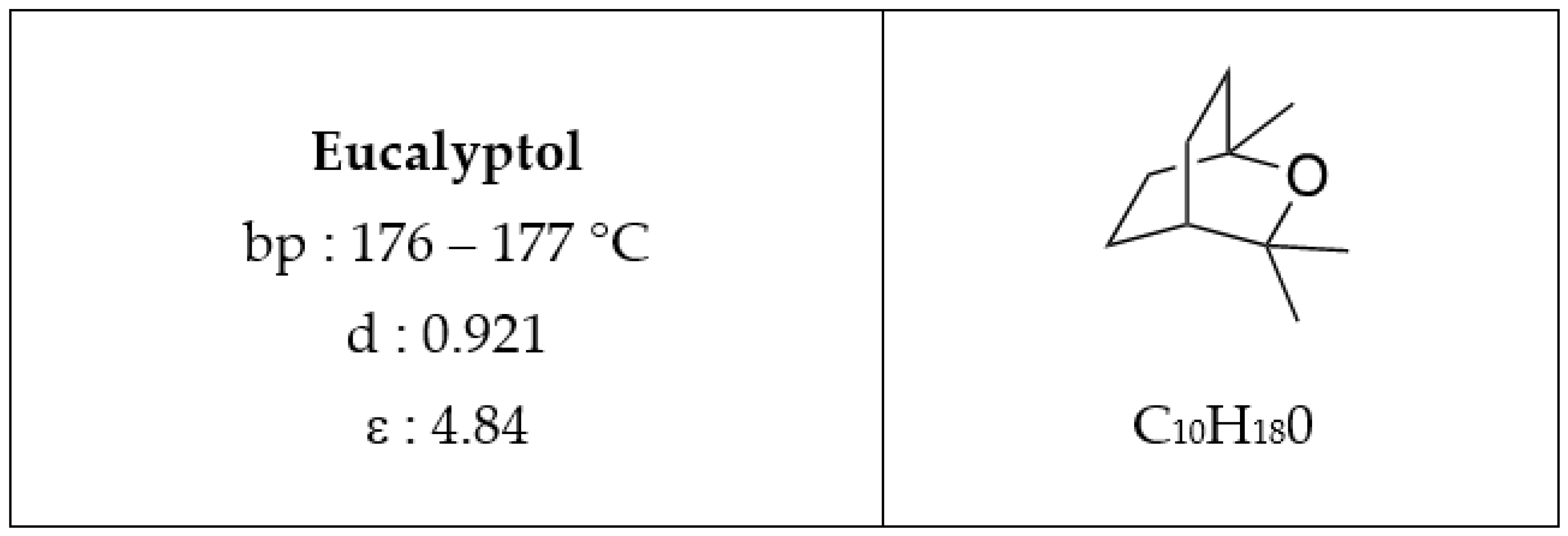
Eucalyptol, an All-Purpose Product
Abstract
:1. Introduction
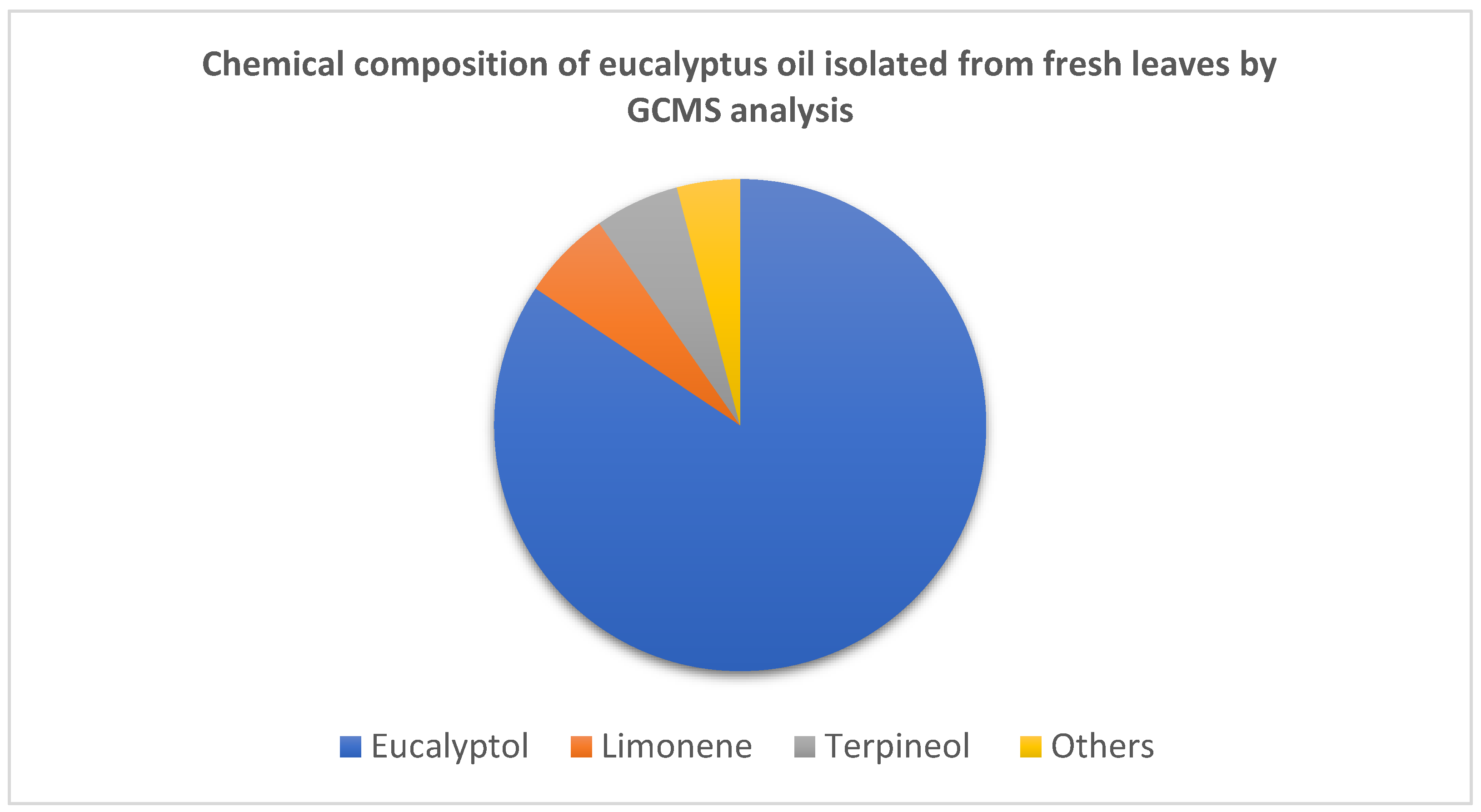
2. Eucalyptus
3. Use of 1,8-Cineole for the Treatment of Disorders
3.1. Cardiovascular Treatments
3.2. Antimicrobial Effects
3.3. Anti-Inflammatory Effects
3.4. Respiratory Disorders
3.5. Toxicity Side Effects
4. Study and Application of Eucalyptol for Greener Coupling Reactions
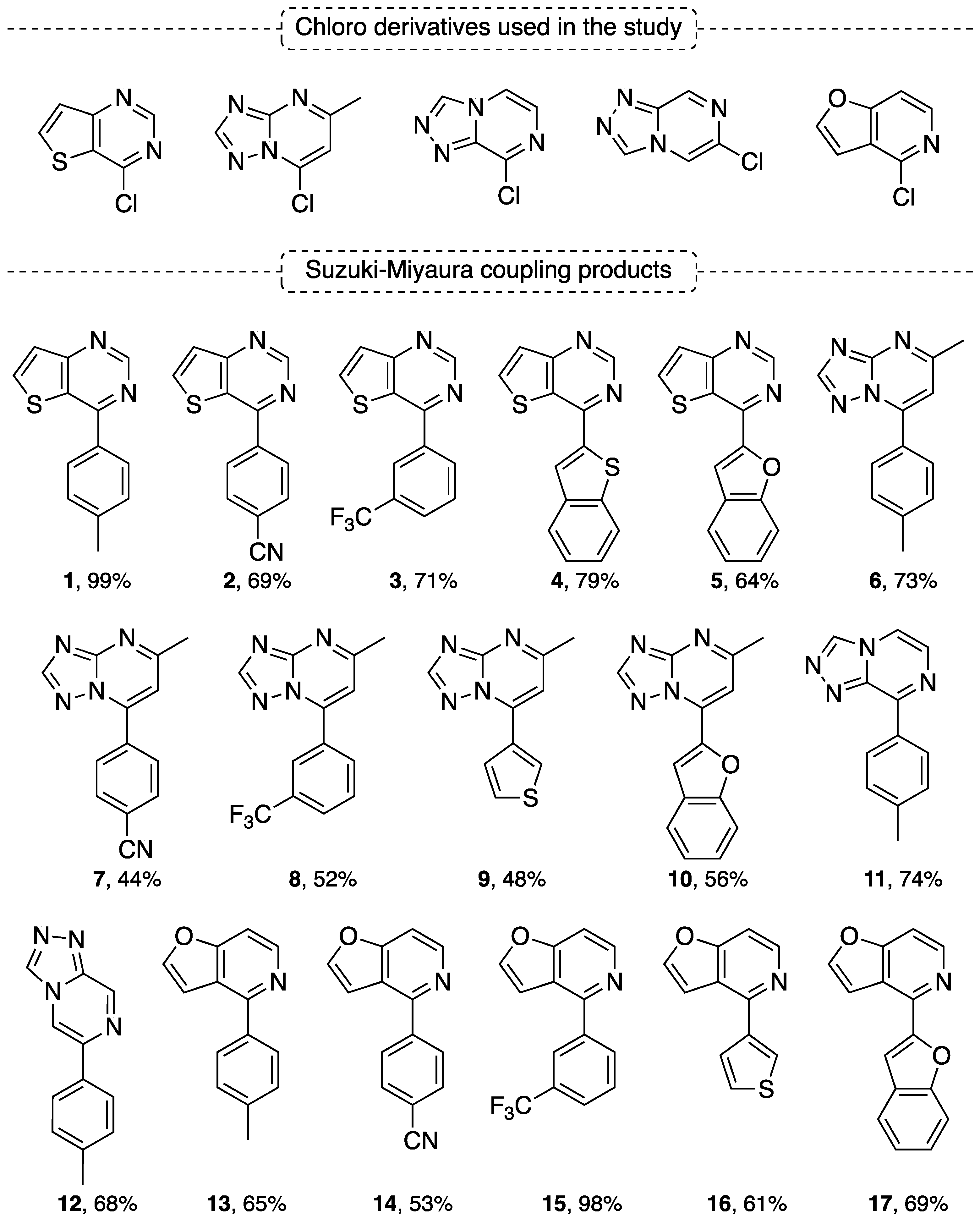
4.1. Suzuki–Miyaura Coupling Reaction
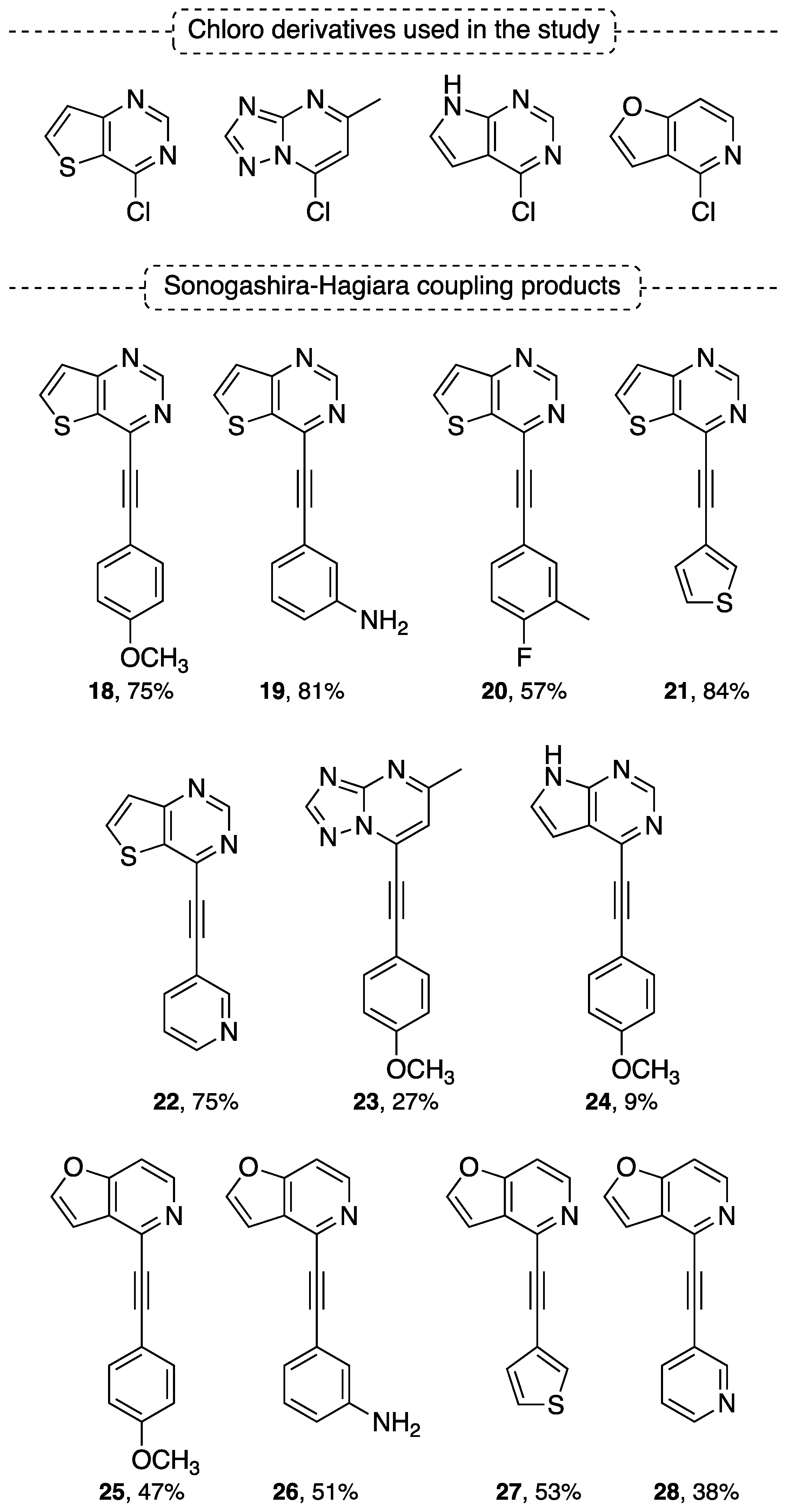
4.2. Sonogashira–Hagihara Coupling Reaction
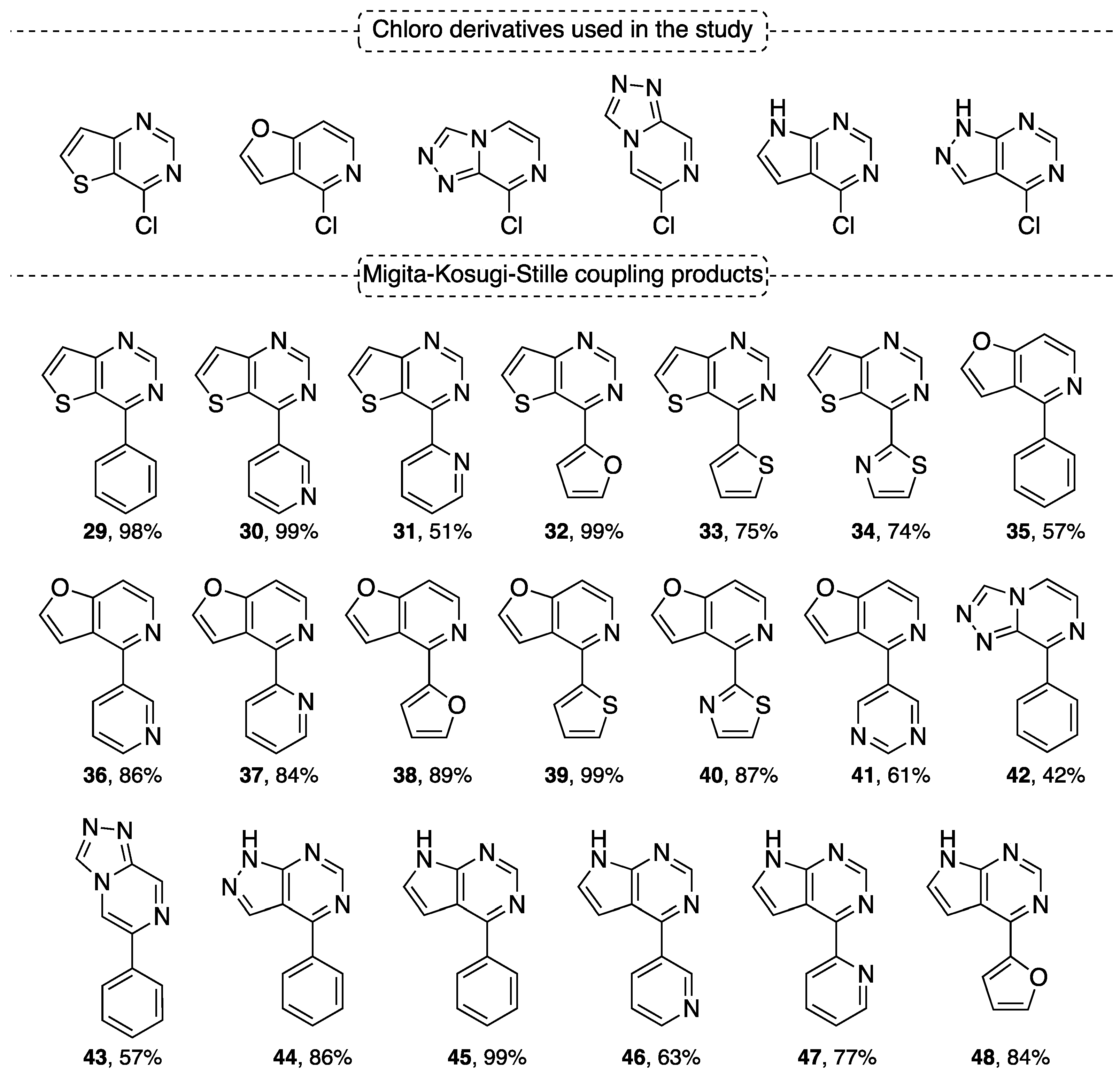
4.3. Migita–Kosugi–Stille Coupling Reaction
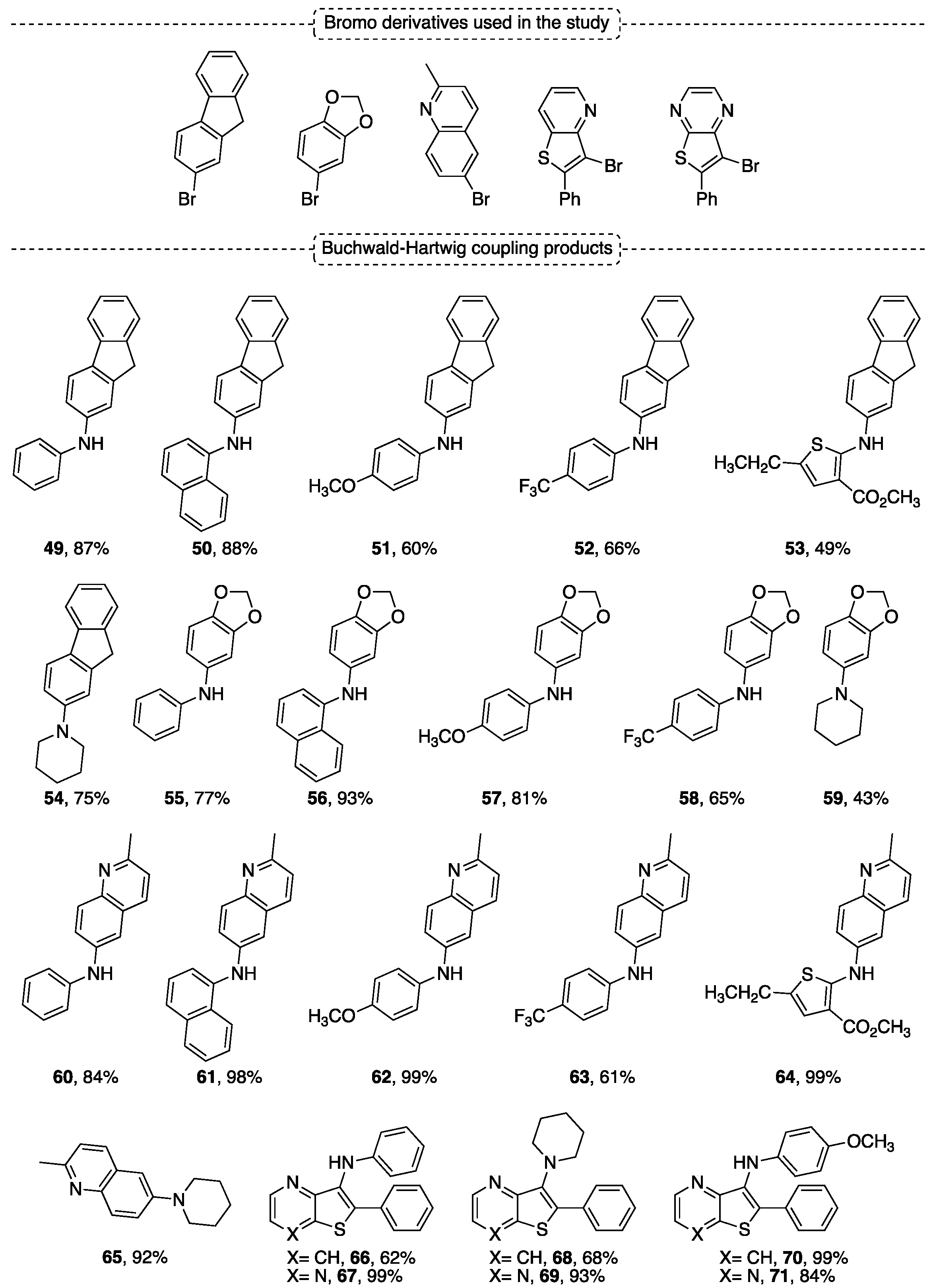
4.4. Buchwald–Hartwig Coupling Reaction
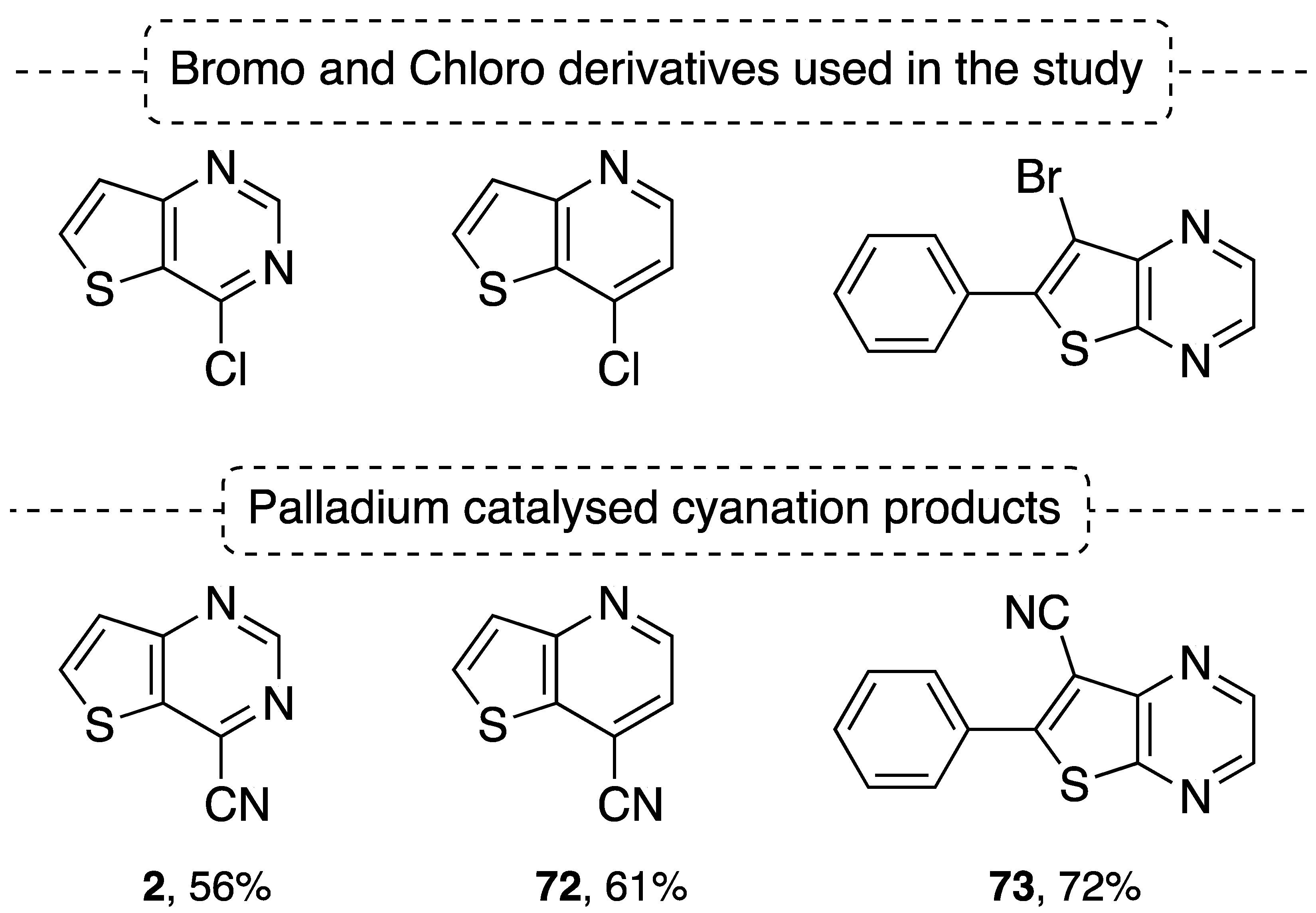
4.5. Palladium Catalyzed Cyanation Reaction
4.6. Hiyama Coupling Reaction
5. Reaction Leading to the Formation of O,S,N-Heterocycles
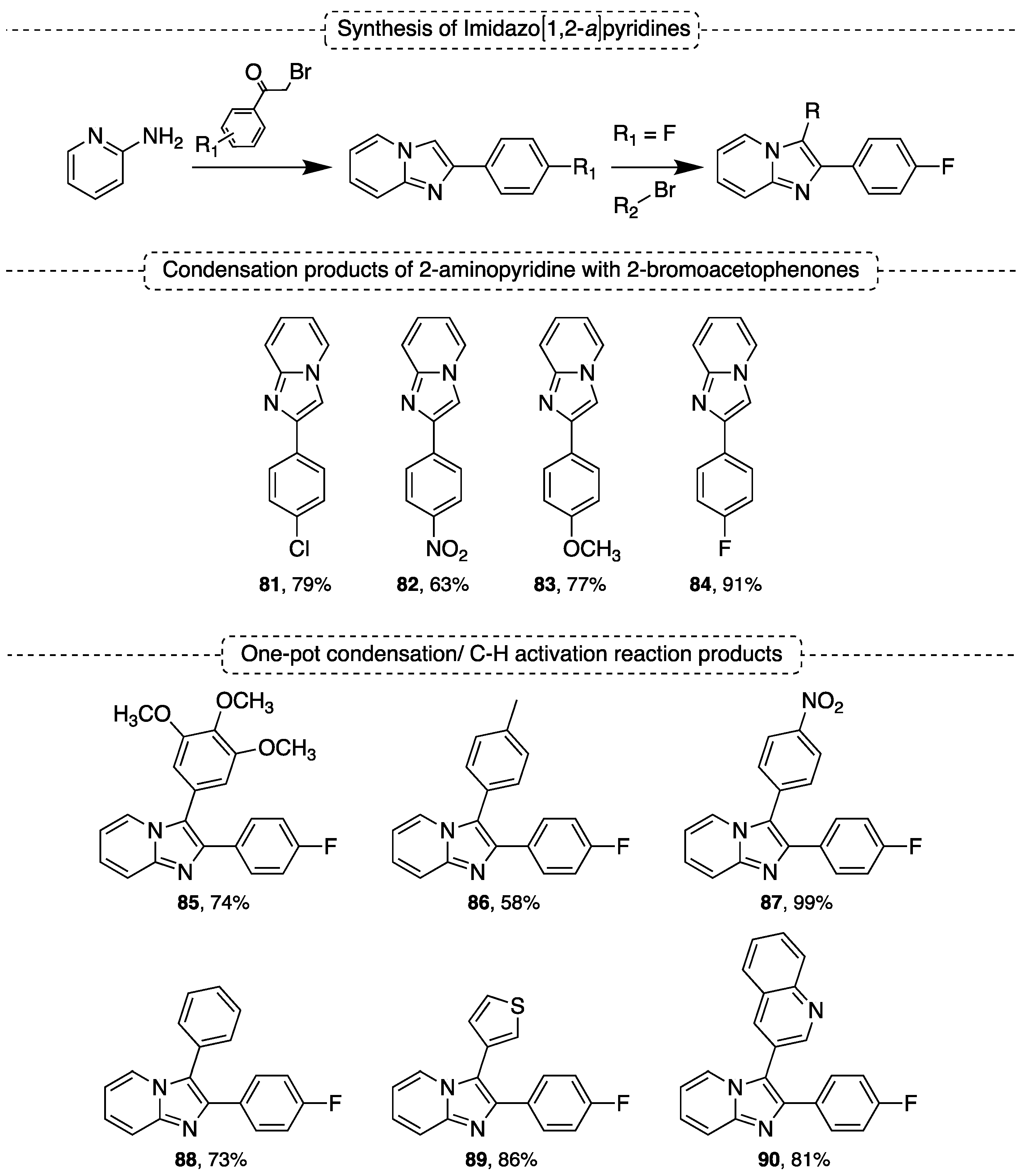
5.1. Synthesis of Imidazo[1,2-a]pyridines
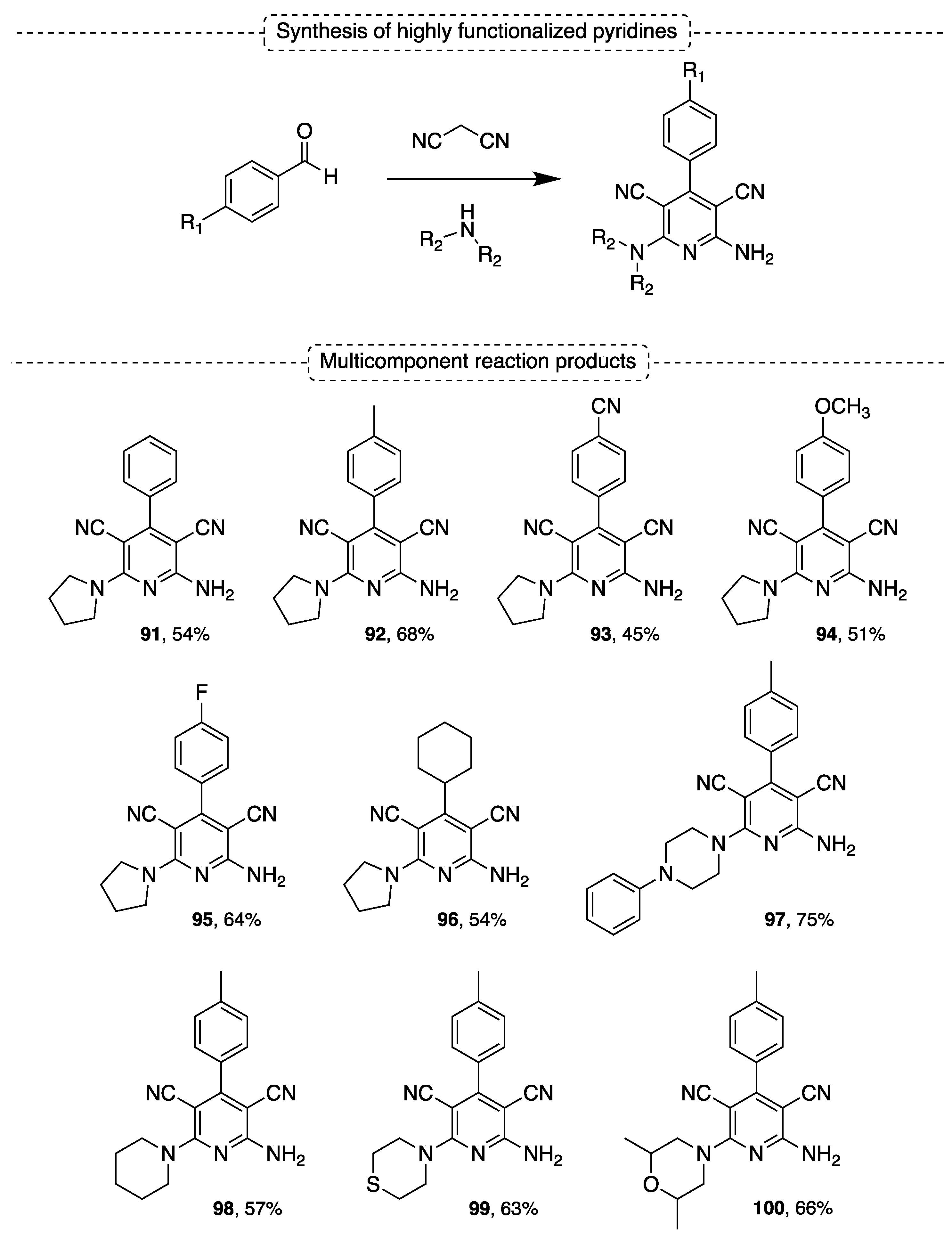
5.2. Synthesis of Highly Functionalized Pyridines
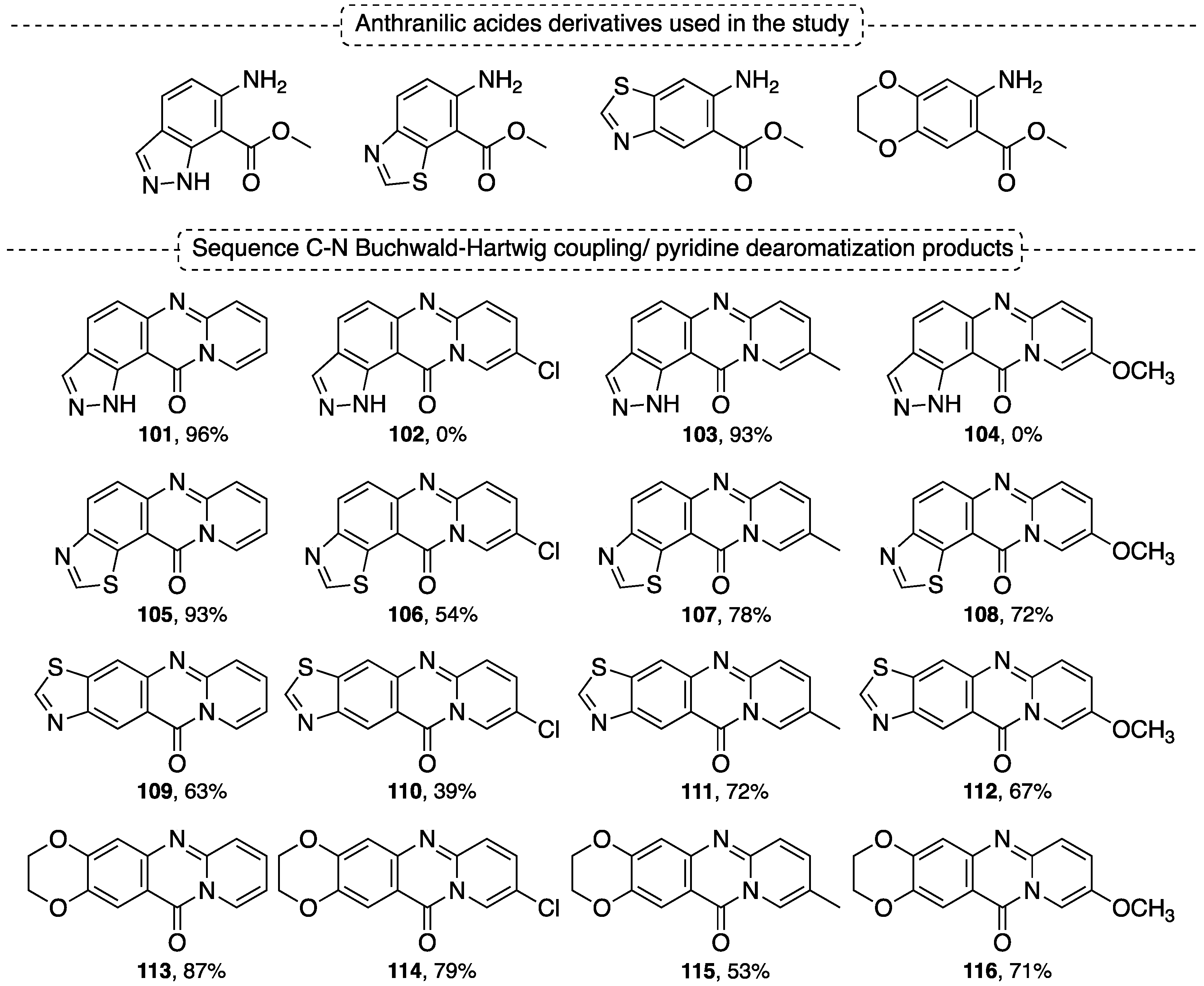
5.3. Synthesis of Pyridoquinazolinone Derivatives
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Boland, D.J.; Brooker, M.I.H.; Chippendale, G.M.; Hall, N.; Hyland, B.P.M.; Johnson, R.D.; Kleinig, D.A.; McDonald, M.W.; Turner, J.D. Forest Trees of Australia; CSIRO Publishing: Collingwood, Australia, 2006. [Google Scholar]
- Hill, K.D.; Johnson, L.A.S. Systematic studies in the eucalypts. 7. A revision of the bloodwoods, genus Corymbia (Myrtaceae). Telopea 1995, 6, 185–504. [Google Scholar] [CrossRef]
- Gilles, M.; Zhao, J.; An, M.; Agboola, S. Chemical composition and antimicrobial properties of EOs of three Australian Eucalyptus species. Food Chem. 2010, 119, 731–737. [Google Scholar] [CrossRef]
- Pereira, V.; Dias, C.; Vasconcelos, M.C.; Rosa, E.; Saavedra, M.J. Antibacterial activity and synergistic effects between Eucalyptus globulus leaf residues (EOs and extracts) and antibiotics against several isolates of respiratory tract infections (Pseudomonas aeruginosa). Ind. Crops Prod. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Bello, M.O.; Olabanji, I.O.; Ibrahim, A.O.; Yekeen, T.A.; Oboh, L.M. Nutraceuticals in leaves of Eucalyptus citriodora and Eucalyptus camandulensis. Food Sci. 2013, 62, 17873–17876. [Google Scholar]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 2011, 126, 228–235. [Google Scholar] [CrossRef]
- Araujo, J.L.; Rietzler, A.C.; Duarte, L.P.; Silva, G.D.F.; Carazza, F.; Filho, S.A.V. Constituents químicos e efeito ecotoxicológico do óleo volátil de folhas de Eucalyptus urograndis (Mirtaceae). Quím. Nova 2010, 33, 1510–1513. [Google Scholar] [CrossRef] [Green Version]
- William, J.E.; Brooker, M.I.H. Eucalypt Ecology: Individuals to Ecosystems; Williams, J.E., Woinarski, J.C.Z., Eds.; Cambridge University Press: Melbourne, Australia, 1997. [Google Scholar]
- Brooker, M.I.; Kleinig, D.A. Field Guide to Eucalyptus, 3rd ed.; Blooming Books: Melbourne, Australia, 2006. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of EOs—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Burt, S. EOs: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Stefanakis, M.K.; Touloupakis, E.; Anastasopoulos, E.; Ghanotakis, D.; Katerinopoulos, H.E.; Makridis, P. Antibacterial activity of EOs from plants of the genus Origanum. Food Control 2013, 34, 539–546. [Google Scholar] [CrossRef]
- Mubita, C.; Syakalima, M.; Chisenga, C.; Munyeme, M.; Bwalya, M.; Chifumpa, G. Antibiograms of faecal Escherichia coli and Enterococci species isolated from pastoralist cattle in the interface areas of the Kafue basin in Zambia. Vet. Arch. 2008, 78, 179–185. [Google Scholar]
- Damjanović-Vratnica, B.; Dakov, T.; Šuković, D.; Damjanović, J. Antimicrobial effect of essential oil isolated from Eucalyptus globulus Labill from Montenegro. Czech J. Food Sci. 2011, 29, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, Y.P.S. Medicinal and Aromatic Plants; Biotechnology in agriculture and forestry; Springer: Berlin, Germany, 1995. [Google Scholar]
- Akin, M.; Aktumsek, A.; Nostro, A. Antibacterial activity and composition of essential oils of Eucalyptus camaldulensis Dehn and Myrtus communis L. growing in northern Cyprus. Afr. J. Biotechnol. 2010, 9, 531–535. [Google Scholar]
- Aparicio, S.; Alcalde, R.; Davila, M.J.; Garcia, B.; Leal, J.M. Properties of 1,8-cineole: A thermophysical and theoretical study. J. Phys. Chem. B 2007, 111, 3167–3177. [Google Scholar] [CrossRef] [PubMed]
