Towards High CO2 Conversions Using Cu/Zn Catalysts Supported on Aluminum Fumarate Metal-Organic Framework for Methanol Synthesis
Abstract
:1. Introduction
2. Results
2.1. Characterization
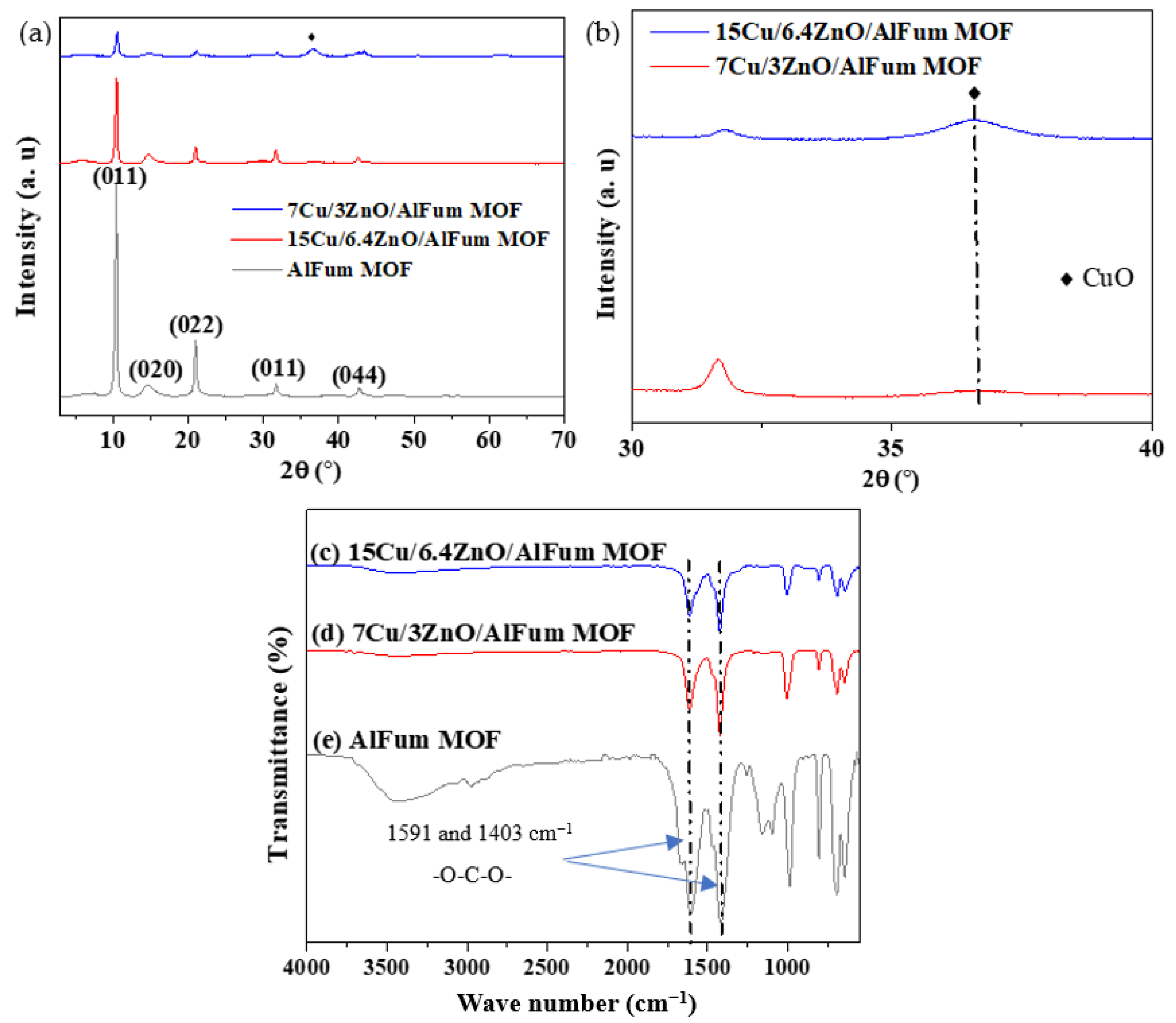
2.1.1. Powder X-ray Diffraction (PXRD) and Fourier Transform Infrared Spectroscopy (FTIR)
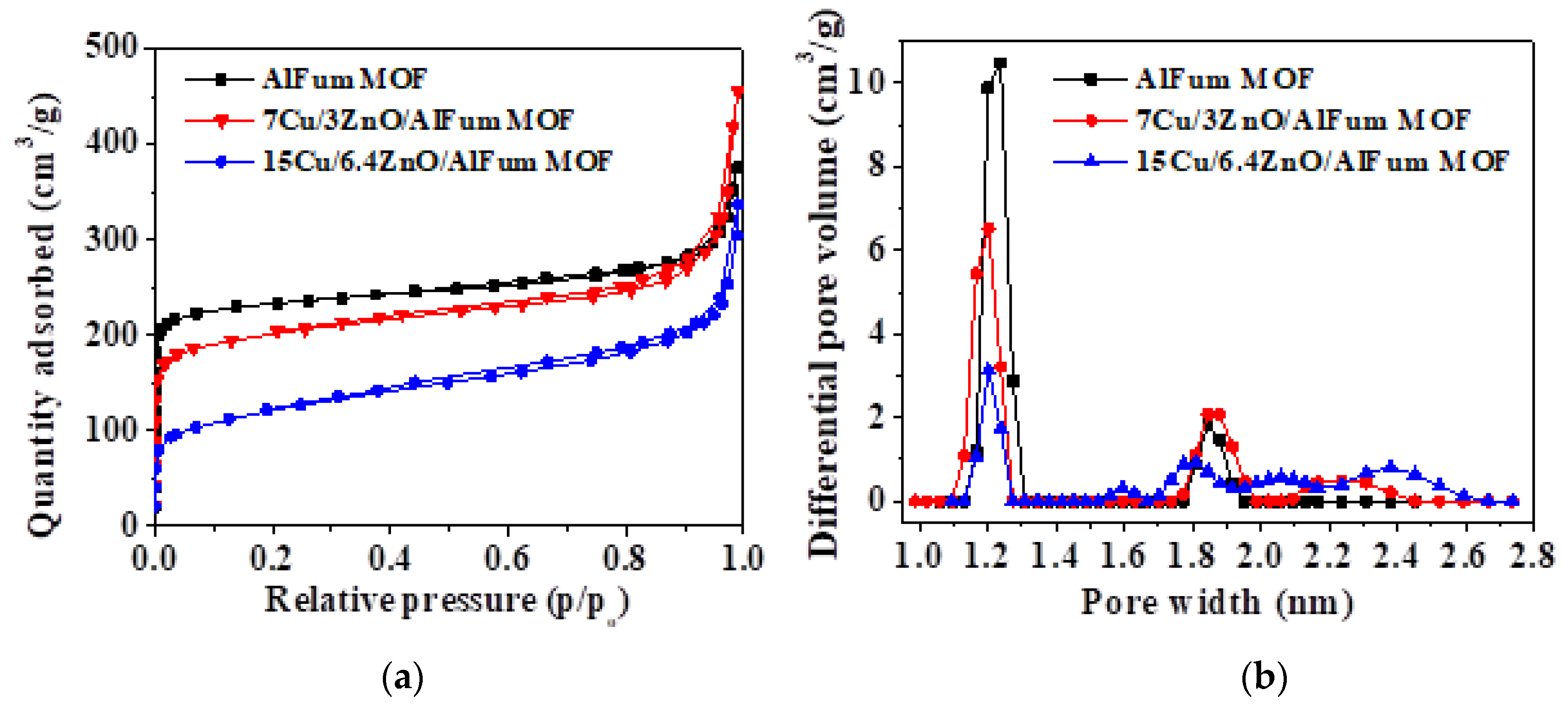
2.1.2. N2 Sorption, and Elemental Loading
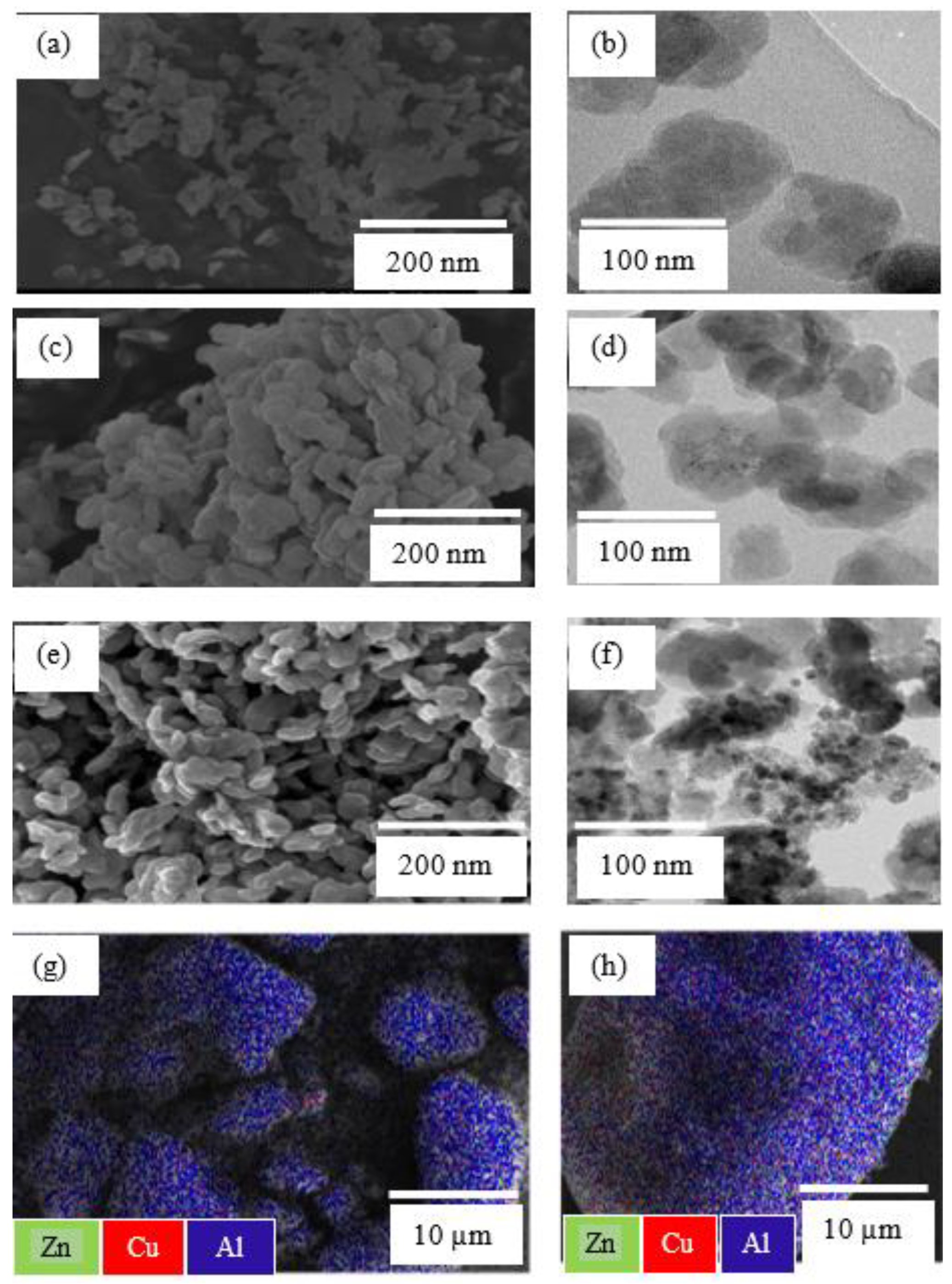
2.1.3. Electron Microscopies
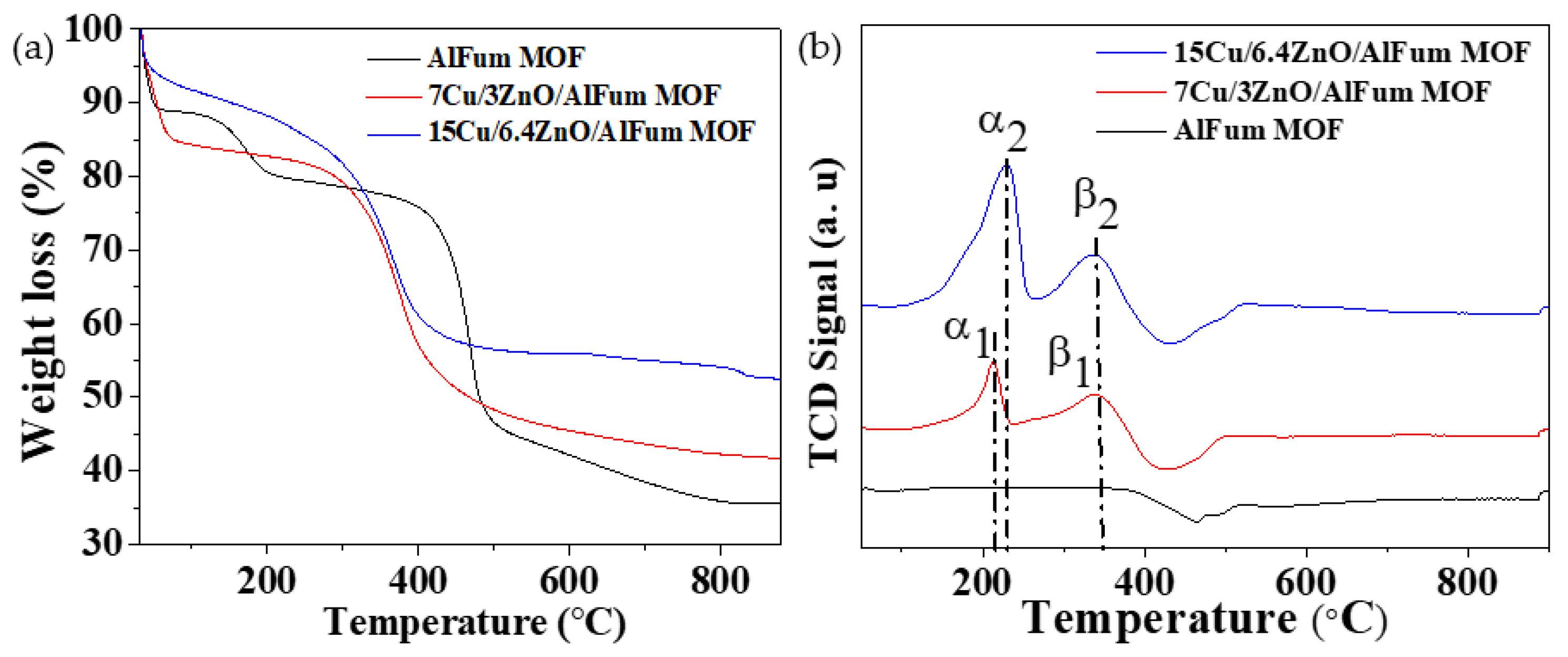
2.1.4. Thermogravimetric Analysis (TGA) and Hydrogen-Temperature Programmed Reduction (H2-TPR)
2.1.5. X-ray Photoelectron Spectroscopy (XPS)
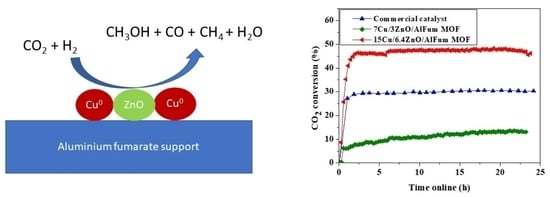
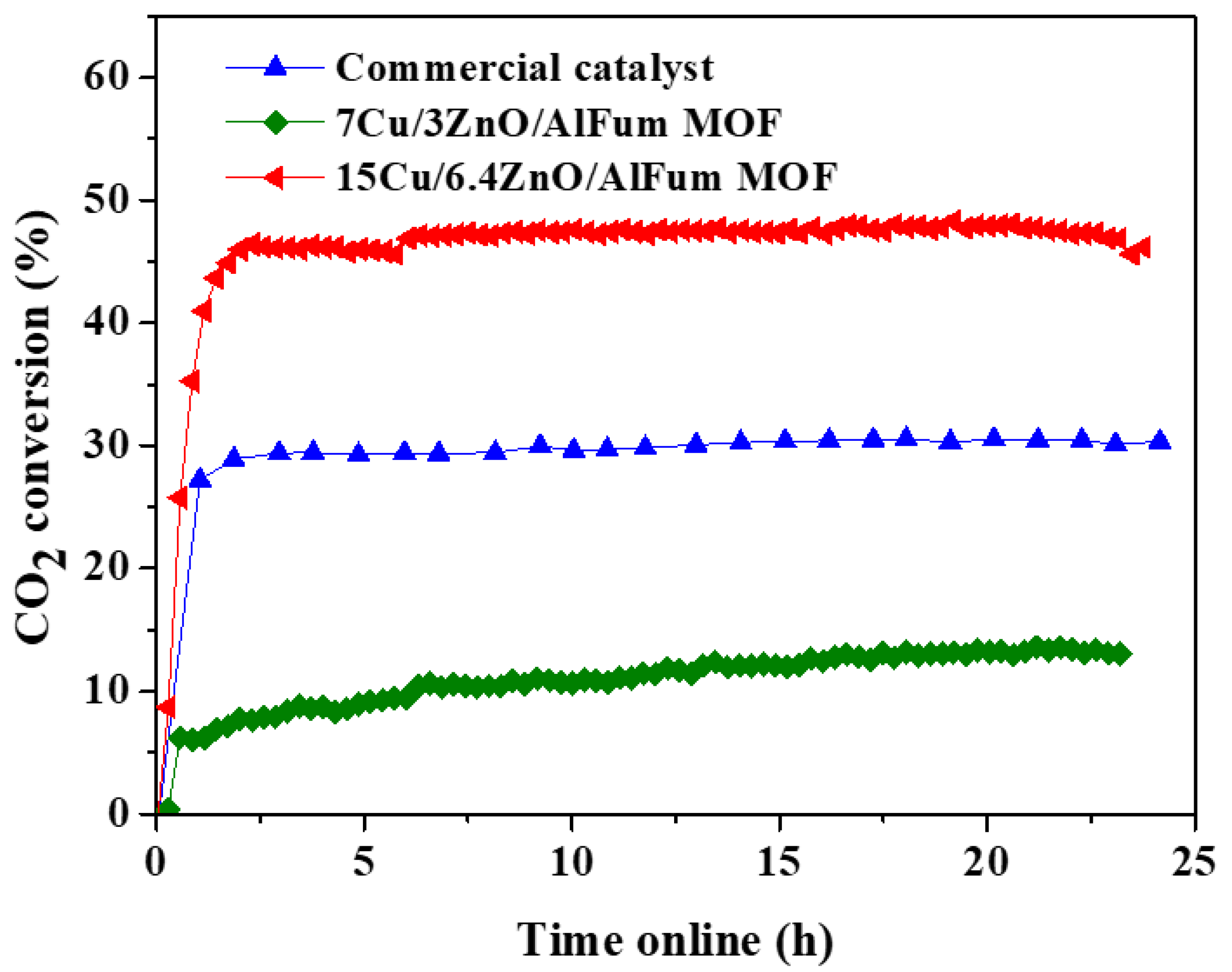
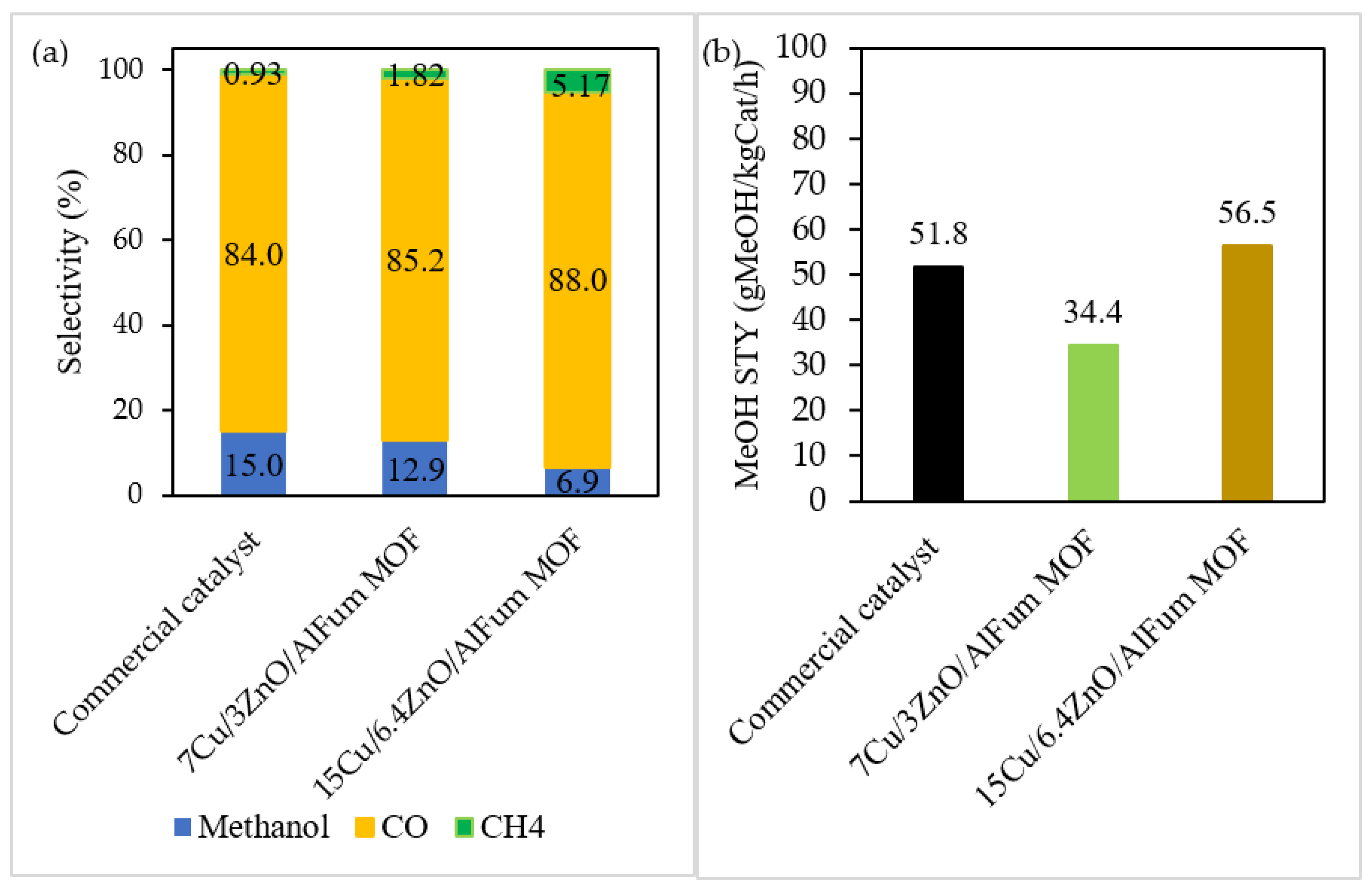
2.2. Catalysts’ Testing and Evaluation
Conversions, Selectivity, and Productivity
3. Methods and Materials
3.1. Reagents
3.2. Synthesis of AlFum MOF
3.3. Synthesis of AlFum MOF-Supported Catalysts
3.4. Characterization
3.5. Catalyst Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef] [PubMed]
- Tursunov, O.; Kustov, L.; Kustov, A. A Brief Review of Carbon Dioxide Hydrogenation to Methanol Over Copper and Iron Based Catalysts. Oil Gas Sci. Technol.-Rev. IFP Energ. Nouv. 2017, 72, 30. [Google Scholar] [CrossRef]
- Fischer, H.; Wahlen, M.; Smith, J.; Mastroianni, D.; Deck, B. Ice core records of atmospheric CO2 around the last three glacial terminations. Science 1999, 283, 1712–1714. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Xiaoding, X.; Moulijn, J.A. Mitigation of CO2 by Chemical Conversion: Plausible Chemical Reactions and Promising Products. Energy Fuels 1996, 10, 305–325. [Google Scholar] [CrossRef]
- Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105. [Google Scholar] [CrossRef]
- Ahouari, H.; Soualah, A.; Le Valant, A.; Pinard, L.; Magnoux, P.; Pouilloux, Y. Methanol synthesis from CO2 hydrogenation over copper based catalysts. React. Kinet. Mech. Catal. 2013, 110, 131–145. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Lekse, J.W.; Baltrus, J.P.; Ohodnicki, P.R.; Howard, B.H.; Deng, X.; Matranga, C. Active Sites and Structure–Activity Relationships of Copper-Based Catalysts for Carbon Dioxide Hydrogenation to Methanol. ACS Catal. 2012, 2, 1667–1676. [Google Scholar] [CrossRef]
- Agarwal, A.S.; Rode, E.; Sridhar, N.; Hill, D. Conversion of CO2 to Value-Added Chemicals: Opportunities and Challenges. In Handbook of Climate Change Mitigation and Adaptation; Chen, W.-Y., Suzuki, T., Lackner, M., Eds.; Springer: New York, NY, NY, USA, 2014; pp. 1–40. [Google Scholar] [CrossRef]
- Etim, U.J.; Song, Y.; Zhong, Z. Improving the Cu/ZnO-Based Catalysts for Carbon Dioxide Hydrogenation to Methanol, and the Use of Methanol As a Renewable Energy Storage Media. Front. Earth Sci. 2020, 8, 545431. [Google Scholar] [CrossRef]
- Qaderi, J. A brief review on the reaction mechanisms of CO2 hydrogenation into methanol. Int. J. Innov. Res. Sci. Stud. 2020, 3, 53–63. [Google Scholar] [CrossRef]
- An, X.; Li, J.; Zuo, Y.; Zhang, Q.; Wang, D.; Wang, J. A Cu/Zn/Al/Zr Fibrous Catalyst that is an Improved CO2 Hydrogenation to Methanol Catalyst. Catal. Lett. 2007, 118, 264–269. [Google Scholar] [CrossRef]
- Spencer, M.S. The role of zinc oxide in Cu/ZnO catalysts for methanol synthesis and the water-gas shift reaction. Top. Catal. 1999, 8, 259. [Google Scholar] [CrossRef]
