A Nickel Coated Copper Substrate as a Hydrogen Evolution Catalyst
Abstract
:1. Introduction
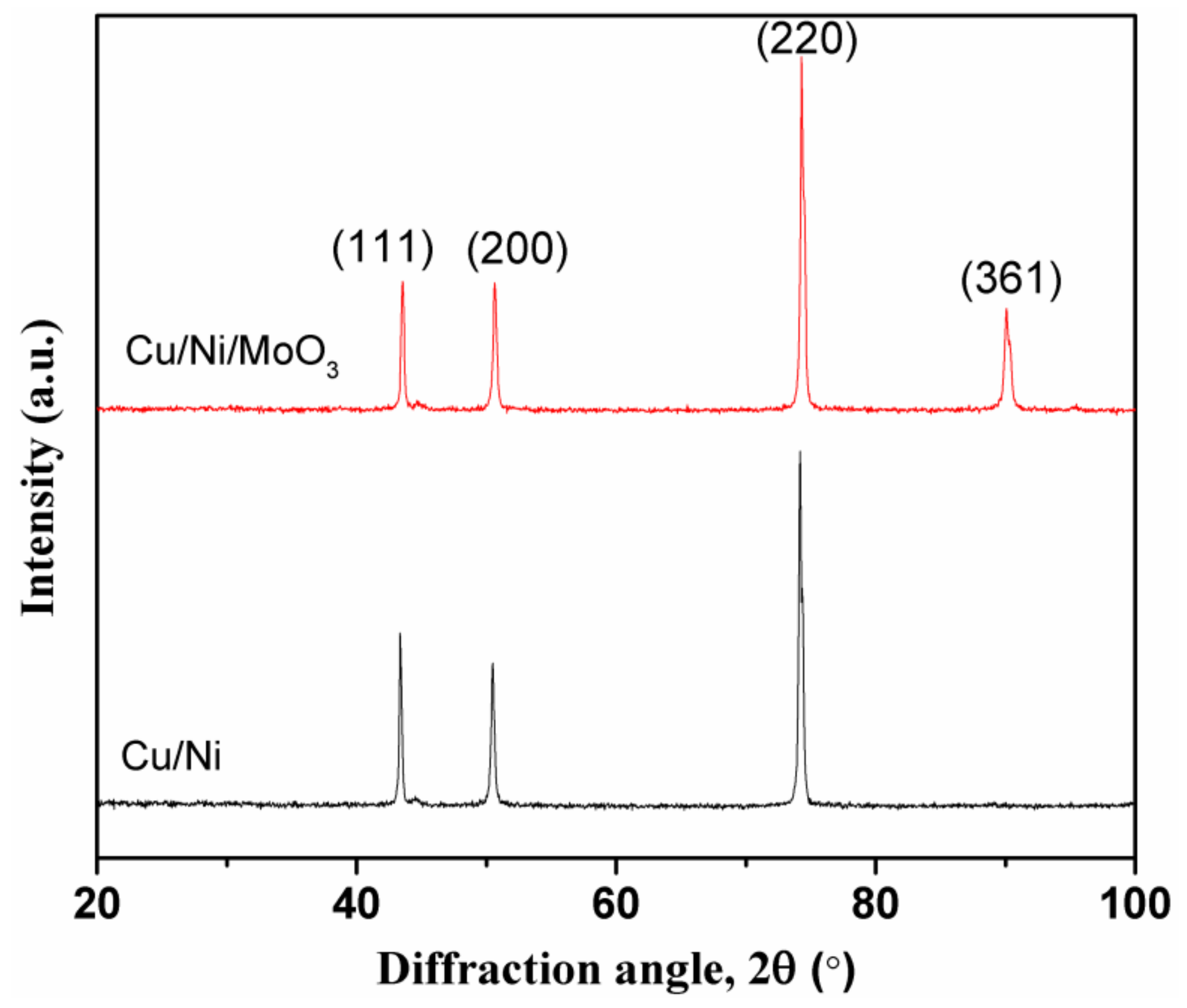
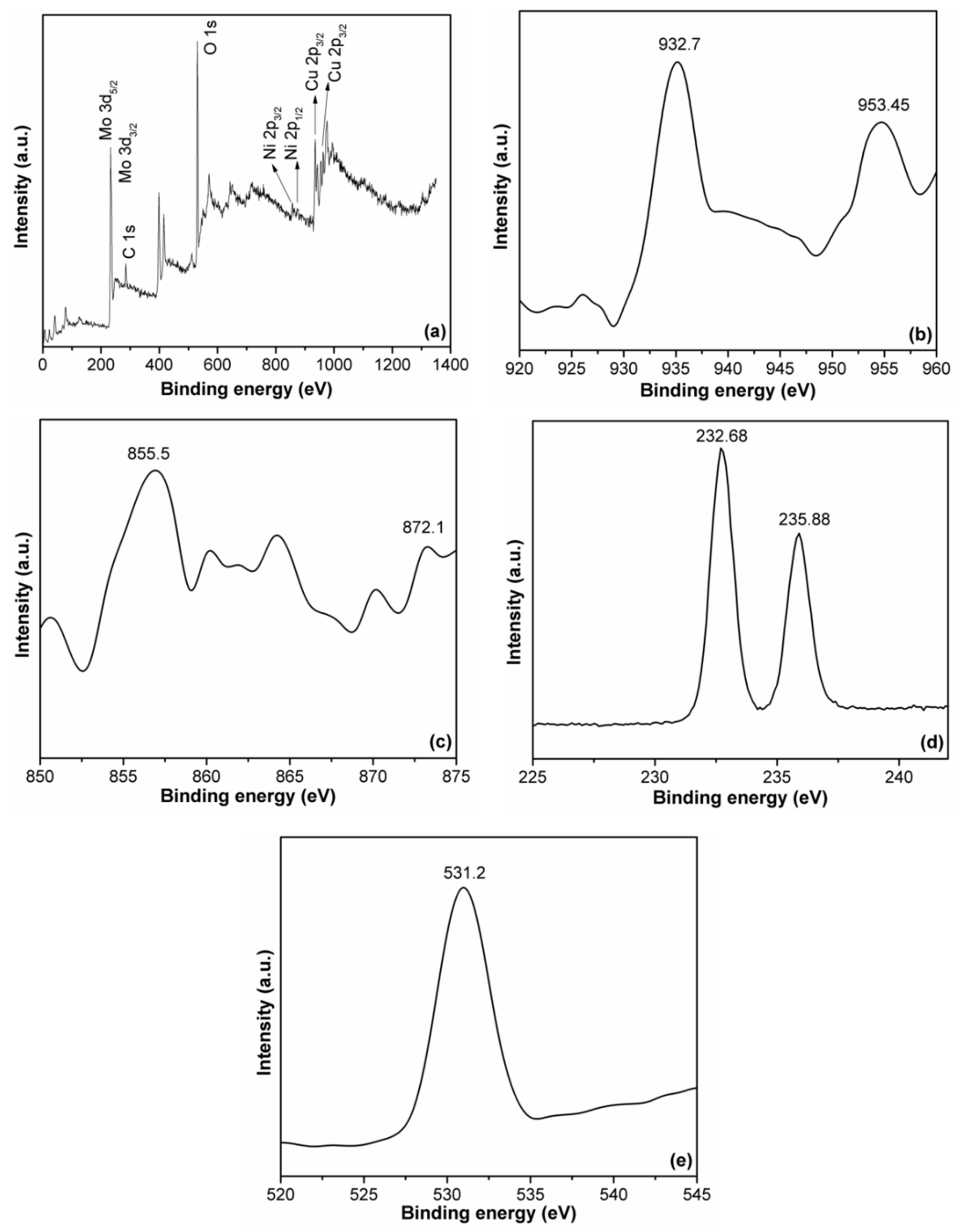
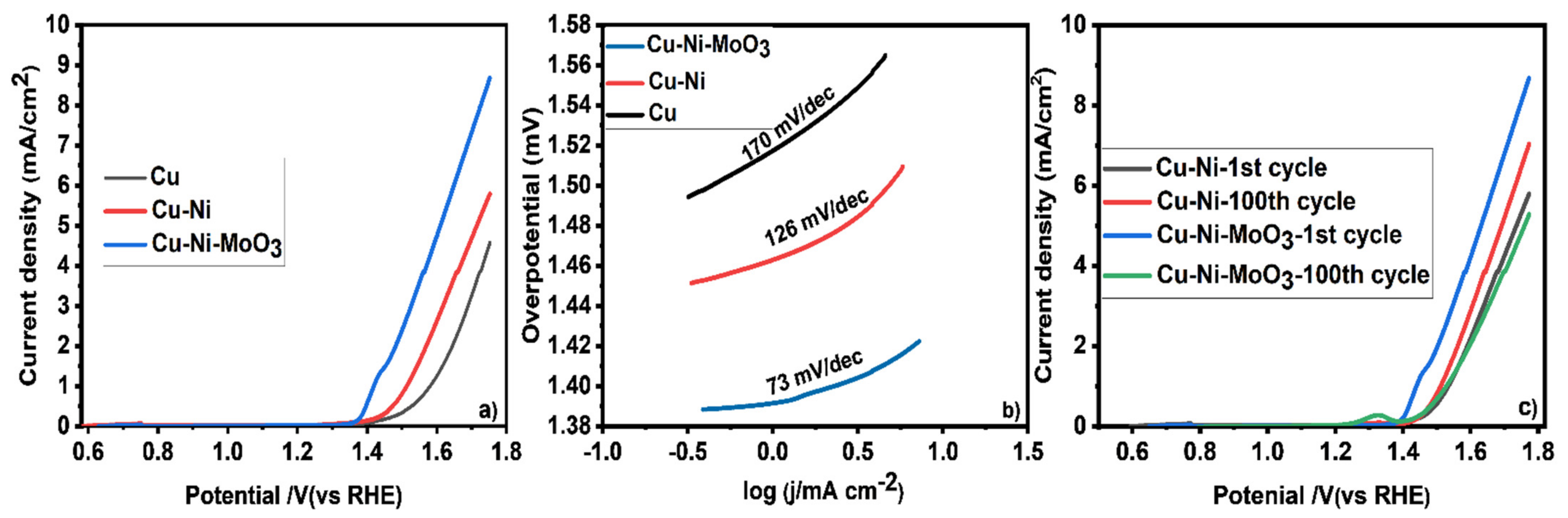
2. Results and Discussion
3. Materials and Methods
3.1. Raw Materials and Preparation of Catalysts
3.2. Characterization of Composition and Structure of Catalysts
3.3. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Liu, P.; Peng, T.; Liu, Q.; Chen, W.; Liu, B.; Yuan, Y.; Zhang, W.; Song, F.; Gu, J.; et al. Mechanically alloyed NiTiO3/transition metal heterostructures: Introducing oxygen vacancies for exceptionally enhanced hydrogen evolution reaction activity. J. Mater. Chem. A 2020, 8, 14908–14914. [Google Scholar] [CrossRef]
- Datta, R.S.; Haque, F.; Mohiuddin, M.; Carey, B.J.; Syed, N.; Zavabeti, A.; Zhang, B.; Khan, H.; Berean, K.J.; Ou, J.Z.; et al. Highly active two dimensional α-MoO3-X for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 24223–24231. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Miao, J.; Xiao, F.X.; Bin Yang, H.; Khoo, S.Y.; Chen, J.; Fan, Z.; Hsu, Y.Y.; Ming Chen, H.; Zhang, H.; Liu, B. Hierarchical Ni-Mo-S nanosheets on carbon fiber cloth: A flexible electrode for efficient hydrogen generation in neutral electrolyte. Sci. Adv. 2015, 1, e1500259. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.R.; Liang, J.X.; Zheng, Y.R.; Xu, Y.F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.H. An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation. Nat. Commun. 2015, 6, 6982. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Ju, H.; Pan, B.; Zhu, J.; Ding, T.; Wang, C.; Yang, Q. Design and Epitaxial Growth of MoSe2-NiSe Vertical Heteronanostructures with Electronic Modulation for Enhanced Hydrogen Evolution Reaction. Chem. Mater. 2016, 28, 1838–1846. [Google Scholar] [CrossRef]
