Chloride-Enhanced Removal of Ammonia Nitrogen and Organic Matter from Landfill Leachate by a Microwave/Peroxymonosulfate System
Abstract
:1. Introduction
2. Results and Discussion
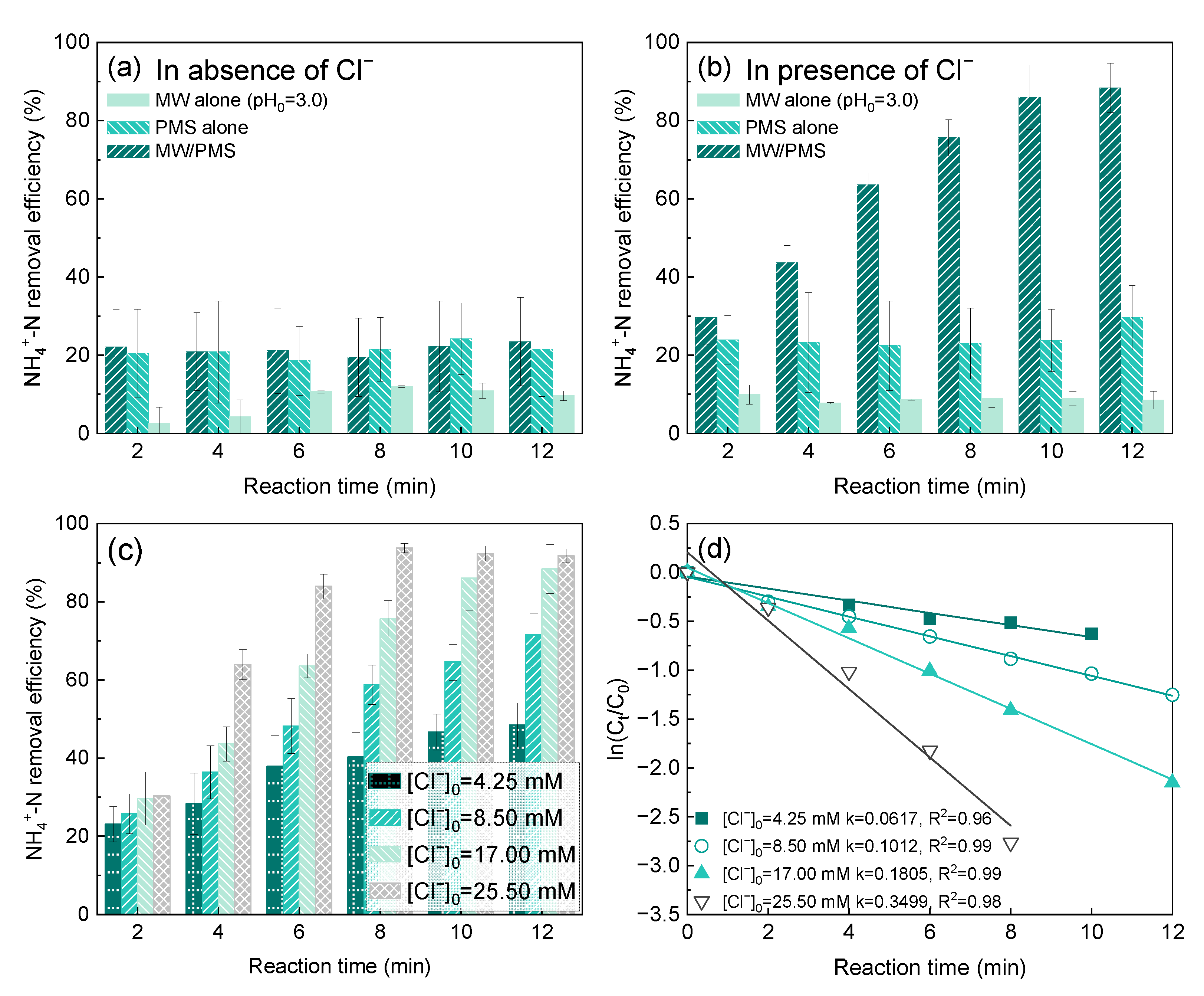
2.1. Effects of Cl− on NH4+-N Removal by MW/PMS
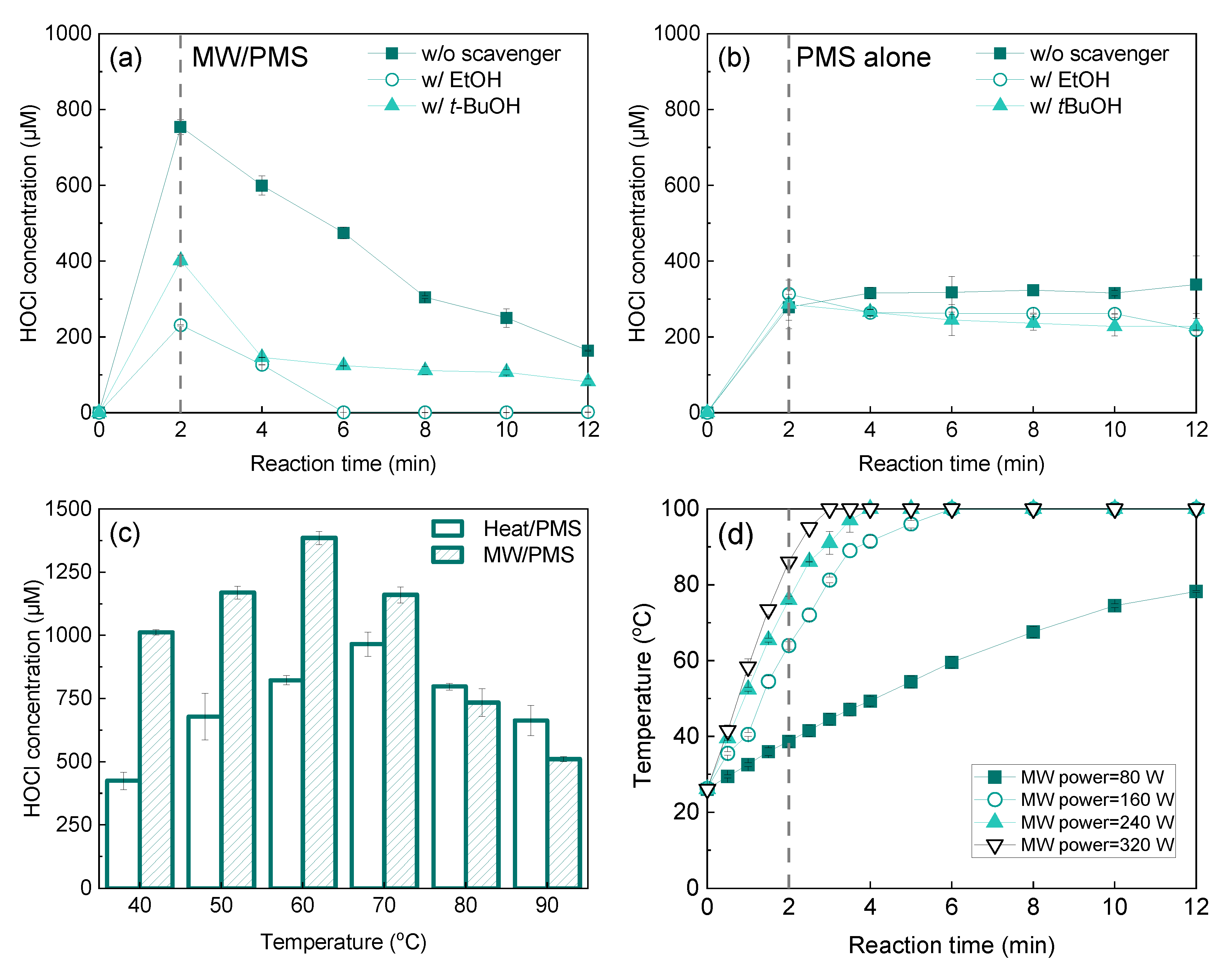
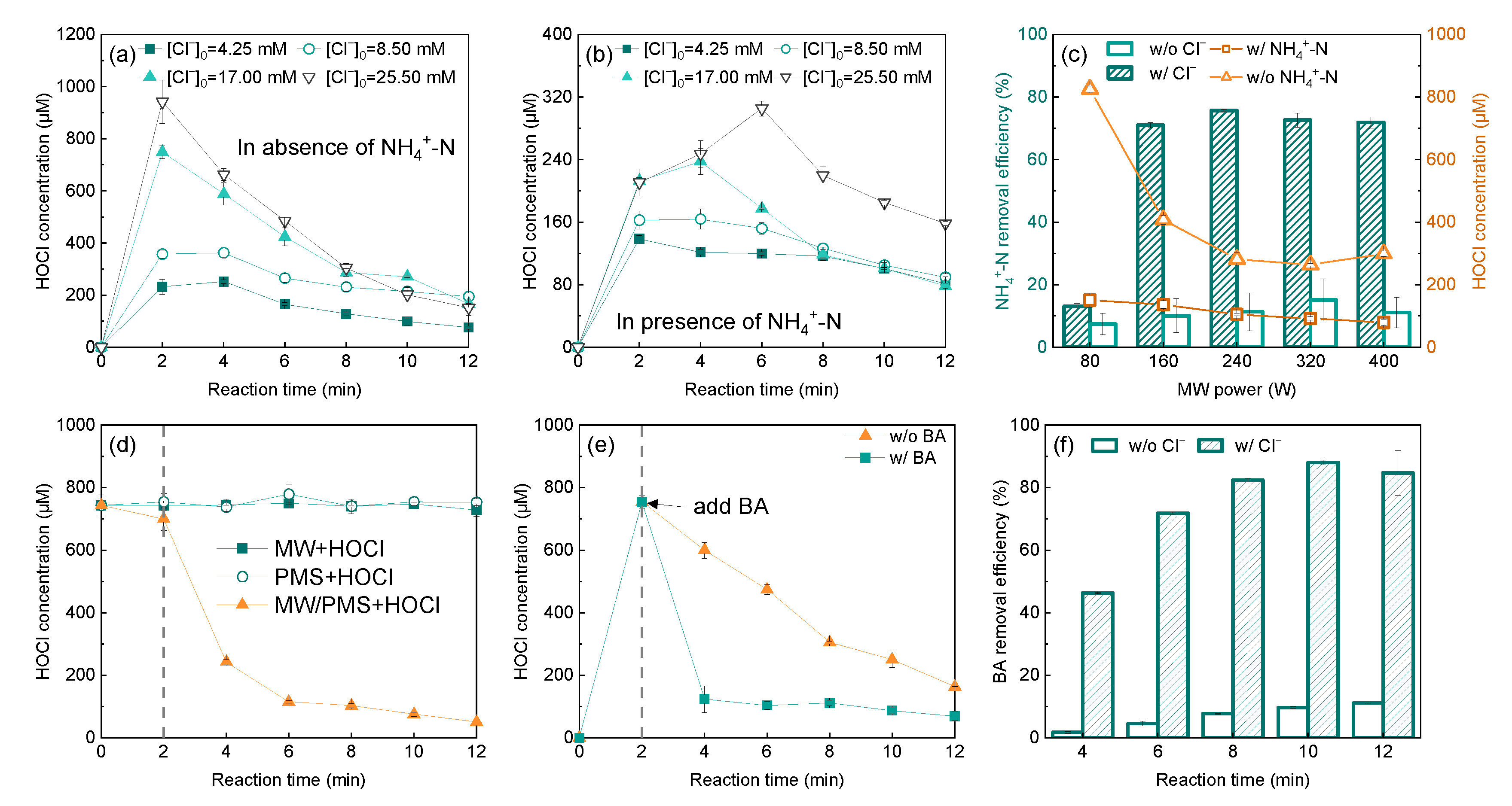
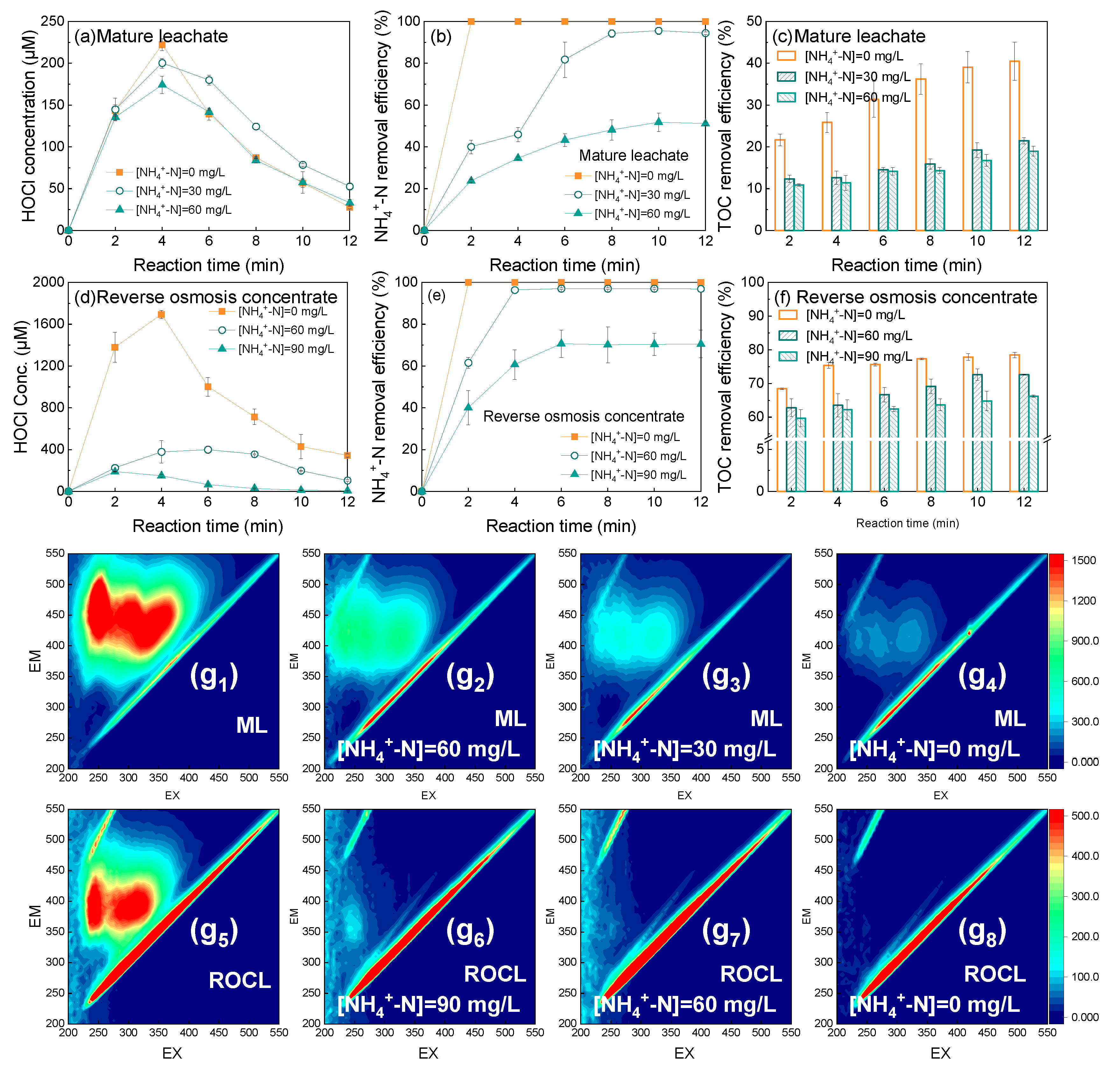
2.2. Formation and Conversion of HOCl in a High-Cl− Background Matrix
2.2.1. Formation Mechanism of HOCl
2.2.2. Conversion of HOCl
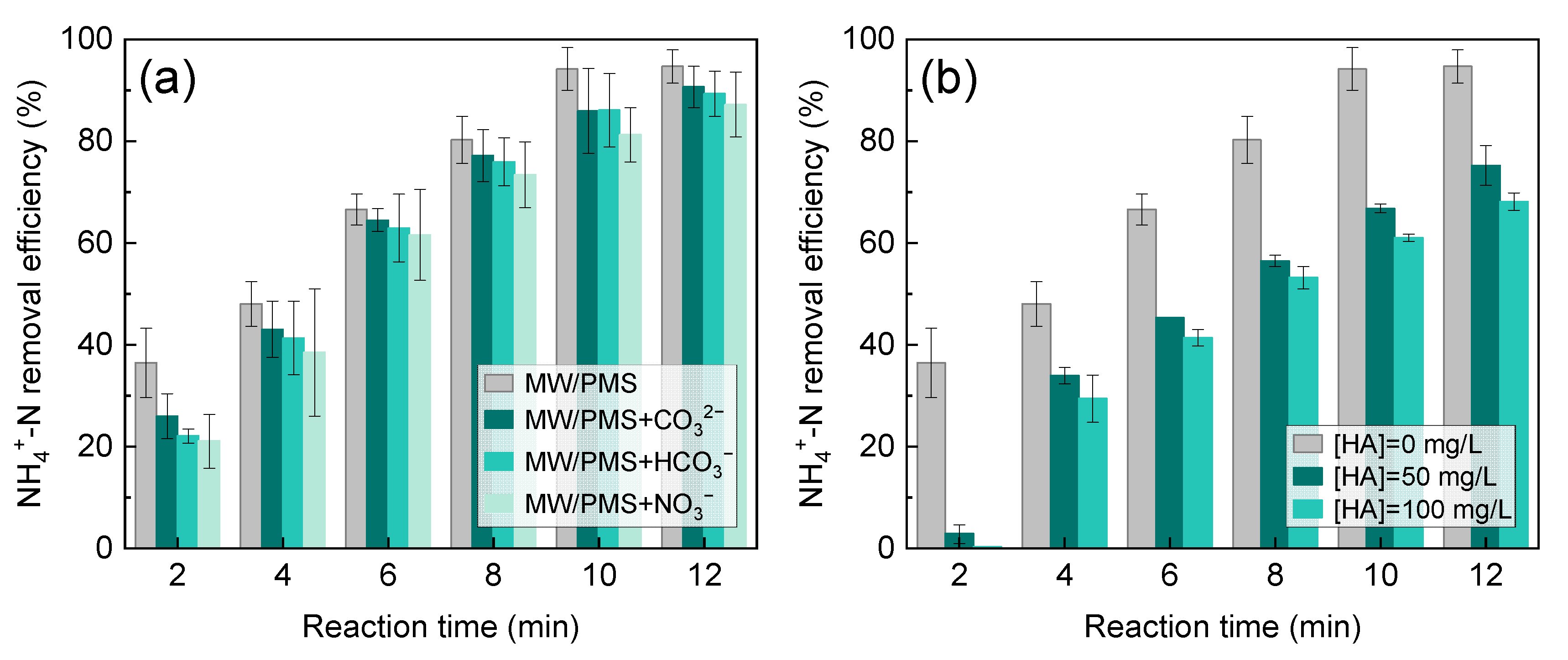
2.3. Influence of the Background Matrix
2.4. Effect of Ammonia Nitrogen on the Removal of Organic Matter from High-Chloride Landfill Leachate by the MW/PMS System
2.4.1. Influence of Ammonia Nitrogen on the Organic Matter Removal Effect
2.4.2. 3D-EEM Analysis
3. Materials and Methods
3.1. Experimental Water Sample and Reagents
3.2. Experimental Procedure
3.3. Analysis Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Zhuo, X.; He, C.; Shi, Q.; Li, Q. Molecular investigation into the transformation of dissolved organic matter in mature landfill leachate during treatment in a combined membrane bioreactor-reverse osmosis process. J. Hazard. Mater. 2020, 397, 122759. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hu, Y. Municipal solid waste (MSW) as a renewable source of energy: Current and future practices in China. Bioresour. Technol. 2010, 101, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Chen, W.; Li, Q.; Zhang, A. Treatment of semi-aerobic aged-refuse biofilter effluent from treating landfill leachate with the Fenton method. Process Saf. Environ. Prot. 2020, 133, 32–40. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Gu, Z.; Li, Q. A review of the characteristics of Fenton and ozonation systems in landfill leachate treatment. Sci. Total Environ. 2021, 762, 143131. [Google Scholar] [CrossRef]