- Dalebout, R.; Visser, N.L.; Pompe, C.E.L.; de Jong, K.P.; de Jongh, P.E. Interplay between carbon dioxide enrichment and zinc oxide promotion of copper catalysts in methanol synthesis. J. Catal. 2020, 392, 150–158. [Google Scholar] [CrossRef]
- Fisher, I.A.; Bell, A.T. In Situ Infrared Study of Methanol Synthesis from H2/CO over Cu/SiO2 and Cu/ZrO2/SiO2. J. Catal. 1998, 178, 153–173. [Google Scholar] [CrossRef]
- Stawowy, M.; Ciesielski, R.; Maniecki, T.; Matus, K.; Łużny, R.; Trawczynski, J.; Silvestre-Albero, J.; Łamacz, A. CO2 Hydrogenation to Methanol over Ce and Zr Containing UiO-66 and Cu/UiO-66. Catalysts 2020, 10, 39. [Google Scholar] [CrossRef]
- Rungtaweevoranit, B.; Baek, J.; Araujo, J.R.; Archanjo, B.S.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Copper Nanocrystals Encapsulated in Zr-based Metal-Organic Frameworks for Highly Selective CO(2) Hydrogenation to Methanol. Nano Lett. 2016, 16, 7645–7649. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Liu, Y.; Zhu, G.; Hungerford, J.T.; Bhattacharyya, S.; Lively, R.P.; Sholl, D.S.; Walton, K.S. Heat-Treatment of Defective UiO-66 from Modulated Synthesis: Adsorption and Stability Studies. J. Phys. Chem. C 2017, 121, 23471–23479. [Google Scholar] [CrossRef]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous catalysis in zeolites, mesoporous silica, and metal-organic frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef] [PubMed]
- Rambau, K.M.; Musyoka, N.M.; Panek, R.; Franus, W.; Wdowin, M.; Manyala, N. Preparation of coal fly ash derived metal organic frameworks and their carbon derivatives. Mater. Today Commun. 2021, 27, 102433. [Google Scholar] [CrossRef]