- Sadlon, A.E.; Lamson, D.W. Immune-modifying and antimicrobial effects of Eucalyptus oil and simple inhalation devices. Altern. Med. Rev. 2010, 15, 33–47. [Google Scholar] [PubMed]
- ESCOP. Eucalypti aetheroleum Eucalyptus Oil. In E/S/C/O/P Monographs, 2nd ed.; Thieme: Stuttgart, Germany, 2003. [Google Scholar]
- Betts, T.J. Solid phase microextraction of volatile constituents from individual fresh Eucalyptus leaves of three species. Planta Med. 2000, 66, 193–195. [Google Scholar] [CrossRef]
- Usman, L.A.; Zubair, M.F.; Adebayo, S.A.; Oladosu, I.A.; NO, M.; Akolade, J.O. Chemical composition of leaf and fruit essential oils of hoslundia opposita vahl grown in nigeria. Am.-Eurasian J. Agric. Environ. Sci. 2010, 8, 40–43. [Google Scholar]
- Emara, S.; Shalaby, A.E. Seasonal variation of fixed and volatile oil percentage of four Eucalyptus spp. related to lamina anatomy. Afr. J. Plant Sci. 2011, 5, 353–359. [Google Scholar]
- Weiss, E.A. Essential Oil Crops; CAB International: Wallingford, UK, 1997. [Google Scholar]
- Sanjib, B. Chapter 3—Cultivation of Essential Oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 19–29. [Google Scholar]
- Mewalal, R.; Rai, D.; Kainer, D.; Chen, F.; Külheim, C.; Peter, G.F.; Tuskan, G.A. Plant-Derived Terpenes: A Feedstock for Specialty Biofuels. Trends Biotechnol. 2017, 35, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Arendt, P.; Pollier, J.; Callewaert, N.; Goossens, A. Synthetic biology for production of natural and new-to-nature terpenoids in photosynthetic organisms. Plant J. 2016, 87, 16–37. [Google Scholar] [CrossRef] [Green Version]
- Pitera, D.J.; Paddon, C.J.; Newman, J.D.; Keasling, J.D. Balancing a heterologous mevalonate pathway for improved isoprenoid production in Escherichia coli. Metab. Eng. 2007, 9, 193–207. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; Bowyer, M.C.; Scarlett, C.J. An Array of Bioactive Compounds From Australian Eucalypts and Their Relevance in Pancreatic Cancer Therapeutics. Pancreas 2018, 47, 690–707. [Google Scholar] [CrossRef] [PubMed]
- Vuonga, Q.V.; Chalmers, A.C.; Bhuyana, D.J.; Bowyera, M.C.; Scarlett, C.J. Botanical, Phytochemical, and Anticancer Properties of the Eucalyptus Species. Chem. Biodivers. 2015, 12, 907–924. [Google Scholar] [CrossRef]
- Amer, A.; Mehlhorn, H. Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol. Res. 2006, 99, 478. [Google Scholar] [CrossRef] [PubMed]
- Sritabutra, D.; Soonwera, M.; Waltanachanobon, S.; Poungjai, S. Evaluation of herbal essential oil as repellents against Aedes aegypti (L.) and Anopheles dirus Peyton & Harrion. Asian Pac. J. Trop. Biomed. 2011, 1, 124–128. [Google Scholar]
- Phasomkusolsil, S.; Soonwera, M. Insect repellent activity of medicinal plant oils against Aedes aegypti (Linn.), Anopheles minimus (Theobald) and Culex quinquefasciatus Say based on protection time and biting rate. Southeast Asian J. Trop. Med. Public Health 2010, 41, 831–840. [Google Scholar]
- Seyoum, A.; Pålsson, K.; Kung’a, S.; Kabiru, E.; Lwande, W.; Killeen, G.; Hassanali, A.; Knols, B.G. Traditional use of mosquito-repellent plants in western Kenya and their evaluation in semi-field experimental huts against Anopheles gambiae: Ethnobotanical studies and application by thermal expulsion and direct burning. Trans. R Soc. Trop. Med. Hyg. 2002, 96, 225–231. [Google Scholar] [CrossRef]
- Rehman, J.U.; Ali, A.; Khan, I.A. Plant based products: Use and development as repellents against mosquitoes: A review. Fitoterapia 2014, 95, 65–74. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Sabo, V.A.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Raftos, D. In vitro Antifungal activity of a new combination of Essential oils against some filamentous Fungi. Middle East J. Sci. Res. 2012, 11, 156–161. [Google Scholar]
- Falahati, M.; Tabrizib, N.O.; Jahaniani, F. Anti dermatophyte activities of Eucalyptus camaldulensis in comparison with Griseofulvin. Iran. J. Pharmacol. Ther. 2005, 4, 80–83. [Google Scholar]
- Mousavi, M.; Mirzargar, S.S.; Ebrahimzadeh, M.H.; Omidbaigi, R.; Khosravi, A.; Bahonar, A. Antifungal and toxicity effects of new combined essential oils on Oncorhynchus mykiss in comparison with malachite green. Iran. J. Vet. Sci. Technol. 2014, 4, 1–8. [Google Scholar]
- Ramezani, H. Fungicidal activity of volatile oil from Eucalyptus citriodora Hook. against Alternaria triticina. Commun. Agric. Appl. Biol. Sci. 2005, 71, 909–914. [Google Scholar]