- Yan, Z.; Lu, Z.; Chen, X.; Jiang, Y.; Huang, Z.; Liu, L.; Fan, G.; Chang, H.; Qu, F.; Liang, H. Membrane distillation treatment of landfill leachate: Characteristics and mechanism of membrane fouling. Sep. Purif. Technol. 2022, 289, 120787. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, K.; Huang, X. Full-scale MBR applications for leachate treatment in China: Practical, technical, and economic features. J. Hazard. Mater. 2020, 389, 122138. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, W.; He, C.; Li, Q. Molecular insights into the transformation of refractory organic matter in landfill leachate nanofiltration concentrates during a flocculation and O3/H2O2 treatment. J. Hazard. Mater. 2022, 435, 128973. [Google Scholar] [CrossRef]
- Chen, W.; Gu, Z.; Ran, G.; Li, Q. Application of membrane separation technology in the treatment of leachate in China: A review. Waste Manag. 2021, 121, 127–140. [Google Scholar] [CrossRef]
- Gu, Z.; Feng, K.; Li, Y.; Li, Q. Microbial characteristics of the leachate contaminated soil of an informal landfill site. Chemosphere 2022, 287, 132155. [Google Scholar] [CrossRef]
- Chen, C.; Feng, H.; Deng, Y. Re-evaluation of sulfate radical based-advanced oxidation processes (SR-AOPs) for treatment of raw municipal landfill leachate. Water Res. 2019, 153, 100–107. [Google Scholar] [CrossRef]
- Chen, S.; He, Z. Sonoelectrochemical activation of peroxymonosulfate: Influencing factors and mechanism of FA degradation, and application on landfill leachate treatment. Chemosphere 2021, 296, 133365. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Gu, Z. Microwave irradiation activated persulfate and hydrogen peroxide for the treatment of mature landfill leachate effluent from a membrane bioreactor. Sep. Purif. Technol. 2020, 250, 117111. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar] [CrossRef]
