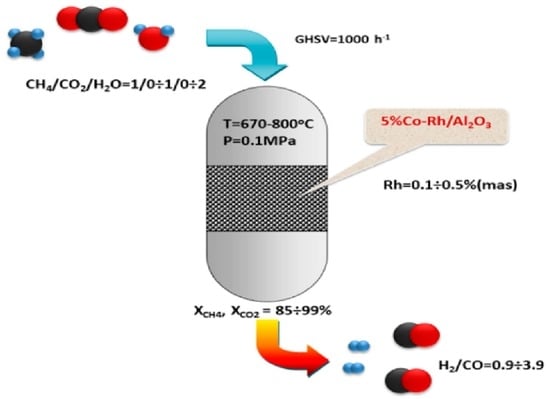
Bimetallic Co-Rh Systems as a Prospective Base for Design of CH4 Reforming Catalysts to Produce Syngas with a Controllable Composition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterisation
2.2. Catalyst Test
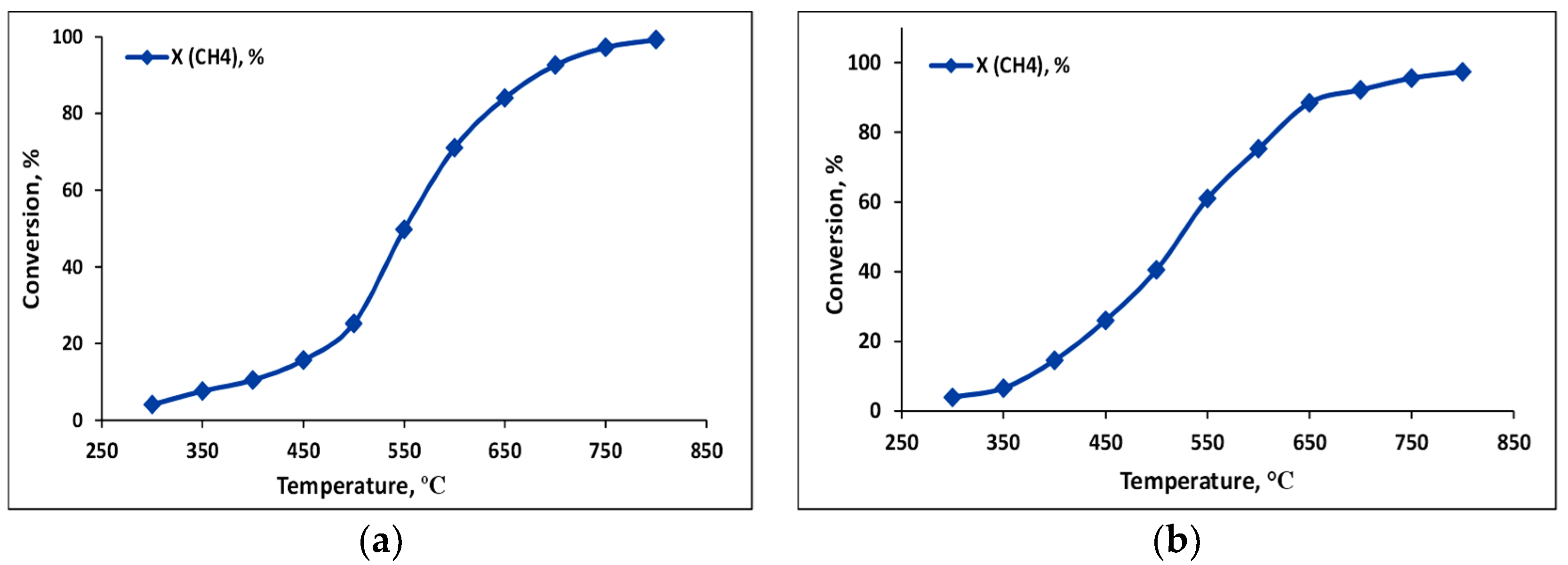
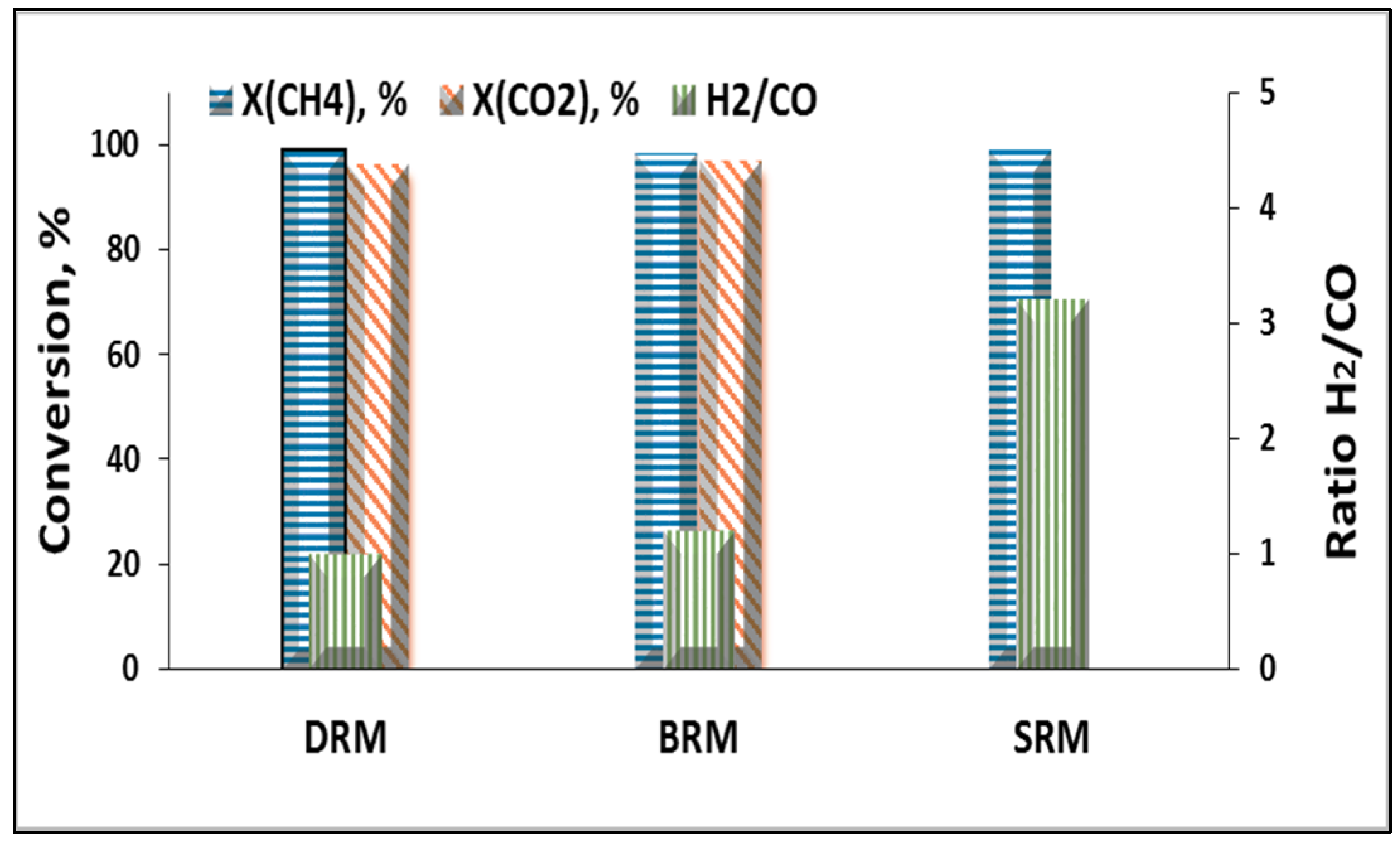
2.2.1. Dry Reforming of Methane
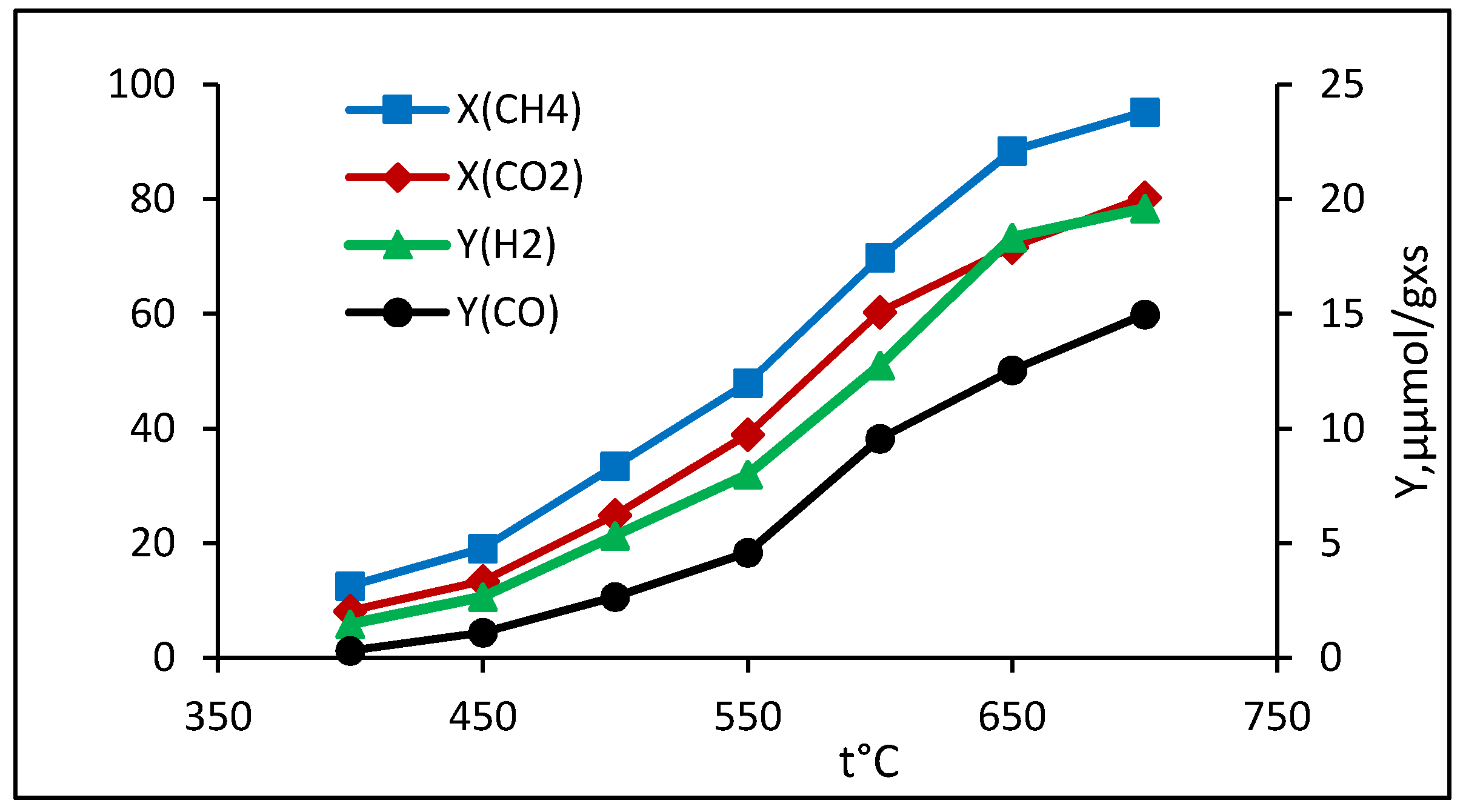
2.2.2. Bireforming of Methane
2.2.3. Steam Reforming of Methane
2.2.4. Stability Test
3. Materials and Methods
3.1. Catalyst Preparation and Characterisation
3.2. Catalyst Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdulrasheed, A.; Jalil, A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Hamid, M.U.S. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sust. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Kumar, N.; Shojaee, M.; Spivey, J.J. Catalytic bi-reforming of methane: From greenhouse gases to syngas. Curr. Opin. Chem. Eng. 2015, 9, 8–15. [Google Scholar] [CrossRef] [Green Version]
- York, A.P.E.; Xiao, T.-C.; Green, M.L.H.; Claridge, J.B. Methane Oxyforming for Synthesis Gas Production. Catal. Rev. 2007, 49, 511–560. [Google Scholar] [CrossRef]
- Budiman, A.W.; Song, S.-H.; Chang, T.-S.; Shin, C.-H.; Choi, M.-J. Dry Reforming of Methane over Cobalt Catalysts: A Literature Review of Catalyst Development. Catal. Surv. Asia 2012, 16, 183–197. [Google Scholar] [CrossRef]
