Coke Deposition and Structural Changes of Pellet V2O5/NaY-SiO2 in Air Regeneration: The Effects of Temperature on Regeneration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Breakthrough Curve of the Fresh and Air Regenerated Vanadia Adsorbents
2.2. Temperature Programmed Oxidation (TPO) of Ethanol-laden Vanadia Adsorbents
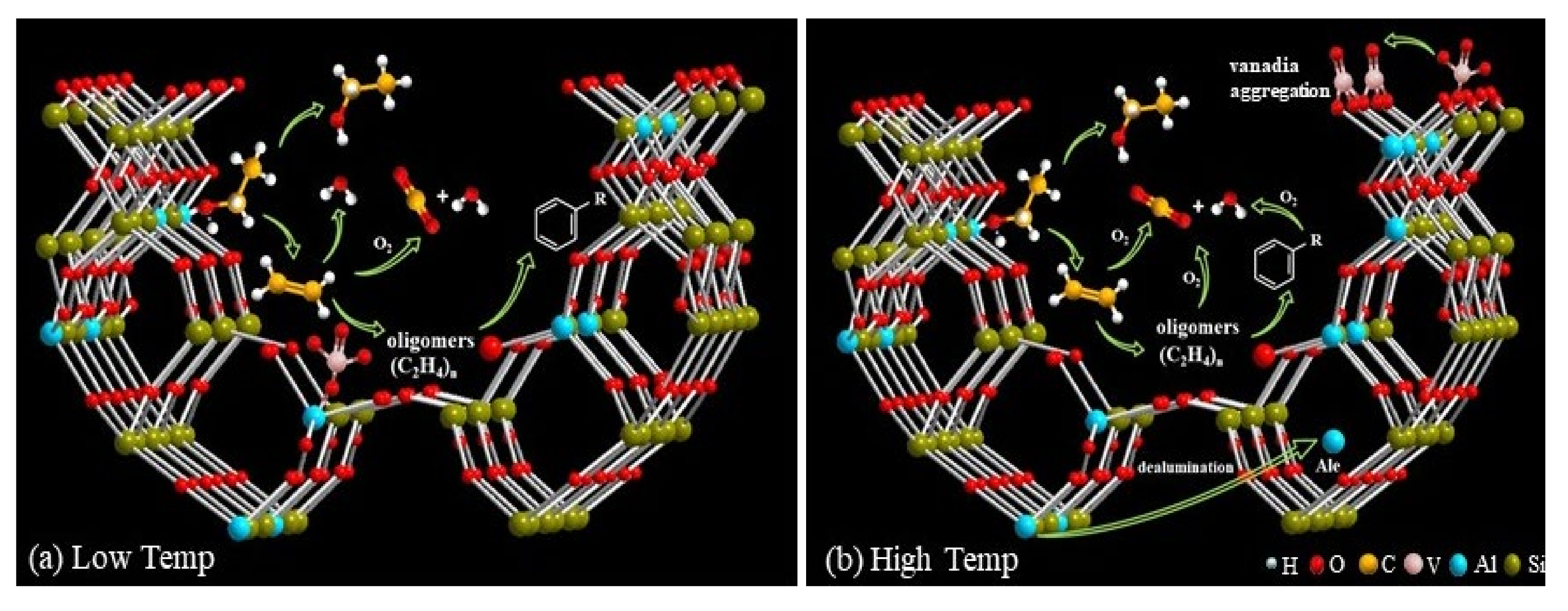
2.3. Causes of Activity Loss during Low-Temperature Regeneration
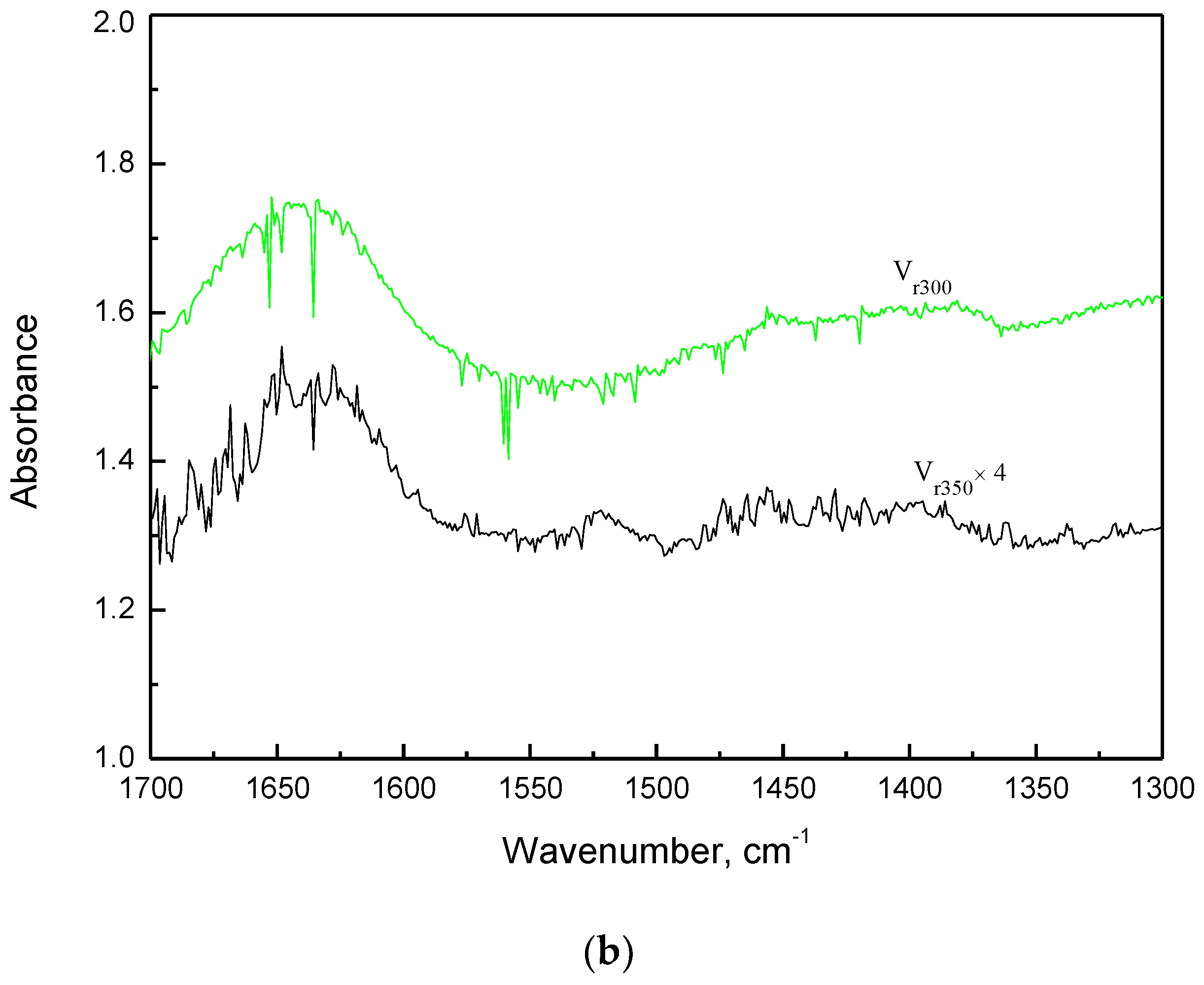
2.3.1. FT-IR Characterization of Air Regenerated Ethanol-laden V2O5/NaY-SiO2
2.3.2. DSC Characterization of Air Regenerated Ethanol-laden V2O5/NaY-SiO2
2.4. Causes of Activity Loss at High-temperature Regeneration
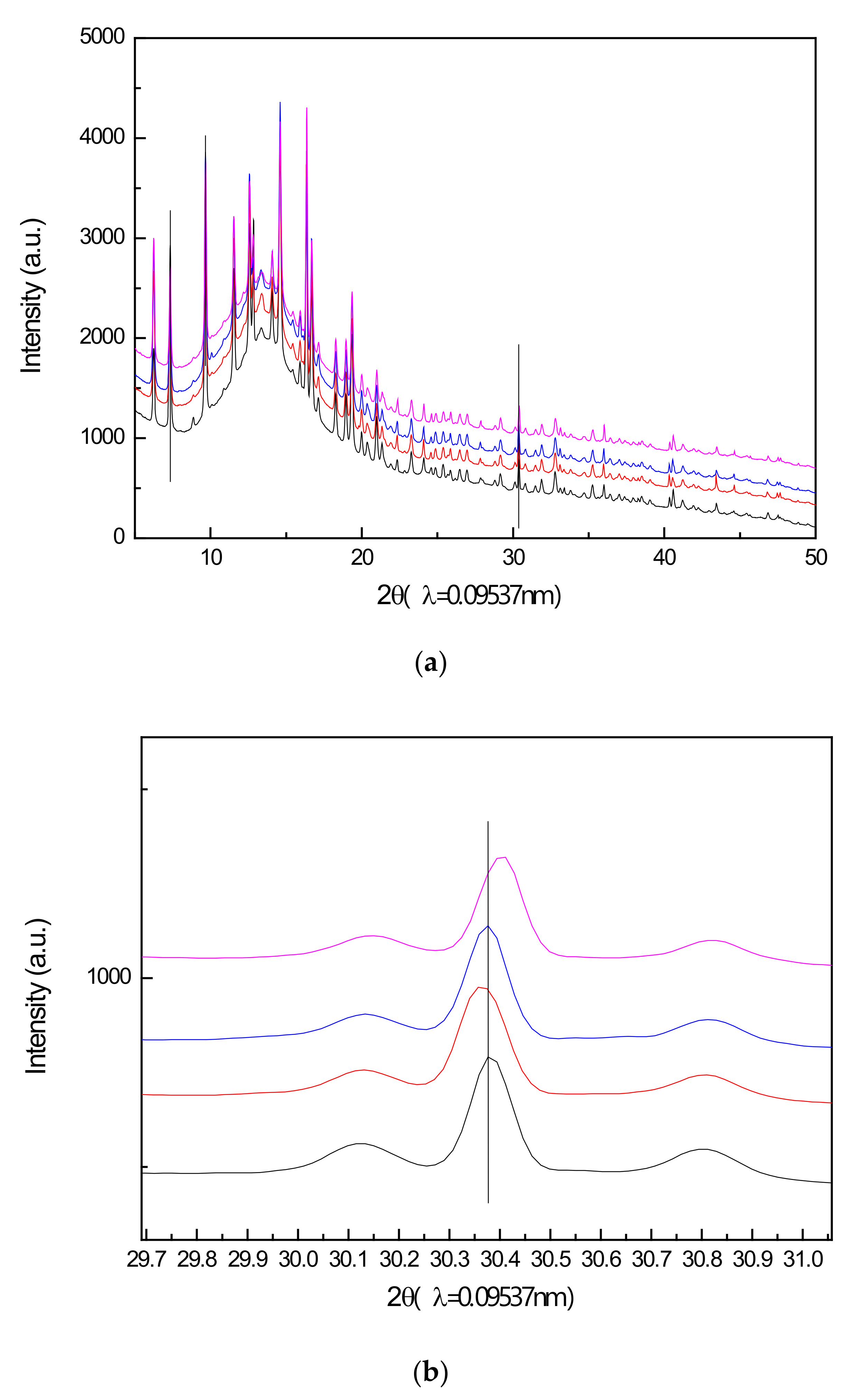
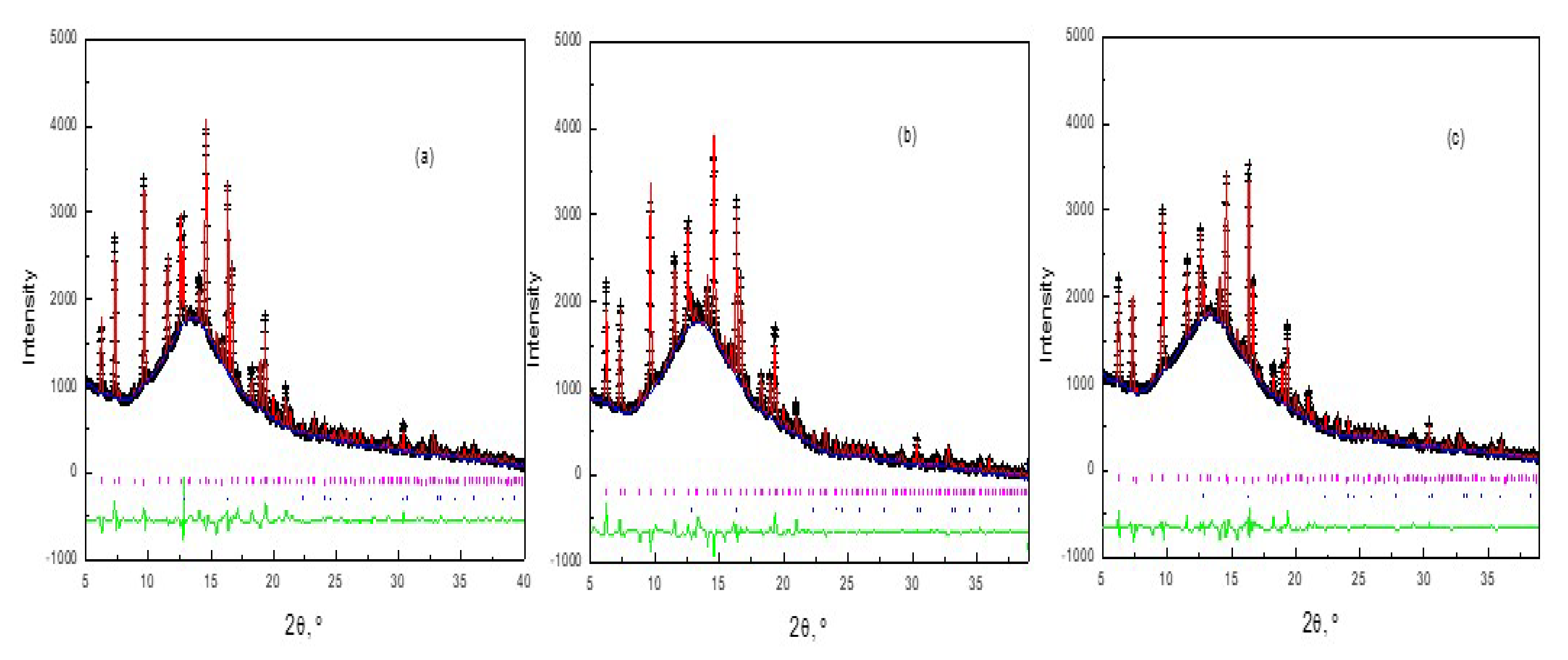
2.4.1. Synchrotron XRPD Characterization of Air Regenerated Ethanol-laden V2O5/NaY-SiO2
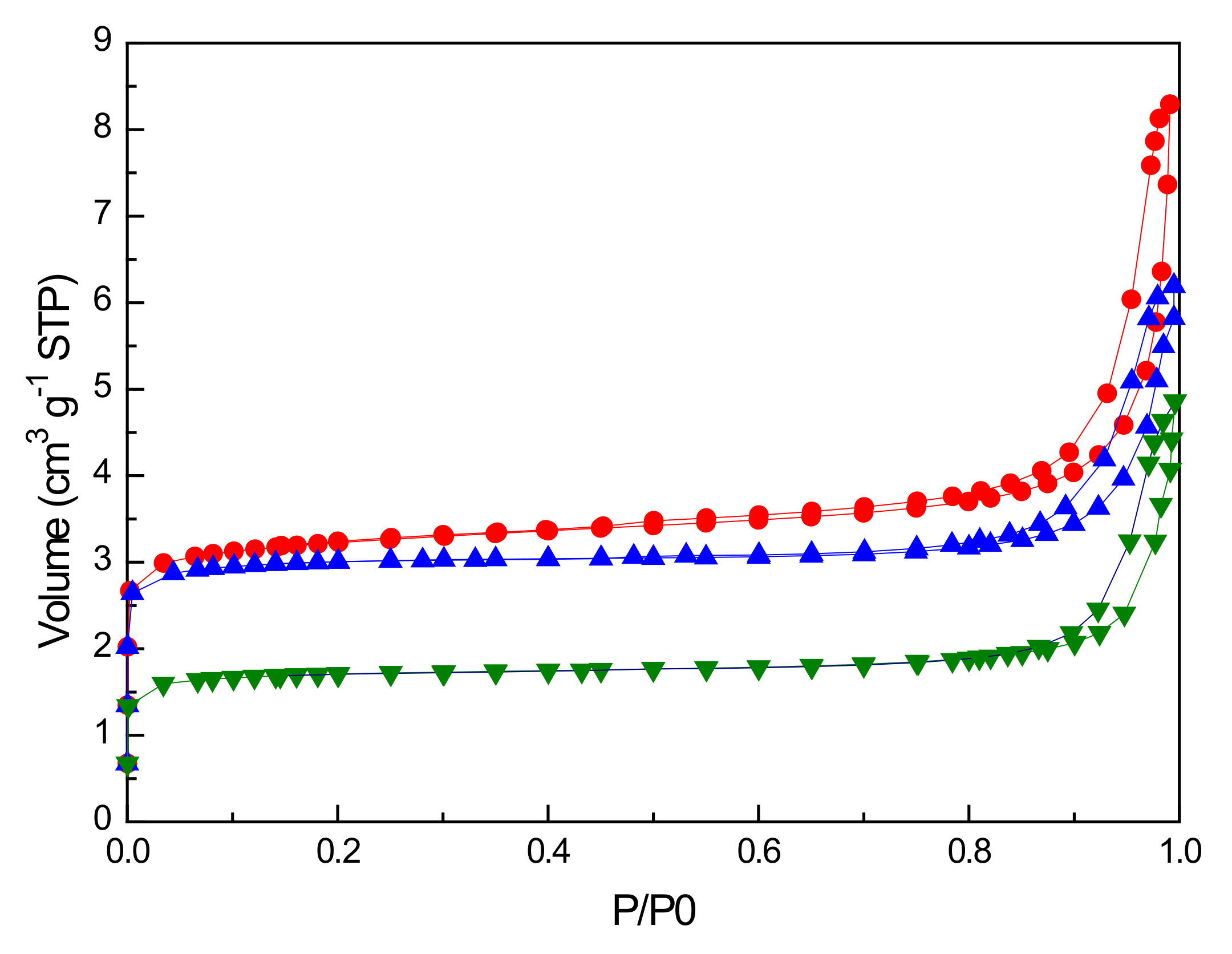
2.4.2. BET Analysis of Air Regenerated Ethanol-laden V2O5/NaY-SiO2
2.4.3. EXAFS Characterization of Air Regenerated Ethanol-laden V2O5/NaY-SiO2
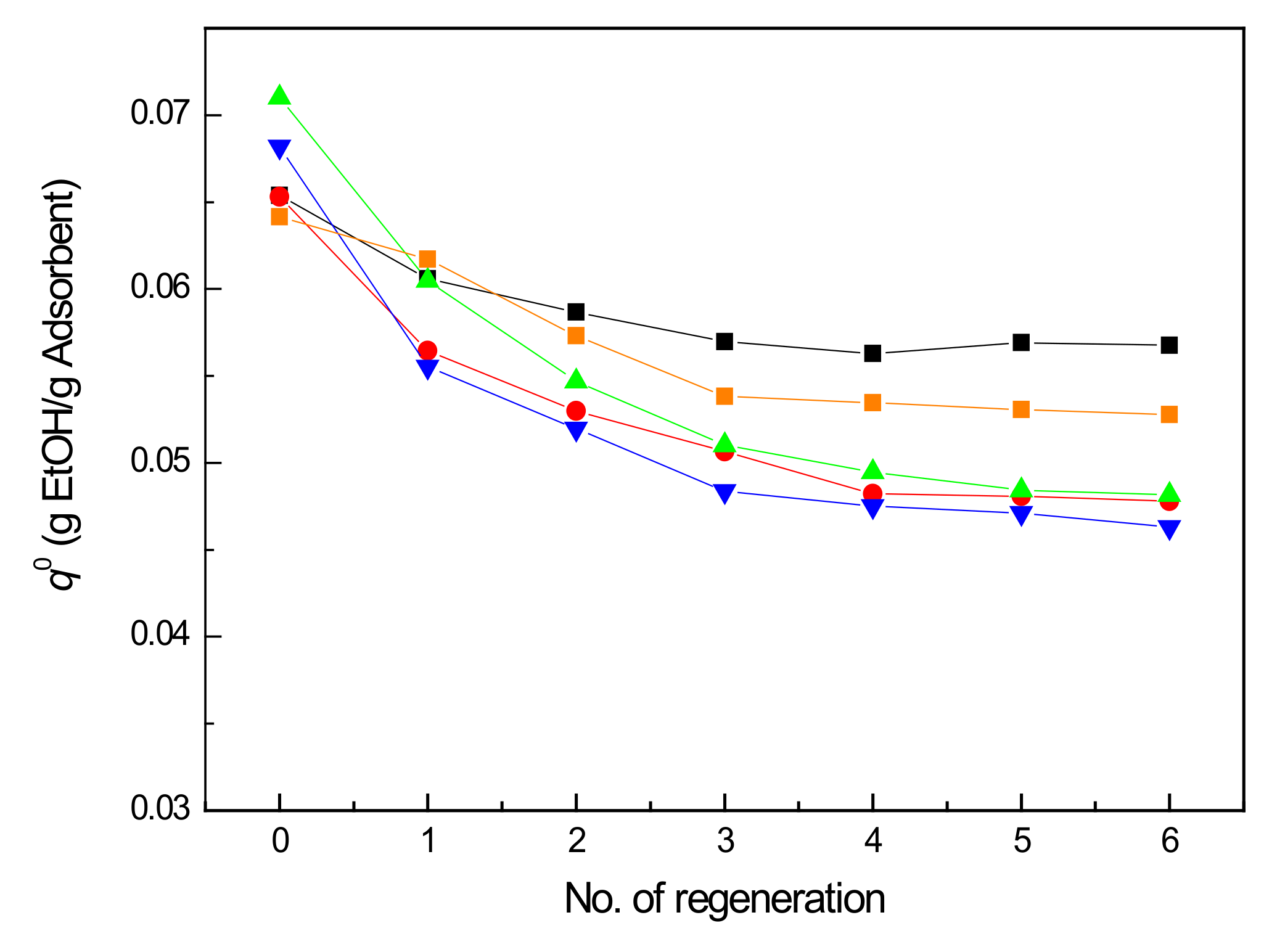
2.5. Cycled Accelerated Aging Tests
3. Materials and Methods
3.1. Preparation of V2O5/NaY-SO2 Pellet
3.2. Breakthrough Curves and Accelerated Cycle Aging Tests
3.3. Synchrotron XRPD
3.4. X-ray Absorption Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chein, H.M.; Chen, T.M. Emission Characteristics of Volatile Organic Compounds from Semiconductor Manufacturing. J. Air Waste Manag. Assoc. 2003, 53, 1029–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.L.; Nie, L.J.; Wang, Y.F.; Wang, G.; Wang, J.H.; Hao, Z.P. Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries. Chin. Sci. Bull. 2013, 58, 724–730. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.I.; Ghoshal, A.K. Removal of volatile organic compounds from polluted air. J. Loss Prev. Proc. Ind. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Cooper, C.D.; Alley, F.C. Air Pollution Control: A Design Approach; Waveland Press Inc.: Long Grove, IL, USA, 1990; Volume 305–409, pp. 353–355. [Google Scholar]