- Hadizadeh, I.; Peivastegan, B.; Hamzehzarghani, H. Antifungal activity of essential oils from some medicinal plants of Iran against Alternaria alternate. Am. J. Appl. Sci. 2009, 6, 857–861. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Ahd, A.A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharmacogn. Mag. 2010, 6, 172–175. [Google Scholar] [CrossRef] [Green Version]
- Safaei-Ghomi, J.; Batooli, H. Chemical composition and antimicrobial activity of the volatile oil of Eucalyptus sargentii Maiden cultivated in central Iran. Int. J. Green Pharm 2010, 4, 174–177. [Google Scholar] [CrossRef]
- Hossein, A.; Fereshte, Z.; Gholamreza, B.; Mehdi, A.; Masoud, Z. Study the antifungal effects of plant medicinal essences on growth of Aspergillus Flavus and Aflatoxin B1 in Pistachio (Pistacia vera L.). J. Zabol. Univ. Med. Sci. Health Serv. 2013, 5, 16–23. [Google Scholar]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Yap, P.S.; Lim, S.H.; Hu, C.P.; Yiap, B.C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 2013, 20, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K. In vitro evaluation of medicinal plant extracts against Pestalotiopsis mangiferae. Hindustan Antibiot. Bull. 1996, 38, 53–56. [Google Scholar] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Cermelli, C.; Fabio, A.; Fabio, G.; Quaglio, P. Effect of eucalyptus essential oil on respiratory bacteria and viruses. Curr. Microbiol. 2008, 56, 89–92. [Google Scholar] [CrossRef]
- Tyski, S.; Bocian, E.; Mikucka, A.; Grzybowska, W. Antibacterial activity of selected commercial products for mouth washing and disinfection, assessed in accordance with PN-EN 1040. Med. Sci. Monit. 2013, 19, 458–466. [Google Scholar] [PubMed] [Green Version]
- Park, J.-W.; Wendt, M.; Heo, G.-J. Antimicrobial activity of essential oil of Eucalyptus globulus against fish pathogenic bacteria. Lab. Anim. Res. 2016, 32, 87–90. [Google Scholar] [CrossRef]
- Martins, C.; Natal-da-Luz, T.; Sousa, J.P.; Gonçalves, M.J.; Salgueiro, L.; Canhoto, C. Effects of essential oils from Eucalyptus globulus leaves on soil organisms involved in leaf degradation. PLoS ONE 2013, 8, e61233. [Google Scholar] [CrossRef] [Green Version]
- Elaissi, A.; Rouis, Z.; Salem, N.A.B.; Mabrouk, S.; Ben Salem, Y.; Salah, K.B.H.; Aouni, M.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Chemical composition of 8 Eucalyptus species’ essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complement. Altern. Med. 2012, 12, 81. [Google Scholar] [CrossRef] [Green Version]
- Ait-Ouazzou, A.; Lorán, S.; Bakkali, M.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R.; Conchello, P. Chemical composition and antimicrobial activity of essential oils of Thymus algeriensis, Eucalyptus globulus and Rosmarinus officinalis from Morocco. J. Sci. Food Agric. 2011, 91, 2643–2651. [Google Scholar] [CrossRef]
- Ndagijimana, A.; Chaitanya, M.V.N.L.; Dhanabal, S.P.; Kabera, J.N. Phytochemical Review on Ocimum sanctum, Zingiber officinale, Rosamarinus officinalis and Eucalypus globules for their antitussive and antioxidant activities. J. Chem. Pharm. Res. 2016, 8, 243–250. [Google Scholar]
- Tomaino, A.; Cimino, F.; Zimbalatti, V.; Venuti, V.; Sulfaro, V.; De Pasquale, A.; Saija, A. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem. 2005, 89, 549–554. [Google Scholar] [CrossRef]
- El-Ghorab, A.; Shaaban, H.A.; El-Massry, K.F.; Shibamoto, T. Chemical composition of volatile extract and biological activities of volatile and less-volatile extracts of juniper berry (Juniperus drupacea L.) fruit. J. Agric. Food Chem. 2008, 56, 5021–5025. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J. Agric. Food Chem. 2010, 58, 7218–7225. [Google Scholar] [CrossRef]
- Serafino, A.; Vallebona, P.; Andreola, F.; Zonfrillo, M.; Mercuri, L.; Federici, M.; Rasi, G.; Garaci, E.; Pierimarchi, P. Stimulatory effect of Eucalyptus essential oil on innate cell-mediated immune response. BMC Immunol. 2008, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Uddin, I.M.; Aulakh, J.S. An approach on phytochemistry and pharmacological studies of Eucalyptus globulus plant parts. Res. J. Mater. Sci. 2017, 5, 1–9. [Google Scholar]
- Barbosa, L.C.A.; Filomeno, C.A.; Teixeira, R.R. Chemical Variability and Biological Activities of Eucalyptus spp. Essential Oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Role of Monoterpenes in Eucalyptus Communities. Curr. Bioact. Compd. 2012, 8, 101–107. [Google Scholar] [CrossRef]
- Zhang, J.; An, M.; Wu, H.; Stanton, R.; Lemerle, D. Chemistry and bioactivity of Eucalyptus essential oils. Allelopath. J 2010, 25, 313–330. [Google Scholar]