- Park, J.-H.; Yeo, S.; Kang, T.-J.; Shin, H.-R.; Heo, I.; Chang, T.-S. Effect of Zn promoter on catalytic activity and stability of Co/ZrO2 catalyst for dry reforming of CH4. J. CO2 Util. 2018, 23, 10–19. [Google Scholar] [CrossRef]
- Yabe, T.; Sekine, Y. Methane conversion using carbon dioxide as an oxidizing agent: A review. Fuel Process. Technol. 2018, 181, 187–198. [Google Scholar] [CrossRef]
- Lau, C.S.; Tsolankis, A.; Wyszynski, M.L. Biogas upgrade to syn-gas (H2-CO) via dry and oxidative reforming. Int. J. Hydrog. Energy 2011, 36, 397–404. [Google Scholar] [CrossRef]
- Xu, B.Q.; Wei, J.M.; Yu, Y.T.; Li, J.L.; Zhu, Q.M. Carbon dioxide reforming of methane over nanocomposite Ni/ZrO2 catalysts. Top. Catal. 2003, 22, 77–85. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Zhang, G.; Liu, J.; Lv, Y.; Zhang, Y. Highly stable activity of cobalt-based catalysts with tungsten carbide-activated carbon support for dry reforming of methane: Role of tungsten carbide. Fuel 2021, 301, 122512. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Jad, G.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Wang, F.Q.; Jing, L.; Cheng, Z.M.; Liang, H.X.; Tan, J.Y. Combination of thermodynamic analysis and regression analysis for steam and dry methane reforming. Int. J. Hydrog. Energy. 2018, 44, 15795–15810. [Google Scholar] [CrossRef]
- Van Hook, J.P. Methane-Steam Reforming. Catal. Rev. 2006, 21, 1–51. [Google Scholar] [CrossRef]
- Angeli, S.D.; Turchetti, L.; Monteleone, G.; Lemonidou, A.A. Catalyst development for steam reforming of methane and model biogas at low temperature. Appl. Catal. B-Environ. 2016, 181, 34–46. [Google Scholar] [CrossRef]