- Heck, R.M.; Farrauto, R.J.; Gulati, S.T. Catalytic Air Pollution Control, Commercial Technology, 2nd ed.; Wiley & Sons: New York, NY, USA, 2002; pp. 281–305. [Google Scholar]
- Campesi, M.A.; Luzi, C.D.; Barreto, G.F.; Martínez, O.M. Evaluation of an adsorption system to concentrate VOC in air streams prior to catalytic incineration. J. Environ. Mang. 2015, 154, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ma, C.; Horlyck, J.; Liu, R.; Yun, J. Development of Pharmaceutical VOCs Elimination by Catalytic Processes in China. Catalysts 2020, 10, 668. [Google Scholar] [CrossRef]
- Ueda, K.; Takata, Y.; Shibahara, T.; Yoshida, Y. Method for Treating Gas and Regenerating Catalyst Portion by Portion. U.S. Patent 5,254,512, 19 October 1993. [Google Scholar]
- Teller, A.J. Integrated Catalytic/Adsorption Process for Destroying Volatile Organic Compounds. U.S. Patent 6,051,199, 18 April 2000. [Google Scholar]
- Yan, T.Y.; Chang, J.-R. Process for Removing Volatile Organic Compounds. U.S. Patent 7,060,236 B2, 13 June 2006. [Google Scholar]
- Zhang, W. Regenerative Air Purification System and Method. U.S. Patent 9,597,627, 21 March 2017. [Google Scholar]
- Meininghaus, C.K.W.; Prins, R. Sorption of volatile organic compounds on hydrophobic zeolites. Microporous Mesoporous Mater. 2000, 35–36, 349–365. [Google Scholar] [CrossRef]