- Tsai, M.L.; Lin, C.C.; Lin, W.C.; Yang, C.H. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils from five selected herbs. Biosci. Biotechnol. Biochem. 2011, 75, 1977–1983. [Google Scholar] [CrossRef] [Green Version]
- Torabi, N.; Mohebali, M.; Shahverdi, A.R.; Rezaiat, S.M.; Edrisian, G.H.H.; Esmaeili, J.; Charehdar, S. Gold nanoparticles for the treatment of cutaneous leishmaniasis caused by Leishmania major Iranian strain: An experimental study on animal model with methanol extract of Eucalyptus camaldulensis. Pharm. Health Sci. J. 2010, 1, 13–16. [Google Scholar]
- Higgins, C.; Palmer, A.; Nixon, R. Eucalyptus oil: Contact allergy and safety. Contact Dermat. 2015, 72, 337–346. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Icaro, C.A.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2017, 13, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Lahlou, S.; Figueiredo, A.F.; Magalhães, P.J.; Leal-Cardoso, J.H. Cardiovascular effects of 1,8-cineole, a terpenoid oxide present in many plant essential oils, in normotensive rats. Can. J. Physiol. Pharmacol. 2002, 80, 1125–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, N.V.; Assreuy, A.M.; Coelho-De-Souza, A.N.; Ceccatto, V.M.; Magalhães, P.J.; Lahlou, S.; Leal-Cardoso, J.H. Endothelium-dependent vasorelaxant effects of the essential oil from aerial parts of Alpinia zerumbet and its main constituent 1,8-cineole in rats. Phytomedicine 2009, 16, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.C.; Damiani, C.E.; Moreira, C.M.; Stefanon, I.; Vassallo, D.V. Eucalyptol, an essential oil, reduces contractile activity in rat cardiac muscle. Braz. J. Med. Biol. Res. 2005, 38, 453–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, H.K.; Kang, P.; Lee, H.S.; Min, S.S.; Seol, G.H. Effects of 1,8-cineole on hypertension induced by chronic exposure to nicotine in rats. J. Pharm. Pharmacol. 2014, 66, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Z.W.; Yin, Z.Q.; Wei, Q.; Jia, R.Y.; Zhou, L.J.; Xu, J.; Song, X.; Zhou, Y.; Du, Y.H.; et al. Antibacterial activity of leaf essential oil and its constituents from Cinnamomum longepaniculatum. Int. J. Clin. Exp. Med. 2014, 7, 1721–1727. [Google Scholar]
- Vlachojannis, C.; Chrubasik-Hausmann, S.; Hellwig, E.; Al-Ahmad, A. A preliminary investigation on the antimicrobial activity of Listerine®, its components, and of mixtures thereof. Phytother. Res. 2015, 29, 1590–1594. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, C.; Al-Ahmad, A.; Hellwig, E.; Chrubasik, S. Listerine® products: An update on the efficacy and safety. Phytother. Res. 2016, 30, 367–373. [Google Scholar] [CrossRef]
- Santos, F.A.; Rao, V.S.N. Antiinflammatory and Antinociceptive Effects of 1,8-Cineole a Terpenoid Oxide Present in many Plant Essential Oils. Phytother. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Juergens, U.R. Anti-inflammatory Properties of the Monoterpene 1.8-cineole: Current Evidence for Co-medication in Inflammatory Airway Diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Galan, D.M.; Ezeudu, N.E.; Garcia, J.; Geronimo, C.A.; Berry, N.M.; Malcolm, B.J. Eucalyptol (1,8-cineole): An underutilized ally in respiratory disorders? J. Essent. Oil Res. 2020, 32, 102–110. [Google Scholar] [CrossRef]
- Juergens, L.J.; Worth, H.; Juergens, U.R. New Perspectives for Mucolytic, Anti-inflammatory and Adjunctive Therapy with 1,8-Cineole in COPD and Asthma: Review on the New Therapeutic Approach. Adv. Ther. 2020, 37, 1737–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juergens, U.R.; Stöber, M.; Vetter, H. Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1.8-cineole) in human blood monocytes in vitro. Eur. J. Med. Res. 1998, 3, 508–510. [Google Scholar]
- Juergens, U.R.; Dethlefsen, U.; Steinkamp, G.; Gillissen, A.; Repges, R.; Vetter, H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: A double-blind placebo-controlled trial. Respir. Med. 2003, 97, 3250–3256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worth, H.; Schacher, C.; Dethlefsen, U. Concomitant therapy with cineole (eucalyptole) reduces exacerbations in COPD: A placebo-controlled double blind trial. Respir. Res. 2009, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Bastos, V.P.D.; Gomes, A.S.; Lima, F.J.B.; Brito, T.S.; Soares, P.M.G.; Pinho, J.P.M.; Silva, C.S.; Santos, A.A.; Souza, M.H.L.P.; Magalhães, P.J.C. Inhaled 1,8-cineole reduces inflammatory parameters in airways of ovalbumin-challenged Guinea pigs. Basic Clin. Pharmacol. Toxicol. 2011, 108, 34–39. [Google Scholar] [CrossRef]
- Theisand, J.G.W.; Koren, G. Camphorated oil: Stillen dangering the lives of Canadian children. Can. Med. Assoc. J. 1995, 152, 1821–1824. [Google Scholar]