- Kolbitsch, P.; Pfeifer, C.; Hofbauer, H. Catalytic steam reforming of model biogas. Fuel 2008, 87, 701–706. [Google Scholar] [CrossRef]
- Koo, K.Y.; Roh, H.S.; Jung, U.H.; Seo, D.J.; Seo, Y.S.; Yoon, W.L. Combined H2O and CO2 reforming of CH4 over nano-sized Ni/MgO-Al2O3 catalysts for synthesis gas production for gas to liquid (GTL): Effect of Mg/Al mixed ratio on coke formation. Catal. Today 2009, 146, 166–171. [Google Scholar] [CrossRef]
- Wu, J.; Qiao, L.-Y.; Zhou, Z.-F.; Cui, G.-J.; Zong, S.-S.; Xu, D.-J.; Ye, R.-P.; Chen, R.P.; Si, R.; Yao, Y.-G. Revealing the Synergistic Effects of Rh and Substituted La2B2O7 (B = Zr or Ti) for Preserving the Reactivity of Catalyst in Dry Reforming of Methane. ACS Catal. 2019, 9, 932–945. [Google Scholar] [CrossRef]
- Park, J.-H.; Yeo, S.Y.; Kang, T.-J.; Heo, I.J.; Lee, K.-Y.; Chang, T.-S. Enhanced stability of Co catalysts supported on phosphorus-modified Al2O3 for dry reforming of CH4. Fuel 2018, 212, 77–87. [Google Scholar] [CrossRef]
- Liu, D.; Cheo, W.N.E.; Lim, Y.W.Y.; Borgna, A.; Lau, R.; Yang, Y. A comparative study on catalyst deactivation of nickel and cobalt incorporated MCM-41 catalysts modified by platinum in methane reforming with carbon dioxide. Catal. Today 2010, 154, 229–236. [Google Scholar] [CrossRef]