- Kim, M.; Anjum, M.A.R.; Lee, M.; Lee, B.J.; Lee, J.S. Activating MoS2 Basal Plane with Ni2P Nanoparticles for Pt-Like Hydrogen Evolution Reaction in Acidic Media. Adv. Funct. Mater. 2019, 29, 1809151. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, Y.; Hu, Q.; Zhou, J.; Feng, R.; Duchesne, P.N.; Zhang, P.; Chen, F.; Han, N.; Li, Y.; et al. Ultrasmall and phase-pure W2C nanoparticles for efficient electrocatalytic and photoelectrochemical hydrogen evolution. Nat. Commun. 2016, 7, 13216. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.Y.; Lou, X.W. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, S.E.; Kim, S. Thermal assessment of copper through silicon via in 3D IC. Microelectron. Eng. 2016, 156, 2–5. [Google Scholar] [CrossRef]
- Hamidah, I.; Solehudin, A.; Hamdani, A.; Hasanah, L.; Khairurrijal, K.; Kurniawan, T.; Mamat, R.; Maryanti, R.; Nandiyanto, A.B.D.; Hammouti, B. Corrosion of copper alloys in KOH, NaOH, NaCl, and HCl electrolyte solutions and its impact to the mechanical properties. Alex. Eng. J. 2021, 60, 2235–2243. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, J.; Liu, Y.; Shen, J.; Wang, H.; Yang, X.; Zhu, Y.; Li, C. Multimetallic Ni-Mo/Cu nanowires as nonprecious and efficient full water splitting catalyst. J. Mater. Chem. A 2017, 5, 4207–4214. [Google Scholar] [CrossRef]
- Sivasakthi, P.; Sangaranarayanan, M.V.; Gurumallesh Prabu, H. Micro-nanoarchitectures of electrodeposited Ni-ITO nanocomposites on copper foil as electrocatalysts for the oxygen evolution reaction. New J. Chem. 2021, 45, 5146–5153. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Li, Y.; Hao, S.; Cao, X.; Yang, G.; Wu, J.; Huang, Y. Self-assembled Cu-Ni bimetal oxide 3D in-plane epitaxial structures for highly efficient oxygen evolution reaction. Appl. Catal. B: Environ. 2019, 244, 56–62. [Google Scholar] [CrossRef]