- Burkhard, P.R.; Burkhardt, K.; Landis, T.; Haenggeli, C.-A. Plant-induced seizures: Reappearance of an old problem. J. Neurol. 1999, 246, 667–670. [Google Scholar] [CrossRef]
- Culić, M.; Keković, G.; Grbić, G.; Martać, L.; Soković, M.; Podgorac, J.; Sekulić, S. Wavelet and fractal analysis of rat brain activity in seizures evoked by camphor essential oil and 1,8-cineole. Gen. Physiol. Biophys. 2009, 28, 33–40. [Google Scholar]
- Doshi, D.; Close, B.R.; Reid, P.F.W. A novel use of Naloxone as a treatment for Eucalyptus oil induced central nervous system depression. Clin. Toxicol. 2011, 49, 768. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.K.; Jiménez-González, C.D.; Constable, J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide—Embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.A.; Brohl, A.; Hallett, J.P. Hallett Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747. [Google Scholar] [CrossRef] [PubMed]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927. [Google Scholar] [CrossRef]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al. Green chemistry tools to influence a medicinal chemistry and research chemistry based organization. Green Chem. 2008, 10, 31. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s Solvent Selection Guide: A Step Toward More Sustainable Processes. Org. Process Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Diorazio, L.J.; Hose, D.R.J.; Adlington, N.K. Toward a More Holistic Framework for Solvent Selection. Org. Process Res. Dev. 2016, 20, 760–773. [Google Scholar] [CrossRef] [Green Version]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Khandelwal, S.; Rajawat, A.; Kumar, M. An Efficient and Environmentally Benign One-Pot Three-Component Domino Protocol for the Synthesis of Structurally Diverse Spiroquinazolines. Curr. Catal. 2015, 4, 214–223. [Google Scholar] [CrossRef]
- Khandelwal, S.; Rajawat, A.; Tailor, Y.K.; Kumar, M. Efficient and Environmentally Benign Diversity Oriented Synthesis of 2, 3- dihydroquinazolin-4(1H)-ones Using GAAS As a Bio-based Green Solvent. Curr. Green Chem. 2015, 2, 156–162. [Google Scholar] [CrossRef]
- Bhat, P.; Shridhar, G.; Ladage, S.; Ravishankar, L. An eco-friendly synthesis of 2-pyrazoline derivatives catalysed by CeCl3⋅7H2O. J. Chem. Sci. 2017, 129, 1441–1448. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, S.P.; Shridhar, G.; Ladage, S.; Ravishankar, L. A Facile Synthesis of Isoxazolone Derivatives Catalyzed by Cerium Chloride Heptahydrate in Ethyl Lactate as a Solvent: A Green Methodology. Curr. Green Chem. 2016, 2, 160–167. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Fang, Q.-S.; Zhou, J.; Song, Z.-B. Reusable proline-based ionic liquid catalyst for the simple synthesis of 2-arylbenzothiazoles in a biomass medium. Res. Chem. Intermed. 2015, 42, 2035–2045. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Zou, S.; Liu, Y. Bio-Based Solvent Mediated Synthesis of Dihydropyrimidinthiones Via Biginelli Reaction. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 791–795. [Google Scholar] [CrossRef]
- Dandia, A.; Jain, A.K.; Laxkar, A.K. Ethyl lactate as a promising bio based green solvent for the synthesis of spiro-oxindole derivatives via 1,3-dipolar cycloaddition reaction. Tetrahedron Lett. 2013, 54, 3929–3932. [Google Scholar] [CrossRef]
- Balijapalli, U.; Thiyagarajan, M.D.; Manickam, S.; Sathiyanarayanan, K.I. Synthesis of T-shaped Oxazolonaphthoimidazo[1,2-a]pyridines using Lactic Acid as Bio–based Green Solvent: An Insight into Photophysical Studies. ChemistrySelect 2016, 1, 2900–2908. [Google Scholar] [CrossRef]
- Wang, S.-F.; Guo, C.-L.; Cui, K.-K.; Zhu, Y.-T.; Ding, J.-X.; Zou, X.-Y.; Li, Y.-H. Lactic acid as an invaluable green solvent for ultrasound-assisted scalable synthesis of pyrrole derivatives. Ultrason. Sonochem. 2015, 26, 81–86. [Google Scholar] [CrossRef]
- Yang, J.; Tana, J.-N.; Gu, Y. Lactic acid as an invaluable bio-based solvent for organic reactions. Green Chem. 2012, 14, 3304–3317. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Li, M.; Peng, J.; Gua, Y. Multicomponent Reactions of β-Ketosulfones and Formaldehyde in a Bio-Based Binary Mixture Solvent System Composed of Meglumine and Gluconic Acid Aqueous Solution. Adv. Synth. Catal. 2012, 354, 688–700. [Google Scholar] [CrossRef]
- Wilson, K.L.; Murray, J.; Jamieson, C.; Watson, A.J.B. Cyrene as a bio-based solvent for HATU mediated amide coupling. Org. Biomol. Chem. 2018, 16, 2851–2854. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wen, W.A. Clean and Practical Catalyst free Synthesis of Keto and Aldoximes as well as the Beckmann Rearrangement by using Ethyl Lactate as an Environmentally Benign Medium. Curr. Green Chem. 2015, 2, 399–402. [Google Scholar] [CrossRef]