- Ferencz, Z.; Baan, K.; Oszko, A.; Konya, Z.; Kecskes, T.; Erdohelyi, A. Dry reforming of CH4 on Rh doped Co/Al2O3 catalysts. Catal. Today 2014, 228, 123–130. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Wang, H.Y. Carbon Deposition and Catalytic Deactivation during CO2 Reforming of CH4 over Co/γ-Al2O3. J. Catal. 2002, 205, 289–293. [Google Scholar] [CrossRef]
- Mei, D.H.; Glezakou, V.A.; Lebarbier, V.; Kovarik, L.; Wan, H.Y.; Albrecht, K.O.; Gerber, M.; Rousseau, R.; Dagle, R.A. Highly active and stable MgAl2O4-supported Rh and Ir catalysts for methane steam reforming: A combined experimental and theoretical study. J. Catal. 2014, 316, 11–23. [Google Scholar] [CrossRef]
- Khani, Y.; Shariatinia, Z.; Bahadoran, F. High catalytic activity and stability of ZnLaAlO 4 supported Ni, Pt and Ru nanocatalysts applied in the dry, steam and combined dry-steam reforming of methane. Chem. Eng. J. 2016, 299, 353–366. [Google Scholar] [CrossRef]
- Köpfle, M.S.N.; Götsch, T.; Grünbacher, M.S.M.; Carbonio, E.A.; Hävecker, M.; Knop-Gericke, A.; Schlicker, D.L.; Doran, A.; Kober, D.D.; Gurlo, A.; et al. Zirconium-Assisted Activation of Palladium To Boost Syngas Production by Methane Dry Reforming. Angew. Chem. Int. Ed. 2018, 57, 14613–14618. [Google Scholar] [CrossRef] [PubMed]
- Drif, A.; Bion, N.; Brahmi, R.; Ojala, S.; Pirault-Roy, L.; Turpeinen, E.; Seelam, P.K.; Keiski, R.L.; Epron, F. Study of the dry reforming of methane and ethanol using Rh catalysts supported on doped alumina. Appl. Catal. A Gen. 2015, 504, 576–584. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Gerdts, R.; Parker, S.F.; Chi, L.; Zhao, Y.; Hill, M.; Guo, J.; Jones, M.O.; Jiang, Z. An in-depth understanding of the bimetallic effects and coked carbon species on an active bimetallic Ni(Co)/Al2O3 dry reforming catalyst. Phys. Chem. Chem. Phys. 2016, 18, 17311–17319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-H.; Chang, T.S. Promotional Efect of Ruthenium Addition to Co/α-Al2O3 Catalyst for Dry Reforming of Methane. Catal. Lett. 2019, 149, 3148–3159. [Google Scholar] [CrossRef]
- Itkulova, S.S.; Zakumbaeva, G.D.; Nurmakanov, Y.Y.; Mukazhanova, A.A.; Yermaganbetova, A.K. Syngas production by bireforming of methane over the Co-based alumina supported catalysts. Catal. Today 2014, 228, 194–198. [Google Scholar] [CrossRef]
- Itkulova, S.S.; Zhunusova, K.Z.; Zakumbaeva, G.D. CO2 Reforming Of CH4 Over Bimetallic Supported Catalysts. Appl. Organomet. Chem. 2000, 14, 850–852. [Google Scholar] [CrossRef]
- Moura, J.S.; Souza, M.O.G.; Bellido, J.D.A.; Assaf, E.M.; Opportus, M.; Reyes, P.; Rangel, M.C. Ethanol Steam Reforming over Rhodium and Cobalt-Based Catalysts: Effect of the Support. Int. J. Hydrog. Energy 2012, 37, 3213–3224. [Google Scholar] [CrossRef]
- Romano, P.N.; de Carvalho Filho, J.F.S.; de Almeida, J.M.A.R.; Sousa-Aguiar, E.F. Screening of mono and bimetallic catalysts for the dry reforming of methane. Catal. Today 2021, in press. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, G.; Xu, Y.; Zhang, Y.; Lv, Y.; Zhang, R. Comparative study on dry reforming of methane over Co-M (M=Ce, Fe, Zr) catalysts supported on N-doped activated carbon. Fuel Proc. Technol. 