- Chang, F.-T.; Lin, Y.-C.; Bai, H.; Pel, B.-S. Adsorption and Desorption Characteristics of Semiconductor Volatile Organic Compounds on the Thermal Swing Honeycomb Zeolite Concentrator. J. Air Waste Manag. Assoc. 2003, 53, 1384–1390. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.Y.; Liu, M.C.; Yang, S.C.; Chang, J.-R. Structure and surface properties of NaY-SiO2 pellets investigated by synchrotron radiation XRPD and FT-IR: Impact of silica binder. Ind. Eng. Chem. Res. 2015, 54, 8456–8468. [Google Scholar] [CrossRef]
- Yeh, C.-Y.; Chen, Y.-T.; Chen, N.-Y.; Chang, J.-R. Air Regeneration of Ethanol-Laden Pellet NaY-SiO2 and Pt/NaY-SiO2: Effects of Air Flow Rate on Pt Morphology and Regeneration Efficiency. Catalysts 2018, 8, 288. [Google Scholar] [CrossRef] [Green Version]
- Chantal Kassargy, C.; Awad, S.; Burnens, G.; Upreti, G.; Kahine, K.; Tazerout, M. Study of the effects of regeneration of USY zeolite on the catalytic cracking of polyethylene. Appl. Catal. B Environ. 2019, 244, 704–708. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B-Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Ribeiro, F.; Silva, J.M.; Silva, E.; Fátima Vaz, M.F.; Oliveira, F.A.C. Catalytic combustion of toluene on Pt zeolite coated cordierite foams. Catal. Today 2011, 176, 93–96. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Oikonmopoulos, E.; Kanistras, D.; Ioannides, T. Complete oxidation of ethanol over alkali-promoted Pt/Al2O3 catalysts. Appl. Catal. B-Environ. 2006, 65, 62–69. [Google Scholar] [CrossRef]
- Song, S.; Zhang, S.; Zhang, X.; Verma, P.; Meicheng Wen, M. Advances in Catalytic Oxidation of Volatile Organic Compounds over Pd-supported Catalysts: Recent Trends and Challenges. Front Mater. 2020, 7, 595667. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Zhang, J.; Zhang, T.; Ye, M.; Liu, Z. Regeneration of catalysts deactivated by coke deposition: A review. Chin. J. Catal. 2020, 41, 1048–1061. [Google Scholar] [CrossRef]