- Guo, R.-Y.; Wang, P.; Wang, G.-D.; Moa, L.-P.; Zhang, Z.-H. One-pot three-component synthesis of functionalized spirooxindoles in gluconic acid aqueous solution. Tetrahedron 2013, 69, 2056–2061. [Google Scholar] [CrossRef]
- Li, B.-L.; Li, P.-H.; Fang, X.-N.; Li, C.-X.; Sun, J.-L.; Mo, L.-P.; Zhang, Z.-H. One-pot four-component synthesis of highly substituted pyrroles in gluconic acid aqueous solution. Tetrahedron 2013, 69, 7011–7018. [Google Scholar] [CrossRef]
- Paul, S.; Das, A.R. An efficient green protocol for the synthesis of coumarin fused highly decorated indenodihydropyridyl and dihydropyridyl derivatives. Tetrahedron Lett. 2012, 53, 2206–2210. [Google Scholar] [CrossRef]
- Babu, G.D.K.; Singh, B. Simulation of Eucalyptus cinerea oil distillation: A study on optimization of 1,8-cineole production. Biochem. Eng. J. 2009, 44, 226–231. [Google Scholar] [CrossRef]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic parameters for solvents of interest in green chemistry. Green Chem. 2012, 14, 1245–1259. [Google Scholar] [CrossRef]
- Pantess, D.A.; Rich, C.V. Aqueous Suzuki Reactions: A Greener Approach to Transition Metal-Mediated Aryl Couplings in the Organic Instructional Laboratory. Chem. Educ. 2009, 14, 258–260. [Google Scholar]
- Vafaeezadeh, M.; Hashemi, M.M. Polyethylene Glycol (PEG) as a Green Solvent for Carbon—Carbon Bond Formation Reactions. J. Mol. Liq. 2015, 207, 73–79. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Norouzi, M. Suzuki, Stille and Heck cross-coupling reactions catalyzed by Fe3O4@PTA–Pd as a recyclable and efficient nanocatalyst in green solvents. New J. Chem. 2016, 40, 6299–6307. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ward, T.R. Recent Advances in the Palladium Catalyzed Suzuki–Miyaura Cross-Coupling Reaction in Water. Catal. Lett. 2016, 146, 820–840. [Google Scholar] [CrossRef] [Green Version]
- Wilson, K.L.; Murray, J.; Jamieson, C.; Watson, A.J.B. Cyrene as a Bio-Based Solvent for the Suzuki-Miyaura Cross-Coupling. Synlett 2018, 29, 650–654. [Google Scholar]
- Campos, J.F.; Scherrmann, M.-C.; Berteina-Raboin, S. Eucalyptol: A new solvent for the synthesis of heterocycles containing oxygen, sulfur and nitrogen. Green Chem. 2019, 21, 1531–1539. [Google Scholar] [CrossRef]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef]
- Chinchilla, R.; Najera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef] [PubMed]
- Loubidi, M.; Moutardier, A.; Campos, J.F.; Berteina-Raboin, S. Pd-catalyzed Suzuki/Sonogashira cross-coupling reaction and the direct sp3 arylation of 7-chloro-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine. Tetrahedron Lett. 2018, 59, 1050–1054. [Google Scholar] [CrossRef]
- Hu, H.; Yang, F.; Wu, Y. Palladacycle-Catalyzed Deacetonative Sonogashira Coupling of Aryl Propargyl Alcohols with Aryl Chlorides. J. Org. Chem. 2013, 78, 10506–10511. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Jiang, H.; Chen, K. Rapid and Efficient Pd-Catalyzed Sonogashira Coupling of Aryl Chlorides. J. Org. Chem. 2008, 73, 6037–6040. [Google Scholar] [CrossRef]
- Yi, C.; Hua, R. Efficient Copper-Free PdCl2(PCy3)2-Catalyzed Sonogashira Coupling of Aryl Chlorides with Terminal Alkynes. J. Org. Chem. 2006, 71, 2535–5996. [Google Scholar] [CrossRef]
- Gelman, D.; Buchwald, S.L. Efficient Palladium-Catalyzed Coupling of Aryl Chlorides and Tosylates with Terminal Alkynes: Use of a Copper Cocatalyst Inhibits the Reaction. Angew. Chem. Int. Ed. 2003, 42, 5993–5996. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol as bio-based solvent for Migita–Kosugi–Stille coupling reaction on O,S,N-heterocycles. Catal. Today 2020, 358, 138–142. [Google Scholar] [CrossRef]
- Bucevicius, J.; Tumkevičius, S. Efficient Synthesis of (Arylethynyl)pyrrolo[2,3-d]pyrimidines by Stille Coupling. Synlett 2015, 26, 810–814. [Google Scholar]
- Karpavičius, K.; Kižys, K.; Leroy, S.; Rousseau, J.; Bucevičius, J.; Tumkevičius, S. Synthesis of (1-substituted 1,2,3-triazol-4-yl)-7-deazapurines. Chemija 2016, 27, 213. [Google Scholar]
- Gribanov, P.S.; Golenko, Y.D.; Topchiy, M.A.; Minaeva, L.I.; Asachenko, A.F.; Nechaev, M.S. Stannylation of Aryl Halides, Stille Cross-Coupling, and One-Pot, Two-Step Stannylation/Stille Cross-Coupling Reactions under Solvent-Free Conditions. Eur. J. Org. Chem. 2018, 1, 120–125. [Google Scholar] [CrossRef]