- Amanollahi, H.; Moussavi, G.; Giannakis, S. Enhanced vacuum UV-based process (VUV/H2O2/PMS) for the effective removal of ammonia from water: Engineering configuration and mechanistic considerations. J. Hazard. Mater. 2021, 402, 123789. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D.; Gonzalez, M.A. Cobalt-Mediated Activation of Peroxymonosulfate and Sulfate Radical Attack on Phenolic Compounds. Implications of Chloride Ions. Environ. Sci. Technol. 2006, 40, 1000–1007. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysios, D.D. Degradation of Organic Contaminants in Water with Sulfate Radicals Generated by the Conjunction of Peroxymonosulfate with Cobalt. Environ. Sci. Technol. 2003, 37, 4749–4797. [Google Scholar] [CrossRef]
- Chan, K.H.; Chu, W. Degradation of atrazine by cobalt-mediated activation of peroxymonosulfate: Different cobalt counteranions in homogenous process and cobalt oxide catalysts in photolytic heterogeneous process. Water Res. 2009, 43, 2513–2521. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Chen, W.; Wang, F.; He, C.; Li, Q. Molecular-level comparison study on microwave irradiation-activated persulfate and hydrogen peroxide processes for the treatment of refractory organics in mature landfill leachate. J. Hazard. Mater. 2020, 397, 122785. [Google Scholar] [CrossRef]
- Xia, H.; Li, C.; Yang, G.; Shi, Z.; Jin, C.; He, W.; Xu, J.; Li, G. A review of microwave-assisted advanced oxidation processes for wastewater treatment. Chemosphere 2022, 287, 131981. [Google Scholar] [CrossRef]
- Dai, Y.; Qi, C.; Cao, H.; Wen, Y.; Zhao, Y.; Xu, C.; Yang, S.; He, H. Enhanced degradation of sulfamethoxazole by microwave-activated peracetic acid under alkaline condition: Influencing factors and mechanism. Sep. Purif. Technol. 2022, 288, 120716. [Google Scholar] [CrossRef]
