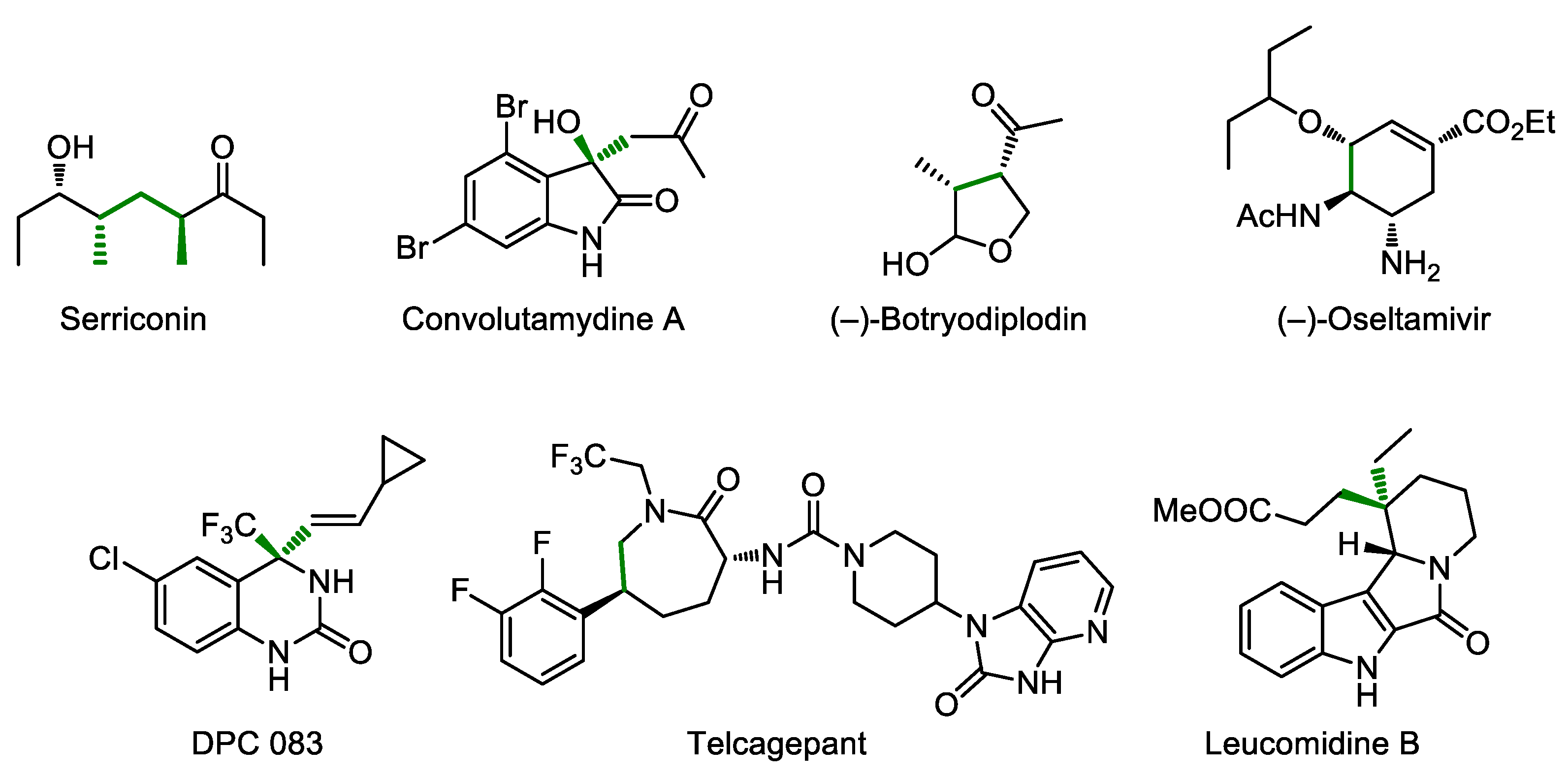
- Marqués-López, E.; Herrera, R.P.; Christmann, M. Asymmetric organocatalysis in total synthesis—A trial by fire. Nat. Prod. Rep. 2010, 27, 1138–1167. [Google Scholar] [CrossRef]
- Alemán, J.; Cabrera, S. Applications of asymmetric organocatalysis in medicinal chemistry. Chem. Soc. Rev. 2013, 42, 774–793. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.-F. Total synthesis of natural and pharmaceutical products powered by organocatalytic reactions. Tetrahedron Lett. 2015, 56, 2133–2140. [Google Scholar] [CrossRef] [Green Version]
- Pellissier, H. Asymmetric Organocatalytic Tandem/Domino Reactions to Access Bioactive Products. Curr. Org. Chem. 2021, 25, 1457–1471. [Google Scholar] [CrossRef]
- Hughes, D.L. Asymmetric Organocatalysis in Drug Development–Highlights of Recent Patent Literature. Org. Process Res. Dev. 2018, 22, 574–584. [Google Scholar] [CrossRef]
- Ward, D.E.; Jheengut, V.; Beye, G.E. Thiopyran Route to Polypropionates: An Efficient Synthesis of Serricornin. J. Org. Chem. 2006, 71, 8989–8992. [Google Scholar] [CrossRef] [PubMed]
- Malkov, A.V.; Kabeshov, M.A.; Bella, M.; Kysilka, O.; Malyshev, D.A.; Pluháčková, K.; Kočovský, P. Vicinal Amino Alcohols as Organocatalysts in Asymmetric Cross-Aldol Reaction of Ketones: Application in the Synthesis of Convolutamydine A. Org. Lett. 2007, 9, 5473–5476. [Google Scholar] [CrossRef]
- Andrey, O.; Vidonne, A.; Alexakis, A. Organocatalytic Michael addition, a convenient tool in total synthesis. First asymmetric synthesis of (−)-botryodiplodin. Tetrahedron Lett. 2003, 44, 7901–7904. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Ishikawa, H.; Koshino, H.; Hayashi, Y. One-Pot Synthesis of (−)-Oseltamivir and Mechanistic Insights into the Organocatalyzed Michael Reaction. Chem. Eur. J. 2013, 19, 17789–17800. [Google Scholar] [CrossRef]
- Zhang, F.-G.; Zhu, X.-Y.; Li, S.; Nie, J.; Ma, J.-A. Highly Enantioselective Organocatalytic Strecker Reaction of Cyclic N-Acyl Trifluoromethylketimines: Synthesis of anti-HIV Drug DPC 083. Chem. Commun. 2012, 48, 11552–11554. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zacuto, M.; Yoshikawa, N.; Desmond, R.; Hoerrner, S.; Itoh, T.; Journet, M.; Humphrey, G.R.; Cowden, C.; Strotman, N.; et al. Asymmetric Synthesis of Telcagepant, a CGRP Receptor Antagonist for the Treatment of Migraine. J. Org. Chem. 2010, 75, 7829–7841. [Google Scholar] [CrossRef] [PubMed]