- Vybornyi, N.J.F.; Skabara, P.J. Scale-up Chemical Synthesis of Thermally-activated Delayed Fluorescence Emitters Based on the Dibenzothiophene-S,S-Dioxide Core. J. Vis. Exp. 2017, 128, 56501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Gong, W.; Tong, Y.; Wei, D.; Wang, Y.; Ding, J.; Hou, H.; Song, Y. Synthesis and properties of benzothiadiazole-pyridine system: The modulation of optical feature. Dyes Pigm. 2017, 137, 135–142. [Google Scholar] [CrossRef]
- Pajtas, D.; Konya, K.; Kiss-Szikszai, A.; Dzubak, P.; Petho, Z.N.; Varga, Z.; Panyi, G.R.; Patonay, T. Optimization of the Synthesis of Flavone–Amino Acid and Flavone–Dipeptide Hybrids via Buchwald–Hartwig Reaction. J. Org. Chem. 2017, 82, 4578–4587. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kim, S.H.; Kim, D.Y.; Kim, C.; Lee, H.W.; Lee, S.E.; Kim, Y.K.; Yoon, S.S. Blue organic light-emitting diodes based on diphenylamino dibenzo[g, p]chrysene derivatives. Thin Solid Films 2017, 636, 8–14. [Google Scholar] [CrossRef]
- Hie, L.; Fine Nathel, N.F.; Hong, X.; Yang, Y.F.; Houk, K.N.; Garg, N.K. Nickel-Catalyzed Activation of Acyl C−O Bonds of Methyl Esters. Angew. Chem. Int. Ed. 2016, 128, 2860–2864. [Google Scholar] [CrossRef]
- Saikia, P.; Sharma, G.; Gogoi, S.; Boruah, R.C. Cascade imination, Buchwald–Hartwig cross coupling and cycloaddition reaction: Synthesis of pyrido[2,3-d]pyrimidines. RSC Adv. 2015, 5, 23210–23212. [Google Scholar] [CrossRef]
- Copin, C.; Massip, S.; Leger, J.M.; Jarry, C.; Buron, F.; Routier, S. SNAr versus Buchwald–Hartwig Amination/Amidation in the Imidazo[2,1-b][1,3,4]thiadiazole Series. Eur. J. Org. Chem. 2015, 71, 6932. [Google Scholar] [CrossRef]
- Schuster, C.; Borger, C.; Julich-Gruner, K.K.; Hesse, R.; Jager, A.; Kaufmann, G.; Schmidt, A.W.; Knolker, H.J. Synthesis of 2-Hydroxy-7-methylcarbazole, Glycozolicine, Mukoline, Mukolidine, Sansoakamine, Clausine-H, and Clausine-K and Structural Revision of Clausine-TY. Eur. J. Org. Chem. 2014, 22, 4741–4752. [Google Scholar] [CrossRef]
- Hesse, R.; Krahl, M.P.; Jager, A.; Kataeva, O.; Schmidt, A.W.; Knolker, H.J. Palladium(II)-Catalyzed Synthesis of the Formylcarbazole Alkaloids Murrayaline A–C, 7-Methoxymukonal, and 7-Methoxy-O-methylmukonal. Eur. J.Org. Chem. 2014, 19, 4014–4028. [Google Scholar] [CrossRef]
- Rao, R.K.; Karthikeyan, I.; Sekar, G. Domino aziridine ring opening and Buchwald–Hartwig type coupling-cyclization by palladium catalyst. Tetrahedron 2012, 68, 9090–9094. [Google Scholar] [CrossRef]
- Fei, X.D.; Zhou, Z.; Li, W.; Zhu, Y.M.; Shen, J.K. Buchwald–Hartwig Coupling/Michael Addition Reactions: One-Pot Synthesis of 1,2-Disubstituted 4-Quinolones from Chalcones and Primary Amines. Eur. J. Org. Chem. 2012, 2012, 3001–3008. [Google Scholar] [CrossRef]
- Krasavin, M. Novel diversely substituted 1-heteroaryl-2-imidazolines for fragment-based drug discovery. Tetrahedron Lett. 2012, 53, 2876–2880. [Google Scholar] [CrossRef] [Green Version]
- Bouhlel, A.; Curti, C.; Khoumeri, O.; Vanelle, P. Efficient one-pot double Buchwald–Hartwig coupling reaction on 5-phenyl-4-phenylsulfonyl-2,3-dihydrofuran derivatives. Tetrahedron Lett. 2011, 52, 1919–1923. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol as a Bio-Based Solvent for Buchwald-Hartwig Reaction on O,S,N-Heterocycles. Catalysts 2019, 9, 840. [Google Scholar] [CrossRef] [Green Version]
- Rosenmund, K.W.; Struck, E. Das am Ringkohlenstoff gebundene Halogen und sein Ersatz durch andere Substituenten. I. Mitteilung: Ersatz des Halogens durch die Carboxylgruppe. Chem. Ber. 1919, 52, 1749–1756. [Google Scholar] [CrossRef] [Green Version]
- Pongratz, A. Untersuchungen über Perylen und seine Derivate. Mon. Chem. 1927, 48, 585–591. [Google Scholar] [CrossRef]
- Von Braun, J.; Manz, G. Fluoranthen und seine Derivate. III. Mitteilung. Eur. J. Org. Chem. 1931, 488, 111–126. [Google Scholar] [CrossRef]
- Connor, J.A.; Leeming, S.W.; Price, R. Influence of substrate structure on copper(I)-assisted cyanide substitution in aryl halides. J. Chem. Soc. Perkin Trans. 1990, 1, 1127–1132. [Google Scholar] [CrossRef]
- Ellis, G.P.; Romney-Alexander, T.M. Cyanation of aromatic halides. Chem. Rev. 1987, 87, 779–794. [Google Scholar] [CrossRef]
- Campos, J.F.; Ferreira, V.; Berteina-Raboin, S. Eucalyptol: A bio-based solvent for the synthesis of O,S,N-Heterocycles. Application to Hiyama Coupling, Cyanation, and Multicomponent Reactions. Catalysts 2021, 11, 222. [Google Scholar] [CrossRef]