- Qi, C.; Chen, H.; Xu, C.; Xu, Z.; Chen, H.; Yang, S.; Li, S.; He, H.; Sun, C. Synthesis and application of magnetic materials-barium ferrite nanomaterial as an effective microwave catalyst for degradation of brilliant green. Chemosphere 2020, 260, 127681. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Lin, C.; Zhang, H.; Li, X.; Ma, J. Activation of peroxymonosulfate by microwave irradiation for degradation of organic contaminants. Chem. Eng. J. 2017, 315, 201–209. [Google Scholar] [CrossRef]
- Benson, S.W. Thermochemistry and Kinetics of Sulfur-Containing Molecules and Radicals. Chemical Reviews 1978, 78, 23–35. [Google Scholar] [CrossRef]
- Chen, W.; Gu, Z.; Guo, S.; Li, Q. Microwave-assisted Fe0-activated persulfate process for treating explosives in production wastewater. Chem. Eng. J. 2020, 391, 123497. [Google Scholar] [CrossRef]
- Chen, W.; Luo, Y.; Ran, G.; Li, Q. An investigation of refractory organics in membrane bioreactor effluent following the treatment of landfill leachate by the O3/H2O2 and MW/PS processes. Waste Manag. 2019, 97, 1–9. [Google Scholar] [CrossRef]
- Chen, W.; Luo, Z.; Wu, C.; Wen, P.; Li, Q. Oxidative removal of recalcitrant organics in shale gas flowback fluid by the microwave-activated persulfate process. Environ. Sci. Pollut. Res. 2019, 26, 684–693. [Google Scholar] [CrossRef]
- Yang, P.; Ji, Y.; Lu, J.; Huang, Q. Formation of Nitrophenolic Byproducts during Heat-Activated Peroxydisulfate Oxidation in the Presence of Natural Organic Matter and Nitrite. Environ. Sci. Technol. 2019, 53, 4255–4264. [Google Scholar] [CrossRef]
- Chen, T.; Yu, Z.; Xu, T.; Xiao, R.; Chu, W.; Yin, D. Formation and degradation mechanisms of CX3R-type oxidation by-products during cobalt catalyzed peroxymonosulfate oxidation: The roles of Co3+ and SO4•-. J. Hazard. Mater. 2021, 405, 124243. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Synergistic effect of PMS activation by Fe0@Fe3O4 anchored on N, S, O co-doped carbon composite for degradation of sulfamethoxazole. Chem. Eng. J. 2022, 427, 131960. [Google Scholar] [CrossRef]