- Gesmanee, S.; Koo-Amornpattana, W. Catalytic hydrogenation of CO2 for methanol production in fixed-bed reactor using Cu-Zn supported on gamma-Al2O3. Energy Procedia 2017, 138, 739–744. [Google Scholar] [CrossRef]
- Karmakar, S.; Dechnik, J.; Janiak, C.; De, S. Aluminium fumarate metal-organic framework: A super adsorbent for fluoride from water. J. Hazard Mater. 2016, 303, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, Q.; Liu, G.; Battaglia, V.S.; Zheng, H. Aluminum fumarate-based metal organic frameworks with tremella-like structure as ultrafast and stable anode for lithium-ion batteries. Nano Energy 2017, 39, 200–210. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, S.; Xiong, W.; Liu, D.; Li, M.; He, B.; Fan, X.; Luo, D. Supported CuO catalysts on metal-organic framework (Cu-UiO-66) for efficient catalytic wet peroxide oxidation of 4-chlorophenol in wastewater. Microporous Mesoporous Mater. 2020, 291, 109703. [Google Scholar] [CrossRef]
- Teo, H.W.B.; Chakraborty, A.; Kitagawa, Y.; Kayal, S. Experimental study of isotherms and kinetics for adsorption of water on Aluminium Fumarate. Int. J. Heat Mass Transf. 2017, 114, 621–627. [Google Scholar] [CrossRef]
- Kobayashi, H.; Taylor, J.M.; Mitsuka, Y.; Ogiwara, N.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kitagawa, H. Charge transfer dependence on CO2 hydrogenation activity to methanol in Cu nanoparticles covered with metal–organic framework systems. Chem. Sci. 2019, 10, 3289–3294. [Google Scholar] [CrossRef]
- Smyrnioti, M.; Ioannides, T. Dimethyl Ether Hydrolysis over WO3/γ-Al2O3 Supported Catalysts. Catalysts 2022, 12, 396. [Google Scholar] [CrossRef]
