Biophotocatalytic Reduction of CO2 in Anaerobic Biogas Produced from Wastewater Treatment Using an Integrated System
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Preliminary Degradation Efficiency
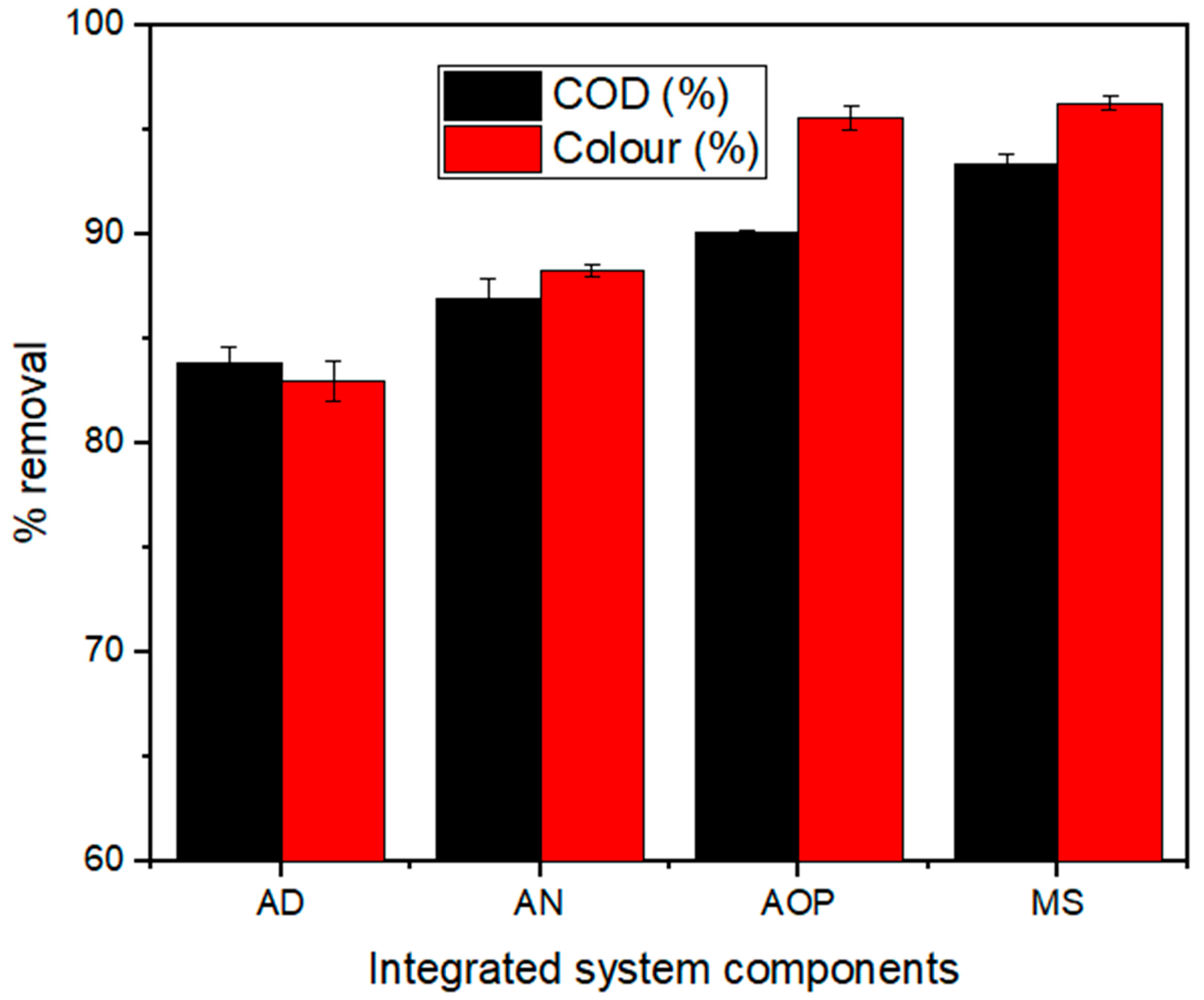
2.2. The Integrated System Decontamination Efficiency
2.3. The Integrated System Degradability Kinetics
2.4. Biogas Production and Energy Estimation
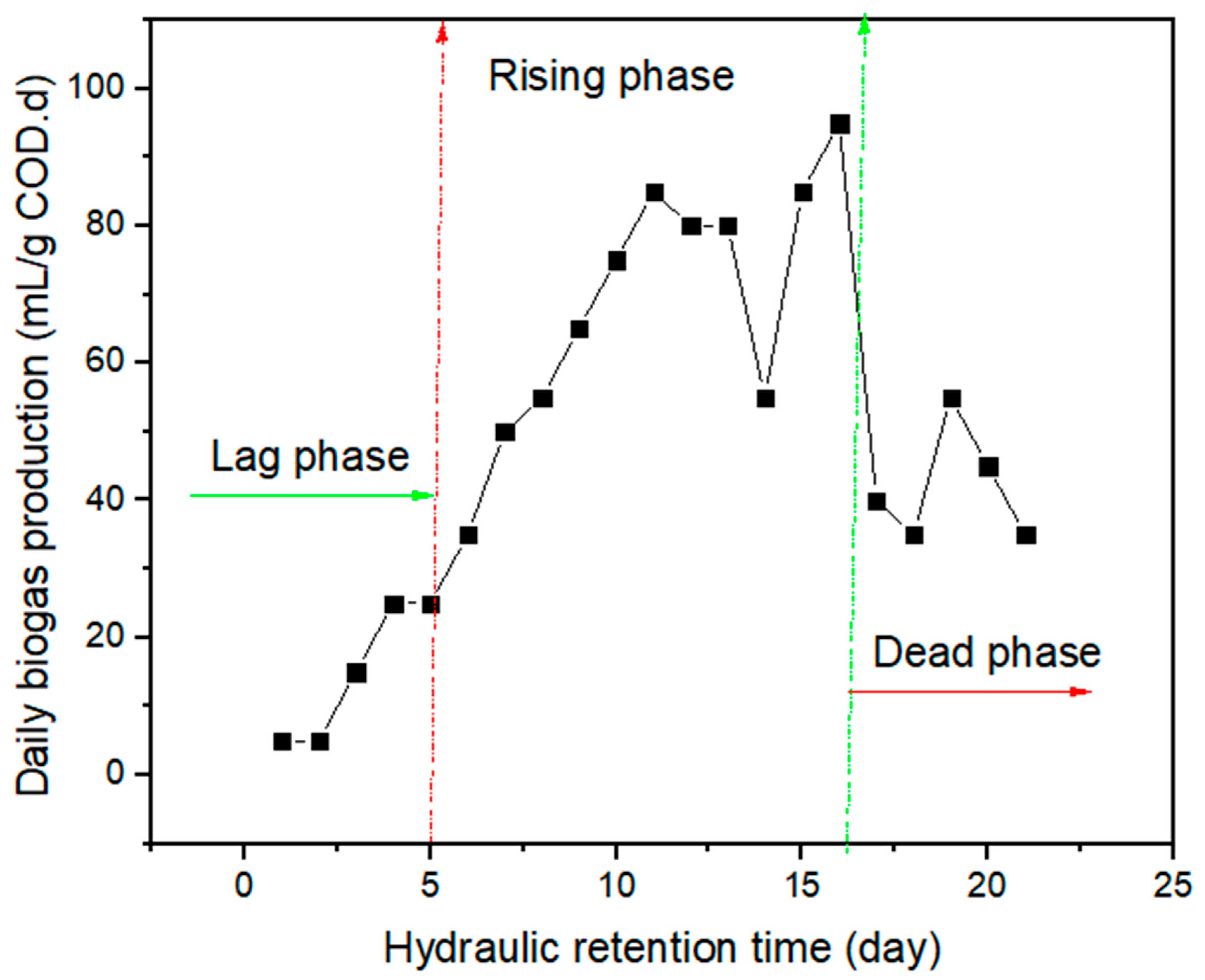
2.4.1. Biogas Production and Methane Composition
2.4.2. Comparative Kinetic Study
2.5. Estimation of Energy and CO2 Emission Reduction
2.5.1. Bioenergy (Ebio) Produced
2.5.2. Energy Utilised (Euv)
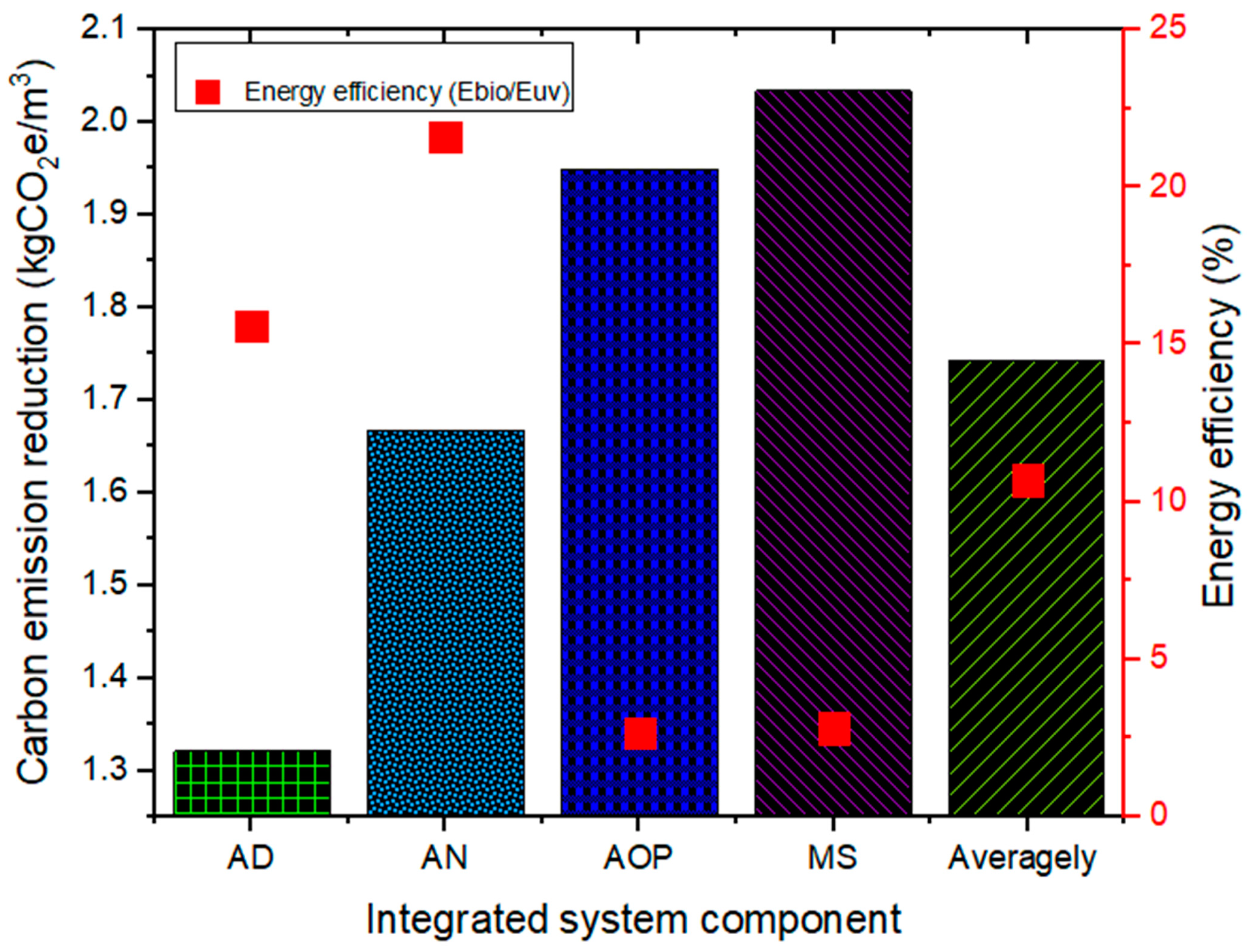
2.5.3. Energy Efficiency and Carbon Emission Reduction (CER)
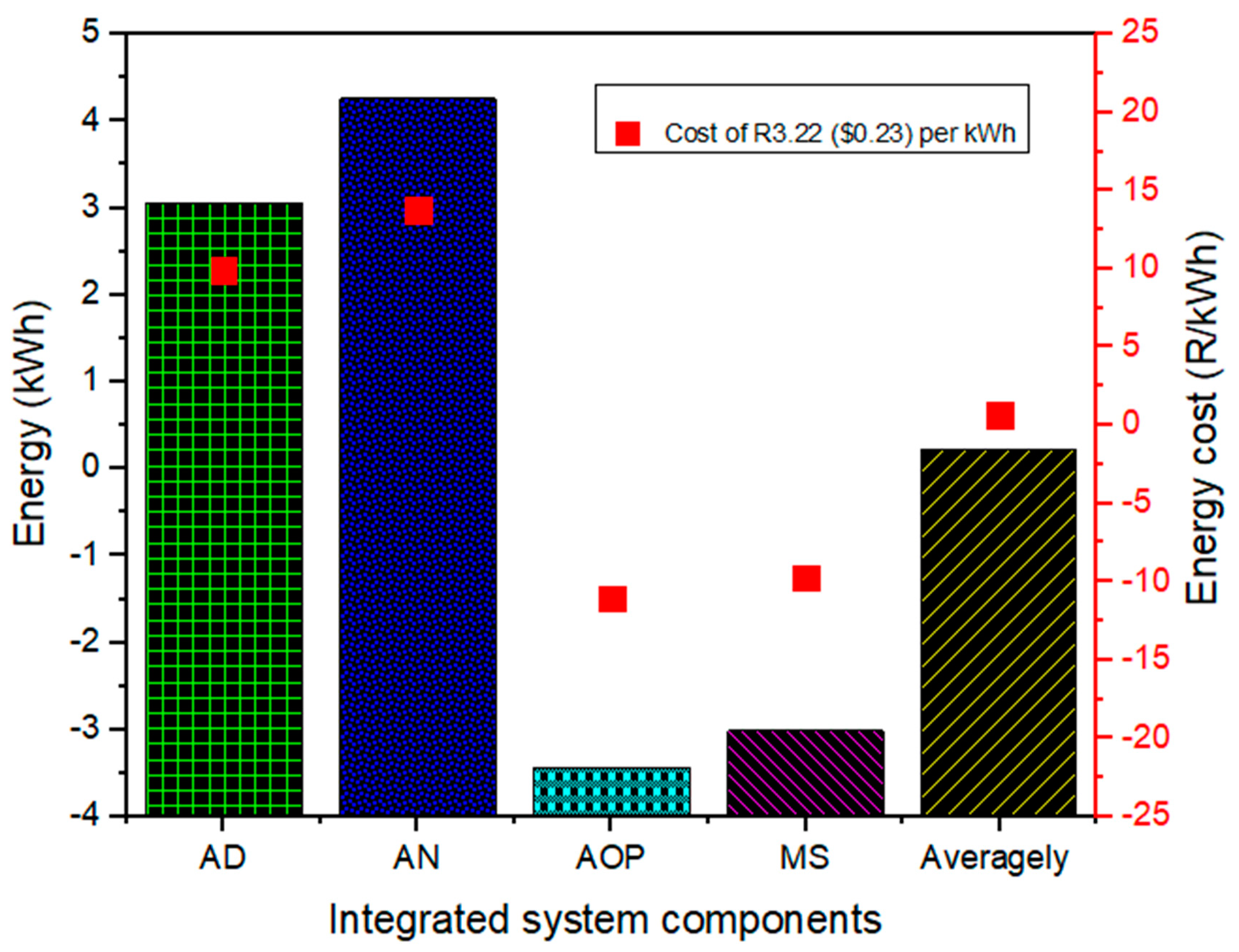
2.5.4. Energy Economy
3. Materials and Methods
3.1. Materials
3.2. Experimental Setup Description and Procedure
3.2.1. Feedstock
3.2.2. Setup—Operation
3.3. Data Collection and Response Analysis
3.3.1. Biogas Production Kinetics
3.3.2. Energy Estimation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, T.K.L.; Ngo, H.H.; Guo, W.; Nguyen, T.L.H.; Chang, S.W.; Nguyen, D.D.; Varjani, S.; Lei, Z.; Deng, L. Environmental impacts and greenhouse gas emissions assessment for energy recovery and material recycle of the wastewater treatment plant. Sci. Total Environ. 2021, 784, 147135. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Asante-Sackey, D.; Armah, E.K.; Rathilal, S. Tapping Wastewater Resource: Why and How? In Handbook of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 125–146. [Google Scholar]