- Gualtierotti, J.-B.; Pasche, D.; Wang, Q.; Zhu, J. Phosphoric acid catalyzed desymmetrization of bicyclic bislactones bearing an all-carbon stereogenic center: Total syntheses of (−)-Rhazinilam and (−)-Leucomidine B. Angew. Chem. Int. Ed. 2014, 53, 9926–9930. [Google Scholar] [CrossRef]
- Han, B.; He, X.-H.; Liu, Y.-Q.; He, G.; Peng, C.; Li, J.-L. Asymmetric organocatalysis: An enabling technology for medicinal chemistry. Chem. Soc. Rev. 2021, 50, 1522–1586. [Google Scholar] [CrossRef] [PubMed]
- Alcaine, A.; Marqués-López, E.; Herrera, R.P. Synthesis of Interesting β-Nitrohydrazides through Thiourea Organocatalyzed aza-Michael Addition. RSC Adv. 2014, 4, 9856–9865. [Google Scholar] [CrossRef] [Green Version]
- Sonsona, I.G.; Alegre-Requena, J.V.; Marqués-López, E.; Gimeno, M.C.; Herrera, R.P. Asymmetric organocatalyzed aza-Henry reaction of hydrazones: Experimental and computational studies. Chem. Eur. J. 2020, 26, 5469–5478. [Google Scholar] [CrossRef]
- Sonsona, I.G.; Marqués-López, E.; Gimeno, M.C.; Herrera, R.P. First aromatic amine organocatalyzed activation of ketones. New J. Chem. 2019, 43, 12233–12240. [Google Scholar] [CrossRef] [Green Version]
- Marqués-López, E.; Herrera, R.P.; Marks, T.; Jacobs, W.C.; Könning, D.; de Figueiredo, R.M.; Christmann, M. Crossed Intramolecular Rauhut-Currier-Type Reactions via Dienamine Activation. Org. Lett. 2009, 11, 4116–4119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marqués-López, E.; Herrera, R.P.; Marks, T.; Jacobs, W.C.; Christmann, M. Enantioselective Rauhut-Currier-Type Cyclizations via Dienamine Activation: Scope and Mechanism. Synthesis 2013, 45, 1016–1028. [Google Scholar] [CrossRef] [Green Version]
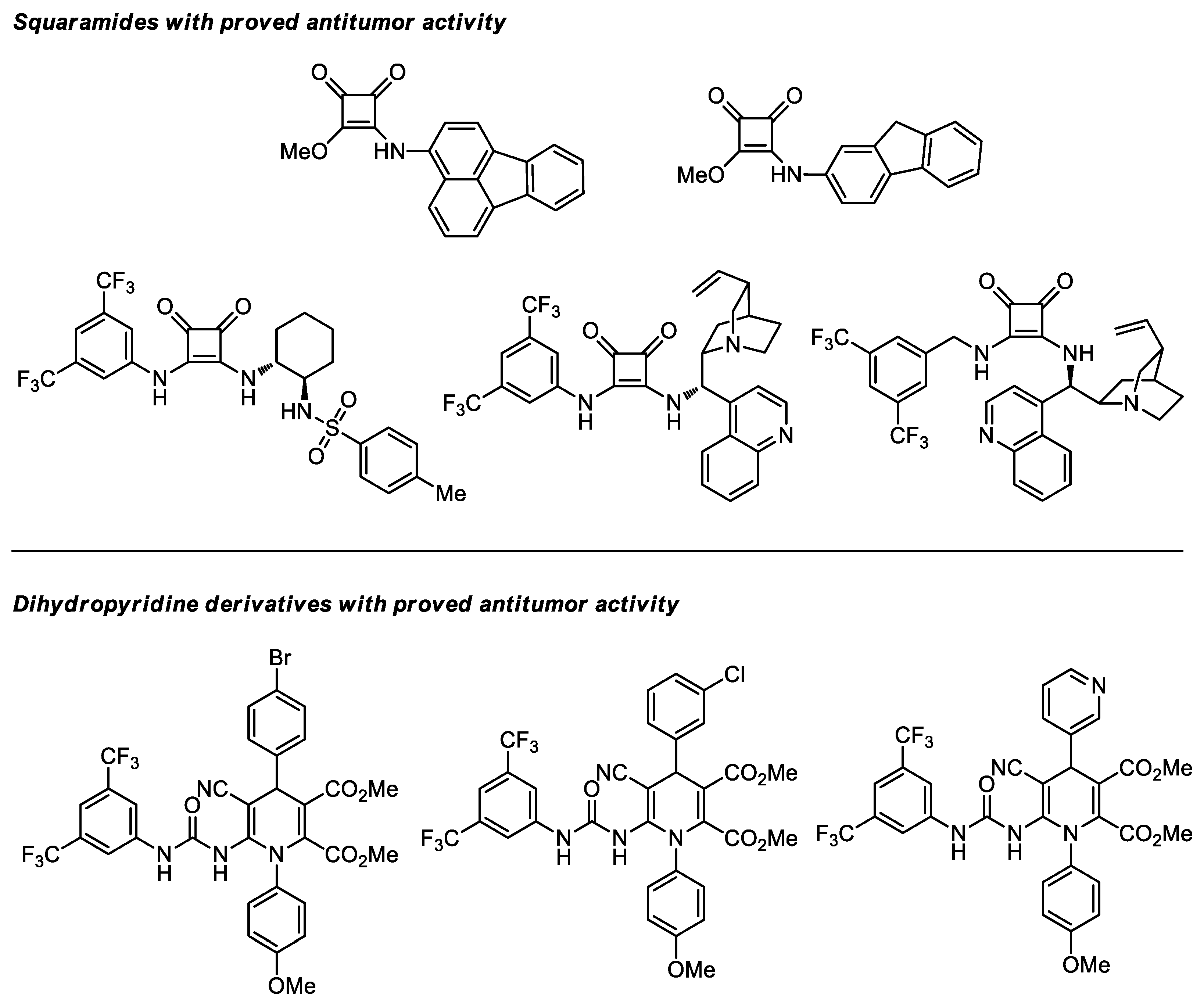
- Sonsona, I.G.; Vicenzi, A.; Guidotti, M.; Bisag, G.D.; Fochi, M.; Herrera, R.P.; Bernardi, L. Investigation of Squaramide Catalysts in the Aldol Reaction en Route to Funapide. Eur. J. Org. Chem. 2022, 2022, e202101254. [Google Scholar] [CrossRef]