- Navascués, N.; Escuin, M.; Rodas, Y.; Irusta, S.; Mallada, R.; Santamaría, J. Combustion of volatile organic compounds at trace concentration levels in zeolite-coated microreactors. Ind. Eng. Chem. Res. 2010, 49, 6941–6947. [Google Scholar] [CrossRef]
- Kosusko, M.; Nunez, C.M. Destruction of Volatile Organic Compounds Using Catalytic Oxidation. Waste Manag. Assoc. 1990, 40, 254–259. [Google Scholar] [CrossRef]
- Özçelik, T.G. VOC Treatment by Catalytic Incineration. J. Multidiscip. Eng. Sci. Tech. 2014, 1, 114–117. [Google Scholar]
- Tomatis, M.; Xu, H.-H.; He, J.; Zhang, X.-D. Recent Development of Catalysts for Removal of Volatile Organic Compounds in Flue Gas by Combustion: A Review. J. Chem. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Spivey, J.J. Complete Catalytic Oxidation of Volatile Organics. Ind. Eng. Chem. Res. 1987, 26, 2165–2180. [Google Scholar] [CrossRef]
- Keller, D.E.; de Groot, F.M.F.; Koningsberger, D.C.; Weckhuysen, B.M. ΛO4 Upside Down: A New Molecular Structure for Supported VO4 Catalysts. J. Phys. Chem. B 2005, 109, 10223–10233. [Google Scholar] [CrossRef]
- Pinard, L.; Hamieh, S.; Canaff, C.; Ferreira Madeira, F.; Batonneau-Gener, I.; Maury, S.; Delpoux, O.; Ben Tayeb, K.; Pouilloux, Y.; Vezin, H. Growth mechanism of coke on HBEA zeolite during ethanol transformation. J. Catal. 2013, 299, 284–297. [Google Scholar] [CrossRef]
- Melzer, M.; Urban, J.; Sack-Kongehl, H.; Weiss, K.; Freund, H.-J.; Schlögl, R. Preparation of Vanadium and Vanadium Oxide Clusters by Means of Inert Gas Aggregation. Catalysis Letters Chin. 2002, 81, 219–221. [Google Scholar] [CrossRef]
- Mahamulkar, S.S.; Yin, K.; Agrawal, P.K.; Davis, R.J.; Jones, C.W.; Malek, A.; Shibata, H. Formation and Oxidation/gasification of Carbonaceous Deposits: A Review. Ind. Eng. Chem. Res. 2016, 55, 9760–9818. [Google Scholar] [CrossRef]