- Kushwaha, J.P. A review on sugar industry wastewater: Sources, treatment technologies, and reuse. Desalination Water Treat. 2015, 53, 309–318. [Google Scholar] [CrossRef]
- Kweinor Tetteh, E.; Opoku Amankwa, M.; Armah, E.K.; Rathilal, S. Fate of COVID-19 Occurrences in Wastewater Systems: Emerging Detection and Treatment Technologies—A Review. Water 2020, 12, 2680. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Mahtab, M.S.; Farooqi, I.H.; Khursheed, A. Zero Fenton Sludge Discharge: A Review on Reuse Approach during Wastewater Treatment by the Advanced Oxidation Process. Int. J. Environ. Sci. Technol. 2021, 1–14. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Naidoo, B.D.; Rathilal, S. Optimization of photo-catalytic degradation of oil refinery wastewater using Box-Behnken design. Environ. Eng. Res. 2019, 24, 711–717. [Google Scholar] [CrossRef]
- Bella, K.; Rao, P.V. Anaerobic Digestion of Dairy Wastewater: Effect of Different Parameters and Co-Digestion Options—A Review. Biomass Convers. Biorefinery 2021, 1–26. [Google Scholar] [CrossRef]
- Ángeles, R.; Vega-Quiel, M.J.; Batista, A.; Fernández-Ramos, O.; Lebrero, R.; Muñoz, R. Influence of biogas supply regime on photosynthetic biogas upgrading performance in an enclosed algal-bacterial photobioreactor. Algal Res. 2021, 57, 102350. [Google Scholar] [CrossRef]