2019, 192, 1–12. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Bartolomeo, E.D. Co and Ni supported on CeO2 as selective bimetallic catalyst for dry reforming of methane. Int. J. Hydrog. Energy 2012, 37, 15992–15999. [Google Scholar] [CrossRef]
- Sarusi, I.; Fodor, K.; Baan, K.; Oszko, A.; Potari, G.; Erdohelyi, A. CO2 reforming of CH4 on doped Rh/Al2O3 catalysts. Catal. Today 2011, 171, 132–139. [Google Scholar] [CrossRef]
- Faroldi, B.; Munera, J.; Falivene, J.M.; Ramos, I.R.; Garcıa, A.G.; Fernandez, L.T.; Carrazan, S.G.; Cornaglia, L. Well-dispersed Rh nanoparticles with high activity for the dry reforming of methane. Int. J. Hydrog. Energy 2017, 42, 16127–16138. [Google Scholar] [CrossRef]
- de Araujo Moreira, T.G.; de Carvalho Filho, J.F.S.; Carvalho, Y.; de Almeida, J.M.A.R.; Nothaft Romano, P.; Falabella Sousa-Aguiar, E. Highly stable low noble metal content rhodium-based catalyst for the dry reforming of methane. Fuel 2021, 287, 119536. [Google Scholar] [CrossRef]
- Nurmakanov, Y.Y.; McCue, A.J.; Anderson, J.A.; Itkulova, S.S.; Kussanova, S.K. Methane reforming by CO2 or CO2-H2O over Co-containing supported catalysts. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Chem. Technol. 2016, 5, 5–11. [Google Scholar]
- Chastain, J.; Moulder, J.F. (Eds.) Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Enzo: ULVAC-PHI Incorp.: Kanagawa, Japan, 1995; 261p. [Google Scholar]