- Liu, M.-C.; Hsieh, C.-C.; Lee, J.-F.; Chang, J.-R. Impact of Pt and V2O5 on Ethanol Removal from Moist Air Using Pellet Silica-Bound NaY. Ind. Eng. Chem. Res. 2015, 54, 8678–8689. [Google Scholar] [CrossRef]
- Bohart, G.S.; Adams, E.Q. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar] [CrossRef] [Green Version]
- Kawazoe, K.; Takeuchi, Y. Mass transfer in adsorption on bidisperse porous material-macro and micro-pore series diffusion model. J. Chem. Eng. Jpn. 1975, 7, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Knox, J.C.; Armin, D.; Ebner, A.D.; LeVan, M.D.; Coker, R.F.; Ritter, J.A. Limitations of Breakthrough Curve Analysis in Fixed-Bed Adsorption. Ind. Eng. Chem. Res. 2016, 55, 4734–4748. [Google Scholar] [CrossRef] [Green Version]
- Kadama, S.A.; Shamzhy, M.V. IR Operando study of ethanol dehydration over M.F.I. zeolite. Catal. Today 2018, 304, 51–57. [Google Scholar] [CrossRef]
- Madeira, F.F.; Gnep, N.S.; Magnoux, P.; Maury, S.; Cadran, N. Ethanol transformation over HFAU, HBEA and HMFI zeolites presenting similar Bronsted acidity. Appl. Catal. A Gen. 2009, 367, 39–46. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Organic chemistry of coke formation. Applied Catalysis A: General. 2001, 212, 83. [Google Scholar] [CrossRef]
- Uslamin, E.A.; Saito, H.; Kosinov, N.; Pidko, E.; Sekine, Y.; Hensen, E.J.M. Aromatization of ethylene over zeolite-based catalysts. Catal. Sci. Technol. 2020, 10, 2774. [Google Scholar] [CrossRef] [Green Version]
- Autthanit, C.; Praserthdam, P.; Jongsomjit, B. Oxidative and non-oxidative dehydrogenation of ethanol to acetaldehyde over different VOx/SBA-15 catalysts. J. Environ. Chem. Eng. 2018, 6, 6516–6529. [Google Scholar] [CrossRef]
- Foo, G.S.; Rogers, A.K.; Yung, M.M.; Sievers, C. Steric Effect and Evolution of Surface Species in the Hydrodeoxygenation of Bio-Oil Model Compounds over Pt/HBEA. ACS Catal. 2016, 6, 1292–1307. [Google Scholar] [CrossRef]