- Wang, S.; Hu, J.; Wang, J. Degradation of sulfamethoxazole using PMS activated by cobalt sulfides encapsulated in nitrogen and sulfur co-doped graphene. Sci. Total Environ. 2022, 827, 154379. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; B, H.; Yang, M.; Liu, R.; Hu, C.; Liu, H.; Qu, J. Anaerobically-digested sludge disintegration by transition metal ions-activated peroxymonosulfate (PMS): Comparison between Co2+, Cu2+, Fe2+ and Mn2+. Sci. Total Environ. 2020, 713, 136530. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Liu, X.; Lin, C.; Zhang, X.; Ma, J.; Tan, H.; Ye, W. Degradation of sulfamethoxazole by microwave-activated persulfate: Kinetics, mechanism and acute toxicity. Chem. Eng. J. 2014, 249, 6–14. [Google Scholar] [CrossRef]
- Ahn, Y.Y.; Choi, J.; Kim, M.; Kim, M.S.; Lee, D.; Bang, W.H.; Yun, E.T.; Lee, H.; Lee, J.H.; Lee, C.; et al. Chloride-Mediated Enhancement in Heat-Induced Activation of Peroxymonosulfate: New Reaction Pathways for Oxidizing Radical Production. Environ. Sci. Technol. 2021, 55, 5382–5392. [Google Scholar] [CrossRef]
- Yu, X.; Bao, Z.; Barker, J.R. Free Radical Reactions Involving Cl•, Cl2-•, and SO4-• in the 248 nm Photolysis of Aqueous Solutions Containing S2O82- and Cl-. J. Phys. Chem. A 2004, 102, 295–308. [Google Scholar] [CrossRef]
- Yuan, R.; Ramjaun, S.N.; Wang, Z.; Liu, J. Effects of chloride ion on degradation of Acid Orange 7 by sulfate radical-based advanced oxidation process: Implications for formation of chlorinated aromatic compounds. J. Hazard. Mater. 2011, 196, 173–179. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, H.; Sun, B.; Zhang, J.; Wang, W. The altered effects of chloride on the treatment efficiency of SO4·−-based AOPs by other background water constituents. Chem. Eng. J. 2022, 441, 135914. [Google Scholar] [CrossRef]