- Felgueiras, M.B.S.; Restivo, J.; Sousa, J.P.S.; Pereira, M.F.R.; Soares, O.S.G.P. Copper Supported on Mesoporous Structured Catalysts for NO Reduction. Catalysts 2022, 12, 170. [Google Scholar] [CrossRef]
- Alvarez, E.; Guillou, N.; Martineau, C.; Bueken, B.; Van de Voorde, B.; Le Guillouzer, C.; Fabry, P.; Nouar, F.; Taulelle, F.; De Vos, D. The structure of the aluminum fumarate metal-organic framework A520. Angew. Chem. 2015, 127, 3735–3739. [Google Scholar] [CrossRef]
- Kayal, S.; Chakraborty, A.; Teo, H.W.B. Green synthesis and characterization of aluminium fumarate metal-organic framework for heat transformation applications. Mater. Lett. 2018, 221, 165–167. [Google Scholar] [CrossRef]
- Jeremias, F.; Fröhlich, D.; Janiak, C.; Henninger, S.K. Advancement of sorption-based heat transformation by a metal coating of highly-stable, hydrophilic aluminium fumarate MOF. RSC Adv. 2014, 4, 24073–24082. [Google Scholar] [CrossRef]
- Coelho, J.A.; Ribeiro, A.M.; Ferreira, A.F.P.; Lucena, S.M.P.; Rodrigues, A.E.; Azevedo, D.C.S.d. Stability of an Al-Fumarate MOF and Its Potential for CO2 Capture from Wet Stream. Ind. Eng. Chem. Res. 2016, 55, 2134–2143. [Google Scholar] [CrossRef]
- Duma, Z.G.; Dyosiba, X.; Moma, J.; Langmi, H.W.; Louis, B.; Parkhomenko, K.; Musyoka, N.M. Thermocatalytic Hydrogenation of CO2 to Methanol Using Cu-ZnO Bimetallic Catalysts Supported on Metal–Organic Frameworks. Catalysts 2022, 12, 401. [Google Scholar] [CrossRef]