- Kosinov, N.; Uslamin, E.A.; Ferdy, J.; Coumans, F.J.A.G.; Wijpkema, A.S.G.; Rohling, R.Y.; Hensen, E.J.M. Structure and Evolution of Confined Carbon Species during Methane Dehydroaromatization over Mo/ZSM-5. ACS Catal. 2018, 8, 8459–8467. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Shao, S.; Xiao, R.; Shen, D.; Zeng, J. Characterization of Coke Deposition in the Catalytic Fast Pyrolysis of Biomass Derivates. Energy Fuels 2014, 28, 52–57. [Google Scholar] [CrossRef]
- Salman, N.; Rüscher, C.H.; Buhl, J.-C.; Lutz, W.; Toufar, H.; Stöcker, M. Effect of temperature and time in the hydrothermal treatment of HY zeolite. Microporous Mesoporous Mater. 2006, 90, 339–346. [Google Scholar] [CrossRef]
- Jia, L.Y.; Farouha, A.; Pinardb, L.; Hedanb, S.; Comparot, J.-D.; Dufour, A.; Tayeb, K.B.; Vezinc, H.; Batiot-Dupeyrat, C. New routes for complete regeneration of coked zeolite. Appl. Catal. B Environ. 2017, 219, 82–91. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley Publishing Company: Reading, MA, USA, 1978; pp. 99–106. [Google Scholar]
- Condon, J.B. Surface Area and Porposity Determinations by Physisorption: Measurements and Theory; Elservier: Amsterdam, The Netherlands, 2006; pp. 1–28. [Google Scholar]
- Lo, B.T.W.; Ye, L.; Tsang, S.E.D. The Contribution of Synchrotron X-Ray Powder Diffraction to Modern Zeolite Applications: A Mini-review and Prospects. Chem 2018, 4, 1778–1808. [Google Scholar] [CrossRef]
- Agostini, G.; Lamberti, C.; Palin, L.; Milanesio, M.; Danilina, N.; Xu, B.; Janousch, M.; Bokhoven, J.A. In situ XAS and XRPD parametric Rietveld refinement to understand dealumination of Y zeolite catalyst. J. Am. Chem. Soc. 2009, 132, 667–678. [Google Scholar] [CrossRef]
- Hammersley, A.P. FIT2D: A multi-purpose data reduction, analysis and visualization program. J. Appl. Cryst. 2016, 49, 646–652. [Google Scholar] [CrossRef]