- Li, C.-X.; Chen, C.-B.; Wang, Y.-J.; Fu, X.-Z.; Cui, S.; Lu, J.-Y.; Li, J.; Liu, H.-Q.; Li, W.-W.; Lau, T.-C. Insights on the pH-dependent roles of peroxymonosulfate and chlorine ions in phenol oxidative transformation. Chem. Eng. J. 2019, 362, 570–575. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, Y.; Li, B.; Feng, L.; Du, Z.; Zhang, L. Reaction kinetics of dissolved black carbon with hydroxyl radical, sulfate radical and reactive chlorine radicals. Sci. Total Environ. 2022, 828, 153984. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Z.; Wang, S.; Liu, J.; Zhang, Y.; Wang, B.; Gong, Z.; Wang, M.; Dong, H.; Shi, J.; et al. Enhanced removal of methylparaben mediated by cobalt/carbon nanotubes (Co/CNTs) activated peroxymonosulfate in chloride-containing water: Reaction kinetics, mechanisms and pathways. Chem. Eng. J. 2021, 409, 128176. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Treatment of membrane filtration concentrate of coking wastewater using PMS/chloridion oxidation process. Chem. Eng. J. 2020, 379, 122361. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, M. Microwave-assisted persulfate/peroxymonosulfate process for environmental remediation. Curr. Opin. Chem. Eng. 2022, 36, 100826. [Google Scholar] [CrossRef]
- Patton, S.D.; Dodd, M.C.; Liu, H. Degradation of 1,4-dioxane by reactive species generated during breakpoint chlorination: Proposed mechanisms and implications for water treatment and reuse. J. Hazard. Mater. Lett. 2022, 3, 100054. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Bai, J.; Li, L.; Chen, S.; Zhou, T.; Wang, J.; Xia, L.; Xu, Q.; Zhou, B. Extremely Efficient Decomposition of Ammonia N to N2 Using ClO(*) from Reactions of HO(*) and HOCl Generated in Situ on a Novel Bifacial Photoelectroanode. Environ. Sci. Technol. 2019, 53, 6945–6953. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Zeng, H.; Zhao, X.; Zhao, F.; Park, Y.; Repo, E.; Thangaraj, S.K.; Janis, J.; Sillanpaa, M. Oxidation of 2,4-dichlorophenol in saline water by unactivated peroxymonosulfate: Mechanism, kinetics and implication for in situ chemical oxidation. Sci. Total Environ. 2020, 728, 138826. [Google Scholar] [CrossRef]