- Fernández-Polanco, D.; Aagesen, E.; Fdz-Polanco, M.; Pérez-Elvira, S.I. Comparative analysis of the thermal hydrolysis integration within WWTPs as a pre-, inter- or post-treatment for anaerobic digestion of sludge. Energy 2021, 223, 120041. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Dong, R. Recent progress towards in-situ biogas upgrading technologies. Sci. Total Environ. 2021, 800, 149667. [Google Scholar] [CrossRef]
- Apollo, S.; Onyango, M.S.; Ochieng, A. An integrated anaerobic digestion and UV photocatalytic treatment of distillery wastewater. J. Hazard. Mater. 2013, 261, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, E.K.; Rathilal, S. Application of Organic Coagulants in Water and Wastewater Treatment. Org. Polym. 2019. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef] [PubMed]
- Kweinor Tetteh, E.; Rathilal, S. Biogas production from wastewater treatment-evaluating anaerobic and biomagnetic systems. Water-Energy Nexus 2021, 4, 165–173. [Google Scholar] [CrossRef]
- Ajay, C.; Mohan, S.; Dinesha, P.; Rosen, M.A. Review of impact of nanoparticle additives on anaerobic digestion and methane generation. Fuel 2020, 277, 118234. [Google Scholar] [CrossRef]
- Xie, S.; Hai, F.I.; Zhan, X.; Guo, W.; Ngo, H.H.; Price, W.E.; Nghiem, L.D. Anaerobic co-digestion: A critical review of mathematical modelling for performance optimization. Bioresour. Technol. 2016, 222, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Kweinor Tetteh, E.; Rathilal, S. Kinetics and nanoparticle catalytic enhancement of biogas production from wastewater using a magnetized biochemical methane potential (Mbmp) system. Catalysts 2020, 10, 1200. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Amo-Duodu, G.; Rathilal, S. Synergistic Effects of Magnetic Nanomaterials on Post-Digestate for Biogas Production. Molecules 2021, 26, 6434. [Google Scholar] [CrossRef]
- Ghofrani-Isfahani, P.; Baniamerian, H.; Tsapekos, P.; Alvarado-Morales, M.; Kasama, T.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Effect of metal oxide based TiO2 nanoparticles on anaerobic digestion process of lignocellulosic substrate. Energy 2020, 191, 116580. [Google Scholar] [CrossRef]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Synergistic Effect of Magnetite and Bioelectrochemical Systems on Anaerobic Digestion. Bioengineering 2021, 8, 198. [Google Scholar] [CrossRef]
- Rojo, E.; Carmona, A.; Soto, C.; Díaz, I.; Fernández-Polanco, M.; Palacio, L.; Muñoz, R.; Bolado, S. Environment and Material Science Technology for Anaerobic Digestion-Based Circular Bioeconomy. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 25–55. [Google Scholar]
- Ge, M.; Hu, Z.; Wei, J.; He, Q.; He, Z. Recent advances in persulfate-assisted TiO2-based photocatalysis for wastewater treatment: Performances, mechanism and perspectives. J. Alloys Compd. 2021, 888, 161625. [Google Scholar] [CrossRef]
- Thakre, K.G.; Barai, D.P.; Bhanvase, B.A. A review of graphene-TiO2 and graphene-ZnO nanocomposite photocatalysts for wastewater treatment. Water Environ. Res. 2021, 93, 2414–2460. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, N.S.; Nassar, H.N. Biosynthesized magnetite nanoparticles as an environmental opulence and sustainable wastewater treatment. Sci. Total Environ. 2021, 774, 145610. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Guidolin, T.; Possolli, N.M.; Polla, M.B.; Wermuth, T.B.; de Oliveira, T.F.; Eller, S.; Montedo, O.R.K.; Arcaro, S.; Cechinel, M.A.P. Photocatalytic pathway on the degradation of methylene blue from aqueous solutions using magnetite nanoparticles. J. Clean. Prod. 2021, 318, 128556. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Moon, G.-h.; Kim, B.; Tachikawa, T.; Majima, T.; Hong, S.; Cho, K.; Kim, W.; Choi, W. Crystal phase-dependent generation of mobile OH radicals on TiO2: Revisiting the photocatalytic oxidation mechanism of anatase and rutile. Appl. Catal. B Environ. 2021, 286, 119905. [Google Scholar] [CrossRef]
- Apollo, S.; Onyango, M.S.; Ochieng, A. Modelling energy efficiency of an integrated anaerobic digestion and photodegradation of distillery effluent using response surface methodology. Environ. Technol. 2016, 37, 2435–2446. [Google Scholar] [CrossRef]
- Durán, I.; Rubiera, F.; Pevida, C. Modeling a biogas upgrading PSA unit with a sustainable activated carbon derived from pine sawdust. Sensitivity analysis on the adsorption of CO2 and CH4 mixtures. Chem. Eng. J. 2021, 428, 132564. [Google Scholar] [CrossRef]
- Krzysztof, Z. Methane fermentation process as anaerobic digestion of biomass: Transformations, stages and microorganisms. Afr. J. Biotechnol. 2012, 11, 4127–4139. [Google Scholar] [CrossRef] [Green Version]
- Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Modelling and optimization of photocatalytic degradation of phenol via TiO2 nanoparticles: An insight into response surface methodology and artificial neural network. J. Photochem. Photobiol. A Chem. 2019, 384, 112039. [Google Scholar] [CrossRef]
- De Andrade Guerra, J.B.S.O.; Berchin, I.I.; Garcia, J.; da Silva Neiva, S.; Jonck, A.V.; Faraco, R.A.; de Amorim, W.S.; Ribeiro, J.M.P. A literature-based study on the water-energy-food nexus for sustainable development. Stoch. Environ. Res. Risk Assess. 2021, 35, 95–116. [Google Scholar] [CrossRef]
- Amo-Duodu, G.; Tetteh, E.K.; Rathilal, S.; Armah, E.K.; Adedeji, J.; Chollom, M.N.; Chetty, M. Effect of Engineered Biomaterials and Magnetite on Wastewater Treatment: Biogas and Kinetic Evaluation. Polymers 2021, 13, 4323. [Google Scholar] [CrossRef] [PubMed]







| Parameter | Feed | AD | AN | AOP | MS |
|---|---|---|---|---|---|
| pH | 7.8 ± 2.3 | 6.83 ± 0.23 | 6.86 ± 0.6 | 6.83 ± 0.2 | 6.9 ± 0.2 |
| Temp (℃) | 28.4 ± 3.6 | 27.88 ± 0.18 | 26.54 ± 0.126 | 26.54 ± 0.15 | 26.04 ± 1.3 |
| Colour (Pt.Co) | 1840 ± 45 | 319.25 ± 1.25 | 215.85 ± 5.23 | 83.17 ± 1.36 | 67.03 ± 2.4 |
| Turbidity (NTU) | 604 ± 13.6 | 102.85 ± 1.25 | 63.25 ± 2.6 | 32.62 ± 1.73 | 23.23 ± 1.73 |
| COD (mg/L) | 1640 ± 24.2 | 265.75 ± 7.24 | 215.75 ± 1.64 | 161.95 ± 1.85 | 108.78 ± 1.65 |
| NH3 (mg/L) | 5.47 ± 1.2 | 7 ± 2.4 | 5.5 ± 1.2 | 4.35 ± 1.3 | 3.5 ± 1.4 |
| TKN (mg/L) | 38.3 ± 1.7 | 8.47 | 7.615 | 5.62 | 3.24 |