- Vaarkamp, M.; Linders, J.C.; Koningsberger, D.C. A new method for parameterization of phase shift and backscattering amplitude. Phys. B 1995, 208–209, 159–160. [Google Scholar] [CrossRef] [Green Version]
- Koningsberger, D.C.; Prins, R. X-ray Absorption: Principles, Applications, Techniques of EXAFS, EXAFS, and XANES; John Wiley and Son: New York, NY, USA, 1988. [Google Scholar]
- Zabinsky, S.I.; Rehr, J.J.; Ankudinov, A.; Albers, R.C.; Eller, M.J. Multiple-scattering calculations of x-ray-absorption spectra. Phys. Rev. B 1995, 52, 2995–3009. [Google Scholar] [CrossRef]
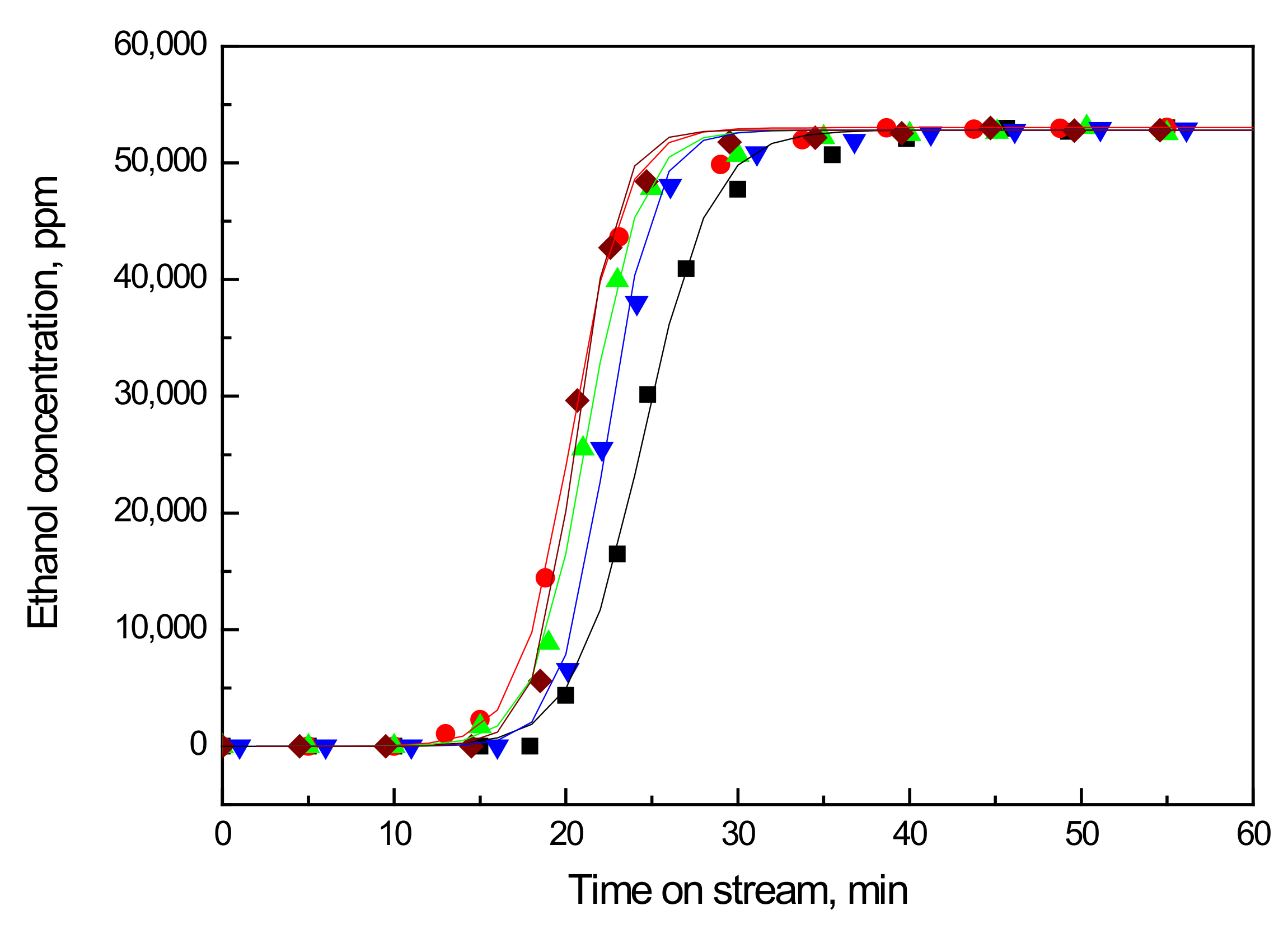
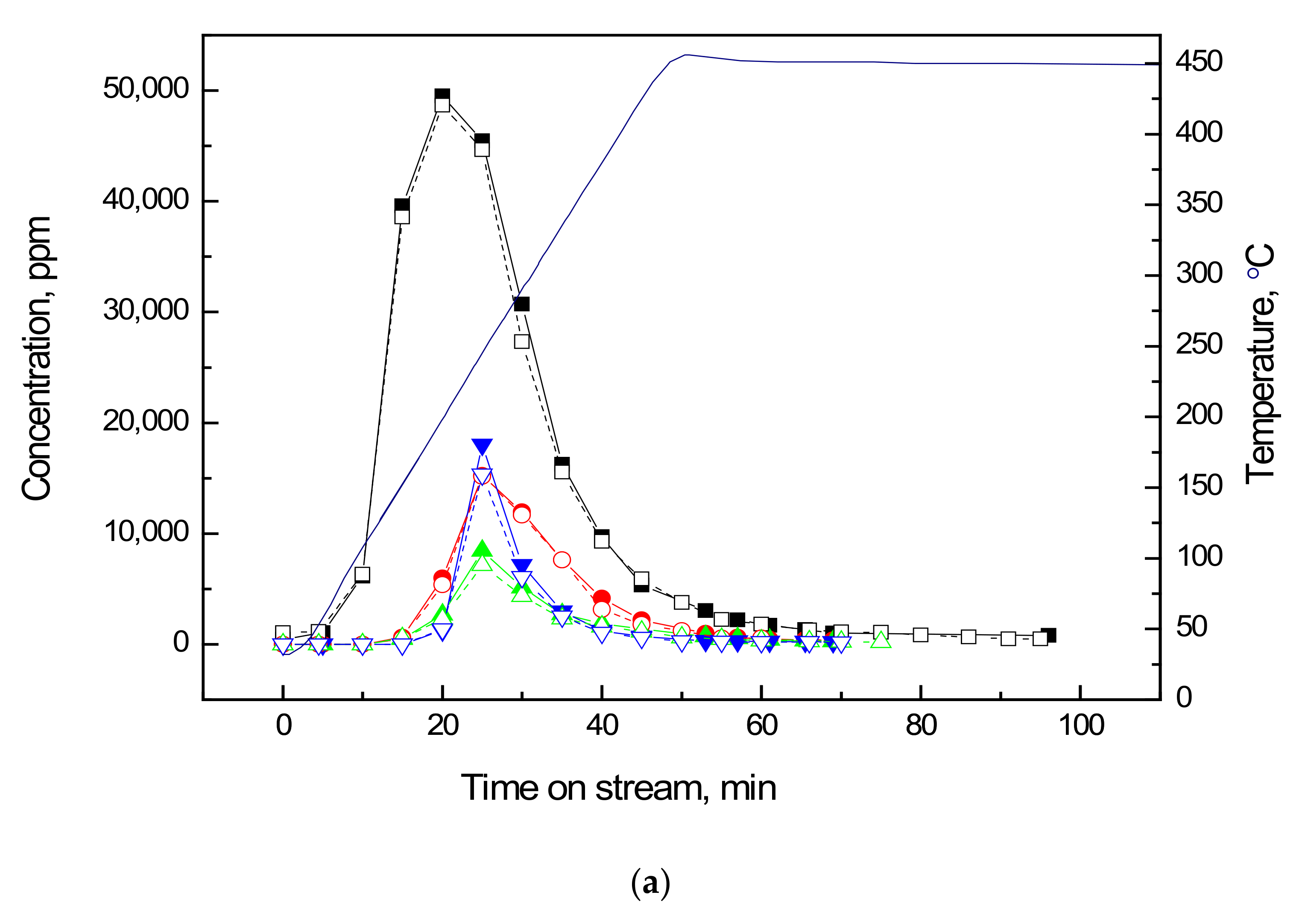
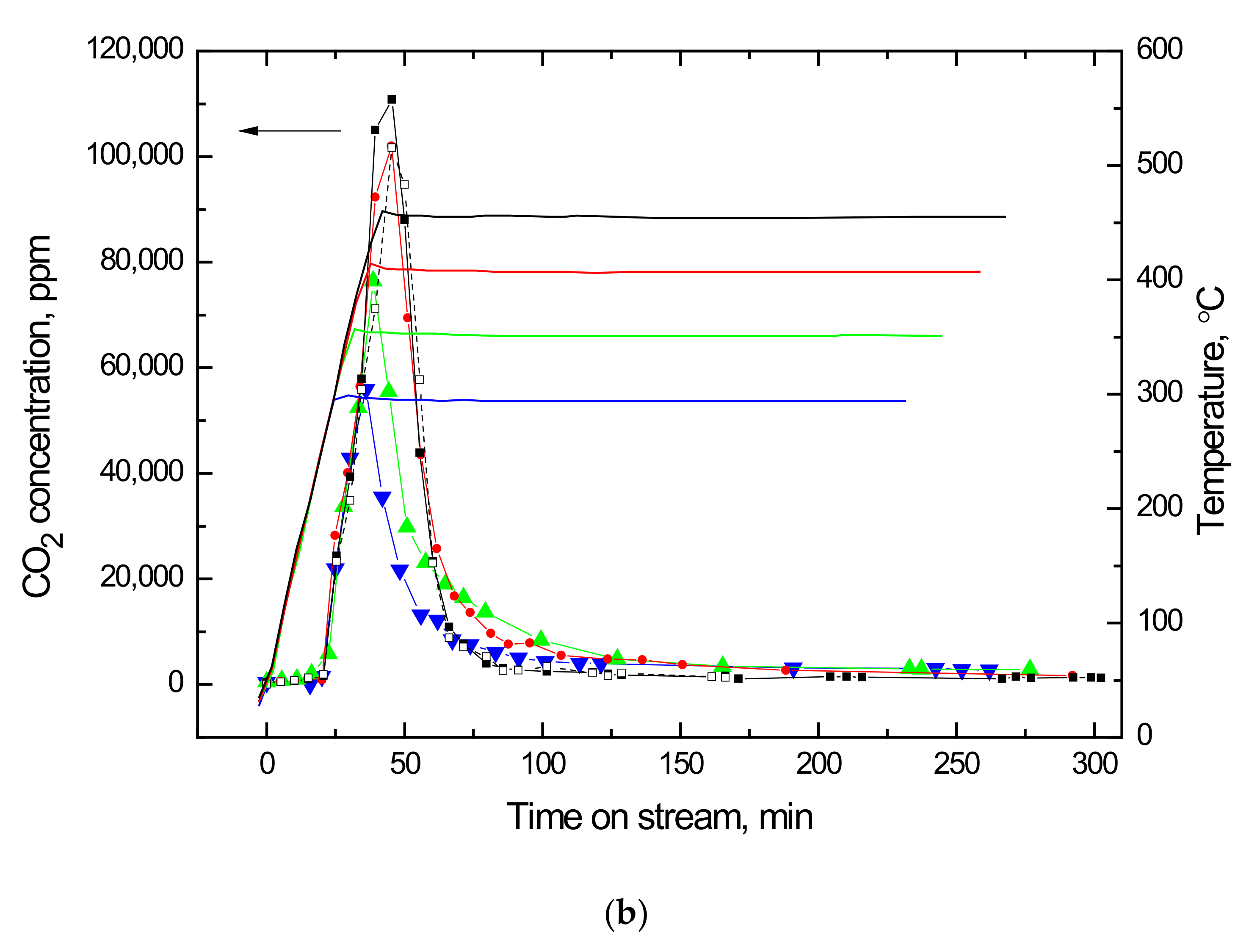
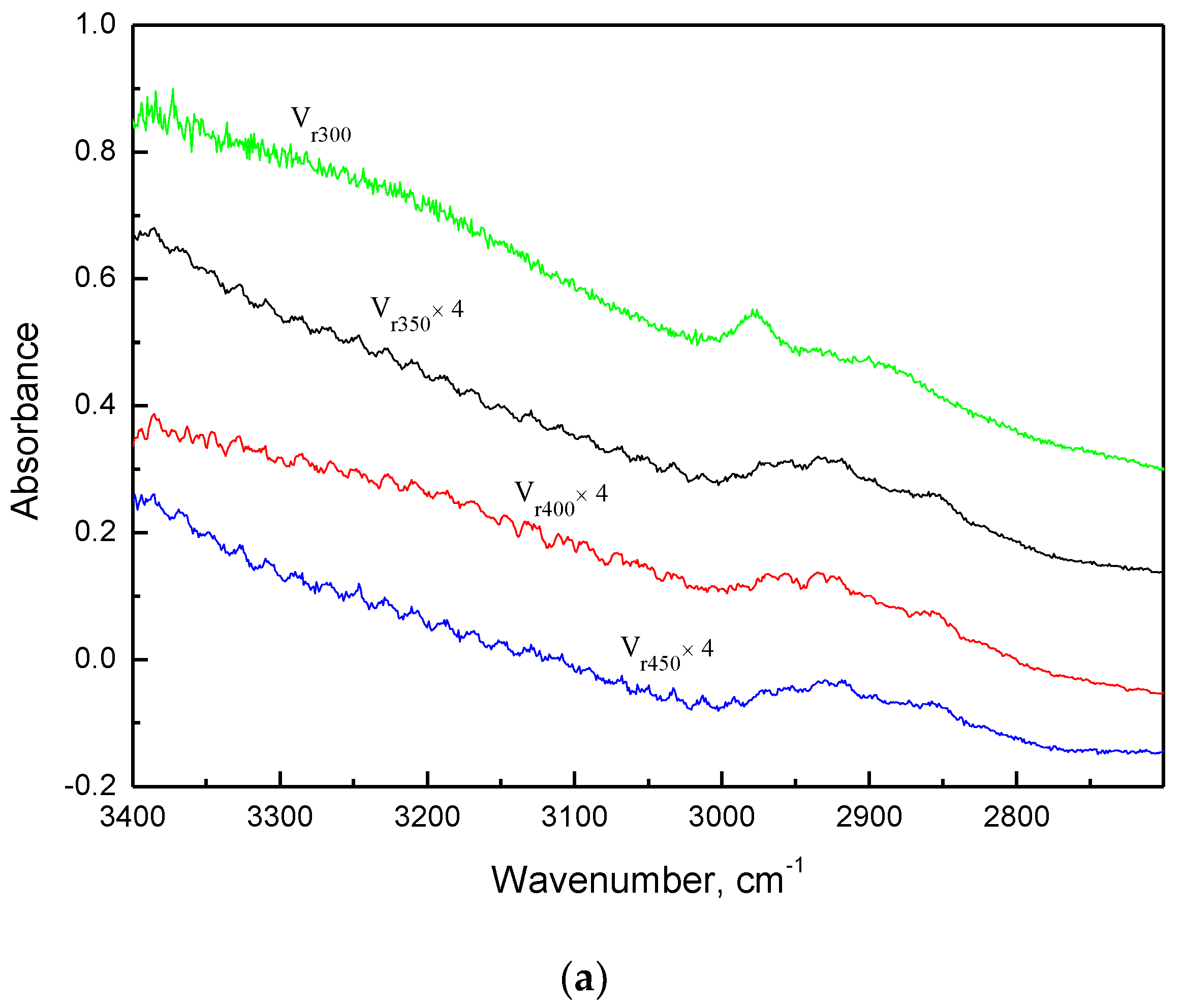


| Adsorbent | τ, 1/min | ξ | q0, mg/g | Regeneration † Efficacy | (η × ka), L/min.mg | η/ηvf |
|---|---|---|---|---|---|---|
| Vf | 0.51 | 12.44 | 61.39 | - | 8.10 | 1.00 |
| Vr300 | 0.57 | 11.70 | 51.48 | 0.84 | 9.09 | 1.12 |
| Vr350 | 0.66 | 13.98 | 53.29 | 0.87 | 10.49 | 1.29 |
| Vr400 | 0.68 | 15.30 | 56.71 | 0.92 | 10.79 | 1.33 |
| Vr450 | 0.82 | 16.83 | 51.67 | 0.84 | 13.03 | 1.61 |
| Temperature °C | Species ppm | |||||
|---|---|---|---|---|---|---|
| Ethanol | Acetaldehyde | Diethyl Ether | Ethylene | CO2 | Acetal | |
| 300 | 27,400 | 11,100 | 4600 | 6300 | 36,300 | 30 |
| 350 | 14,300 | 6800 | 2400 | 2400 | 45,700 | 60 |
| 400 | 8000 | 3400 | 1700 | 1200 | 87,200 | 80 |
| 450 | 3700 | 1400 | 1000 | 500 | 110,800 | 40 |
| Sample Name | Vr450 | Vr400 | Vf |
|---|---|---|---|
| Crystal system | Cubic | Cubic | Cubic |
| Space group | F d -3 m | F d -3 m | F d -3 m |
| Lattice parameter, a (Å) | 24.4652(6) | 24.5048(6) | 24.5084(5) |
| Vol (Å3) | 14643 | 14714 | 14721 |