- Ngamlerdpokin, K.; Tantavichet, N. Electrodeposition of nickel-copper alloys to use as a cathode for hydrogen evolution in an alkaline media. Int. J. Hydrog. Energy 2014, 39, 2505–2515. [Google Scholar] [CrossRef]
- Solmaz, R.; Döner, A.; Kardaş, G. Electrochemical deposition and characterization of NiCu coatings as cathode materials for hydrogen evolution reaction. Electrochem. Commun. 2008, 10, 1909–1911. [Google Scholar] [CrossRef]
- Solmaz, R.; Döner, A.; Kardaş, G. The stability of hydrogen evolution activity and corrosion behavior of NiCu coatings with long-term electrolysis in alkaline solution. Int. J. Hydrog. Energy 2009, 34, 2089–2094. [Google Scholar] [CrossRef]
- Damian, A.; Omanovic, S. Ni and Ni-Mo hydrogen evolution electrocatalysts electrodeposited in a polyaniline matrix. J. Power Sources 2006, 158, 464–476. [Google Scholar] [CrossRef]
- Gomez Vidales, A.; Omanovic, S. Evaluation of nickel-molybdenum-oxides as cathodes for hydrogen evolution by water electrolysis in acidic, alkaline, and neutral media. Electrochim. Acta 2018, 262, 115–123. [Google Scholar] [CrossRef]
- Navarro-Flores, E.; Chong, Z.; Omanovic, S. Characterization of Ni, NiMo, NiW and NiFe electroactive coatings as electrocatalysts for hydrogen evolution in an acidic medium. J. Mol. Catal. A: Chem. 2005, 226, 179–197. [Google Scholar] [CrossRef]
- Kawashima, A.; Akiyama, E.; Habazaki, H.; Hashimoto, K. Characterization of sputter-deposited Ni-Mo and Ni-W alloy electrocatalysts for hydrogen evolution in alkaline solution. Mater. Sci. Eng. A 1997, 226–228, 905–909. [Google Scholar] [CrossRef]