- Jacobs, G.; Ji, Y.; Davis, B.; Cronauer, D.C.; Kropf, A.J.; Marshall, C.L. Fischer-Tropsch synthesis: Temperature programmed EXAFS/XANES investigation of the influence of support type, cobalt loading, and noble metal promoter addition to the reduction behavior of cobalt oxide particles. Appl. Catal. A-Gen. 2007, 333, 177–191. [Google Scholar] [CrossRef]
- Itkulova, S.; Nurmakanov, Y.Y.; Kussanova, S.K.; Boleubayev, Y.A. Production of a hydrogen-enriched syngas by combined CO2-steam reforming of methane over Co-based catalysts supported on alumina modified with zirconia. Catal. Today 2018, 299, 272–279. [Google Scholar] [CrossRef]









| Catalyst | BET Surface Area, m2/g | Duration of Testing, h | |
|---|---|---|---|
| Fresh | Spent | ||
| 5%Co-Rh(98:2)/Al2O3 | 144.5 | 126.8 | 200 |
| 5%Co-Rh(95:5)/Al2O3 | 156.5 | 147.6 | 60 |
| 5%Co-Rh(9:1)/Al2O3 | 154.1 | 142.7 | 25 |
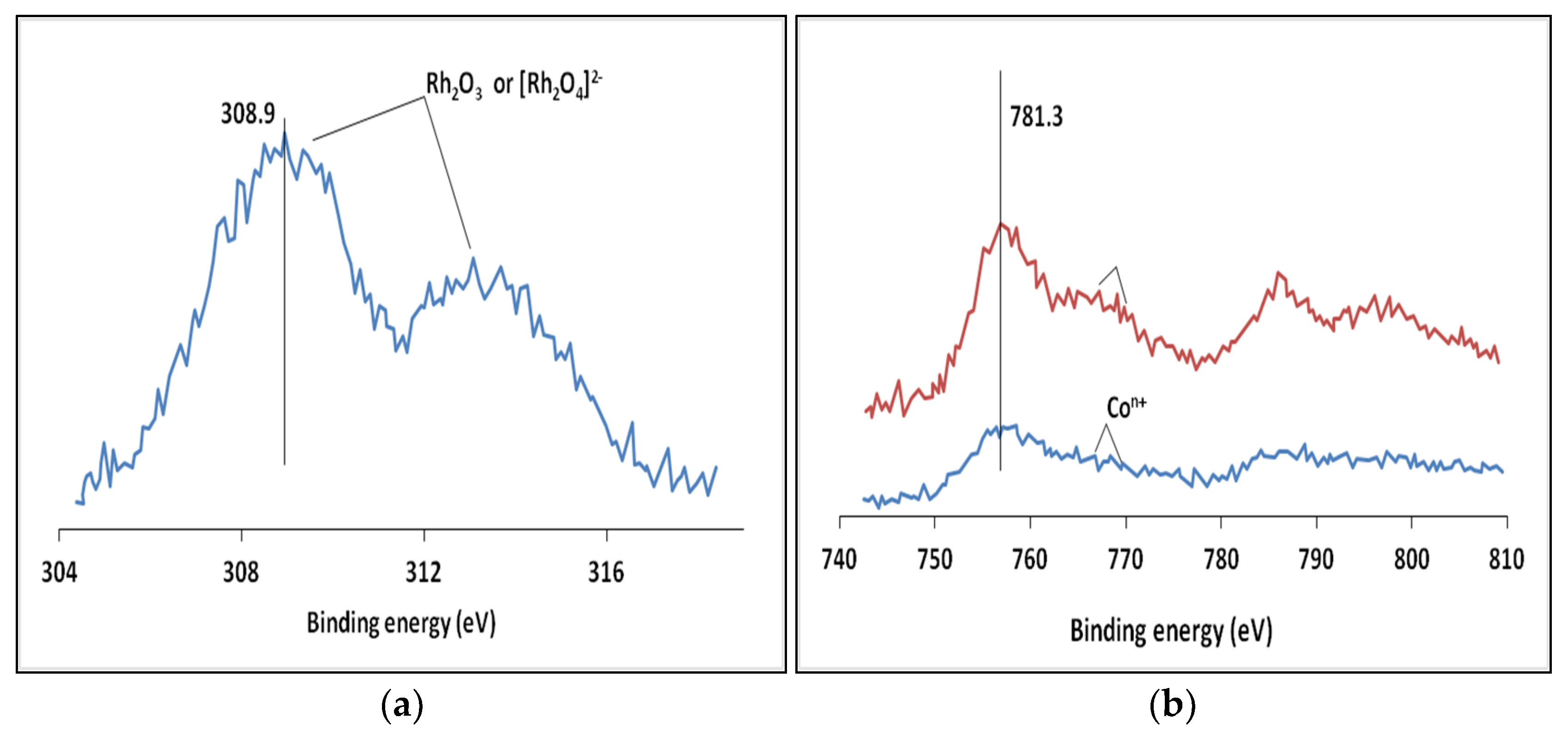
| Element, Level | Ebind, eV |
|---|---|
| C 1s | 284.4 |
| O ls | 531.2 |
| Al 2p | 74.3 |
| Co 2p | 781.3 |
| Rh 3d | 308.9 |
| Process | Feed, CH4:CO2:H2O | Co:Rh, mass. | Extent of Conversion, % | Yield, µmol/gcat×s | Ratio of H2/CO | ||
|---|---|---|---|---|---|---|---|
| CH4 | CO2 | H2 | CO | ||||
| DRM | 1:1:0 | 98:2 | 87.6 | 86.8 | 16.2 * | 16.4 * | 0.99 |
| 95:5 | 90.3 | 90.6 | 3.2 | 3.2 | 1.0 | ||
| 9:1 | 94.4 | 91.1 | 3.2 | 3.2 | 1.0 | ||
| BRM | 1:1:1 | 98:2 | 95.2 | 80.3 | 19.6 * | 15.0 * | 1.3 |
| 95:5 | 94.3 | 90.6 | 4.3 | 3.1 | 1.4 | ||
| 9:1 | 99.0 | 92.6 | 4.5 | 2.5 | 1.8 | ||
| SRM | 1:0:1 | 95:5 | 92.6 | - | 4.7 | 1.5 | 3.1 |
| 9:1 | 92.2 | - | 4.7 | 1.3 | 3.6 | ||