- Kamyar, N.; Khani, Y.; Amini, M.M.; Bahadoran, F.; Safari, N. Copper-based catalysts over A520-MOF derived aluminum spinels for hydrogen production by methanol steam reforming: The role of spinal support on the performance. Int. J. Hydrog Energy 2020, 45, 21341–21353. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T. Effect of synthesis parameters on catalytic properties of CuO-CeO2. Appl. Catal. B Environ. 2006, 67, 1–11. [Google Scholar] [CrossRef]
- Djinovic, P.; Batista, J.; Levec, J.; Pintar, A. Influence of morphological, redox and surface acidity properties on WGS activity of CuO–CeO2 catalysts. J. Chem. Eng. Jpn. 2009, 42, s3–s9. [Google Scholar] [CrossRef]
- Peng, B.; Feng, C.; Liu, S.; Zhang, R. Synthesis of CuO catalyst derived from HKUST-1 temple for the low-temperature NH3-SCR process. Catal. Today 2018, 314, 122–128. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, L.; Yang, R.T. Activity, propene poisoning resistance and hydrothermal stability of copper exchanged chabazite-like zeolite catalysts for SCR of NO with ammonia in comparison to Cu/ZSM-5. Appl. Catal. A Gen. 2012, 427–428, 24–34. [Google Scholar] [CrossRef]
- An, B.; Zhang, J.; Cheng, K.; Ji, P.; Wang, C.; Lin, W. Confinement of Ultrasmall Cu/ZnOx Nanoparticles in Metal-Organic Frameworks for Selective Methanol Synthesis from Catalytic Hydrogenation of CO2. J. Am. Chem. Soc. 2017, 139, 3834–3840. [Google Scholar] [CrossRef]
- Farrusseng, D.; Daniel, C.; Hamill, C.; Casaban, J.; Didriksen, T.; Blom, R.; Velte, A.; Fueldner, G.; Gantenbein, P.; Persdorf, P.; et al. Adsorber heat exchanger using Al-fumarate beads for heat-pump applications—A transport study. Faraday Discuss. 2021, 225, 384–402. [Google Scholar] [CrossRef]
- Shi, Z.; Tan, Q.; Tian, C.; Pan, Y.; Sun, X.; Zhang, J.; Wu, D. CO2 hydrogenation to methanol over Cu-In intermetallic catalysts: Effect of reduction temperature. J. Catal. 2019, 379, 78–89. [Google Scholar] [CrossRef]