| NO3 (mg/L) | 7.05 ± 2.3 | −7.24 | −6.84 | −5.03 | −2.94 |
| TN (mg/L) | 45.35 ± 8.7 | 1.23 | 0.778 | 0.587 | 0.295 |
| Water Parameter | Colour | COD | ||
|---|---|---|---|---|
| Integrated system component | k (d−1) | R2 | k (d−1) | R2 |
| AD | 1.7 | 0.853 | 1.8 | 0.965 |
| AN | 2.14 | 0.865 | 2.02 | 0.873 |
| AOP | 3.12 | 0.896 | 2.33 | 0.965 |
| Ms | 3.38 | 0.951 | 2.717 | 0.986 |
| Model Parameters | First-Order | Modified Gompertz |
|---|---|---|
| Y(t) (mL/gCOD) | 1220 | 1220 |
| Ym (mL/gCOD) | 1180.04 | 1210.74 |
| ʎ (days) | n/a | 10.50 |
| k (1/day) | 5.7 × 10−5 | 0.171 |
| R2 | 0.988 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetteh, E.K.; Rathilal, S. Biophotocatalytic Reduction of CO2 in Anaerobic Biogas Produced from Wastewater Treatment Using an Integrated System. Catalysts 2022, 12, 76. https://doi.org/10.3390/catal12010076
Tetteh EK, Rathilal S. Biophotocatalytic Reduction of CO2 in Anaerobic Biogas Produced from Wastewater Treatment Using an Integrated System. Catalysts. 2022; 12(1):76. https://doi.org/10.3390/catal12010076
Chicago/Turabian StyleTetteh, Emmanuel Kweinor, and Sudesh Rathilal. 2022. "Biophotocatalytic Reduction of CO2 in Anaerobic Biogas Produced from Wastewater Treatment Using an Integrated System" Catalysts 12, no. 1: 76. https://doi.org/10.3390/catal12010076
APA StyleTetteh, E. K., & Rathilal, S. (2022). Biophotocatalytic Reduction of CO2 in Anaerobic Biogas Produced from Wastewater Treatment Using an Integrated System. Catalysts, 12(1), 76. https://doi.org/10.3390/catal12010076