| Steam in a Feed, Vol. Part | Co-Rh(95:5) | Co-Rh(9:1) | ||||
|---|---|---|---|---|---|---|
| XCH4, % | XCO2, % | H2/CO | XCH4, % | XCO2, % | H2/CO | |
| 0 | 90.3 | 90.6 | 1.0 | 94.4 | 89.8 | 1.0 |
| 0.5 | 91.1 | 91.5 | 1.2 | 94.8 | 90.0 | 1.2 |
| 1 | 94.3 | 90.6 | 1.4 | 99.0 | 92.6 | 1.8 |
| 1.5 | 97.9 | 90.6 | 1.6 | 99.2 | 90.4 | 2.1 |
| 2 | 98.3 | 88.7 | 2.7 | 98.3 | 81.3 | 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itkulova, S.S.; Valishevskiy, K.A.; Boleubayev, Y.A. Bimetallic Co-Rh Systems as a Prospective Base for Design of CH4 Reforming Catalysts to Produce Syngas with a Controllable Composition. Catalysts 2022, 12, 105. https://doi.org/10.3390/catal12010105
Itkulova SS, Valishevskiy KA, Boleubayev YA. Bimetallic Co-Rh Systems as a Prospective Base for Design of CH4 Reforming Catalysts to Produce Syngas with a Controllable Composition. Catalysts. 2022; 12(1):105. https://doi.org/10.3390/catal12010105
Chicago/Turabian StyleItkulova, Sholpan S., Kirill A. Valishevskiy, and Yerzhan A. Boleubayev. 2022. "Bimetallic Co-Rh Systems as a Prospective Base for Design of CH4 Reforming Catalysts to Produce Syngas with a Controllable Composition" Catalysts 12, no. 1: 105. https://doi.org/10.3390/catal12010105
APA StyleItkulova, S. S., Valishevskiy, K. A., & Boleubayev, Y. A. (2022). Bimetallic Co-Rh Systems as a Prospective Base for Design of CH4 Reforming Catalysts to Produce Syngas with a Controllable Composition. Catalysts, 12(1), 105. https://doi.org/10.3390/catal12010105