| Lattice parameters of SiO2 (Å) | a = 4.9016(2) c = 5.3916(4) | a = 4.9114(3) c = 5.4030(6) | a = 4.9096(3) c = 5.4038(6) |
| wRp | 0.031 | 0.045 | 0.041 |
| Rp | 0.022 | 0.030 | 0.028 |
| X2 | 0.664 | 1.348 | 1.094 |
| Grain size calculated by Scherrer equation (nm) | 47.57 | 46.5 | 46.2 |
| Shell | N | R | 1000 × Δσ2 | ΔE0 | EXAFS |
|---|---|---|---|---|---|
| (Å) | (Å2) | (eV) | Reference | ||
| Vf | |||||
| V-OS | 1.0 | 1.60 ± 0.01 | −2 ± 2 | −1.6 ± 0.4 | V-O |
| V-Ol | 3.0 | 1.73 ± 0.02 | 2 ± 1 | 13.4 ± 0.2 | V-O |
| V-Alsup | 0.62 ± 0.04 | 2.66 ± 0.01 | 14 ± 2 | −4.92 | V-Al |
| Vr400 | |||||
| V-OS | 1.0 | 1.59 ± 0.01 | −4 ± 2 | −13 ± 1 | V-O |
| V-Ol | 4.0 | 1.70 ± 0.01 | 0.2 ± 0.1 | 12 ± 1 | V-O |
| V-Alsup | 0.4 ± 0.1 | 2.48 ± 0.02 | 3 ± 1 | −13 ± 3 | V-Al |
| V-V | 0.3 ± 0.1 | 2.91 ± 0.03 | 0 ± 1 | −2 ± 4 | V-V |
| Vr450 | |||||
| V-Os | 1.0 | 1.59 ± 0.01 | −4 ± 2 | −14 ± 1 | V-O |
| V-Ol | 4.0 | 1.70 ± 0.01 | 0 ± 1 | 11 ± 1 | V-O |
| V-Alsup | 0.2 ± 0.1 | 2.46 ± 0.05 | 3 ± 1 | −12 ± 1 | V-Al |
| V-V | 0.5 ± 0.1 | 2.92 ± 0.03 | 0 ± 3 | −2 ± 3 | V-V |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-C.; Tsai, J.-S.; Sheu, H.-S.; Chang, J.-R. Coke Deposition and Structural Changes of Pellet V2O5/NaY-SiO2 in Air Regeneration: The Effects of Temperature on Regeneration. Catalysts 2022, 12, 95. https://doi.org/10.3390/catal12010095
Hsieh C-C, Tsai J-S, Sheu H-S, Chang J-R. Coke Deposition and Structural Changes of Pellet V2O5/NaY-SiO2 in Air Regeneration: The Effects of Temperature on Regeneration. Catalysts. 2022; 12(1):95. https://doi.org/10.3390/catal12010095
Chicago/Turabian StyleHsieh, Chu-Chin, Jyong-Sian Tsai, Hwo-Shuenn Sheu, and Jen-Ray Chang. 2022. "Coke Deposition and Structural Changes of Pellet V2O5/NaY-SiO2 in Air Regeneration: The Effects of Temperature on Regeneration" Catalysts 12, no. 1: 95. https://doi.org/10.3390/catal12010095