- García, A.C.; Moral-Vico, J.; Abo Markeb, A.; Sánchez, A. Conversion of Carbon Dioxide into Methanol Using Cu–Zn Nanostructured Materials as Catalysts. Nanomaterials 2022, 12, 999. [Google Scholar] [CrossRef] [PubMed]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Angelo, L.; Kobl, K.; Tejada, L.M.M.; Zimmermann, Y.; Parkhomenko, K.; Roger, A.-C. Study of CuZnMOx oxides (M=Al, Zr, Ce, CeZr) for the catalytic hydrogenation of CO2 into methanol. Comptes Rendus Chim. 2015, 18, 250–260. [Google Scholar] [CrossRef]
- Joo, O.-S.; Jung, K.-D.; Moon, I.; Rozovskii, A.Y.; Lin, G.I.; Han, S.-H.; Uhm, S.-J. Carbon Dioxide Hydrogenation To Form Methanol via a Reverse-Water-Gas-Shift Reaction (the CAMERE Process). Ind. Eng. Chem. Res. 1999, 38, 1808–1812. [Google Scholar] [CrossRef]
- Bonura, G.; Cordaro, M.; Cannilla, C.; Arena, F.; Frusteri, F. The changing nature of the active site of Cu-Zn-Zr catalysts for the CO2 hydrogenation reaction to methanol. Appl. Catal. B Environ. 2014, 152–153, 152–161. [Google Scholar] [CrossRef]
- Gaab, M.; Trukhan, N.; Maurer, S.; Gummaraju, R.; Müller, U. The progression of Al-based metal-organic frameworks—From academic research to industrial production and applications. Microporous Mesoporous Mater. 2012, 157, 131–136. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.Y.; Tsung, C.-K.; Yamada, Y.; Yang, P.; Somorjai, G.A. Thermally stable Pt/mesoporous silica core–shell nanocatalysts for high-temperature reactions. Nat. Mater. 2009, 8, 126–131. [Google Scholar] [CrossRef]

| Sample | SBET (m2/g) | Pore Volume (cm3/g) | d (nm) | Elemental Loading (Weight %) | |