- Lente, G.b.; Kalma’r, J.z.; Baranyai, Z.; Kun, A.z.; Ildiko’Ke´k; Bajusz, D.v.; Taka´cs, M.; Veres, L.; Fa´bia´n, I.n. One- Versus Two-Electron Oxidation with Peroxomonosulfate Ion: Reactions with Iron(II), Vanadium(IV), Halide Ions, and Photoreaction with Cerium(III). Inorg. Chem. 2009, 48, 1763–1773. [Google Scholar] [CrossRef]
- Deborde, M.; von Gunten, U. Reactions of chlorine with inorganic and organic compounds during water treatment-Kinetics and mechanisms: A critical review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Xiang, Y.; Ling, L.; Fang, J.; Shang, C.; Dionysiou, D.D. Bromate formation in bromide-containing water through the cobalt-mediated activation of peroxymonosulfate. Water Res. 2015, 83, 132–140. [Google Scholar] [CrossRef]
- Qian, Y.; Guo, X.; Zhang, Y.; Peng, Y.; Sun, P.; Huang, C.H.; Niu, J.; Zhou, X.; Crittenden, J.C. Perfluorooctanoic Acid Degradation Using UV-Persulfate Process: Modeling of the Degradation and Chlorate Formation. Environ. Sci. Technol. 2016, 50, 772–781. [Google Scholar] [CrossRef]
- Sbardella, L.; Velo-Gala, I.; Comas, J.; Rodriguez-Roda Layret, I.; Fenu, A.; Gernjak, W. The impact of wastewater matrix on the degradation of pharmaceutically active compounds by oxidation processes including ultraviolet radiation and sulfate radicals. J. Hazard. Mater. 2019, 380, 120869. [Google Scholar] [CrossRef]
- Jackson, A.; Gaffney, J.W.; Boult, S. Subsurface Interactions of Fe(II) with Humic Acid or Landfill Leachate Do Not Control Subsequent Iron(III) (Hydr)oxide Production at the Surface. Environ. Sci. Technol. 2012, 46, 7543–7550. [Google Scholar] [CrossRef]