- Campos, J.F.; Queiroz, M.-J.R.P.; Berteina-Raboin, S. The First Catalytic Direct C–H Arylation on C2 and C3 of Thiophene Ring Applied to Thieno-Pyridines, -Pyrimidines and -Pyrazines. Catalysts 2018, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.F.; Queiroz, M.-J.R.P.; Berteina-Raboin, S. Synthesis of New Thieno[3, 2-b]pyridines and Thieno[2,3-b]pyrazines by Palladium Cross-Coupling. ChemistrySelect 2017, 24, 6945–6948. [Google Scholar] [CrossRef]
- Li, J.J. Hiyama Cross-Coupling Reaction in: Name Reactions; Springer: Berlin/Heidelberg, Germany, 2003; pp. 187–188. [Google Scholar]
- Li, J.-H.; Deng, C.-L.; Liu, W.-J.; Xie, Y.-X. Pd(OAc)2/DABCO as an Inexpensive and Efficient Catalytic System for Hiyama Cross-Coupling Reactions of Aryl Halides with Aryltrimethoxysilanes. Synthesis 2005, 18, 3039–3044. [Google Scholar] [CrossRef]
- Molander, G.A.; Iannazzo, L. Palladium-Catalyzed Hiyama Cross-Coupling of Aryltrifluorosilanes with Aryl and Heteroaryl Chlorides. J. Org. Chem. 2011, 76, 9182–9187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monguchi, Y.; Yanase, T.; Mori, S.; Sajiki, H. A Practical Protocol for the Hiyama Cross-Coupling Reaction Catalyzed by Palladium on Carbon. Synthesis 2013, 45, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Raders, S.M.; Kingston, J.V.; Verkade, J.G. Advantageous Use of tBu2P-N=P(iBuNCH2CH2)3N in the Hiyama Coupling of Aryl Bromides and Chlorides. J. Org. Chem. 2010, 75, 1744–1747. [Google Scholar] [CrossRef]
- Srimani, D.; Bej, A.; Sarkar, A. Palladium Nanoparticle Catalyzed Hiyama Coupling Reaction of Benzyl Halides. J. Org. Chem. 2010, 75, 4296–4299. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Luxami, V.; Paul, K. Imidazo[1,2-a]pyridines: Promising Drug Candidate for Antitumor Therapy. Curr. Top. Med. Chem. 2016, 16, 3590–3616. [Google Scholar] [CrossRef]
- Deep, A.; Bhatia, R.K.; Kaur, R.; Kumar, S.; Jain, U.K.; Singh, H.; Batra, S.; Kaushik, D.; Deb, P.K. Imidazo[1,2-a]pyridine Scaffold as Prospective Therapeutic Agents. Curr. Top. Med. Chem. 2017, 17, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Hiebel, M.-A.; Fall, Y.; Scherrmann, M.-C.; Berteina-Raboin, S. Straightforward Synthesis of Various 2,3-Diarylimidazo[1,2-a]pyridines in PEG400 Medium through One-Pot Condensation and C–H Arylation. Eur. J. Org. Chem. 2014, 21, 4643–4650. [Google Scholar] [CrossRef]
- Altaf, A.A.; Shahzad, A.; Gul, Z.; Rasool, N.; Badshah, A.; Lal, B.; Khan, E. A Review on the Medicinal Importance of Pyridine Derivatives. J. Drug Des. Med. Chem. 2015, 1, 1–11. [Google Scholar]
- Fuentes, L.; Vaquero, J.J.; Soto, J.L. Heterocycle synthesis. XVI. Reaction of malononitrile with benzylidenemalononitriles in presence of amines. An. Quim. Ser. C 1980, 76, 68–69. [Google Scholar]
- Raghukumar, V.; Thirumalai, D.; Ramakrishnan, V.T.; Karunakara, V.; Ramamurthy, P. Synthesis of nicotinonitrile derivatives as a new class of Non-linear optical materials. Tetrahedron 2003, 59, 3761–3768. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, D.K.; Khan, A.T. Synthesis of fully-substituted pyridines and dihydropyridines in a highly chemoselective manner utilizing a multicomponent reaction (MCR) strategy. RSC Adv. 2014, 4, 53752–53760. [Google Scholar] [CrossRef]
- Chen, J.; Natte, K.; Spannenberg, A.; Neumann, H.; Langer, P.; Beller, M.; Wu, X.-F. Base-controlled selectivity in the synthesis of linear and angular fused quinazolinones by a palladium-catalyzed carbonylation/nucleophilic aromatic substitution sequence. Angew. Chem. Int. Ed. 2014, 53, 7579–7583. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: The advances continue. Eur. J. Med. Chem. 2015, 90, 124–169. [Google Scholar] [CrossRef]
- Campos, J.F.; Pacheco-Benichou, A.; Fruit, C.; Besson, T.; Berteina-Raboin, S. Synthesis of Benzo-Fused 11H-Pyrido[2,1-b]quinazolin-11-ones by a Buchwald-Hartwig Coupling/Pyridine Dearomatization Sequence in Eucalyptol. Synthesis 2020, 52, 3071. [Google Scholar]
- Aounzou, M.; Campos, J.F.; Loubidi, M.; Berteina-Raboin, S. First Metal-Free Synthesis of Tetracyclic Pyrido and Pyrazino Thienopyrimidinone Molecules. Molecules 2018, 23, 1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, J.F.; Berteina-Raboin, S. Eucalyptol, an All-Purpose Product. Catalysts 2022, 12, 48. https://doi.org/10.3390/catal12010048
Campos JF, Berteina-Raboin S. Eucalyptol, an All-Purpose Product. Catalysts. 2022; 12(1):48. https://doi.org/10.3390/catal12010048
Chicago/Turabian StyleCampos, Joana F., and Sabine Berteina-Raboin. 2022. "Eucalyptol, an All-Purpose Product" Catalysts 12, no. 1: 48. https://doi.org/10.3390/catal12010048
APA StyleCampos, J. F., & Berteina-Raboin, S. (2022). Eucalyptol, an All-Purpose Product. Catalysts, 12(1), 48. https://doi.org/10.3390/catal12010048