- Highfield, J.G.; Claude, E.; Oguro, K. Electrocatalytic synergism in Ni/Mo cathodes for hydrogen evolution in acid medium: A new model. Electrochim. Acta 1999, 44, 2805–2814. [Google Scholar] [CrossRef]
- Jakšić, J.M.; Vojnović, M.V.; Krstajić, N.V. Kinetic analysis of hydrogen evolution at Ni-Mo alloy electrodes. Electrochim. Acta 2000, 45, 4151–4158. [Google Scholar] [CrossRef]
- Krstajić, N.V.; Gajić-Krstajić, L.; Lačnjevac, U.; Jović, B.M.; Mora, S.; Jović, V.D. Non-noble metal composite cathodes for hydrogen evolution. Part I: The Ni-MoOx coatings electrodeposited from Watt’s type bath containing MoO3 powder particles. Int. J. Hydrog. Energy 2011, 36, 6441–6449. [Google Scholar] [CrossRef]
- Krstajić, N.V.; Lačnjevac, U.; Jović, B.M.; Mora, S.; Jović, V.D. Non-noble metal composite cathodes for hydrogen evolution. Part II: The Ni-MoO2 coatings electrodeposited from nickel chloride-ammonium chloride bath containing MoO2 powder particles. Int. J. Hydrog. Energy 2011, 36, 6450–6461. [Google Scholar] [CrossRef]
- Harinipriya, S.; Sangaranarayanan, M.V. Influence of the work function on electron transfer processes at metals: Application to the hydrogen evolution reaction. Langmuir 2002, 18, 5572–5578. [Google Scholar] [CrossRef]
- Tilak, B.V. High Performance Electrode Materials for the Hydrogen Evolution Reaction From Alkaline Media. Electrochem. Soc. Ext. Abstr. 1983, 97, 359–393. [Google Scholar] [CrossRef]
- Mckone, J.R.; Sadtler, B.F.; Werlang, C.A.; Lewis, N.S.; Gray, H.B. Ni−Mo Nanopowders for Efficient Electrochemical Hydrogen Evolution. ACS Catal. 2013, 3, 166–169. [Google Scholar] [CrossRef]
- Sharma, L.; Khushwaha, H.S.; Mathur, A.; Halder, A. Role of molybdenum in Ni-MoO2 catalysts supported on reduced graphene oxide for temperature dependent hydrogen evolution reaction. J. Solid State Chem. 2018, 265, 208–217. [Google Scholar] [CrossRef]