- Feng, K.; Mu, S.; Bai, J.; Li, Q. Microwave-enhanced iron–carbon-activated hydrogen peroxide process for the advanced treatment of semi-aerobic aged refuse biofilter effluent. Environ. Sci. Water Res. Technol. 2021, 7, 2321–2334. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, W.; Li, Q.; Wang, Y.; Wu, C.; Zhang, A. Degradation of recalcitrant organics in landfill concentrated leachate by a microwave-activated peroxydisulfate process. RSC Adv. 2018, 8, 32461–32469. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Q.; Luo, C.; Yao, J.; Qiu, Z.; Li, Q. Hydroxyl radical-based and sulfate radical-based photocatalytic advanced oxidation processes for treatment of refractory organic matter in semi-aerobic aged refuse biofilter effluent arising from treating landfill leachate. Chemosphere 2020, 243, 125390. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, A.; Gu, Z.; Li, Q. Enhanced degradation of refractory organics in concentrated landfill leachate by Fe0/H2O2 coupled with microwave irradiation. Chem. Eng. J. 2018, 354, 680–691. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, K.; Li, Q. Chloride-Enhanced Removal of Ammonia Nitrogen and Organic Matter from Landfill Leachate by a Microwave/Peroxymonosulfate System. Catalysts 2022, 12, 1078. https://doi.org/10.3390/catal12101078
Feng K, Li Q. Chloride-Enhanced Removal of Ammonia Nitrogen and Organic Matter from Landfill Leachate by a Microwave/Peroxymonosulfate System. Catalysts. 2022; 12(10):1078. https://doi.org/10.3390/catal12101078
Chicago/Turabian StyleFeng, Ke, and Qibin Li. 2022. "Chloride-Enhanced Removal of Ammonia Nitrogen and Organic Matter from Landfill Leachate by a Microwave/Peroxymonosulfate System" Catalysts 12, no. 10: 1078. https://doi.org/10.3390/catal12101078
APA StyleFeng, K., & Li, Q. (2022). Chloride-Enhanced Removal of Ammonia Nitrogen and Organic Matter from Landfill Leachate by a Microwave/Peroxymonosulfate System. Catalysts, 12(10), 1078. https://doi.org/10.3390/catal12101078