|---|---|---|---|---|---|
| Cu | Zn | ||||
| AlFum MOF | 910 | 0.344 | 1.27 | - | - |
| 7Cu/3ZnO/AlFum MOF | 757 | 0.300 | 1.23 | 11.2 (7) | 3.12 (3) |
| 15.4Cu/6.4ZnO/AlFum MOF | 416 | 0.148 | 1.30 | 18.2 (15) | 5.77 (6.4) |
| Product Distribution (mol%) | ||||
|---|---|---|---|---|
| Catalyst | MeOH | CO | CH4 | H2O |
| Commercial | 12.8 | 36.0 | 2.50 | 48.7 |
| 7Cu/3ZnO/AlFum MOF | 6.50 | 42.8 | 0.9 | 49.8 |
| 15Cu/6.4ZnO/AlFum MOF | 3.48 | 44.6 | 2.62 | 49.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duma, Z.G.; Moma, J.; Langmi, H.W.; Louis, B.; Parkhomenko, K.; Musyoka, N.M. Towards High CO2 Conversions Using Cu/Zn Catalysts Supported on Aluminum Fumarate Metal-Organic Framework for Methanol Synthesis. Catalysts 2022, 12, 1104. https://doi.org/10.3390/catal12101104
Duma ZG, Moma J, Langmi HW, Louis B, Parkhomenko K, Musyoka NM. Towards High CO2 Conversions Using Cu/Zn Catalysts Supported on Aluminum Fumarate Metal-Organic Framework for Methanol Synthesis. Catalysts. 2022; 12(10):1104. https://doi.org/10.3390/catal12101104
Chicago/Turabian StyleDuma, Zama G., John Moma, Henrietta W. Langmi, Benoit Louis, Ksenia Parkhomenko, and Nicholas M. Musyoka. 2022. "Towards High CO2 Conversions Using Cu/Zn Catalysts Supported on Aluminum Fumarate Metal-Organic Framework for Methanol Synthesis" Catalysts 12, no. 10: 1104. https://doi.org/10.3390/catal12101104
APA StyleDuma, Z. G., Moma, J., Langmi, H. W., Louis, B., Parkhomenko, K., & Musyoka, N. M. (2022). Towards High CO2 Conversions Using Cu/Zn Catalysts Supported on Aluminum Fumarate Metal-Organic Framework for Methanol Synthesis. Catalysts, 12(10), 1104. https://doi.org/10.3390/catal12101104