- Xu, Y.; Lai, K.; Gu, C.; Jiang, T.; Shen, X.; Zeng, S.; Ho, A.H.P.; Ang, D.S.; Zhou, J. Electrical Tuning of MoOx/Ag Hybrids and Investigation of their Surface-Enhanced Raman Scattering Performance. Phys. Status Solidi-Rapid Res. Lett. 2021, 15, 2000499. [Google Scholar] [CrossRef]
- Crist, B. A Review of XPS Data-Banks. XPS Int. 2007, 1, 1–52. [Google Scholar]
- Santosh, K.C.; Longo, R.C.; Addou, R.; Wallace, R.M.; Cho, K. Electronic properties of MoS2/MoOx interfaces: Implications in Tunnel Field Effect Transistors and Hole Contacts. Sci. Rep. 2016, 6, 33562. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Friend, C.M.; Kaxiras, E. The chemical nature of surface point defects on MoO3(010): Adsorption of hydrogen and methyl. J. Am. Chem. Soc. 2001, 123, 2224–2230. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.; Lei, F.; Xie, Y. Atomically-thin two-dimensional sheets for understanding active sites in catalysis. Chem. Soc. Rev. 2015, 44, 623–636. [Google Scholar] [CrossRef]
- Kim, H.S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3-x. Nat. Mater. 2017, 16, 454–462. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Kats, E.; Carey, B.; Balendhran, S. Proton intercalated two-dimensional WO3 nano-flakes with enhanced charge-carrier mobility at room temperature. Nanoscale 2014, 6, 15029–15036. [Google Scholar] [CrossRef]
- Liang, H.; Li, L.; Meng, F.; Dang, L.; Zhuo, J.; Forticaux, A.; Wang, Z.; Jin, S. Porous Two-Dimensional Nanosheets Converted from Layered Double Hydroxides and Their Applications in Electrocatalytic Water Splitting. Chem. Mater. 2015, 27, 5702–5711. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, H.X.; Wang, Z.L.; Meng, F.L.; Zhang, X.B. Integrated Three-Dimensional Carbon Paper/Carbon Tubes/Cobalt-Sulfide Sheets as an Efficient Electrode for Overall Water Splitting. ACS Nano 2016, 10, 2342–2348. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Li, L.H.; Xing, T.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. Toward design of synergistically active carbon-based catalysts for electrocatalytic hydrogen evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lee, C.Y.; Schmuki, P. Solar water splitting: Preserving the beneficial small feature size in porous α-Fe2O3 photoelectrodes during annealing. J. Mater. Chem. A 2013, 1, 212–215. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuppam, P.K.R.; Kimbulapitiya, K.M.M.D.K.; Vuppala, S.; Wang, K.; Reddy, G.P.; Pande, K.P.; Lee, P.-T.; Chueh, Y.-L. A Nickel Coated Copper Substrate as a Hydrogen Evolution Catalyst. Catalysts 2022, 12, 58. https://doi.org/10.3390/catal12010058
Kuppam PKR, Kimbulapitiya KMMDK, Vuppala S, Wang K, Reddy GP, Pande KP, Lee P-T, Chueh Y-L. A Nickel Coated Copper Substrate as a Hydrogen Evolution Catalyst. Catalysts. 2022; 12(1):58. https://doi.org/10.3390/catal12010058
Chicago/Turabian StyleKuppam, Poshan Kumar Reddy, K. M. M. D. K. Kimbulapitiya, Srikanth Vuppala, Kuangye Wang, G. Phaneendra Reddy, Krishna P. Pande, Po-Tsung Lee, and Yun-Lun Chueh. 2022. "A Nickel Coated Copper Substrate as a Hydrogen Evolution Catalyst" Catalysts 12, no. 1: 58. https://doi.org/10.3390/catal12010058
APA StyleKuppam, P. K. R., Kimbulapitiya, K. M. M. D. K., Vuppala, S., Wang, K., Reddy, G. P., Pande, K. P., Lee, P.-T., & Chueh, Y.-L. (2022). A Nickel Coated Copper Substrate as a Hydrogen Evolution Catalyst. Catalysts, 12(1), 58. https://doi.org/10.3390/catal12010058