- Fernández-Moreira, V.; Alegre-Requena, J.V.; Herrera, R.P.; Marzo, I.; Gimeno, M.C. Synthesis of luminescent squaramide monoesters: Cytotoxicity and cell imaging studies in HeLa cells. RSC Adv. 2016, 6, 14171–14177. [Google Scholar] [CrossRef] [Green Version]
- Quintana, M.; Alegre-Requena, J.V.; Marqués-López, E.; Herrera, R.P.; Triola, G. Squaramides with cytotoxic activity against human gastric carcinoma cells HGC-27: Synthesis and mechanism of action. Med. Chem. Commun. 2016, 7, 550–561. [Google Scholar] [CrossRef] [Green Version]
- Auria-Luna, F.; Marqués-López, E.; Romanos, E.; Fernández-Moreira, V.; Gimeno, M.C.; Marzo, I.; Herrera, R.P. Novel ureido-dihydropyridine scaffolds as theranostic agents. Bioorg. Chem. 2020, 105, 104364. [Google Scholar] [CrossRef] [PubMed]
- Liu, F. The Upside of Downsizing: Asymmetric Trifunctional Organocatalysts as Small Enzyme Mimics for Cooperative Enhancement of Both Rate and Enantioselectivity with Regulation. Chirality 2013, 25, 675–683. [Google Scholar] [CrossRef]
- D’Souza, V.T.; Bender, M.L. Miniature Organic Models of Enzymes. Acc. Chem. Res. 1987, 20, 146–152. [Google Scholar] [CrossRef]
- Gimeno, M.C.; Herrera, R.P. Hydrogen Bonding and Internal or External Lewis or Brønsted Acid Assisted (Thio)urea Catalysts. Eur. J. Org. Chem. 2020, 2020, 1057–1068. [Google Scholar] [CrossRef]
- Miyabe, H.; Takemoto, Y. Discovery and Application of Asymmetric Reaction by Multi-Functional Thioureas. Bull. Chem. Soc. Jpn. 2008, 81, 785–795. [Google Scholar] [CrossRef] [Green Version]
- Connon, S.J. Asymmetric catalysis with bifunctional cinchona alkaloid-based urea and thiourea organocatalysts. Chem. Commun. 2008, 2499–2510. [Google Scholar] [CrossRef] [Green Version]
- Sonsona, I.G.; Marqués-López, E.; Herrera, R.P. The aminoindanol core as a key scaffold in bifunctional organocatalysts. Beilstein J. Org. Chem. 2016, 12, 505–523. [Google Scholar] [CrossRef] [Green Version]
- Kenny, R.; Liu, F. Trifunctional Organocatalysts: Catalytic Proficiency by Cooperative Activation. Eur. J. Org. Chem. 2015, 2015, 5304–5319. [Google Scholar] [CrossRef]
- Kenny, R.; Liu, F. Cooperative Trifunctional Organocatalysts for Proficient Proton Transfer Reactions. Chem. Rec. 2017, 17, 535–553. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Candeias, N.R.; Barbas, C.F., III. Construction of bispirooxindoles containing three quaternary stereocentres in a cascade using a single multifunctional organocatalyst. Nat. Chem. 2011, 3, 473–477. [Google Scholar] [CrossRef]
- Zhong, N.-J.; Liu, L.; Wang, D.; Chen, Y.-J. Enantioselective tandem reaction of chromone-derived Morita–Baylis–Hillman carbonates with benzylamines catalyzed by a trifunctional organocatalyst: The synthesis of chiral 3-aminomethylene-flavanones. Chem. Commun. 2013, 49, 3697–3699. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-H.; Li, X.; Liu, D.; Cui, W.-C.; Ju, X.; Wang, S.; Yao, Z.-J. Target-oriented multifunctional organocatalysis for enantioselective synthesis of bicyclo[3.3.1]nona-2,6-dien-9-ones. A formal asymmetric synthesis of huperzine A. Tetrahedron 2012, 68, 6240–6248. [Google Scholar] [CrossRef]
- Garnier, J.-M.; Liu, F. Trifunctional organocatalyst-promoted counterion catalysis for fast and enantioselective aza-Morita–Baylis–Hillman reactions at ambient temperature. Org. Biomol. Chem. 2009, 7, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Marqués-López, E.; Herrera, R.P. Asymmetric Organocatalysis in Total Synthesis. In Comprehensive Enantioselective Organocatalysis; Dalko, P., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; Chapter 13; pp. 1359–1383. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardevines, S.; Marqués-López, E.; Herrera, R.P. Horizons in Asymmetric Organocatalysis: En Route to the Sustainability and New Applications. Catalysts 2022, 12, 101. https://doi.org/10.3390/catal12010101
Ardevines S, Marqués-López E, Herrera RP. Horizons in Asymmetric Organocatalysis: En Route to the Sustainability and New Applications. Catalysts. 2022; 12(1):101. https://doi.org/10.3390/catal12010101
Chicago/Turabian StyleArdevines, Sandra, Eugenia Marqués-López, and Raquel P. Herrera. 2022. "Horizons in Asymmetric Organocatalysis: En Route to the Sustainability and New Applications" Catalysts 12, no. 1: 101. https://doi.org/10.3390/catal12010101
APA StyleArdevines, S., Marqués-López, E., & Herrera, R. P. (2022). Horizons in Asymmetric Organocatalysis: En Route to the Sustainability and New Applications. Catalysts, 12(1), 101. https://doi.org/10.3390/catal12010101